Abstract
Background
Non-invasive measurements of endothelial function predict future adverse cardiovascular events, but offer limited opportunities for mechanistic insights into phenotypic observations. Subcutaneous adipose arterioles, accessible through minimally invasive methods, provide an opportunity for complimentary mechanistic studies. Limited data relating subcutaneous arteriolar endothelial function, cardiovascular risk factors, and non-invasive measurements of endothelial function currently exist.
Methods
44 subjects underwent non-invasive studies of endothelial function [brachial reactivity (FMD) and digital pulse arterial tonometry (PAT)] and measurements of endothelial dependent vasodilation of gluteal subcutaneous arterioles to acetylcholine. Arteriolar endothelial function was measured 1) percent vasodilation to maximal acetylcholine dose (10−5 M) and 2) total area under the curve (AUC) for the entire acetylcholine dose-response curve (Total AUC- Ach, doses 10−10–10−5 M).
Results
Acetylcholine responses were almost completely nitric oxide (NO) dependent. Total AUC-Ach predicted FMD and PAT, but maximal acetylcholine vasodilation was not associated with these measures. A history of hypertension, diabetes, smoking, and LDL cholesterol levels were independent predictors of Total AUC-Ach. In regression models, Total AUC-Ach independently predicted FMD.
Conclusions
Acetylcholine vasodilator responses in human gluteal subcutaneous arterioles are nitric oxide synthase dependent, and correlate with cardiac risk factors and in vivo measures of endothelial function. These data suggest subcutaneous arterioles offer an opportunity for translational studies of mechanisms of modulating NO bioavailability relevant to in vivo endothelial function measures.
Keywords: Arterioles, Endothelial Function, Nitric Oxide, Flow Mediated Dilation, Cardiovascular Risk
Introduction
Endothelial dysfunction involves the systemic expression of an abnormal pro-thrombotic, pro-vasoconstrictive, and pro-inflammatory vascular phenotype that predicts increased cardiovascular risk.1, 2 Early studies demonstrating the existence of endothelial dysfunction and its relationship to cardiovascular risk factors and atherosclerosis were performed directly in the coronary circulation.3–5 However, given the risk and expense of these studies and the systemic nature of endothelial dysfunction, non-invasive methods for measurement endothelial function emerged, including flow mediated dilation of the brachial artery (FMD) and digital pulse arterial tonometry (PAT).6–8 Important correlations of FMD and PAT with cardiovascular risk factors, coronary endothelial dysfunction, and future cardiovascular risk have buoyed the use of these methodologies in cardiovascular research.1, 9, 10
While convenient, non-invasive methods such as FMD and PAT employed to follow the effects of disease states and interventions do not allow for significant mechanistic insights into how diseases and interventions alter endothelial function. Circulating markers provide some insights but these surrogates do not necessarily reflect activity at the tissue level. Subcutaneous adipose arterioles are easily accessible through minimally invasive means.11 Recent data suggest, similarly to FMD and PAT,12, 13 acetylcholine induced vasodilation of subcutaneous arterioles may depend on niric oxide (NO) production from endothelium-derived NO synthase.14 However, data regarding the relative contributions of traditional cardiovascular risk factors to endothelium-dependent vasodilation in subcutaneous arterioles is lacking. Further, data designed to determine associations between endothelial function in human subcutaneous arterioles and non-invasive measures of endothelial function are limited.15 Data linking endothelial function in these two vascular beds would suggest studies of subcutaneous arterioles in conjunction with FMD and PAT measurements could provide mechanistic insights into phenotypical alterations in FMD and PAT. We hypothesized that acetylcholine induced endothelium-dependent vasodilation of gluteal subcutaneous arterioles would correlate with traditional cardiovascular risk factors and both FMD and PAT in humans with a range of cardiovascular risk profiles.
Methods
Study population
Subjects undergoing PAT and/or FMD with concomitant subcutaneous arteriolar vasodilation data from cross-sectional studies of endothelial function performed at the Medical College of Wisconsin from 2007–2011 were included in the study population (data not published to date). Criteria for qualifying as having hypertension, diabetes, and/or hypercholesterolemia are described in the Online Supplement. Subjects with a history of coronary artery disease, peripheral vascular disease, cerebrovascular disease, or chronic renal or liver disease were excluded. All study protocols were approved by Institutional Review Board of the Medical College of Wisconsin and informed consent was obtained from all participants prior to any study procedures.
Study protocol
All studies were performed in the Adult Translation Research Unit at the Medical College of Wisconsin from 7–10 AM. All subjects fasted for at least 6 hours prior to their study procedures. Current smokers refrained from smoking for 24 hours prior to their study visits. A medical history as well as subject height, weight, and waist circumference were measured and recorded. Blood pressure and heart rate measurements were made in triplicate and averaged for a final result. Prior to in vivo endothelial function tests, subjects laid in a supine position in a quiet, dimly lit, temperature controlled (22–24°C) room for 20 minutes.
