Abstract
Background
Dialysis grafts fail due to recurrent stenosis and thrombosis. Vasoactive and pro-thrombotic substances affecting intimal hyperplasia or thrombosis may modify graft outcomes.
Study design
Genetic polymorphisms association study of patients enrolled in a multi-center, randomized clinical trial.
Setting and participants
354 Dialysis Access Consortium (DAC) Study patients receiving a new graft with DNA samples obtained. Subjects were randomized to treatment with aspirin+dipyridamole vs placebo.
Predictor
DNA sequence polymorphisms for the following candidate genes and their interaction with the study intervention: methylenetetrahydrofolate reductase (MTHFR), heme oxygenase 1 (HO-1), Factor V (F5), transforming growth factor β1 (TGF-β1), Klotho, nitric oxide synthase (NOS), and angiotensin converting enzyme (ACE).
Outcome
Graft failure (>50% stenosis, angioplasty, thrombosis, surgical intervention or permanent loss of function).
Results
During a median patient follow-up of 34.3 months, 304 grafts failed. After adjusting for clinical factors (patient age, gender, access location, diabetes, cardiovascular disease, baseline aspirin use, body mass index, timing of graft placement, and study treatment) and genetic ancestral background, SNP rs6019 of the Factor V gene was significantly associated with graft failure in a dominant model (HR of 1.70 [95% CI, 1.32–2.19; p<0.001] for G/C and G/G genotypes vs C/C genotypes). There was no significant association between graft failure and polymorphisms of MTHFR, HO-1, TGF-β1, Klotho, NOS, or ACE.
Limitations
Small sample size
Conclusion
Factor V Leiden is associated with an increased risk of graft failure. Anticoagulation may reduce graft failure in patients with the G/C or G/G genotypes.
Index words: Arteriovenous access, arteriovenous graft, thrombosis, genetic polymorphism
Arteriovenous grafts for hemodialysis have limited cumulative survival, and are prone to recurrent stenosis and thrombosis 1. Pathologic studies have demonstrated aggressive intimal hyperplasia, which develops most commonly at the graft-vein anastomosis, and leads to graft stenosis with superimposed thrombosis 2. The cellular mechanisms leading to intimal hyperplasia and thrombosis are incompletely understood, but involve a complex interaction among factors affecting inflammation and thrombosis 3. The frequency of stenosis and thrombosis varies substantially among patients, suggesting that underlying differences in vascular tissue expression of vasoactive and pro-thrombotic substances contribute to the graft outcomes. The expression of these substances may, in turn, be affected by genetic polymorphisms.
Like graft failure, arteriovenous fistula failure is also attributed to intimal hyperplasia and thrombosis. Several retrospective analyses have evaluated associations between fistula failure and genetic polymorphisms 6. In particular, fistula failure has been associated with polymorphisms of methylentetrahydrofolate reductase (MTHFR) 7, heme oxygenase 1 (HO-1; encoded by the HMOX1 gene)8, Factor V 9, transforming growth factor β1 (TGFβ1, encoded by the TGFB1 gene), and Klotho (encoded by KL) 12, but not with angiotensin converting enzyme (ACE)13. In addition, polymorphisms of nitric oxide synthase (NOS) have been associated with coronary artery re-stenosis, an event also associated with intimal hyperplasia 14. In contrast, there are no publications evaluating the association of graft outcomes with genetic polymorphisms.
The Dialysis Access Consortium (DAC) graft study was a multi-center, randomized, double blind, clinical trial evaluating the effect of dipyridamole with aspirin on graft survival 15. This study enrolled a large number of patients receiving new grafts and obtained prospective assessment of graft outcomes. A subset of these patients had DNA samples obtained at the time of enrollment. The current study evaluated the association between graft outcomes and the polymorphisms of several biologically plausible genes in this patient population.
Methods
Clinical data collection
The DAC Study enrolled adult patients on hemodialysis or patients with advanced chronic kidney disease who received a new arteriovenous graft. The major exclusion criteria were an increased risk of hemorrhage; active peptic ulcer disease, gastritis or esophagitis; thrombocytopenia; advanced liver disease; and requirement for an anticoagulant or antiplatelet agent other than aspirin. The participants were randomized to receive aspirin and dipyridamole (n=321) or placebo (n=328) and followed prospectively for a median of 34.3 months). All DAC Study subjects were invited to participate in an ancillary research study whereby a blood sample was obtained for subsequent DNA extraction. Each subject provided separate informed consent for the ancillary study that was approved by the local Institutional Review Board of the individual clinical center. Of the 649 randomized study patients, 354 (or 54.5%) consented to participate in this ancillary study. The blood samples were shipped on dry ice to a central repository, where they were processed to extract DNA and stored for future genetic assays. Clinical and demographic information on the research subjects was collected prospectively by the clinical centers. The primary study endpoint was loss of primary unassisted graft patency (graft failure), defined as >50% stenosis (documented by angiogram), angioplasty, thrombosis, surgical intervention, or permanent loss of function.
