Abstract
To assess the potency of regulatory T (Treg) cells induced against an irrelevant Ag, mice were orally vaccinated with Salmonella expressing E. coli colonization factor antigen I fimbriae. Isolated CD25+ and CD25− CD4+ T cells were adoptively transferred to naive mice, and Treg cells effectively protected against experimental autoimmune encephalomyelitis (EAE), unlike Treg cells from Salmonella vector-immunized mice. This protection was abrogated upon in vivo neutralization of TGF-β, resulting in elevated IL-17 and loss of IL-4 and IL-10 production. Thus, Treg cells induced to irrelevant Ags offer a novel approach to treat autoimmune diseases independent of auto-Ag.
Keywords: Salmonella, Treg cells, experimental autoimmune encephalomyelitis, TGF-β
1. Introduction
Experimental autoimmune encephalomyelitis (EAE) serves as a rodent model for multiple sclerosis (MS) and shares some aspects of this human neurodegenerative disease and T cell dependency (Sospedra and Martin, 2005; Baxter, 2007). EAE can be actively induced upon immunization with encephalitogenic peptides (Sospedra and Martin, 2005; Baxter, 2007) or by passive transfer of myelin auto-Ag reactive T cells (Linington et al., 1993; Miller et al., 1995; Williams et al., 2011). EAE pathology is characterized by the massive CNS infiltration of myelin-reactive CD4+ T cells (Olsson, 1996; Fife et al, 2000), along with other inflammatory cells, such as macrophage and neutrophils (Juedes et al., 2000; Kroenke 2008; Li et al., 2011), causing CNS inflammation and variable paralysis.
The major CNS-invading CD4+ T cells are T helper type 1 cells (Th1) and Th17 cells, which produce inflammatory cytokines IFN-γ and IL-17, respectively. Although IFN-γ can contribute to disease (Olsson et al., 1996; Karpuss et al., 1998; Juedes et al., 2000), Th17 cells have been found to be the major encephalitogenic T cells (Hofstetter et al., 2005; Komiyama et al., 2006; Sutton et al., 2006), which can be induced by IL-23 (Cua et al., 2003). In fact, IL-23p19−/− mice have been found to be resistant to EAE (Cua et al., 2003; Langrish et al., 2005). IL-23 exerts its role in EAE through proliferation of Th17 cells (Aggarwal et al, 2003; Langrish et al., 2005), and adoptively transferred IL-23-driven Th17 cells have been found to be highly potent in promoting EAE (Langrish et al., 2005). Recent findings have shown that IL-23 also stimulates GM-CSF-producing autoreactive CD4+ T cells, which in turn induce Th17 cells, causing an exacerbation of disease because of myeloid cell recruitment to the CNS (Codarri et al. 2011; El-Behi et al. 2011). Alternatively, Th17 cells can also be induced upon concomitant stimulation by TGF-β and IL-6 (Veldhoen et al., 2006; Kimura et al., 2006; Okamoto et al. 2010).
Stimulation of anti-inflammatory Th2 cells has been shown to confer protection against EAE. This Th2 cell-dependence is demonstrated by the production of the anti-inflammatory cytokines, IL-4 (Falcone et al., 1998; Inobe et al., 1998) and IL-13 (Offner et al., 2005; Ochoa-Repáraz et al., 2008). However, regulatory T cells are the predominant T cell subset responsible for protection via IL-10 (Stephens et al., 2009; Faria et al., 2003), TGF-β (Faria et al., 2003; Chen et al., 2003; Zheng et al., 2008), and now IL-35 (Kochetkova et al., 2010; Collison et al. 2010). These regulatory T cells can vary phenotypically (Stephens et al. 2009; Kochetkova et al. 2010; Sakaguchi et al., 2010; Campbell and Koch, 2011), but most often the inducible regulatory CD4+ T cells express CD25 and FoxP3 (Stephens et al., 2009; Campbell and Koch, 2011) and are commonly referred to as Treg cells. While Treg cells are induced during EAE (McGeachy et al., 2005; Rynda-Apple et al., 2011), these often lack the ability to ameliorate disease. Thus, alternative means to induce Treg cells are sought. Along these lines, Ag-specific Treg cells are preferential, and tolerization mechanisms surely enhance their development (Rynda et al., 2008; Rynda et al., 2010; Rynda-Apple et al., 2011; Getts et al., 2011). Alternatively, we (Jun et al. 2005; Ochoa-Repáraz et al., 2007; Ochoa-Repáraz et al., 2008; Kochetkova et al. 2008; Kochetkova et al. 2010) and others (Falcone and Bloom, 1997; Fiori et al. 1997) have shown that stimulation of bystander immunity can be effective in treating autoimmunity. Specifically, the expression of enterotoxigenic E. coli (ETEC) colonization factor antigen 1 (CFA/I) by an attenuated Salmonella Typhimurium vaccine vector (Salmonella-CFA/I) alters Salmonella’s properties. When macrophages are infected by the Salmonella-CFA/I, the stimulation of inflammatory cytokines is suppressed (Pascual et al., 2002). Upon oral immunization, the observed anti-inflammatory properties of Salmonella-CFA/I could, via bystander immunity, prevent (Jun et al., 2005; Ochoa-Repáraz et al., 2007) or treat EAE (Ochoa-Repáraz et al., 2008) or collagen-induced arthritis (Kochetkova et al., 2008). Subsequently, it has been learned that Salmonella-CFA/I could stimulate diverse subsets of regulatory T cells, depending on the disease model: Treg cells for EAE (Ochoa-Repáraz et al., 2007) and CD39+CD4+ T cells for arthritis (Kochetkova et al., 2008). An advantage of this immunization approach is that it eliminates the need to develop Ag-specific tolerogens for each disease and instead can utilize this nonspecific approach to render Ag-specific Treg cells, as well as Th2 cells during EAE challenge (Jun et al., 2005; Ochoa-Repáraz et al., 2008) without loss of anti-Salmonella protection (Walters et al., 2005). Both the Th2 and Treg cells, when adoptively transferred into EAE-challenged mice, could confer protection, but the level of protection is significantly greater using the vaccine-induced Treg cells. Since production of TGF-β is similar for both CD4+ T cell subsets (Ochoa-Repáraz et al., 2008), we inquired into the significance of this finding. From the evidence presented here, clearly the production of TGF-β by the Treg cells has a greater impact than Th2 cells. Adoptive transfer of vaccine-induced Treg cells and subsequent TGF-β neutralization rendered the complete loss of protection. Similar adoptive transfer of Th2 cells only conferred partial protection against EAE and also showed loss of efficacy as a consequence of TGF-β neutralization. Nonetheless, these results demonstrate that the production of TGF-β is essential for protection mediated by Salmonella-CFA/I-induced Treg and Th2 cells.