Measurements of In Vivo Endothelial Function
Brachial artery images were captured prior to and following blood pressure cuff inflation and analyzed as previously described to determine the extent of resting and post-hyperemic shear and flow-mediated dilation (FMD) in the brachial artery.16 FMD was recorded both as the absolute change in brachial diameter (FMDmm) and the percent change in diameter (FMD%). In a subset of 40 subjects, measurement of endothelium-independent vasodilation (NMD%) was performed following administration of 0.4 mg of sublingual nitroglycerin as previously described.16
Digital PAT measurements were performed concomitantly with FMD measurements using EndoPAT 2000 (Itamar medical Ltd, Caesarea, Israel). EndoPAT results were recorded and are reported the methodology suggested by the Framingham study as most strongly correlated with cardiovascular risk factors.17 Greater methodological details are found in the Online Supplement.
Gluteal Adipose Biopsy and Measurement of Arteriolar Endothelial Function
Subcutaneous arterioles were obtained by gluteal adipose biopsy under local anesthesia (1% lidocaine) using sterile technique. A small (~ 1–1.5 cm) horizontal incision was made in the upper external gluteal quadrant and gluteal subcutaneous adipose tissue was taken from the point located at margin superior to gluteus maximus muscle approximately 3–5 cm cephalad of the greater trochanter. Adipose tissue (approximately 1.5 × 1.0 × 1.0 cm3 in size) was removed by sharp dissection. The incision was closed with an absorbable suture and Steristrips. The fat sample was transferred immediately into cold HEPES buffer (4°C) and taken for immediate analysis. Endothelium dependent vasodilation of adipose arterioles dissected from these samples under light microscopy was measured by video microscopy previously described.18 Greater methodological detail can be found in the Online Supplement. Our overall success rate in obtaining arterioles suitable and viable for study is ~75%.
Vasodilation was recorded as a percentage change from baseline diameter measured following endothelin-1 pre-constriction (at least 50% constriction with endothelin-1 was used as a marker of vessel viability). We plotted the percent vasodilation at each dose of acetylcholine from 10−10 to 10−5 M and calculated the area under the entire dose-response curve (Total AUC-Ach) for each arteriole. Endothelium independent dilation determined at the end of each experiment with papaverine (0.2 mM). Following washout, re-equilibration and repeat pre-constriction, a subset of 16 vessels were incubated with 100μM L-NG-Nitroarginine methyl ester (L-NAME, nitric oxide synthase inhibitor) and exposed to increasing doses of Ach from 10−10 to 10−5 M to determine the contribution of nitric oxide synthase to vasodilation of these arterioles.
Statistical analysis
The statistical analysis was done using SPSS 19.0 (SPSS Inc, Chicago, IL) and SigmaStat 12.0. Full details on the statistical analyses can be found in the Online Supplement. P values of <0.05 were considered statistically significant.
Results
Participants
From our studies, 47 subjects had adipose tissue arterioles available for vasoreactivity experiments. 3 subjects from this group were excluded as they did not have FMD or PAT data for analysis. 34 patients had both FMD measurements as well as arteriolar vasoreactivity data, while PAT measures were obtained in 34 subjects in this group. The baseline characteristics of the all 44 subjects are listed in Table 1. In the healthy subject group, there was 1 subject who reported diet-controlled high cholesterol and 2 current smokers. Systolic blood pressure and heart rate were significantly lower in the healthy group, and there was a strong trend toward a lower BMI in healthy group. There were no significant differences in these demographic variables between individuals who underwent both FMD and PAT versus those who underwent FMD alone (data not shown). A total of 8 subjects (18%) were on concomitant HMG CoA reductase therapy, all in the group of subjects with type 2 diabetes and/or hypertension. HMG CoA reductase therapy was the only form of lipid lowering therapy in this study population.
Table 1.
Baseline Subject Characteristics
| Metric | All Subjects (n=44) | Healthy (n=23) | Prevalent DM and/or HTN (n=21) | P-Value |
|---|---|---|---|---|
| Age | 48±8 | 47±7 | 49±9 | 0.45 |
| Sex (% Women) | 55 | 57 | 52 | 0.78 |
| History of HTN (%) | 27 | 0 | 57 | <0.001 |
| History of DM (%) | 30 | 0 | 62 | <0.001 |
| History of hyperlipidemia (%) | 32 | 4 | 62 | <0.001 |
| Current smokers (#) | 6 | 2 | 4 | 0.45 |
| Body mass index (kg/m2) | 31.0±6.8 | 29.2±5.5 | 33.1±7.6 | 0.05 |
| LDL cholesterol (mg/dL) | 99±26 | 103±23 | 94±29 | 0.23 |
| HDL cholesterol mg/dL) | 52±17 | 52±13 | 52±21 | 0.97 |
| Total Cholesterol (mg/dL) | 171±31 | 172±29 | 170±34 | 0.82 |
| Total:HDL Cholesterol Ratio | 3.5±1.0 | 3.5±1.0 | 3.5±1.0 | 0.91 |
| Triglycerides (mg/dL) | 102±8 | 84±7 | 121±13 | 0.17 |
| Systolic Blood Pressure (mmHg) | 128±20 | 122±18 | 134±21 | 0.04 |
| Diastolic Blood pressure (mmHg) | 75±13 | 73±12 | 77±15 | 0.33 |
| Heart Rate (beats/min) | 64±9 | 61±7 | 68±10 | 0.02 |
Data reported as mean ± S.D. P-values represent comparisons between healthy subjects and subjects with hypertension and/or diabetes.