Genotyping of candidate gene single nucleotide polymorphisms (SNPs)
A total of 192 SNPs were genotyped in the subject sample, including 144 ancestry informative markers (AIMs) and 48 SNPs in nine candidate genes. We selected SNPs for several candidate genes for which previous studies had reported associations between fistula failure and genetic polymorphisms. The laboratory personnel performing the SNP analysis were not aware of the subjects’ clinical information or graft outcomes. Genotyping for the study was performed using the GoldenGate assay on the BeadXpress system (Illumina, Inc.). Briefly, the GoldenGate assay involves biotin labeling of genomic DNA followed by capture of the labeled DNA onto streptavidin-coated sepharose beads. Assay oligonucleotides anneal to the target DNA of interest and are extended and ligated to produce a sequence that contains universal priming sequences on either end, which is then amplified and hybridized to holographically-labeled silica bars that form arrays with up to 30-fold redundancy of each target to be interrogated. Once the array has been visualized with the BeadXpress reader, wavelength and intensity values of the fluorescence are used to determine genotype. Allele detection and genotype calling were performed using the GenomeStudio software v3 (Illumina, Inc.).
Admixture Estimation
AIMs were used to obtain individual estimates of ancestral genetic admixture known to account for the biodiversity of the sample and to reduce potential confounding from population stratification. The markers included for the admixture estimation for this study are a subset of those described recently 16. The markers were chosen based on large frequency differences among West African, Amerindian and European ancestral populations that intermixed during the colonization of the US. Maximum likelihood estimation was used to translate the information from the AIMs into estimates of West African, Amerindian and European ancestral estimates for each participant, based on the method from Hanis et al 17. This method estimates the logarithms of the individual locus probabilities at all loci, computes the probability of the observed genotype for every possible admixture proportion from 0 to 100, and determines the maximum likelihood estimate of ancestry for each parental population for every individual. The range of West African, Amerindian and European ancestral estimates is from 0 to 100, but the sum of the three estimates equals 100.
Quality Control Analysis
A lab replicate was run on each sample plate for quality control, and both sample-specific and sample-independent controls internal to the assay were evaluated to verify data quality. Of the 192 SNPs that were genotyped in the subject sample, a total of 18 SNP’s were coded to zero due to poor clustering and/or multi-clusters. One SNP was eliminated due to low call rate (<0.90) and 22 SNPs were eliminated from analysis due to low minor allele frequency (MAF<0.01). The remaining 151 SNPs consisted of the AIMs and 21 candidate polymorphisms.
Statistical Analysis
Standard descriptive statistics were used for baseline demographic and clinical characteristics. Cumulative incidence curves comparing rates of loss of primary unassisted graft patency (graft failure) between the genotypes for each significant associated SNP were prepared using the Kaplan-Meier method.
Association studies of the outcome and SNP genotype were first done for each SNP separately. Cox proportional hazards regression was used to relate the primary and secondary outcomes to each individual SNP after controlling for baseline covariates and for the estimates of genetic admixture for the control of population stratification. Four genetic models were considered: co-dominant model (nominal genotype effect), dominant model (mutant genotype vs. wild-type homozygote), recessive model (mutant homozygote vs. other genotype), and additive model (assuming linear-trend effect of the genotypes). The genetic model was picked based on the AIC for each SNP. P-values were obtained through a likelihood ratio test of the reduced model (the model without the SNP genotype) vs. the full model (the model with the SNP genotype as a predictor). The following pre-specified baseline covariates were included: age, gender, diabetes history, cardiovascular disease, body mass index, baseline aspirin use, timing of graft surgery (before or after initiation of dialysis), graft location, and study treatment.
The two-dimensional, i.e., pair-wise, SNP-SNP interaction (epistasis) analysis for association studies was performed by adding an interaction term to the basic Cox model. All analyses were accomplished in R environment (http://www.r-project.org/). The genetic association study with the time-to-event outcome was done by applying the R packages of Design and SNPassoc. Multiple testing adjustments for individual SNP analysis were done by controlling false discovery rate)18 across all SNPs of their best models within each gene. For epistasis (gene-gene interaction), the multiple testing adjustments were done for each SNP by adjusting across the rest of 20 SNPs.
Results
A subset of 354 DAC graft study patients with DNA samples available for measurement of genetic polymorphisms formed the subject of the current investigation. The clinical features of this study population are summarized in Table 1. Their mean age was 57.5 years, and about 60% were female. By self-report, 73% of the subjects were black. Their mean BMI was 30.8 kg/m2. Diabetes was present in nearly two-thirds of the patient, and cardiovascular disease in 41%. About 40% of the subjects were on aspirin prior to study enrollment. The graft was placed after initiation of dialysis in 71% of patients and in 29% prior to starting dialysis. The graft location was equally distributed between the forearm and upper arm. About half of this study population received the active drug, and half were on placebo. The demographic and clinical characteristics of the patient population participating in the genetic study were very similar to those of the entire DAC graft study population 15, including age (57.5 vs 57.5 yrs); female sex (62 vs 61%), black race (73 vs 72%), diabetes (63 vs 63%), cardiovascular disease (41 vs 41%), and active study drug (47 vs 49%).