2. Materials and methods
2.1 Mice and oral immunizations
Female SJL mice (6 wks-old) were obtained from The Jackson Laboratory (Bar Harbor, MA) or Frederick Cancer Research Facility, National Cancer Institute, (Frederick, MD). All mice were maintained at the Montana State University Animal Resource Center in individual ventilated cages under HEPA-filtered barrier conditions and fed sterile food and water ad libitum. All procedures were compliant with institutional policies for animal health and well-being.
Mice were pretreated with a 50% saturated sodium bicarbonate solution by intragastric gavage and then orally immunized with 5x109 CFU Salmonella Typhimurium-CFA/I (strain H696) or its isogenic Salmonella vector (H647; lacks the cfa/I operon), as previously described (Jun et al., 2005).
2.2 Adoptive transfer, EAE challenge, and TGF-β neutralization
To isolate Treg cells, CD4+CD25− and CD4+CD25+ T cells were obtained from spleens and head and neck lymph nodes (HNLNs) from H696- or H647-vaccinated mice. On day 14 post-immunization, CD4+ T cell subsets were isolated by sequential application of lymphocytes to magnetic beads using a Dynal® Mouse CD4 Negative Isolation Kit (Invitrogen-Life Technologies, Carlsbad, CA), and to isolate CD25+ T cells, CELLection™ Biotin Binder Kit (Invitrogen-Life Technologies); > 98% and 96% cell purity were obtained, respectively.
To induce EAE, SJL females were given s.c 200 μg of the encephalitogenic PLP peptide (PLP139–151; HSLGKWLGHPDKF, HPLC-purified to >90%; Biosynthesis, Inc., Lewisville, TX) in 200 μl of complete Freund’s adjuvant. On days 0 and 2, mice received i.p. 200 ng of Bordetella pertussis toxin (List Biological Laboratories; Campbell, CA), as previously described (Jun et al., 2005; Ochoa-Repáraz et al., 2007; Ochoa-Repáraz et al., 2008).
To determine the mechanism of protection induced by H696 vaccine, naïve SJL mice were given CD25− CD4+ or CD25+ CD4+ T cells (5 × 105/mouse) derived from H696- or H647-immunized mice via tail vein injection (day -1). EAE was induced 1 day after adoptive transfer (day 0). To neutralize TGF-β in vivo, mice were given i.p. anti-TGF-β mAb (0.5 mg/dose) purified from hybridoma cell line (clone 1D11.16.8; American Type Culture Collection, Manassas, VA) on days -1 and 4 of EAE induction; for isotype control Ab, mouse IgG1 (MOPC-21 myeloma; 0.5mg/dose; Abcam, Cambridge, MA) was given. Mice were monitored and scored daily for disease progression (Jun et al., 2005): 0, normal; 1, a limp tail; 2, hind limb weakness; 3, hind limb paresis; 4, quadriplegia; 5, death.
2.3 Cytokine ELISA
To assess the types of protective cytokines induced, spleens and HNLNs were harvested at the peak of the disease, and total mononuclear cells (5 × 106 cells/ml) were cultured without or with 30 μg/ml of PLP139–151 for 3 days in a complete medium consisting of RPMI 1640 medium with the supplements (Invitrogen-Life Technologies): 1 mM sodium pyruvate, 1 mM nonessential amino acids, penicillin/streptomycin (10 U/ml), and 10% FBS (Atlanta Biologicals, Atlanta, GA). IL-4, IL-10, IL-13, IL-17, IFN-γ, and TGF-β were measured from culture supernatants, as previously described (Ochoa-Repáraz et al., 2007). Briefly, wells were coated with mouse cytokine-specific mAbs: IFN-γ (10 μg/ml; BD Pharmingen, San Diego, CA), IL-17 (2 μg/ml; BD Pharmingen), IL-4 (2 μg/ml; BD Pharmingen), IL-13 (4 μg/ml; R&D Systems, Minneapolis, MN), TGF-β (10 μg/ml; R&D Systems), and IL-10 (2 μg/ml; BD Pharmingen). Following blocking with PBS plus 1% BSA for 2hr at 37°C, supernatants were incubated overnight at 4°C. For detection, biotinylated cytokine-specific mAbs were added to wells for 1.5 hr at 37°C: IFN-γ (0.5 μg/ml; BD Pharmingen), IL-17 (0.5 μg/ml; BD Pharmingen), IL-4 (0.2 μg/ml; BD Pharmingen), IL-13 (0.2 μg/ml; R&D Systems), TGF-β (0.5 μg/ml; R&D Systems), and IL-10 (0.3 μg/ml; BD Pharmingen). Following washing, 1:1000 HRP-goat anti-biotin Ab (Vector Laboratories, Burlingame, CA) was added to each well for 1 hr at room temperature, and developed with ABTS peroxidase substrate (Moss Inc., Pasadena, CA).
2.4 Flow cytometry analysis
Immunofluorescent staining for cell surface expression of CD4 and CD25 and intracellular Foxp3 was achieved using fluorochrome-labeled mAbs: CD4-FITC (BD Pharmingen), CD25-APC, and Foxp3-PE (eBioscience, San Diego, CA). For intracellular Foxp3 staining, cells were stained for cell surface Ags and then fixed with 2% paraformaldehyde, permeabilized with 0.2% saponin, and stained with anti-FoxP3-PE, similar to that previously described (Kochetkova et al. 2011). Cells were acquired using a LSR-II flow cytometer (Becton Dickinson, San Jose, CA) and analyzed with Flow Jo software (Tree Star Inc. Ashland, OR).
2.5 Statistical analysis
The Student t test was used to evaluate differences between variations in cytokine levels and flow cytometry data. The ANOVA followed by a posthoc Tukey test was applied to show differences in clinical scores in treated versus PBS mice. Results were considered statistically significant if p-value was less than 0.05.