Measurements of In Vivo Endothelial Function and Endothelial Function in 1st Order Arterioles and Mechanism of Acetylcholine Induced Vasodilation in Subcutaneous Arterioles
Our findings are summarized in Table 2. As expected, both in vivo (FMD%, FMDmm) and in vitro endothelial function were impaired in patients with diabetes and/or hypertension compared to healthy subjects. We observed a trend toward decreased PAT in patients with diabetes and/or hypertension which did not reach statistical significance in this study. Neither FMD% nor FMDmm correlated with PAT (r=0.006 and 0.028, p=0.97 and 0.88 for FMD% and FMDmm, respectively). There were no differences between groups with respect to the concentration of endothelin-1 required for vessel pre-constriction prior to acetylcholine studies (0.72±0.15 nM vs. 0.84±0.12 nM for healthy subjects and diabetic and/or hypertensive subjects, respectively P=0.70).
Table 2.
Measurements of Endothelial Function
| Measurement | All Subjects (n=44) | Healthy (n=28) | Prevalent DM and/or HTN (n=27) | P-Value |
|---|---|---|---|---|
| Baseline Diameter (mm) | 3.8±1 | 3.8±0.1 | 3.8±0.1 | 0.77 |
| Percent Flow Mediated Dilation (FMD%) | 5.0±0.3 | 5.7±0.4 | 4.2±0.5 | 0.01 |
| Absolute Flow Mediated Dilation (FMDmm) | 0.19±0.01 | 0.22±0.02 | 0.15±0.02 | 0.01 |
| Peak Hyperemic Shear (dynes/cm2) | 73±4 | 74±6 | 72±7 | 0.77 |
| Nitroglycerin Mediated Dilation | 21±1 | 23±1 | 20±2 | 0.10 |
| In PAT | 0.52±0.11 | 0.76±0.14 | 0.43±0.10 | 0.18 |
| Arteriolar Diameter (μM) | 109±8 | 121±14 | 113±12 | 0.12 |
| Peak Percent Ach-Induced Dilation (Ach 10−5 M) | 55±3 | 72±3 | 38±3 | <0.001 |
| Total Ach-Induced AUC (Ach 10−10–10−5 M) | 144±10 | 193±11 | 92±9 | <0.001 |
All data are reported as mean ± S.E. P-values represent comparisons between healthy subjects and subjects with hypertension and/or diabetes.
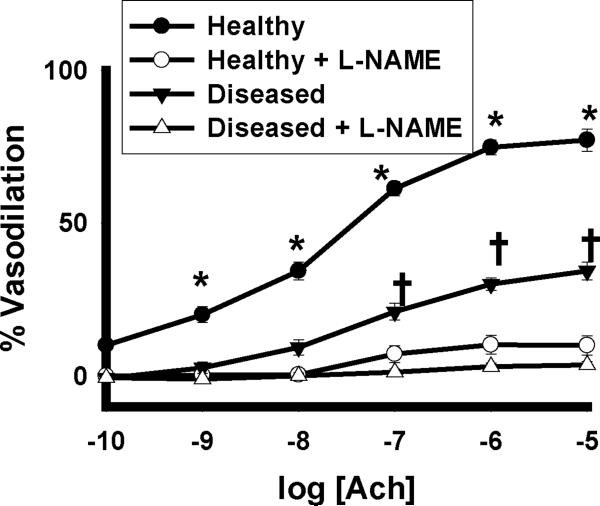
Illustration of the vasodilatory response to acetycholine in the absence and presence of 100µM L-NAME is depicted in Figure 1. L-NAME reduced the vasodilator response to acetylcholine in these vessels by approximately 95% in both subjects with and without diabetes and/or hypertension (P<0.001 overall for healthy subjects vs. subjects with diabetes and/or hypertension in the absence of L-NG-Nitroarginine methyl ester). All vessels dilated over 95% to 0.2 mM papaverine, with no significant differences between healthy and diabetes and/or hypertension study groups (data not shown).
Figure 1. NOS Dependence of Ach Vasodilation in Human Gluteal Subcutaneous Adipose Arterioles.