Table 1.
Clinical patient characteristics of the study population
| Variable | Overall | rs6019 genotypes | ||
|---|---|---|---|---|
| C/C | C/G | G/G | ||
| Age (y) | 57.5 ± 13.5 | 58.5±14.1 | 56.5±12.2 | 56.4±13.9 |
| Female sex | 218 (62%) | 112(60%) | 80(62%) | 27(68%) |
| Black race | 256 (73%) | 101(54%) | 116(91%) | 0(0%) |
| Diabetes mellitus | 222 (63%) | 116(62%) | 83(65%) | 23(57%) |
| Dialysis started before graft | 252 (71%) | 127(68%) | 99(77%) | 26(65%) |
| Graft located in forearm | 175 (49%) | 95(51%) | 60(47%) | 20(50%) |
| Prior aspirin use | 146 (41%) | 78(42%) | 50(39%) | 18(45%) |
| Cardiovascular disease | 144 (41%) | 71(38%) | 54(43%) | 19(48%) |
| BMI, kg/m2 | 30.8 ± 8.6 | 30.6±8.5 | 30.7±8.5 | 32.5±9.1 |
| Active study drug | 165 (47%) | 84(45%) | 62(49%) | 19(48%) |
Note: continuous variables given as mean ± standard deviation; categorical variables as number (percentage).
BMI, body mass index; rs, reference single - nucleotide polymorphism identification number
During study followup the primary graft endpoint (>50% stenosis, angioplasty, thrombosis, surgical revision, or permanent loss of function) occurred in 86% of the subjects. On univariate analysis, only two of the pre-specified clinical factors were associated with decreased primary graft failure: graft placement prior to initiation of dialysis and active study drug (Table 2). Graft failure was not significantly associated with patient age, sex, diabetes, graft location, cardiovascular disease, baseline aspirin use or body mass index. On multivariate analysis in a model including the demographic and clinical factors, and adjusted for patient admixture to control for population stratification, graft failure was more likely in patients with graft surgery after initiation of dialysis (HR, 1.38; 95% CI, 1.05–1.82; p=0.02) and less likely in patients randomized to the active drug (HR, 0.76; 95% CI, 0.60–0.96; p=0.02)(Table 2).
Table 2.
Association between phenotype and the primary outcome
| Univariate Analysis* | Multivariate Analysis** | Interaction with rs6019*** | ||||
|---|---|---|---|---|---|---|
| HR (95% CI) | p-value | HR (95%CI) | p-value | Exp of interaction coefficient (95% CI) | p-value | |
| Diabetes (Yes vs no) | 1.03 (0.81–1.30) | 0.8 | 1.09(0.84,1.42) | 0.5 | 0.50(0.29,0.86) | 0.02 |
| Dialysis_before graft (Yes vs no) | 1.33 (1.04–1.71) | 0.02 | 1.38(1.05,1.82) | 0.02 | 1.59(0.90,2.81) | |
| Study Group (Active drug vs placebo) | 0.79 (0.63–0.99) | 0.04 | 0.76(0.60,0.96) | 0.02 | 0.72(0.45,1.17) | |
| Baseline aspirin (Yes vs no) | 0.93 (0.74–1.17) | 0.6 | 0.88(0.68,1.14) | 0.3 | 0.97(0.58,1.65) | |
| Gender (Female vs male) | 0.99 (0.78–1.25) | 0.9 | 0.99(0.77,1.27) | 0.9 | 0.67(0.40,1.11) | |
| Cardiovascular disease (Yes vs no) | 1.06 (0.84–1.34) | 0.6 | 1.04(0.81,1.33) | 0.8 | 1.24(0.74,2.06) | |
| Access location (Upper arm vs forearm) | 1.14 (0.91–1.42) | 0.3 | 1.07(0.83,1.37) | 0.6 | 0.92(0.56,1.51) | |
| Age (per 5 yr) | 1.00 (0.97–1.01) | 0.6 | 1.00(0.99,1.01) | 0.9 | 1.01(0.99,1.03) | 0.6 |
| BMI (per 5 kg/m2) | 1.00 (0.97–1.03) | 0.9 | 1.01(0.99,1.02) | 0.3 | 1.02(0.99,1.06) | 0.2 |
BMI, body mass index; HR, hazard ratio; CI, confidence interval; rs, reference single-nucleotide polymorphism identification number; exp, exponent.
the cox proportional hazard model was fitted by considering each phenotype as a covariate.
the cox proportional hazard model was fitted by considering all phenotypes as covariates simultaneously.
obtained through multivariate analysis where the cox proportional hazard model was fitted by considering all phenotypes as covariates and their interaction with SNP rs6019 simultaneously.