3. Results
3.1 Adoptive transfer of CD25+CD4+ T cells from orally Salmonella-CFA/I immunized mice protects against EAE in a TGF-β-dependent fashion
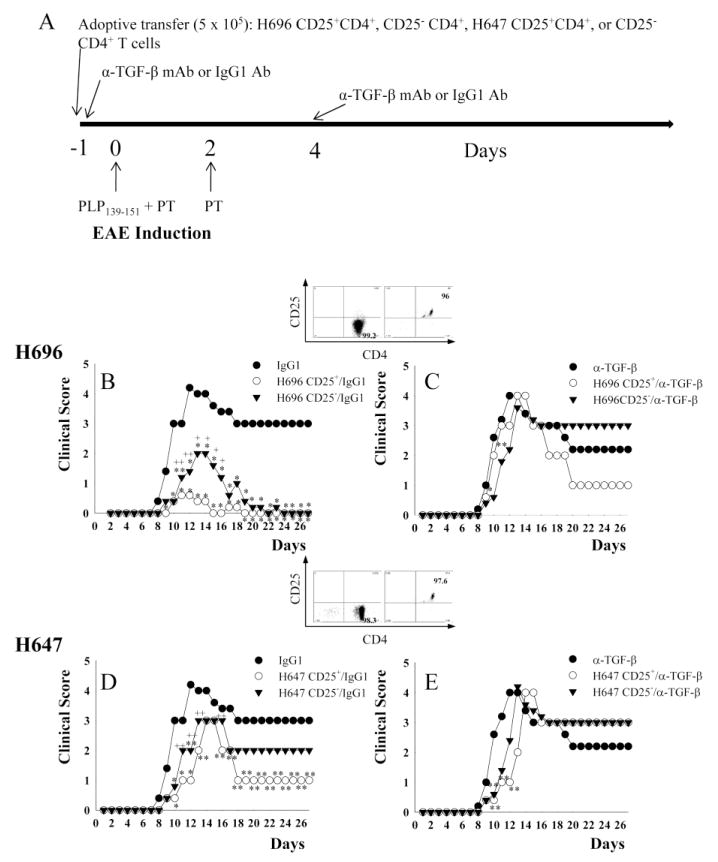
In our previous study, regulatory CD25+CD4+ Treg cells obtained from EAE-challenged mice treated with Salmonella-CFA/I (strain H696) were found to produce elevated levels of TGF-β in contrast to minimal production by Treg cells isolated from isogenic Salmonella (strain H647)-vaccinated mice (Ochoa-Repáraz et al., 2008). To test the hypothesis that TGF-β-producing Treg cells are protective against EAE, groups of SJL mice were orally immunized with Salmonella-CFA/I or its isogenic H647 strain (Fig. 1A). Two wks later, CD25+CD4+ and CD25−CD4+ T cells were isolated to > 98% and 96% purity, respectively, from Salmonella vector- or Salmonella-CFA/I-immunized mice and adoptively transferred into naive mice (day -1) (Fig. 1A-E). Following adoptive transfer, mice were challenged with PLP139–151 (day 0) and treated with anti-TGF-β mAb to neutralize their TGF-β or its isotype control Ab (day -1 and 4) (Fig. 1A). In vivo neutralization of TGF-β in mice given H696-induced CD25+CD4+ T cells abrogated protection against EAE and showed no significant differences from PBS/IgG1-dosed EAE control mice at the peak of the disease (Fig. 1B and C); mice treated with the same Treg cells, but with isotype IgG control Ab, were protected against EAE with minimal clinical disease and showed a delayed onset of disease with no relapse of the disease during the observation period (Table 1, Fig. 1B). In the absence of TGF-β neutralization, H647-induced CD25+CD4+ T cells failed to confer protection against EAE, although the clinical severity was significantly less than in TGF-β-neutralized mice (p < 0.05) (Fig. 1D and E). Thus, these results show a critical role for TGF-β derived from H696-induced CD25+CD4+ T cells and qualitative differences in the protective capacity of these Treg cells against EAE.
Fig. 1.
Adoptive transfer of Salmonella-CFA/I-induced CD25+CD4+ T cells confers protection against EAE, but is abrogated upon in vivo neutralization of TGF-β. CD25+CD4+ and CD25−CD4+ T cells obtained from mice previously orally immunized with Salmonella-CFA/I (strain H696) or Salmonella vector (strain H647) were adoptively transferred into naive SJL mice. A, Schematic of adoptive transfer, EAE challenge with PLP139–151, and in vivo neutralization with anti-TGF-β mAb or isotype MOPC 21 IgG1 control Ab is shown; PT, pertussis toxin. EAE clinical scores following adoptive transfer, EAE challenge, and in vivo treatment with IgG1 Ab or anti-TGF-β mAb of recipients given B,C, CD25+CD4+ and CD25− CD4+ T cells (purity plots in inset) from H696-immunized mice; and D, E, CD25+CD4+ and CD25− CD4+ T cells (purity plots in inset) from H647-immunized mice are shown. Data depict mean clinical scores combined from three experiments: *, p < 0.001; **, p < 0.05 versus PBS/IgG1or PBS/α-TGF-β mAb-treated mice; +, p < 0.001; ++, p < 0.05 for CD25+ versus CD25−.
Table 1.
Adoptive transfer of Salmonella/CFA-I (H696)-induced CD25+CD4+ T cells to naïve recipients confers protection against EAE in a TGF-β-dependent fashion.
| Adoptive Transfer/Treatment a | Day of Onset b | EAE c/Total | Max. Score d | C.S. e | |
|---|---|---|---|---|---|
| PBS | IgG1 | 7.4±0.4 | 15/15 | 5 | 57.8 |
| α-TGF-β | 7.8±1.4 | 15/15 | 5 | 46.8 | |
| H696 | CD25+/ IgG1 | 11.4±0.6 *, †, ++ | 9/15 | 1 | 2.8 *, †, + |
| CD25+/α-TGF-β | 7.8±1.8 | 15/15 | 5 | 49.8 | |
| CD25−/ IgG1 | 9±1.2 | 15/15 | 4 | 12.2 † | |
| CD25−/α-TGF-β | 8.4±0.6 | 15/15 | 5 | 51.2 | |
| H647 | CD25+/ IgG1 | 8.8±1.3 | 15/15 | 5 | 28 **, †† |
| CD25+/α-TGF-β | 8.2±0.4 | 15/15 | 5 | 50.6 | |
| CD25−/ IgG1 | 8.4±1.6 | 15/15 | 5 | 39.2 | |
| CD25−/α-TGF-β | 8.2±0.6 | 15/15 | 5 | 52.2 |
Two wks after immunization with H696 or H647, cell-sorted CD25+CD4+ and CD25−CD4+ T cells were adoptively transferred (5x105) into naïve recipients 1 day prior to EAE induction. EAE was induced as described in Materials and Methods on day 0. Mice were treated with anti-TGF-β mAb or its isotype Ab on the day of adoptive transfer and 4 days post-EAE challenge
Mean day ± SD of clinical disease onset. *, p < 0.001 versus PBS/IgG1; †, p < 0.001 versus CD25+/α-TGF-β; ++, p < 0.05 versus CD25−/IgG1.
Number of mice with EAE/total number of mice in group.
Maximum (Max.) daily clinical score.
Cumulative disease scores (C.S.) were calculated as the sum of all clinical score from disease onset to the days of sacrifice, divided by the number of mice in each group. *, p < 0.001; **, p < 0.05 versus PBS/IgG1; †, p < 0.001; ††, p < 0.05 versus CD25+/α-TGF-β; +, p < 0.001 versus CD25−/IgG1.