In a subset of 16 subjects, L-NAME virtually eliminated the vasodilatory response of human gluteal subcutaneous arterioles in subjects with and without diabetes and hypertension(P<0.001 overall by 3 way ANOVA, *-P≤0.001 for healthy subjects with and without L-NAME at the indicated doses, †- P<0.001 for subjects with diabetes and hypertension with and without L-NAME at the indicated doses). Ach- acetylcholine.
Strengths of Associations Between Measures of In Vivo Endothelial Function and Measurements of Endothelial Function in Subcutaneous Arterioles
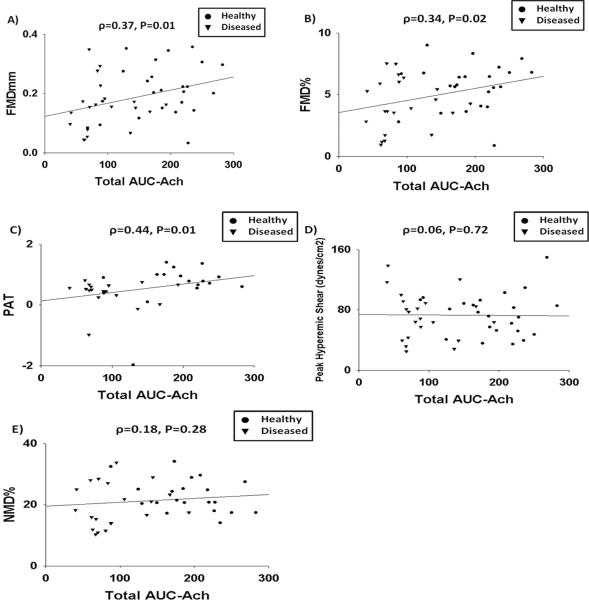
Given the >95% nitric oxide synthase dependence of arteriolar vasodilation in both subjects with and with diabetes and/or hypertension (Figure 1), all subjects were grouped together for analyses of associations to in vivo measurements of endothelial function. Total AUC-Ach was significantly associated with FMD%, FMDmm, and PAT, but not with peak hyperemic shear or NMD% (Figure 2). We found no associations between maximal acetylcholine vasodilation and FMD% (r=0.24, P=0.12), FMDmm (r=0.25, P=0.11), PAT (r=0.18, P=0.34), peak hyperemic shear (r=0.05, P=0.73), or NMD% (r=0.22, P=0.18). There were no associations between any measurement of arteriolar endothelial function and resting brachial diameter (ρ=0.04, P=0.79 and r=0.003, P=0.99 for Total AUC-Ach and maximal acetylcholine dilation, respectively) or resting shear (ρ=0.00, P=1.00 and r=0.04, P=0.81, for Total AUC-Ach and maximal Ach dilation, respectively).
Figure 2. Correlations Between Total AUC-Ach and Measurements of in vivo Measures of Endothelium-Dependent and Endothelium-Independent Vasodilation.
Total AUC-Ach correlated significantly with FMDmm (A), FMD% (B), and PAT (C) but not with peak hyperemic shear (D) or NMD% (E).
In a stepwise multivariable linear regressions including age, sex, systolic blood pressure, BMI, smoking, lipid lowering therapy, and Total AUC-Ach, only Total AUC-Ach emerged as an independent predictors of both FMDmm [Model R2=0.17, β=0.31 (P=0.006)] and FMD% [Model R2=0.15, β=0.39 (P=0.01)]. The model for PAT was not significant (Model R2=0.40, P=0.06).
Associations of Total AUC-Ach with Cardiovascular Risk Factors
Overall, we found Total AUC-Ach was inversely associated with the presence of diabetes mellitus, a history of hypertension, a history of high cholesterol, and current lipid lowering therapy (Table 3). Body mass index and heart rate trended toward an inverse correlation with Total AUC-Ach. In a stepwise multivariable model including all of these variables except lipid lowering therapy (likely a marker for a history of high cholesterol rather than a biological effect), only histories of diabetes and hypertension remained significant predictors.
Table 3.
Univariable and Multivariable Predictors of Total Acetylcholine-induced Arteriolar Vasodilation AUC (10−10 to 10−5 M)
| Univariate |
Multivariate* |
|||
|---|---|---|---|---|
| ρ | P-value | β | P-value | |
| Age | −0.07 | 0.64 | -- | -- |
| Sex (F=0, M=1) | −0.08 | 0.63 | -- | -- |
| History of Diabetes Mellitus | −0.60 | <0.001 | − 0.55 | <0.001 |
| History of Hypertension | −0.42 | 0.004 | − 0.37 | 0.003 |
| History of High Cholesterol | −0.52 | <0.001 | −0.14 | 0.73 |
| Smoking Status (1=current/past, 0=never) | 0.09 | 0.57 | -- | -- |
| Family history of CAD | −0.16 | 0.31 | -- | -- |
| Body Mass Index | −0.29 | 0.06 | −0.06 | 0.49 |
| HDL (≤40 mg/dl) | −0.06 | 0.70 | -- | -- |
| LDL (≥130 mg/dl) | 0.18 | 0.24 | -- | -- |
| Triglycerides (≥150 mg/dl) | −0.25 | 0.10 | -- | -- |
| Total Cholesterol (≥200 mg/dl) | 0.04 | 0.79 | -- | -- |
| Lipid Lowering Therapy† | −0.41 | 0.006 | -- | -- |
| Heart Rate | −0.27 | 0.09 | 0.06 | 0.42 |
Model R2=0.46.