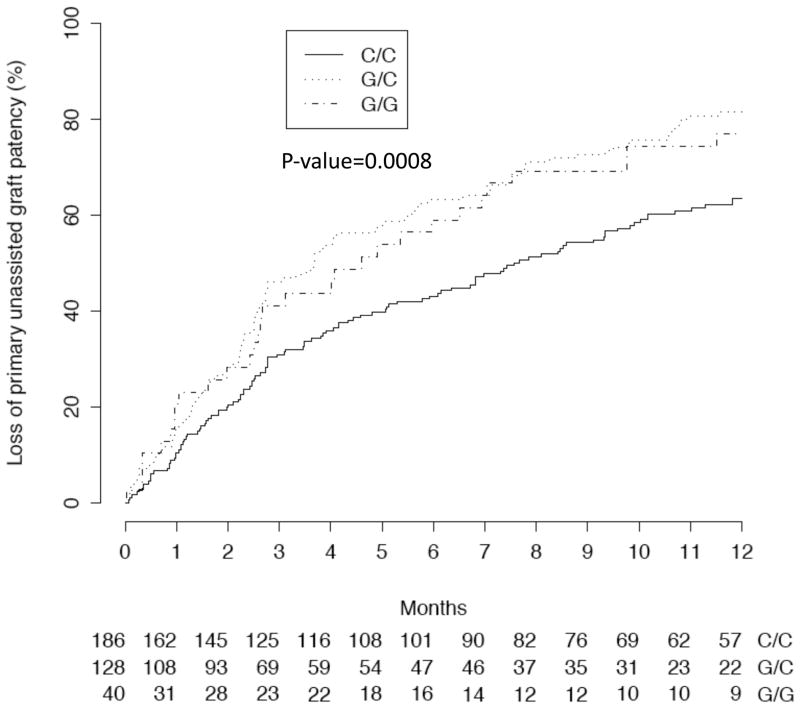
The association between individual SNPs (Table 3) and graft outcomes was analyzed after adjustment for the clinical factors and patient admixture. In this analysis, the rs6019 SNP of Factor V (F5) was associated with graft failure in a dominant model (HR of 1.70 [95% CI, 1.32–2.19; p<0.001] for G/C and G/G genotypes vs C/C genotypes)(Figure 1). Because the distribution of rs6019 polymorphisms varied substantially by patient race (Table 1), we repeated this analysis after restricting it to the 256 black participants. In this patient subgroup, rs6019 was also associated with graft failure in a dominant model (HR of 1.86 [95% CI, 1.40–2.47; p<0.001] for G/C and G/G genotypes vs C/C genotypes). The association between SNP rs6019 of Factor V and graft thrombosis in the black patients failed to reach statistical significance in a dominant model (HR, 1.23; 95% CI, 0.81–1.88; p=0.3).
Table 3.
Genotype data summary.
| Gene | Major Allele | |||
|---|---|---|---|---|
| SNP | Alleles | Frequency (%) | HWE p-value* | |
| MTHFR | rs2274976 | G/A | 96.7 | 0.3 |
| MTHFR | rs1801131 | A/C | 79.3 | 0.4 |
| MTHFR | rs1801133 | G/A | 84 | 0.001 |
| F5 | rs6030 | A/G | 76.8 | 0.05 |
| F5 | rs13306334 | G/A | 98.6 | NA |
| F5 | rs6032 | A/G | 78.9 | 0.1 |
| F5 | rs4525 | A/G | 79.5 | 0.05 |
| F5 | rs4524 | A/G | 79.2 | 0.1 |
| F5 | rs6019 | C/G | 70.7 | 0.01 |
| F5 | rs9332485 | G/A | 96.5 | 0.4 |
| KL | rs9536314 | A/C | 81.2 | 0.08 |
| KL | rs9527025 | G/C | 81.2 | 0.08 |
| KL | rs3752472 | G/A | 97.3 | NA |
| NOS2A | rs2297518 | G/A | 87 | 0.6 |
| NOS2A | rs3730017 | G/A | 84.6 | 0.8 |
| ACE | rs4303 | C/A | 94.2 | 0.1 |
| ACE | rs12709426 | A/G | 97.7 | 0.2 |
| TGFB1 | rs1800472 | G/A | 98.9 | 0.04 |
| TGFB1 | rs1800471 | G/C | 93.1 | 0.7 |
| TGFB1 | rs1982073 | A/G | 56.4 | 0.9 |
| HMOX1 | rs2071747 | C/G | 96.6 | NA |
MTHFR, methylenetatrahydrofolate reductase; F5, factor V Leiden; KL, Klotho; NOS2A, nitric oxide synthase; ACE, angiotensin-converting enzyme; TGFB1, transforming growth factor β1; HMOX1, heme oxygenase 1; HWE: Hardy-Weinberg equilibrium; SNP, single-nucleotide polymorphism.
p value*: HWE test was conducted by Chi-square test with p-value < 0.05 means the null hypothesis of Hardy-Weinberg equilibrium does not hold.
Fig 1.
