Abstract
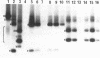
We have used a polymerase chain reaction (PCR) procedure to analyse low abundance complementary sense RNAs of Digitaria streak virus (DSV) from infected leaves of Digitaria setigera. This study has confirmed that both spliced and unspliced RNAs are synthesised by the same transcription unit. The position of the intron has been proven from sequencing cDNAs corresponding to the spliced RNA. Although the majority of cDNAs have 3' ends at coordinate 1063, downstream from a consensus polyadenylation sequence, a minor population of RNAs with heterogeneous 3' ends has also been identified. Two major RNA species with alternative splice sites or 3' ends, previously identified by nuclease S1 protection assays, could not be detected, but a cDNA species was observed with an apparent 90bp insertion at the 5' end of the intron. In transgenic tobacco containing integrated dimers of DSV DNA, the major unspliced RNA could readily be detected, but no spliced RNA was present. This may be a reason why DSV DNA did not replicate in tobacco. In addition, neither the minor population of heterogeneous RNAs nor the cDNA species with the insertion could be detected. The failure of the intron to be spliced in tobacco and its low activity in Digitaria is discussed in relation to recent studies on RNA splicing in plants and has led us to the conclusion that the geminivirus introns may be intrinsically inefficient.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Accotto G. P., Donson J., Mullineaux P. M. Mapping of Digitaria streak virus transcripts reveals different RNA species from the same transcription unit. EMBO J. 1989 Apr;8(4):1033–1039. doi: 10.1002/j.1460-2075.1989.tb03470.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker B., Schell J., Lörz H., Fedoroff N. Transposition of the maize controlling element "Activator" in tobacco. Proc Natl Acad Sci U S A. 1986 Jul;83(13):4844–4848. doi: 10.1073/pnas.83.13.4844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevan M. Binary Agrobacterium vectors for plant transformation. Nucleic Acids Res. 1984 Nov 26;12(22):8711–8721. doi: 10.1093/nar/12.22.8711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks-Gunn J., Warren M. P. Mother-daughter differences in menarcheal age in adolescent girls attending national dance company schools and non-dancers. Ann Hum Biol. 1988 Jan-Feb;15(1):35–43. doi: 10.1080/03014468800009441. [DOI] [PubMed] [Google Scholar]
- Dean C., Tamaki S., Dunsmuir P., Favreau M., Katayama C., Dooner H., Bedbrook J. mRNA transcripts of several plant genes are polyadenylated at multiple sites in vivo. Nucleic Acids Res. 1986 Mar 11;14(5):2229–2240. doi: 10.1093/nar/14.5.2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donson J., Accotto G. P., Boulton M. I., Mullineaux P. M., Davies J. W. The nucleotide sequence of a geminivirus from Digitaria sanguinalis. Virology. 1987 Nov;161(1):160–169. doi: 10.1016/0042-6822(87)90182-6. [DOI] [PubMed] [Google Scholar]
- Donson J., Gunn H. V., Woolston C. J., Pinner M. S., Boulton M. I., Mullineaux P. M., Davies J. W. Agrobacterium-mediated infectivity of cloned digitaria streak virus DNA. Virology. 1988 Jan;162(1):248–250. doi: 10.1016/0042-6822(88)90416-3. [DOI] [PubMed] [Google Scholar]
- Egeland D. B., Sturtevant A. P., Schuler M. A. Molecular analysis of dicot and monocot small nuclear RNA populations. Plant Cell. 1989 Jun;1(6):633–643. doi: 10.1105/tpc.1.6.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis J. G., Llewellyn D. J., Dennis E. S., Peacock W. J. Maize Adh-1 promoter sequences control anaerobic regulation: addition of upstream promoter elements from constitutive genes is necessary for expression in tobacco. EMBO J. 1987 Jan;6(1):11–16. doi: 10.1002/j.1460-2075.1987.tb04711.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favaloro J., Treisman R., Kamen R. Transcription maps of polyoma virus-specific RNA: analysis by two-dimensional nuclease S1 gel mapping. Methods Enzymol. 1980;65(1):718–749. doi: 10.1016/s0076-6879(80)65070-8. [DOI] [PubMed] [Google Scholar]
- Frohman M. A., Dush M. K., Martin G. R. Rapid production of full-length cDNAs from rare transcripts: amplification using a single gene-specific oligonucleotide primer. Proc Natl Acad Sci U S A. 1988 Dec;85(23):8998–9002. doi: 10.1073/pnas.85.23.8998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gierl A., Lütticke S., Saedler H. TnpA product encoded by the transposable element En-1 of Zea mays is a DNA binding protein. EMBO J. 1988 Dec 20;7(13):4045–4053. doi: 10.1002/j.1460-2075.1988.tb03298.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodall G. J., Filipowicz W. The AU-rich sequences present in the introns of plant nuclear pre-mRNAs are required for splicing. Cell. 1989 Aug 11;58(3):473–483. doi: 10.1016/0092-8674(89)90428-5. [DOI] [PubMed] [Google Scholar]
- Goodall G. J., Filipowicz W. The minimum functional length of pre-mRNA introns in monocots and dicots. Plant Mol Biol. 1990 May;14(5):727–733. doi: 10.1007/BF00016505. [DOI] [PubMed] [Google Scholar]