Multivariable model includes history of diabetes mellitus, history of hypertension, history of high cholesterol, body mass index, and heart rate.
Discussion
This study reports several novel findings. First, in subjects with a wide range of cardiovascular risk, endothelium-dependent vasodilation to acetylcholine in intact gluteal subcutaneous arterioles is associated traditional cardiovascular risk factors, including hypertension, diabetes, and hypercholesterolemia. Second, in humans without established coronary artery disease, our data establish the NOS dependence of the acetylcholine induced vasodilation in human gluteal subcutaneous arterioles. Third, in subcutaneous gluteal arterioles, the ex-vivo endothelium-dependent response to acetylcholine is positively associated with common in vivo measurements endothelial function, FMD and PAT. These data suggest ex vivo measurements of subcutaneous arteriolar function generally reflect in vivo measurements of endothelial function within an individual. These findings are consistent with the systemic nature of endothelial dysfunction and suggest studies of subcutaneous arterioles could be leveraged to provide mechanistic insights into in vivo measurements of endothelial function.
Prior work has established that traditional cardiovascular risk factors impair in vivo conduit and microvascular function.1, 10 From these data, researchers have inferred impairment occurring at the arteriolar level given the systemic nature of endothelial dysfunction. However, only recently has data emerged demonstrating similar impairments in small artery endothelial function with traditional risk factors. Initial work with subcutaneous arterioles demonstrated both adverse structural remodeling and impairment of endothelium-dependent vasodilation to acetylcholine in patients with hypertension, obesity, and type 2 diabetes.14, 19, 20 Our data extend these prior findings by demonstrating measurements of subcutaneous arteriolar endothelial function using acetylcholine correlate with in vivo measurements of endothelial function in group of individuals with a range of cardiovascular risk.
While human arterioles have been used in multiple small studies evaluating structural alterations in relationship to cardiovascular risk factors,19, 21–25 only two prior studies relate in vivo measurements of endothelial function with endothelial function measured directly in arterioles.15, 26 In the 1st study, a strong correlation between vasodilation of subcutaneous arterioles to the maximal acetycholine dose (10−4 M) measured by pressurized myography and FMD% was shown in 16 patients with hypertension. The strength of this correlation was significantly attenuated by exclusion of 4 subjects with significantly impaired FMD%. In a second study of 33 subjects, 25 with established coronary artery disease, FMD% significantly correlated with peak flow-induced dilation of abdominal fat pad subcutaneous arterioles measured by pressurized myography (r=0.46, P<0.01). However, the acetylcholine vasodilatory response in vessels from patients with coronary artery disease was paradoxically significantly more robust than that in healthy controls. We found associations between FMD and PAT and acetylcholine induced vasodilation over the full range of acetylcholine doses, but no correlation between either in vivo measurement or peak response to acetylcholine. Differences between prior work and our results may relate to differences in vessel size (on average ~½ the luminal diameter compared to both prior studies), technique for measuring vasodilation (pressurized video microscopy vs. myography), the source of fat,27 or potentially a shift in the balance of paracrine factors (e.g. EDHF, prostaglandins, NO, and hydrogen peroxide) responsible for endothelium dependent vasodilation in patients with established coronary artery disease.28, 29 Our data also significantly extends the previously reported findings by showing the association between acetylcholine-induced arteriolar vasodilation and in vivo measures of endothelial function in a population comprised of ≥50% healthy subjects.
We found that increased NO production is the primary mechanism acetylcholine induced vasodilation in subcutaneous gluteal arterioles in our study population. Taken together with the established mechanistic links between PAT, FMD, and NO bioavailability,2, 12, 13 we believe the association seen between PAT, FMD, and subcutaneous arteriolar vasodilation most likely relates to similar alterations in eNOS dependent NO bioavailability. Our hypothesis is supported by prior data demonstrating reduced NO bioavailability in subcutaneous vessels of patients with hypertension or type 2 diabetes in parallel with in vivo data from separate studies showing consistent impairment in brachial FMD and PAT in the setting of either risk factor.1, 14, 20, 30 Interestingly, the relatively modest correlations we report here between FMD and acetylcholine induced vasodilation of gluteal adipose arterioles may relate to emerging data suggesting FMD in certain populations may not be as reliant on NO production as previously suspected.31, 32 Future studies comparing NO production in subcutaneous arterioles and the NO dependent portions of the FMD and PAT responses in humans are necessary.