The capacity of CD25−CD4+ T cells to protect against EAE was also assessed (Fig. 1B-D). H696-induced CD25−CD4+ T cells conferred protection against EAE, albeit not as effectively as the induced Treg cells, but nonetheless with significantly diminished clinical scores when compared to anti-TGF-β mAb-treated group (p < 0.05) or PBS/IgG1-treated disease control (p < 0.001). This evidence suggests H696-induced CD25− CD4+ T cells do partially contribute to protection via TGF-β, since neutralization of this cytokine also abrogated protection (Table 1, Fig. 1C). CD25−CD4+ T cells derived from mice immunized with Salmonella vector were unable to confer protection, and TGF-β neutralization exacerbated EAE (Table 1, Fig. 1D and E). Collectively, these data show the importance of TGF-β in conferring protection against EAE by the Salmonella-CFA/I-induced CD25+ and CD25−CD4+ T cells.
3.2 In vivo TGF-β neutralization aborts EAE protection due to elevated Th1 and Th17 responses
To examine how TGF-β neutralization resulted in loss of protection, Ag-specific cytokine analysis was performed at the peak of the EAE (day 14 after EAE challenge). Total splenic and HNLN mononuclear cells were isolated from each of the treatment groups (Table 1) and restimulated with PLP139–151 for 3 days (Figs. 2 – 4). While HNLN H696- and H647-induced Treg cells significantly suppressed IFN-γ production relative to the PBS-treated mice, in vivo TGF-β neutralization failed to reverse splenic IFN-γ production (Fig. 2A). In vivo TGF-β neutralization of mice adoptively transferred with H696-induced Treg cells did enhance IL-17 production in HNLNs similar to PBS-dosed mice (Fig. 2B); IL-17 production was also enhanced by splenic Treg cells neutralized of their TGF-β. Mice adoptively transferred with H647-induced Treg cells showed similar levels of IL-17 as PBS-dosed mice, and TGF-β neutralization had no impact on IL-17 production (Fig. 2B). Examination of Th2 (Fig. 3) and regulatory cytokines (Fig. 4) produced by mice treated with H696-induced Treg cells revealed HNLN and splenic IL-4 and TGF-β levels were significantly enhanced relative to production by mononuclear cells from PBS-dosed mice, and TGF-β neutralization suppressed this production. Mice adoptively transferred with H647-induced Treg cells produced minimal to no IL-4 or TGF-β (Figs. 3 and 4). Minimal IL-13 was produced in these mice adoptively transferred with H696- or H647-induced Treg cells and treated or untreated with anti-TGF-β mAb (Fig. 3). Mice adoptively transferred with H696-induced Treg cells/IgG1 showed enhanced HNLN and splenic IL-10 production relative to PBS-treated mice, and in vivo TGF-β neutralization abrogated this response; minimal to no IL-10 was induced in mice adoptively transferred with H647-induced Treg cells (Fig. 4B).
Fig. 2.
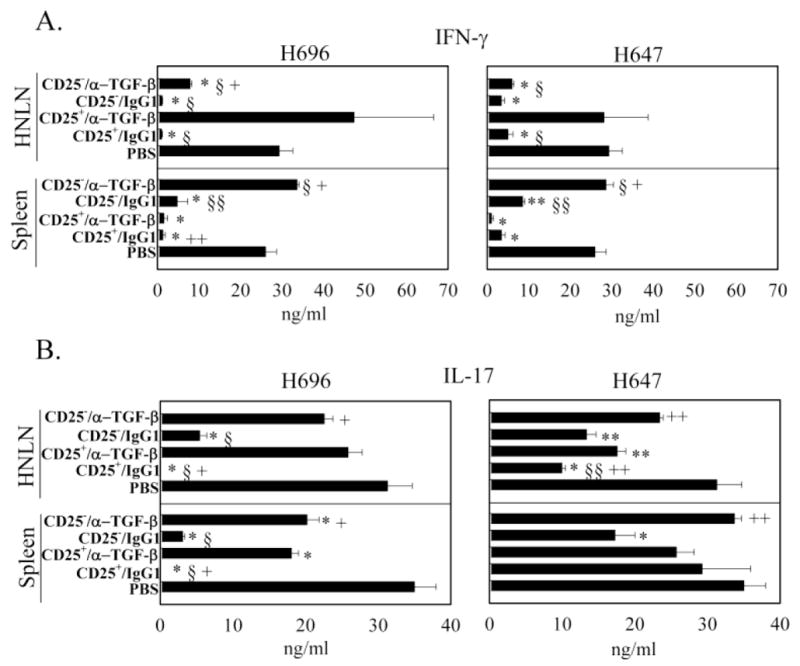
Adoptively transferred Salmonella-CFA/I-induced CD25+CD4+ T cells suppress IFN-γ and IL-17 production. At the peak of EAE, A, B, HNLN (top panels) and splenic (bottom panels) mononuclear cells were PLP139–151-restimulated for 3 days, and A, IFN-γ and B, IL-17 production in culture supernatants were measured by cytokine-specific ELISA. Cytokine production by recipients adoptively transferred with CD4+ T cell subsets from Salmonella-CFA/I (H696; left), and Salmonella vector (H647; right) was measured for the various treatment groups: PBS, CD25+CD4+ T cells plus IgG1, CD25+CD4+ T cells plus anti-TGF-β mAb, CD25− CD4+ T cells plus IgG1, and CD25− CD4+ T cells plus anti-TGF-β mAb. Data depict the mean ± SEM of triplicate wells combined from two experiments: *, p < 0.001; **, p < 0.05 versus PBS; §, p < 0.001; §§, p < 0.05 versus CD25+/α-TGF-β and +, p < 0.001; ++, p < 0.05 versus CD25−/IgG1.
Fig. 4.