- Grunstein M., Hogness D. S. Colony hybridization: a method for the isolation of cloned DNAs that contain a specific gene. Proc Natl Acad Sci U S A. 1975 Oct;72(10):3961–3965. doi: 10.1073/pnas.72.10.3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerineau F., Brooks L., Meadows J., Lucy A., Robinson C., Mullineaux P. Sulfonamide resistance gene for plant transformation. Plant Mol Biol. 1990 Jul;15(1):127–136. doi: 10.1007/BF00017730. [DOI] [PubMed] [Google Scholar]
- Hayes R. J., Buck K. W. Replication of tomato golden mosaic virus DNA B in transgenic plants expressing open reading frames (ORFs) of DNA A: requirement of ORF AL2 for production of single-stranded DNA. Nucleic Acids Res. 1989 Dec 25;17(24):10213–10222. doi: 10.1093/nar/17.24.10213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howarth A. J., Vandemark G. J. Phylogeny of geminiviruses. J Gen Virol. 1989 Oct;70(Pt 10):2717–2727. doi: 10.1099/0022-1317-70-10-2717. [DOI] [PubMed] [Google Scholar]
- Jones J. D., Dunsmuir P., Bedbrook J. High level expression of introduced chimaeric genes in regenerated transformed plants. EMBO J. 1985 Oct;4(10):2411–2418. doi: 10.1002/j.1460-2075.1985.tb03949.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keith B., Chua N. H. Monocot and dicot pre-mRNAs are processed with different efficiencies in transgenic tobacco. EMBO J. 1986 Oct;5(10):2419–2425. doi: 10.1002/j.1460-2075.1986.tb04516.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunze R., Stochaj U., Laufs J., Starlinger P. Transcription of transposable element Activator (Ac) of Zea mays L. EMBO J. 1987 Jun;6(6):1555–1563. doi: 10.1002/j.1460-2075.1987.tb02400.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamppa G., Nagy F., Chua N. H. Light-regulated and organ-specific expression of a wheat Cab gene in transgenic tobacco. Nature. 1985 Aug 22;316(6030):750–752. doi: 10.1038/316750a0. [DOI] [PubMed] [Google Scholar]
- Matzke M. A., Susani M., Binns A. N., Lewis E. D., Rubenstein I., Matzke A. J. Transcription of a zein gene introduced into sunflower using a Ti plasmid vector. EMBO J. 1984 Jul;3(7):1525–1531. doi: 10.1002/j.1460-2075.1984.tb02006.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris-Krsinich B. A., Mullineaux P. M., Donson J., Boulton M. I., Markham P. G., Short M. N., Davies J. W. Bidirectional transcription of maize streak virus DNA and identification of the coat protein gene. Nucleic Acids Res. 1985 Oct 25;13(20):7237–7256. doi: 10.1093/nar/13.20.7237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ow D. W., DE Wet J. R., Helinski D. R., Howell S. H., Wood K. V., Deluca M. Transient and stable expression of the firefly luciferase gene in plant cells and transgenic plants. Science. 1986 Nov 14;234(4778):856–859. doi: 10.1126/science.234.4778.856. [DOI] [PubMed] [Google Scholar]
- Pereira A., Saedler H. Transpositional behavior of the maize En/Spm element in transgenic tobacco. EMBO J. 1989 May;8(5):1315–1321. doi: 10.1002/j.1460-2075.1989.tb03511.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proudfoot N. J., Brownlee G. G. 3' non-coding region sequences in eukaryotic messenger RNA. Nature. 1976 Sep 16;263(5574):211–214. doi: 10.1038/263211a0. [DOI] [PubMed] [Google Scholar]
- Rogers S. G., Bisaro D. M., Horsch R. B., Fraley R. T., Hoffmann N. L., Brand L., Elmer J. S., Lloyd A. M. Tomato golden mosaic virus A component DNA replicates autonomously in transgenic plants. Cell. 1986 May 23;45(4):593–600. doi: 10.1016/0092-8674(86)90291-6. [DOI] [PubMed] [Google Scholar]
- Sanger F., Coulson A. R., Barrell B. G., Smith A. J., Roe B. A. Cloning in single-stranded bacteriophage as an aid to rapid DNA sequencing. J Mol Biol. 1980 Oct 25;143(2):161–178. doi: 10.1016/0022-2836(80)90196-5. [DOI] [PubMed] [Google Scholar]
- Schalk H. J., Matzeit V., Schiller B., Schell J., Gronenborn B. Wheat dwarf virus, a geminivirus of graminaceous plants needs splicing for replication. EMBO J. 1989 Feb;8(2):359–364. doi: 10.1002/j.1460-2075.1989.tb03385.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley J. The molecular biology of geminiviruses. Adv Virus Res. 1985;30:139–177. doi: 10.1016/s0065-3527(08)60450-9. [DOI] [PubMed] [Google Scholar]
- Sunter G., Gardiner W. E., Bisaro D. M. Identification of tomato golden mosaic virus-specific RNAs in infected plants. Virology. 1989 May;170(1):243–250. doi: 10.1016/0042-6822(89)90372-3. [DOI] [PubMed] [Google Scholar]
- Townsend R., Stanley J., Curson S. J., Short M. N. Major polyadenylated transcripts of cassava latent virus and location of the gene encoding coat protein. EMBO J. 1985 Jan;4(1):33–37. doi: 10.1002/j.1460-2075.1985.tb02313.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- de Wet J. R., Wood K. V., Helinski D. R., DeLuca M. Cloning of firefly luciferase cDNA and the expression of active luciferase in Escherichia coli. Proc Natl Acad Sci U S A. 1985 Dec;82(23):7870–7873. doi: 10.1073/pnas.82.23.7870. [DOI] [PMC free article] [PubMed] [Google Scholar]