Our data showed negative univariate correlation between Total AUC-Ach and LDL. This finding most likely relates to confounding by indication rather than a true association. Individuals on HMG CoA reductase therapy had significantly lower LDL levels than those not on these medications (82±9 mg/dL vs. 102±4 mg/dL, P=0.045) and univariate analysis revealed a known history of high cholesterol correlated negatively with Total AUC-Ach.
Our data have several limitations. First, pharmacological exposures can influence measurements acetylcholine induced vasodilation in subcutaneous arterioles.1, 11 However, we would expect these changes to occur in parallel in the arterioles and in vivo measurements making medications an unlikely cause of significant variation. Second, the correlations we found were relatively modest (ranging between 0.34 and 0.44). However, the correlation sizes are similar in magnitude to studies showing parallel impairments in coronary circulation with FMD (r=0.36) and PAT (r=0.41). These moderate size of these correlations are reasonable given the variability of in vivo endothelial function measurements.8, 9 While we did not find any correlation between either baseline and peak hyperemic shear and acetylcholine induced vasodilation, we cannot exclude an association arteriolar vasodilation and other measurements of shear such as shear AUC. Finally, the association between FMD and acetylcholine induced vasodilation of gluteal arterioles cannot be easily extrapolated to FMD of other conduit vessels.33 Balanced against these limitations is the novelty of our findings related to the association of endothelial function in subcutaneous vessels to traditional cardiovascular risk factors and associations of measurements of non-invasive in vivo endothelial function in individuals without established coronary artery disease. Further we identified the likely mechanism of endothelium dependent vasodilation in gluteal subcutaneous arterioles.
In the absence of coronary artery disease, endothelium dependent vasodilation to acetylcholine in human gluteal subcutaneous arterioles is 1) associated with common cardiovascular risk factors 2) primarily dependent of eNOS activity and 3) associated with concomitant measurements of in vivo endothelial function. These data support the concept of endothelial dysfunction as a systemic illness. Associations between eNOS dependent vasodilation of arterioles with FMD and PAT suggest subcutaneous arterioles offer an opportunity for translational studies of mechanisms of modulating NO bioavailability relevant to in vivo measures endothelial function.
Supplementary Material
Acknowledgements
Dr Widlansky is supported by K23HL089326, the Elsa Shoeneich Medical Research Fund (Greater Milwaukee Foundation), and a T. Franklin Williams Scholars Award provided by Atlantic Philanthropies, the American Heart Association (Grant-in-Aid 10GRNT3880044), the John A. Hartford Foundation, and the Association of Specialty Physicians. This work was supported by the Clinical Translational Research Institute at the Medical College of Wisconsin and NIH (HL081587). Drs. Kizhakekuttu, Dharmashankar, and Wang received supported from a Ruth L. Kirschstein NIH T32 training grant (HL007792-15). Dr. Gutterman is supported by HL094971 and HL080704. The contents of this manuscript are the responsibility of the authors and do not necessarily represent the official views of any of the above funding sources.
Footnotes
Disclosures: No Relevant Conflicts
Supplementary information is available at http://360 www.nature.com/ajh
Reference List
- (1).Widlansky ME, Gokce N, Keaney JF, Jr, Vita JA. The clinical implications of endothelial dysfunction. J Am Coll Cardiol. 2003;42:1149–1160. doi: 10.1016/s0735-1097(03)00994-x. [DOI] [PubMed] [Google Scholar]
- (2).Thijssen DH, Black MA, Pyke KE, Padilla J, Atkinson G, Harris RA, Parker BA, Widlansky ME, Tschakovsky ME, Green DJ. Assessment of flow mediated dilation (FMD) in humans: a methodological and technical guideline. Am J Physiol Heart Circ Physiol. 2010 doi: 10.1152/ajpheart.00471.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Ludmer PL, Selwyn AP, Shook TL, Wayne RR, Mudge GH, Alexander RW, Ganz P. Paradoxical vasoconstriction induced by acetylcholine in atherosclerotic coronary arteries. N Engl J Med. 1986;315:1046–1051. doi: 10.1056/NEJM198610233151702. [DOI] [PubMed] [Google Scholar]