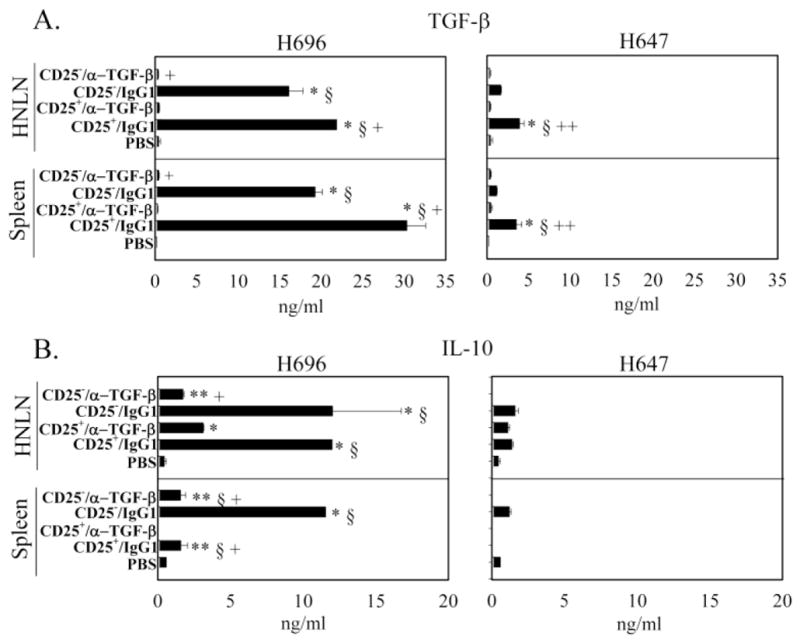
Adoptively transferred Salmonella-CFA/I-induced CD25+CD4+ T cells enhance TGF-β and IL-10 production. At the peak of EAE, A, B, HNLN (top panels) and splenic (bottom panels) mononuclear cells were restimulated as described in Fig. 2, and A, TGF-β and B, IL-10 production in culture supernatants were measured by cytokine-specific ELISA. Cytokine production by recipients adoptively transferred with CD4+ T cell subsets from Salmonella-CFA/I (H696; left), and Salmonella vector (H647; right) was measured for the various treatment groups: PBS, CD25+CD4+ T cells plus IgG1, CD25+CD4+ T cells plus anti-TGF-β mAb, CD25− CD4+ T cells plus IgG1, and CD25− CD4+ T cells plus anti-TGF-β mAb. Data depict the mean ± SEM of triplicate wells combined from two experiments: *, p < 0.001; **, p < 0.05 versus PBS; §, p < 0.001; §§, p < 0.05 versus CD25+/α-TGF-β and +, p < 0.001; ++, p < 0.05 versus CD25−/IgG1.
Fig. 3.
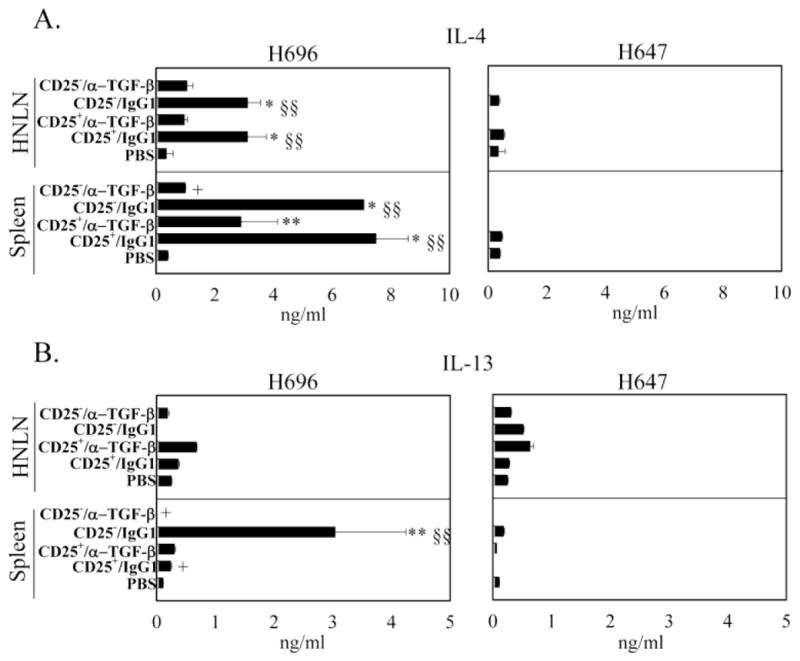
Adoptively transferred Salmonella-CFA/I-induced CD25+CD4+ T cells enhance IL-4 and CD25− CD4+ T cells enhance IL-13 production. At the peak of EAE, A, B, HNLN (top panels) and splenic (bottom panels) mononuclear cells were restimulated as described in Fig. 2, and A, IL-4 and B, IL-13 production in culture supernatants were measured by cytokine-specific ELISA. Cytokine production by recipients adoptively transferred with CD4+ T cell subsets from Salmonella-CFA/I (H696; left), and Salmonella vector (H647; right) was measured for the various treatment groups: PBS, CD25+CD4+ T cells plus IgG1, CD25+CD4+ T cells plus anti-TGF-β mAb, CD25− CD4+ T cells plus IgG1, and CD25− CD4+ T cells plus anti-TGF-β mAb. Data depict the mean ± SEM of triplicate wells combined from two experiments: *, p < 0.001; **, p < 0.05 versus PBS, §, p < 0.001; §§, p < 0.05 versus CD25+/α-TGF-β and +, p < 0.001; ++, p < 0.05 versus CD25−/IgG1.
Mice adoptively transferred with H696-induced CD25−CD4+ T cells showed suppressed production of HNLN and splenic IFN-γ, and this suppression was reversed by the in vivo TGF-β neutralization (Fig. 2A-B). However, only H696-induced CD25−CD4+ T cells were able to effectively suppress HNLN and splenic IL-17 production, unlike those induced by H647 vaccine, which showed only partial suppression (Fig. 2B). The observed suppression of inflammatory cytokines mediated by the H696-induced CD25−CD4+ T cells appeared to be attributed to elevated production of HNLN and splenic Th2-type, IL-4 (Fig. 3A) and regulatory cytokines, IL-10 and TGF-β (Fig. 4A and B). Mice adoptively transferred with H647-induced CD25− CD4+ T cells, and co-administered isotype control Ab or anti-TGF-β mAb produced minimal to no IL-4, IL-13, IL-10, or TGF-β (Figs. 3 and 4). Thus, these data show that Salmonella-CFA/I stimulates regulatory and Th2-type cytokine-producing CD25+CD4+ and CD25−CD4+ T cells, suppressing Th1- and Th17-type cells, and in vivo TGF-β neutralization diminishes the production of these anti-inflammatory cytokines, compromising protection to EAE.
3.3 TGF-β neutralization dampens the frequency of Foxp3+ CD4+ T cells
Since Foxp3 transcription factor is expressed by Treg cells, differential Foxp3+ cell frequencies were determined as a consequence of in vivo TGF-β neutralization (Fig. 5). CD4+ T cells were sorted from individual HNLNs of each experimental group at the peak of the EAE and evaluated for Foxp3 expression by FACS. A dramatic reduction in numbers of Foxp3+CD4+ T cells were found in mice given H696-induced CD25+CD4+ T cells and subjected to TGF-β neutralization relative to mice given the same cells, but treated with isotype control Ab (Fig. 5 and Table 2). Likewise, mice given H696-induced CD25− CD4+ T cells and subjected to TGF-β neutralization showed depressed frequency of Foxp3+CD4+ T cells relative to isotype control Ab treatment (Fig. 5 and Table 2). Similar reductions were found in the frequency of Foxp3+CD4+ T cells in mice given CD25+CD4+ T cells derived from H647-immunized mice and subjected to anti-TGF-β mAb treatment (Fig. 5 and Table 2). However, mice given H647-induced CD25− CD4+ T cells and treated with either anti-TGF-β mAb or isotype control Ab did not alter the frequency of Foxp3+CD4+ T cells (Fig. 5 and Table 2). Hence, adoptive transfer with Salmonella-CFA/I-induced CD25+CD4+ T cells in the absence of TGF-β neutralization resulted in the best stimulation of Foxp3+CD4+ T cells, further emphasizing the importance of TGF-β for the expansion and/or maintenance of these Treg cells.