Time to graft failure (>50% stenosis, angioplasty, thrombosis, surgical revision, or permanent loss of function) among patients with Factor V Leiden (F5) polymorphisms. SNP rs6019 of F5 was associated with graft failure in a dominant model (HR of 1.70 [95% CI, 1.32- 2.19; p<0.001] for G/C and G/G vs CC genotypes).
We also evaluated whether there was an interaction between the clinical variables and rs6019 in affecting the primary study outcome (Table 2). This analysis revealed a significant interaction between diabetes and rs6019 in affecting graft failure. Using non-diabetic patients with the C/C genotype as the reference group, the hazard ratio of graft failure was 1.45 (95% CI, 1.01–2.10) for diabetics with the C/C genotype; 2.66 (95% CI, 1.77–4.00) for non-diabetics with the G/C or G/G genotypes; and 1.97 (95% CI, 1.35–2.88) for diabetics with the G/C or G/G genotypes. In contrast, there was no significant interaction between the study drug and rs6019 on graft failure (p=0.2). Finally, there was no significant association between graft failure and polymorphisms of MTHFR, HO-1, TGFβ1, Klotho, NOS, or ACE (Table 4).
Table 4.
Association between each SNP and the primary outcome after adjusting for all covariates.
| Gene | SNP | Position | Genotype | No. | HR (95% CI) | p-value | FDR |
|---|---|---|---|---|---|---|---|
| MTHFR | rs2274976 | 11773513 | G/G | 330 | 0.04 | 0.12 | |
| A/G-A/A | 22 | 0.57 (0.33–0.97) | |||||
| MTHFR | rs1801131 | 11777062 | A/A | 219 | 0.2 | 0.22 | |
| C/A-C/C | 135 | 0.84 (0.65–1.07) | |||||
| MTHFR | rs1801133 | 11778964 | G/G | 259 | 0.6 | 0.61 | |
| A/G-A/A | 95 | 1.08 (0.80–1.47) | |||||
| F5 | rs6030 | 167765598 | A/A | 215 | 0.05 | 0.08 | |
| G/A-G/G | 139 | 0.79 (0.62–1.00) | |||||
| F5 | rs6032 | 167778178 | A/A | 225 | 0.05 | 0.08 | |
| G/A-G/G | 129 | 0.78 (0.61–1.00) | |||||
| F5 | rs4525 | 167778357 | A/A | 225 | 0.08 | 0.09 | |
| G/A-G/G | 122 | 0.80 (0.62–1.03) | |||||
| F5 | rs4524 | 167778378 | A/A | 223 | 0.04 | 0.08 | |
| G/A-G/G | 126 | 0.77 (0.60–0.99) | |||||
| F5 | rs6019 | 167808136 | C/C | 186 | <0.0001 | <0.001 | |
| G/C-G/G | 168 | 1.70 (1.32–2.19) | |||||
| F5 | rs9332485 | 167822205 | G/G | 330 | 0.7 | 0.73 | |
| A/G-A/A | 24 | 0.92 (0.58–1.45) | |||||
| KL | rs9536314 | 32526137 | A/A | 227 | 0.4 | 0.39 | |
| C/A-C/C | 127 | 1.12 (0.88–1.43) | |||||
| KL | rs9527025 | 32526192 | G/G | 227 | 0.4 | 0.39 | |
| C/G-C/C | 127 | 1.12 (0.88–1.43) | |||||
| NOS2A | rs2297518 | 23120723 | G/G | 269 | 0.3 | 0.53 | |
| A/G-A/A | 85 | 0.85 (0.65–1.13) | |||||
| NOS2A | rs3730017 | 23133228 | G/G | 254 | 0.5 | 0.53 | |
| A/G-A/A | 100 | 0.92 (0.70–1.19) | |||||
| ACE | rs4303 | 58911554 | C/C | 315 | 0.9 | 0.91 | |
| A/C-A/A | 38 | 1.02 (0.70–1.49) | |||||
| ACE | rs12709426 | 58915487 | A/A | 339 | 0.2 | 0.38 | |
| G/A-G/G | 15 | 1.42 (0.84–2.43) | |||||
| TGFB1 | rs1800472 | 46539699 | G/G | 346 | 0.5 | 0.72 | |
| A/G-A/A | 7 | 0.74 (0.32–1.69) | |||||
| TGFB1 | rs1800471 | 46550715 | G/G | 305 | 0.8 | 0.80 | |
| C/G-C/C | 46 | 0.95 (0.68–1.34) | |||||
| TGFB1 | rs1982073 | 46550760 | A/A | 114 | 0.2 | 0.52 | |
| G/A-G/G | 240 | 0.84 (0.65–1.07) |
MTHFR, methylenetatrahydrofolate reductase; F5, factor V Leiden; KL, Klotho; NOS2A, nitric oxide synthase; ACE, angiotensin-converting enzyme; TGFB1, transforming growth factor β1; HMOX1, heme oxygenase 1; HWE: Hardy-Weinberg equilibrium; rs, reference single-nucleotide polymorphism identification number; HR, hazard ratio; CI, confidence interval; SNP, single-nucleotide polymorphism; FDR, false discovery rate.