- (4).Cox DA, Vita JA, Treasure CB, Fish RD, Alexander RW, Ganz P, Selwyn AP. Atherosclerosis impairs flow-mediated dilation of coronary arteries in humans. Circulation. 1989;80:458–465. doi: 10.1161/01.cir.80.3.458. [DOI] [PubMed] [Google Scholar]
- (5).Vita JA, Treasure CB, Nabel EG, McLenachan JM, Fish RD, Yeung AC, Vekshtein VI, Selwyn AP, Ganz P. Coronary vasomotor response to acetylcholine relates to risk factors for coronary artery disease. Circulation. 1990;81:491–497. doi: 10.1161/01.cir.81.2.491. [DOI] [PubMed] [Google Scholar]
- (6).Celermajer DS, Sorensen KE, Gooch VM, Spiegelhalter DJ, Miller OI, Sullivan ID, Lloyd JK, Deanfield JE. Non-invasive detection of endothelial dysfunction in children and adults at risk of atherosclerosis. Lancet. 1992;340:1111–1115. doi: 10.1016/0140-6736(92)93147-f. [DOI] [PubMed] [Google Scholar]
- (7).Bonetti PO, Holmes DR, Lerman A, Barsness GW. Enhanced external counterpulsation for ischemic heart disease. What's behind the curtain? J Am Coll Cardiol. 2003;41:1918–1925. doi: 10.1016/s0735-1097(03)00428-5. [DOI] [PubMed] [Google Scholar]
- (8).Bonetti PO, Pumper GM, Higano ST, Holmes DR, Kuvin JT, Lerman A. Noninvasive identification of patients with early coronary atherosclerosis by assessment of digital reactive hyperemia. J Am Coll Cardiol. 2004;44:2137–2141. doi: 10.1016/j.jacc.2004.08.062. [DOI] [PubMed] [Google Scholar]
- (9).Anderson TJ, Uehata A, Gerhard MD, Meredith IT, Knab S, Delagrange D, Lieberman EH, Ganz P, Creager MA, Yeung AC. Close relation of endothelial function in the human coronary and peripheral circulations. J Am Coll Cardiol. 1995;26:1235–1241. doi: 10.1016/0735-1097(95)00327-4. [DOI] [PubMed] [Google Scholar]
- (10).Hamburg NM, Benjamin EJ. Assessment of endothelial function using digital pulse amplitude tonometry. Trends Cardiovasc Med. 2009;19:6–11. doi: 10.1016/j.tcm.2009.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Schiffrin EL. Structure and function of small arteries of essential hypertensive patients following chronic treatment with once-a-day nifedipine. Cardiology. 1997;88(Suppl 3):20–26. doi: 10.1159/000177502. [DOI] [PubMed] [Google Scholar]
- (12).Joannides R, Haefeli WE, Linder L, Richard V, Bakkali EH, Thuillez C, Luscher TF. Nitric oxide is responsible for flow-dependent dilatation of human peripheral conduit arteries in vivo. Circulation. 1995;91:1314–1319. doi: 10.1161/01.cir.91.5.1314. [DOI] [PubMed] [Google Scholar]
- (13).Nohria A, Gerhard-Herman M, Creager MA, Hurley S, Mitra D, Ganz P. Role of nitric oxide in the regulation of digital pulse volume amplitude in humans. J Appl Physiol. 2006;101:545–548. doi: 10.1152/japplphysiol.01285.2005. [DOI] [PubMed] [Google Scholar]
- (14).Georgescu A, Popov D, Constantin A, Nemecz M, Alexandru N, Cochior D, Tudor A. Dysfunction of human subcutaneous fat arterioles in obesity alone or obesity associated with Type 2 diabetes. Clin Sci (Lond) 2011;120:463–472. doi: 10.1042/CS20100355. [DOI] [PubMed] [Google Scholar]
- (15).Agewall S, Henareh L, Kublickiene K. Endothelial function in conduit and resistance arteries in men with coronary disease. Atherosclerosis. 2006;184:130–136. doi: 10.1016/j.atherosclerosis.2005.03.025. [DOI] [PubMed] [Google Scholar]
- (16).Kizhakekuttu TJ, Gutterman DD, Phillips SA, Jurva JW, Arthur EI, Das E, Widlansky ME. Measuring FMD in the Brachial Artery: How important is QRS-gating? J Appl Physiol. 2010 doi: 10.1152/japplphysiol.00532.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Hamburg NM, Keyes MJ, Larson MG, Vasan RS, Schnabel R, Pryde MM, Mitchell GF, Sheffy J, Vita JA, Benjamin EJ. Cross-sectional relations of digital vascular function to cardiovascular risk factors in the Framingham Heart Study. Circulation. 2008;117:2467–2474. doi: 10.1161/CIRCULATIONAHA.107.748574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Strande JL, Widlansky ME, Tsopanoglou NE, Su J, Wang J, Hsu A, Routhu KV, Baker JE. Parstatin: a cryptic peptide involved in cardioprotection after ischaemia and reperfusion injury. Cardiovasc Res. 2009;83:325–334. doi: 10.1093/cvr/cvp122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Rizzoni D, Porteri E, Castellano M, Bettoni G, Muiesan ML, Tiberio G, Giulini SM, Rossi G, Bernini G, gabiti-Rosei E. Endothelial dysfunction in hypertension is independent from the etiology and from vascular structure. Hypertension. 1998;31:335–341. doi: 10.1161/01.hyp.31.1.335. [DOI] [PubMed] [Google Scholar]