Fig. 5.
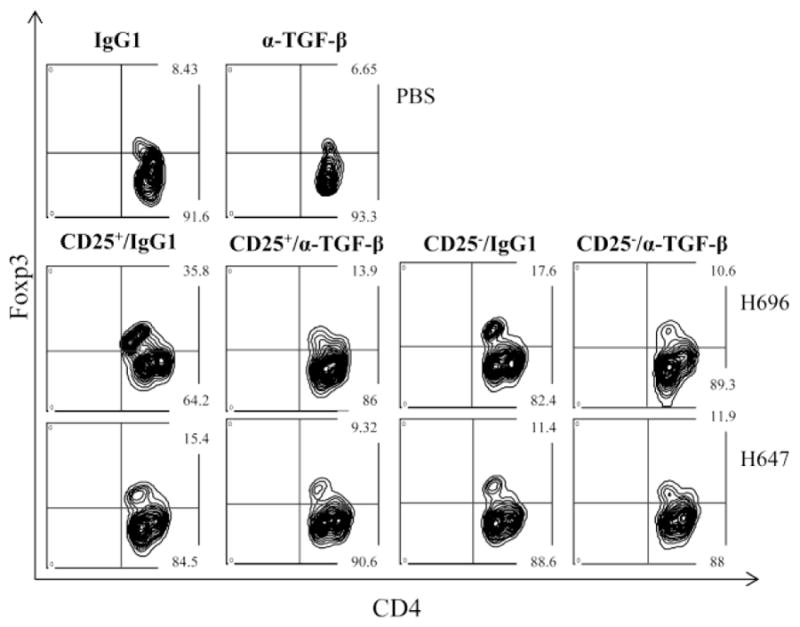
In vivo TGF-β neutralization reduces the expression of Foxp3. At the peak of EAE, FACS analysis for Foxp3 expression on CD4+ T cells was performed on cells obtained from mice adoptively transferred with Salmonella-CFA/I (H696)- or Salmonella vector (H647)-induced CD25+CD4+ and CD25− CD4+ T cells and co-administered IgG1 or anti-TGF-β mAb.
Table 2.
Adoptive transfer of Salmonella-CFA/I-induced CD25+CD4+ T cells enhances Foxp3+ frequency in the recipient micea.
| Treatment b | % Fox3+/CD4+ c | Average Clinical Score d | |
|---|---|---|---|
| PBS | IgG1 | 8.2 ± 2.4 | 4.2 ± 0.5 |
| α-TGF-β | 6.2 ± 3.6 | 4.4 ± 0.6 | |
| H696 | CD25+/IgG1 | 37.2 ± 3.6 *, †, + | 0.4 ± 0.2 *, †, + |
| CD25+/α-TGF-β | 12.2 ± 3.2 | 4.0 ± 0.5 | |
| CD25−/IgG1 | 16.5 ± 3.1 ** | 2.4 ± 0.8 **, †† | |
| CD25−/α-TGF-β | 11.4 ± 2.8 | 4.2 ± 0.5 | |
| H647 | CD25+/IgG1 | 14.5 ± 2.7 †† | 3.8 ± 0.6 |
| CD25+/α-TGF-β | 8.54 ± 2.2 | 4.4 ± 0.8 | |
| CD25−/IgG1 | 11.8 ± 2.5 | 3.8 ± 0.2 | |
| CD25−/α-TGF-β | 10.3 ± 3.8 | 4.2 ± 0.6 |
Naïve SJL recipients were adoptively transferred with cell-sorted CD25+CD4+ and CD25−CD4+ T cells from mice previously (2 wks earlier) immunized with H696 or H647 vaccines one day prior to EAE.
Mice were treated twice with anti-TGF-β mAb or its isotype IgG1 control Ab on the same day of adoptive transfer and 4 days post-EAE challenge.
At the peak of EAE, CD4+ T cells were purified from HNLNs obtained from individual mice and measured Foxp3+ frequency by FACS. Seven mice/group were used and data represent mean ± SEM. *, p < 0.001; **, p < 0.05 versus PBS/IgG1; †, p < 0.001; ††, p < 0.05 versus CD25+/α-TGF-β; +, p < 0.001 versus CD25−/IgG1.
Upon termination of experiment, clinical scores were measured, and the data represent mean clinical score ± SEM. *, p < 0.001; **, p < 0.05 versus PBS/IgG1; †, p < 0.001; ††, p < 0.05 versus CD25+/α-TGF-β; +, p < 0.001 versus CD25−/IgG1.
4. Discussion
Encephalitogenic T cells are believed to be activated in the periphery and subsequently migrate into the CNS to initiate EAE (Olsson, 1996; Fife et al., 2000). Reactivation of infiltrating autoreactive T cells by CNS resident APCs stimulates inflammatory cytokine production to facilitate massive influx of nonspecific T cells, macrophages, B cells, and neutrophils, exacerbating further CNS tissue damage and paralysis (Juedes et al., 2000; Kroenke, 2008; Li et al., 2011). Cytokines play a crucial role in the initiation, expansion, and regulation of EAE. Initially, IFN-γ, produced by myelin-specific Th1 cells, was considered as a major contributor of the EAE, and ways to neutralize Th1 cell activity were actively pursued for treating disease, especially since EAE could be induced in naive mice adoptively transferred with myelin-specific Th1 cells (Linington et al., 1993; Miller et al., 1995; Williams et al., 2011). However, studies with IFN-γ−/− (Ferber et al., 1996) or IFN-γR−/− mice (Willenborg et al., 1996) or neutralization of IFN-γ (Billiau et al., 1998; Duong et al., 1994) or abrogation of IFN-γ-mediated signaling (Langrish et al., 2004) showed IFN-γ was not critical in the development of EAE. In a similar vein, IL-12, a major stimulator of IFN-γ production and originally thought to be as a crucial proinflammatory cytokine for EAE, presented conflicting results in IL-12p35−/− and IL-12p40−/− mice: IL-12p35−/− mice were susceptible to EAE, as opposed to IL-12p40−/− mice, which were resistant to EAE (Gran et al., 2002; Becher et al., 2002). These results implicated other sources of inflammatory mediators involving IL-12p40. It was subsequently found that IL-23 comprising IL-23p19 and IL-12 p40 subunits supported the development of EAE through the proliferation of Th17 cells (Langrish et al., 2005), and as such, IL-23p19−/− mice were protected from EAE development (Cua et al., 2003). Thus, IL-23 is critical for EAE induction and subsequently has been found essential for encephalitogenic T cells homing to the CNS and their survival in the CNS (Gyülvészi et al., 2009), and the exacerbation of encephalitogenic effects by Th17 cells within the CNS became more pronounced when infiltrating APCs respond to GM-CSF stimulation (Codarri et al. 2011; El-Behi et al. 2011).