We also evaluated pair-wise SNP-SNP interactions on primary graft patency. The only significant interaction was between SNP rs3730017 for NOS and SNP rs1801131 for MTHFR (p=0.003). We observed that if the individuals carrying G/G for SNP rs3730017, the SNP rs1801131 does not have statistically significant association with graft failure (HR, 1.06; 95% CI, 0.79–1.43; p>0.05); however, if the patients carrying either A/G or A/A for SNP rs3730017, the SNP rs1801131 is significantly associated with the graft failure, i.e., the primary graft failure was less likely in patients with minor allele C of SNP rs1801131 (HR, 0.52; 95% CI, 0.32–0.85; p<0.05).
Discussion
Analysis of the DAC Study sub-population demonstrated an association between graft failure and treatment with extended-release dipyridamole/aspirin, as previously demonstrated in the DAC trial 15. In addition, placement of the graft after initiation of dialysis was associated with higher graft failure. Although this association has not been previously reported for grafts, the Dialysis outcomes and Practice Pattern Study (DOPPS) reported a similar finding for patients with dialysis fistulas 19. We cannot, however, exclude the possibility that earlier cannulation contributed to the shortened patency of grafts placed after dialysis initiation.
Graft failure is mediated by intimal hyperplasia and thrombosis, suggesting that factors affecting inflammation and thrombosis may affect the risk of graft failure. These factors in turn may be regulated by polymorphisms of biologically plausible compounds. To investigate the role of these genetic factors, we evaluated the association between various SNPs and graft outcomes, after adjustment for pre-specified clinical factors and estimates of genetic admixture. SNP rs6019 polymorphisms of Factor V, a pro-thrombotic compound, were independently associated with graft failure. Whereas activated Factor V is normally inactivated by activated protein C, factor V Leiden (a single nucleotide mutation) is resistant to inactivation by protein C and therefore has a longer half-life 20. As a result, homozygotes and heterozygotes for Factor V Leiden have an increased risk of thromboembolism 21. Given that thrombosis is an important mechanism of graft failure, the association between Factor V Leiden and graft failure in the present study makes physiologic sense, and is consistent with a previous retrospective study that observed an association between Factor V Leiden polymorphisms and fistula failure 9. The lack of significant association between SNP rs6019 of Factor V Leiden and graft thrombosis in the current study was likely a reflection of the lower event rate, as graft failure occurred in 86% of subjects, whereas thrombosis occurred in only 40%. Of interest, some studies have observed an increased risk of thromboembolic diseases in patients with chronic kidney disease 22, which could potentially be related to Factor V Leiden. Unfortunately, we did not track thromboembolic events in the DAC graft study population, so are unable to assess a possible association.
This association between SNP rs6019 and graft failure suggests the potential for drugs that interfere with thrombosis to prevent graft failure. One might postulate that such drugs would be particularly effective in preventing graft failure in patients with the SNP that increases risk of thrombosis. However, this is not straightforward, given the lack of interaction between Factor V polymorphism and study drug on primary graft outcome. Moreover, two previous randomized studies found no benefit of warfarin or aspirin-clopidogrel on graft thrombosis. It is possible, however, that anticoagulation may be useful in preventing graft failure in the subset of patients with Factor V Leiden expression, but addressing this question would require a randomized clinical trial.
In contrast to retrospective studies evaluating associations between fistula outcomes and genetic polymorphisms, we did not observe an association of graft failure with polymorphisms for several candidate genes, including MTHFR, HO-1, TGFβ1, Klotho, NOS, or ACE. We did, however, observe a significant interaction between polymorphisms of the nitric oxide synthase and MTHFR genes on graft failure. The former affects inflammation (i.e., intimal hyperplasia), whereas the latter affects thrombosis. This interaction is consistent with the combined contribution of both physiologic factors to graft failure 3.
The current study has several strengths, including prospective data collection, multi-center trial design, the use of pre-specified clinical factors, and the inclusion of genetic admixture to control for population stratification. In addition, our study enrolled a large number of black patients, whereas previous genetic association studies with arteriovenous access outcomes included only white or Asian patients. Our study also has some limitations. The DAC graft study was designed as a clinical trial, rather than a genetic associations study. As a result, it was not well-powered to detect genetic associations with graft failure. In a post-hoc power calculation we had sufficient power to detect an association between the rs6019 SNP and graft failure (power 0.92 at the significance level of 0.001). However, the frequency of minor alleles was relatively small for some of the SNPs (Table 3), and the study population relatively small, raising the potential that a significant association may have been missed. There may also be additional candidate genes affecting graft failure, which were not included in the present study.
In conclusion our study observed an association between rs6019 Factor V polymorphisms and primary graft patency. Patients with the high-risk Factor V Leiden genotype, who accounted for 47% of our study population, are at increased risk for graft failure and may be ideal subjects for clinical trials of pharmacologic interventions, such as prophylactic anticoagulation.