- (20).Rizzoni D, Porteri E, Guelfi D, Muiesan ML, Valentini U, Cimino A, Girelli A, Rodella L, Bianchi R, Sleiman I, Rosei EA. Structural alterations in subcutaneous small arteries of normotensive and hypertensive patients with non-insulin-dependent diabetes mellitus. Circulation. 2001;103:1238–1244. doi: 10.1161/01.cir.103.9.1238. [DOI] [PubMed] [Google Scholar]
- (21).Schiffrin EL, Deng LY. Comparison of effects of angiotensin I-converting enzyme inhibition and beta blockade for 2 years on function of small arteries from hypertensive patients. Hypertension. 1995;25:699–703. doi: 10.1161/01.hyp.25.4.699. [DOI] [PubMed] [Google Scholar]
- (22).Rizzoni D, Muiesan ML, Porteri E, Salvetti M, Castellano M, Bettoni G, Tiberio G, Giulini SM, Monteduro C, Garavelli G, Agabiti-Rosei E. Relations between cardiac and vascular structure in patients with primary and secondary hypertension. J Am Coll Cardiol. 1998;32:985–992. doi: 10.1016/s0735-1097(98)00322-2. [DOI] [PubMed] [Google Scholar]
- (23).Rizzoni D, Porteri E, Boari GE, De Ciuceis C, Sleiman I, Muiesan ML, Castellano M, Miclini M, Agabiti-Rosei E. Prognostic significance of small-artery structure in hypertension. Circulation. 2003;108:2230–2235. doi: 10.1161/01.CIR.0000095031.51492.C5. [DOI] [PubMed] [Google Scholar]
- (24).De CC, Porteri E, Rizzoni D, Corbellini C, La BE, Boari GE, Pilu A, Mittempergher F, Di BE, Casella C, Nascimbeni R, Rosei CA, Ruggeri G, Caimi L, Rosei EA. Effects of weight loss on structural and functional alterations of subcutaneous small arteries in obese patients. Hypertension. 2011;58:29–36. doi: 10.1161/HYPERTENSIONAHA.111.171082. [DOI] [PubMed] [Google Scholar]
- (25).Intengan HD, Deng LY, Li JS, Schiffrin EL. Mechanics and composition of human subcutaneous resistance arteries in essential hypertension. Hypertension. 1999;33:569–574. doi: 10.1161/01.hyp.33.1.569. [DOI] [PubMed] [Google Scholar]
- (26).Park JB, Charbonneau F, Schiffrin EL. Correlation of endothelial function in large and small arteries in human essential hypertension. J Hypertens. 2001;19:415–420. doi: 10.1097/00004872-200103000-00009. [DOI] [PubMed] [Google Scholar]
- (27).Sato A, Miura H, Liu Y, Somberg LB, Otterson MF, Demeure MJ, Schulte WJ, Eberhardt LM, Loberiza FR, Sakuma I, Gutterman DD. Effect of gender on endothelium-dependent dilation to bradykinin in human adipose microvessels. Am J Physiol Heart Circ Physiol. 2002;283:H845–H852. doi: 10.1152/ajpheart.00160.2002. [DOI] [PubMed] [Google Scholar]
- (28).Miura H, Bosnjak JJ, Ning G, Saito T, Miura M, Gutterman DD. Role for hydrogen peroxide in flow-induced dilation of human coronary arterioles. Circ Res. 2003;92:e31–e40. doi: 10.1161/01.res.0000054200.44505.ab. [DOI] [PubMed] [Google Scholar]
- (29).Phillips SA, Hatoum OA, Gutterman DD. The mechanism of flow-induced dilation in human adipose arterioles involves hydrogen peroxide during CAD. Am J Physiol Heart Circ Physiol. 2007;292:H93–100. doi: 10.1152/ajpheart.00819.2006. [DOI] [PubMed] [Google Scholar]
- (30).Tabit CE, Chung WB, Hamburg NM, Vita JA. Endothelial dysfunction in diabetes mellitus: molecular mechanisms and clinical implications. Rev Endocr Metab Disord. 2010;11:61–74. doi: 10.1007/s11154-010-9134-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Pyke K, Green DJ, Weisbrod C, Best M, Dembo L, O'Driscoll G, Tschakovsky M. Nitric oxide is not obligatory for radial artery flow-mediated dilation following release of 5 or 10 min distal occlusion. Am J Physiol Heart Circ Physiol. 2010;298:H119–H126. doi: 10.1152/ajpheart.00571.2009. [DOI] [PubMed] [Google Scholar]
- (32).Parker BA, Tschakovsky ME, Augeri AL, Polk DM, Thompson PD, Kiernan FJ. Heterogenous vasodilator pathways underlie flow-mediated dilation in men and women. Am J Physiol Heart Circ Physiol. 2011;301:H1118–H1126. doi: 10.1152/ajpheart.00400.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Thijssen DH, Green DJ, Hopman MT. Blood vessel remodeling and physical inactivity in humans. J Appl Physiol. 2011;111:1836–1845. doi: 10.1152/japplphysiol.00394.2011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.