Th2 cells, through the production of anti-inflammatory cytokines IL-4 (Falcone et al., 1998; Inobe et al., 1998) and IL-13 (Offner et al., 2005; Ochoa-Repáraz et al., 2008), have been shown to be protective against EAE. However, adoptive transfer of Th2 cells into Rag-1−/−, but not immunocompetent, mice could induce EAE (Lafaille et al., 1997). Alternatively, Treg cells are the major suppressive T cells, characterized by their expression of CD25 and Foxp3. Foxp3+CD25+ Treg cells suppress effector T cell proliferation by cell-cell contact inhibition (TRAIL, CTLA-4) (Ren et al., 2007; Annunziato et al., 2002) and cytokine-mediated mechanisms (IL-10, TGF-β, and IL-35) (Maloy et al., 2001; Kochetkova et al., 2010). To exploit Treg cells in treating EAE, a number of approaches have been tested, including vaccination with altered peptide ligands (Young et al., 2000), isolating Treg cells from diseased animals (McGeachy et al., 2005), in vitro Treg cell stimulation with cognate Ag (Yu et al., 2005), polyclonal activation with anti-CD3 and IL-2 treatment (Taylor et al., 2002; Vieira et al., 2004), or irrelevant Ag immunization, as we previously have shown (Jun et al., 2005; Ochoa-Repáraz et al., 2007). Obtaining Treg cells from diseased patients has the disadvantage of not knowing the TCR specificity. Polyclonally expanded Treg cells have adverse clinical consequences, resulting in cessation of their evaluation in humans (Marshall, 2006). To circumvent the lack of understanding the etiology of MS, one alternative approach is to stimulate Treg cells to a defined Ag unrelated to suspect Ag-specificities of encephalitogenic T cells. Salmonella-CFA/I vaccine offers such an alternative strategy capable of deriving Ag-specific Treg cells independent of myelin proteins. Studies here show adoptive transfer of CD25+CD4+ T cells from Salmonella-CFA/I-vaccinated mice confers protection against EAE. Previous studies have shown both CD25−CD4+ and CD25+CD4+ T cells from Salmonella-CFA/I-vaccinated mice produce elevated levels of TGF-β when compared to the same T cells derived from Salmonella vector-immunized mice (Ochoa-Repáraz et al., 2008). Since TGF-β has been known to suppress encephalitogenic T effector cells and confer protection against EAE (Gorelik et al., 2002; Faria et al., 2003; Chen et al., 2003), we queried the significance of TGF-β production by Salmonella-CFA/I induced Treg cells upon the protection of EAE.
An adoptive transfer experiment was performed to assess the protective capacity of TGF-β produced by CD25+CD4+ T cells obtained from Salmonella-CFA/I-immunized mice. Mice given CD25+CD4+ T cells obtained from Salmonella-CFA/I-vaccinated mice were protected against EAE (p < 0.001: Salmonella-CFA/I vs. Salmonella vector). While adoptive transfer of CD25+CD4+ T cells obtained from Salmonella vector-vaccinated mice was effective in reducing EAE relative to PBS/IgG-treated EAE control group (p<0.05), it was not as efficacious as for those recipients given Salmonella-CFA/I-induced CD25+CD4+ T cells. These results implicate differences in potency by the respective CD25+CD4+ T cells in suppressing EAE. In vivo neutralization of TGF-β upon adoptive transfer of CD25+CD4+ T cells obtained from both Salmonella-CFA/I- and Salmonella vector-immunized mice completely reversed protection to EAE. Thus, these data show the importance of TGF-β production by Salmonella-CFA/I-induced Treg cells upon EAE accomplished independently of auto-Ag. Moreover, we hypothesize these induce Treg cells stimulate myelin-specific Treg cells in a bystander-dependent fashion, since upon in vitro PLP139–151 restimulation, enhanced TGF-β production was observed in mice adoptively transferred with CD25+CD4+ T cells from Salmonella-CFA/I-vaccinated mice. Since Salmonella-CFA/I-induced CD25−CD4+ T cells also showed elevated TGF-β production relative to Salmonella vector-immunized mice, TGF-β production by CD25−CD4+ T cells was assessed for its contribution to protection against EAE. Mice given Salmonella-CFA/I-induced CD25−CD4+ T cells were only partially protected against EAE, unlike with Salmonella vector-induced CD25−CD4+ T cells, which failed to confer protection. While the contribution from TGF-β produced by Salmonella-CFA/I-derived CD25−CD4+ T cells was not as apparent, in vivo TGF-β neutralization abrogated this protection. Thus, as with the induced Treg cells, these results show that enhanced TGF-β production by Salmonella-CFA/I-induced CD25−CD4+ T cells can, in part, contribute to protection against EAE in addition to other Th2 cytokines (Jun et al., 2005; Ochoa-Repáraz et al., 2007; Ochoa-Repáraz et al., 2008).
From the in vivo neutralization studies, the relevance of TGF-β produced by the Salmonella-CFA/I-induced CD25+CD4+ T cells for protection against EAE is clear. TGF-β has been previously found to protect against EAE by suppressing encephalitogenic T effector cells (Gorelik et al., 2002; Faria et al., 2003; Chen et al., 2003) and to expand or sustain functional Treg cells (Liu et al., 2008; Lu et al., 2010; Gabryšová et al., 2011). From the in vitro peptide restimulation assays, mice adoptively transferred with Treg cells showed the greatest production of TGF-β, unlike the same cells derived from Salmonella vector-immunized mice. Suppression of inflammatory cytokines was also evident by the dampened IFN-γ and IL-17 production and their subsequent reversal when mice were treated with anti-TGF-β mAb. Otherwise, no such suppression of IL-17 was evident in mice adoptively transferred with Salmonella-vector-induced CD25+CD4+ T cells, although some degree of IFN-γ suppression was achieved. Given these findings, the elevated TGF-β production, suppressing both Th1 and Th17 cells, mediated the observed protection against EAE by both Salmonella-CFA/I-induced CD25+CD4+ and CD25−CD4+ T cells. However, IL-4 may have a supportive role in combination with TGF-β, as IL-4 was elevated for both CD25+CD4+ and CD25− CD4+ T cells, similar to that previously found (Ochoa-Repáraz et al., 2008; Rynda et al., 2010). Still, others have also found IL-4 enhances TGF-β-producing Treg cell function (Faria and Weiner, 2005) and stimulates the conversion of Foxp3+ CD25+CD4+ T cells (Skapenko et al., 2005). The role of IL-13 was less evident. Perhaps how the CFA/I fimbrial Ags are presented may dictate the stimulation of IL-13 since periplasmic/intracellular CFA/I fimbriae expression did augment IL-13’s protective capacity against EAE (Ochoa-Repáraz et al., 2008). Others have shown IL-13 can be protective against EAE (Offner et al., 2005).