Acknowledgments
A list of the DAC Study Investigators follows. Boston University Medical Center - L. Dember, J. Kaufman, M. Hawley, A. Lauer, P. LeSage, R. Nathan, E. Holmberg; Baystate Medical Center - G. Braden, M. Ryan, A. Berkowitz; Charleston Area Medical Center - A. Rahman, B. Lucas Jr, R. Santos, B. Reyes; Duke University Medical Center - A. Greenberg, M. Berkoben, E. Kovalik, J. Lawson, J. Middleton, S, Schwab, D. Schumm, S. Adams, K. Gitter, T. Cantaffa, A. Quarles; Emory University - J. Work, S. Rhodes; Maine Medical Center - J. Himmelfarb, J. Whiting, J. Kane, S. Freedman, R. Violette, H. Cyr-Alves, K. Garrison;Saint Louis University - K. Martin, P Schmitz, V. Jenkins; Tyler (Texas) Nephrology Associates - J. Cotton Jr., E. Husband; University of Alabama at Birmingham - M. Allon, M. Robbin, M. Lockhart, B. Casey, J. Newsome; University of Iowa, Iowa City, IA - B. Dixon, B. Franzwa, L. Hunsicker, J. Hoballah, D. Katz, W. Sharp, T. Kresowik, Y. Wu, S Rayhill; Renal Core Associates (Peoria, IL) - T. Pflederer, K. DuPage, K. Welch, F. Darras, A. Banqero, B. Ketel, A. Wounded Arrow, C. Grant, J. Deeb, L. Pyszka, Covenant Medical Center (Waterloo, IA) - M. Slavin, D. Wedeking; University of Texas Southwestern - M. Vazquez, I. Davidson, R. Toto, L. Littmon, C. Ying, T. Lightfoot, H. Quinones, R. Saxena, P. Clagett, J. Valentine, B. Dolmatch, J. Thompson; Baylor University Medical Center - A. Fenves, G. Pearl; Vanderbilt University Medical Center - A. Ikizler, P. Egbert; Vascular Surgery Associates (Baton Rouge, LA.) - J. McNeil, D. Holmes, W. Freiberger; Washington University in Saint Louis - J. Delmez, D. Windus, D. Coyne, M. Rothstein, S. Shenoy, R. Creaghan, B. Lluka; National Institute of Diabetes and Digestive and Kidney Diseases - J. Kusek, C. Meyers; Steering Committee Chair - H. Feldman (U. Pennsylvania); Data Coordinating Center (Cleveland Clinic Foundation) - G. Beck, J. Gassman, T. Greene, B. Hu, S. Bi, A. Liu, M. Radeva, L. Tuason, B. Weiss; Data and Safety Monitoring Board (DSMB) - N. Levin (Chair), A. Besarab, G. Chertow, M. Diener-West, T. Louis, W. McClellan, C. Stehman-Breen.
We are indebted to the study coordinators and patients who participated in the DAC Graft trial.
Support: The current study was funded in part by NIH grant K23 DK075385 to Dr. Maya and by NIH grant 5R01DK085027 to Dr. Allon. The original DAC trial was funded by NIDDK. Boehringer-Ingelheim Pharmaceuticals, Inc., Ridgefield, CT provided additional funding as well as the study drugs.
Footnotes
Financial Disclosure: The authors declare that they have no other relevant financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Allon M. Current management of vascular access. Clin J Am Soc Nephrol. 2007;2:786–800. doi: 10.2215/CJN.00860207. [DOI] [PubMed] [Google Scholar]
- 2.Roy-Chaudhury P, Kelly BS, Miller MA, et al. Venous neointimal hyperplasia in polytetrafluoroethylene dialysis grafts. Kidney Int. 2001;59:2325–34. doi: 10.1046/j.1523-1755.2001.00750.x. [DOI] [PubMed] [Google Scholar]
- 3.Roy-Chaudhury P, Sukhatme VP, Cheung AK. Hemodialysis vascular access dysfunction: A cellular and molecular viewpoint. J Am Soc Nephrol. 2006;17:1112–27. doi: 10.1681/ASN.2005050615. [DOI] [PubMed] [Google Scholar]
- 4.Roy-Chaudhury P, Arend L, Zhang J, et al. Neointimal hyperplasia in early arteriovenous fistula failure. Am J Kidney Dis. 2007;50:782–90. doi: 10.1053/j.ajkd.2007.07.019. [DOI] [PubMed] [Google Scholar]
- 5.Chang CJ, Ko PJ, Hsu LA, et al. Highly increased cell proliferation activity in restenotic hemodialysis vascular access after percutaneous transluminal angioplasty: Implication in prevention of stenosis. Am J Kidney Dis. 2004;43:74–84. doi: 10.1053/j.ajkd.2003.09.015. [DOI] [PubMed] [Google Scholar]
- 6.Girndt M, Heine GH, Ulrich C, Kohler H. Gene polymorphism association studies in dialysis: vascular access. Semin Dial. 2007;20:63–7. doi: 10.1111/j.1525-139X.2007.00243.x. [DOI] [PubMed] [Google Scholar]
- 7.Fukasawa M, Matsushita K, Kamiyama M, et al. The methylentetrahydrofolate C677T point mutation is a risk factor for vascular access thrombosis in hemodialysis patients. Am J Kidney Dis. 2003;41:637–42. doi: 10.1053/ajkd.2003.50125. [DOI] [PubMed] [Google Scholar]
- 8.Lin CC, Yang WC, Lin SJ, et al. Length polymorphism in heme-oxygenase-1 is associated with arteriovenous fistula patency in hemodialysis patients. Kidney Int. 2006;69:165–72. doi: 10.1038/sj.ki.5000019. [DOI] [PubMed] [Google Scholar]
- 9.Knoll GA, Wells PS, Young D, et al. Thrombophilia and the risk for hemodialysis vascular access thrombosis. J Am Soc Nephrol. 2005;16:1108–14. doi: 10.1681/ASN.2004110999. [DOI] [PubMed] [Google Scholar]
- 10.Heine GH, Ulrich C, Sester U, Sester M, Kohler H, Girndt M. Transforming growth factor b1 genotype polymorphisms determine AV fistula patency in hemodialysis patients. Kidney Int. 2003;64:1101–7. doi: 10.1046/j.1523-1755.2003.00176.x. [DOI] [PubMed] [Google Scholar]
- 11.Lazo-Langner A, Knoll GA, Wells PS, Carson N, Rodger MA. The risk of dialysis access thrombosis is related to the transforming growth factor-beta1 production haplotype and is modified by polymorphisms in the plasminogen activator inhibitor-type 1 gene. Blood. 2006;108:4052–8. doi: 10.1182/blood-2006-06-028902. [DOI] [PubMed] [Google Scholar]
- 12.Kim Y, Jeong SJ, Lee HS, et al. Polymorphism in the promoter region of the klotho gene (G-395A) is associated with early dysfunction in vascular access in hemodialysis patients. Korean J Intern Med. 2008;23:201–7. doi: 10.3904/kjim.2008.23.4.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heine GH, Ulrich C, Kohler H, Girndt M. Is AV fistula patency associated with angiotensin-converting enzyme (ACE) polymorphism and ACE inhibitor intake? Am J Nephrol. 2004;24:461–168. doi: 10.1159/000080464. [DOI] [PubMed] [Google Scholar]
- 14.Petrovic D, Peterlin B. Genetic markers of restenosis after coronary angioplasty and after stent implantation. Med Sci Monit. 2005;11:127–35. [PubMed] [Google Scholar]
- 15.Dixon BS, Beck GJ, Vazquez MA, et al. Effect of dipyridamole plus aspirin on hemodialysis graft patency. N Engl J Med. 2009;360:2191–201. doi: 10.1056/NEJMoa0805840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Klimentidis YC, Divers J, Casazza K, Beasley TM, Allison DB, Fernandez JR. Ancestry informative markers on chromosomes 2, 8, and 15 are associated with insulin-related traits in a racially diverse sample of children. Hum Genomics. 2011;5:79–89. doi: 10.1186/1479-7364-5-2-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hanis CL, Chakraborty R, Ferrell RE, Schull WJ. Individual admixture estimates: disease associations and individual risk of diabetes and gallbladder disease among Mexican-Americans in Starr County, Texas. Am J Phys Anthropol. 1986;70:433–41. doi: 10.1002/ajpa.1330700404. [DOI] [PubMed] [Google Scholar]
- 18.Bejamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J Royal Statistical Society Series B. 1995;57:289–300. [Google Scholar]
- 19.Rayner HC, Pisoni Rl, Gillespie BW, et al. Creation, cannulation, and survival of arteriovenous fistulae: Data from the Dialysis Outcomes and Practice Patterns Study. Kidney Int. 2003;63:323–30. doi: 10.1046/j.1523-1755.2003.00724.x. [DOI] [PubMed] [Google Scholar]
- 20.Bertina RM, Koeleman BP, Koster T, et al. Mutation in blood coagulation factor V associated with resistance to activated protein C. Nature. 1994;369:64–7. doi: 10.1038/369064a0. [DOI] [PubMed] [Google Scholar]
- 21.Ridker PM, Hennekens CH, Lindpaintner K, Stampfer MJ, Eisenberg PR, Miletich JP. Mutation in the gene coding for coagulation factor V and the risk of myocardial infarction, stroke, and venous thrombosis in apparently healthy men. N Engl J Med. 1995;332:912. doi: 10.1056/NEJM199504063321403. [DOI] [PubMed] [Google Scholar]
- 22.Wattanakit K, Cushman M, Stehman-Breen CO, Heckbert SR, Folsom AR. Chronic kidney disease increases risk for venous thromboembolism. J Am Soc Nephrol. 2008;19:135–40. doi: 10.1681/ASN.2007030308. [DOI] [PMC free article] [PubMed] [Google Scholar]