In addition to TGF-β’s suppressive capacity, its essential role has been shown in the induction of Foxp3 expression and maintenance of Treg (natural Treg and inducible Treg) cells (Liu et al., 2008; Lu et al., 2010; Gabryšová et al., 2011). To this end, the influence of TGF-β upon the frequency of Foxp3+ T cells was measured. TGF-β clearly had a role in the development/expansion of Foxp3+ T cells because in vivo neutralization of TGF-β dampened the frequency of Foxp3+ T cells by more than 3-fold (p < 0.001). Adoptive transfer of Salmonella vector-induced CD25+CD4+ T cells did not have the impact upon the frequency of Foxp3+ T cells as did adoptive transfer of Treg cells obtained from Salmonella-CFA/I-vaccinated mice. TGF-β neutralization of the adoptively transferred Treg cells from Salmonella vector-immunized mice abrogated what few Foxp3+ T cells were present. Thus, these data show TGF-β is important for the stimulation and maintenance of Treg cells induced by Salmonella-CFA/I.
In summary, the results show vaccination with irrelevant Ags promotes stimulation of Treg cells. In this case, CFA/I fimbriae offer an alternative strategy to adapt Treg cells to perpetuate the expansion of myelin-specific Treg cells to suppress autoimmune disease. Salmonella-CFA/I can elicit elevated levels of Treg cells within two weeks of vaccination and, as shown here, when adoptively transferred, can confer protection against EAE in a bystander fashion. The vaccine-induced Treg cells produced TGF-β, which is important for subsequent stimulation of PLP-specific Treg cells. The potency of these Treg cells was ablated upon in vivo TGF-β neutralization. Although the Salmonella-CFA/I-induced CD25−CD4+ T cells also produced TGF-β, their potency was less than the observed Treg cells. Nonetheless, these CD25− CD4+ T cells may also be a Treg cell subset, albeit independent of Foxp3. Since IL-4 was produced by both T cell subsets, IL-4 may be acting to enhance their regulatory function, as others have suggested (Faria and Weiner, 2005; Skapenko et al., 2005; Ochoa-Repáraz et al., 2008; Rynda et al., 2010). The impact of these Treg cells was largely attributed to the fimbriae since the Salmonella vector exhibited much less capacity to promote the development and expansion of Treg cells and was clearly less capable of protecting against EAE. Moreover, these TGF-β-producing Treg cells potently suppressed the development and expansion of Th1 and Th17 cells. In conclusion, the described studies demonstrate the effectiveness of Salmonella-CFA/I-induced TGF-β-producing Treg cells to protect against autoimmune disease.
Acknowledgments
We thank Ms. Nancy Kommers for her assistance in preparing the manuscript. This work is supported by U.S. Public Health Service Grant R01 AI-41123 and, in part, by Montana Agricultural Experiment Station and U.S. Department of Agriculture Formula Funds. The Department of Immunology and Infectious Diseases’ flow cytometry facility was, in part, supported by NIH/National Center for Research Resources, Centers of Biomedical Excellence P20 RR-020185, and an equipment grant from the M.J. Murdock Charitable Trust.
Abbreviations
- CFA/I
colonization factor antigen I
- EAE
experimental autoimmune encephalomyelitis
- HNLNs
head and neck lymph nodes
- MS
multiple sclerosis
- PLP
proteolipid protein
- Treg cell
regulatory T cell
Footnotes
Disclosure
The authors have no financial conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aggarwal S, Ghilardi N, Xie MH, de Sauvage FJ, Gurney AL. Interleukin-23 promotes a distinct CD4 T cell activation state characterized by the reduction of interleukin-17. J Biol Chem. 2003;278:1910–1914. doi: 10.1074/jbc.M207577200. [DOI] [PubMed] [Google Scholar]
- Annunziato F, Cosmi L, Liotta F, Lazzeri E, Manetti R, Vanini V, Romagnani P, Maggi E, Romagnani S. Phenotype, localization, and mechanism of suppression of CD4+CD25+ human thymocytes. J Exp Med. 2002;196:379–387. doi: 10.1084/jem.20020110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrat FJ, Cua DJ, Boonstra A, Richards DF, Crain C, Savelkoul HF, de Waal-Malefyt R, Coffman RL, Hawrylowicz CM, O’Garra A. In vitro generation of interleukin 10-producing regulatory CD4+ T cells is induced by immunosuppressive drugs and inhibited by T helper type 1 (Th1)- and Th2-inducing cytokines. J Exp Med. 2002;195:603–616. doi: 10.1084/jem.20011629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxter AG. The origin and application of experimental autoimmune encephalomyelitis. Nat Rev Immunol. 2007;7:904–912. doi: 10.1038/nri2190. [DOI] [PubMed] [Google Scholar]
- Becher B, Durell BG, Noelle RJ. Experimental autoimmune encephalitis and inflammation in the absence of interleukin-12. J Clin Invest. 2002;110:493–497. doi: 10.1172/JCI15751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bettelli E, Das MP, Howard ED, Weiner HL, Sobel RA, Kuchroo VK. IL-10 is critical in the regulation of autoimmune encephalomyelitis as demonstrated by studies of IL-10- and IL-4-deficient and transgenic mice. J Immunol. 1998;161:3299–3306. [PubMed] [Google Scholar]
- Billiau A, Heremans H, Vandekerckhove F, Dijkmans R, Sobis H, Meulepas E, Carton H. Enhancement of experimental allergic encephalomyelitis in mice by antibodies against IFN-gamma. J Immunol. 1988;140:1506–1510. [PubMed] [Google Scholar]
- Campbell DJ, Koch MA. Phenotypical and functional specialization of FOXP3+ regulatory T cells. Nat Rev Immunol. 2011;11:119–130. doi: 10.1038/nri2916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, Jin W, Hardegen N, Lei KJ, Li L, Marinos N, McGrady G, Wahl SM. Conversion of peripheral CD4+CD25− naive T cells into CD4+CD25+ regulatory T cells by TGF-β induction of transcription factor FoxP3. J Exp Med. 2003;198:1875–1886. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu CQ, Wittmer S, Dalton DK. Failure to suppress the expansion of the activated CD4 T cell population in interferon-γ-deficient mice leads to exacerbation of experimental autoimmune encephalomyelitis. J Exp Med. 2000;192:123–128. doi: 10.1084/jem.192.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Codarri L, Gyülvészi G, Tosevski V, Hesske L, Fontana A, Magnenat L, Suter T, Becher B. RORγt drives production of the cytokine GM-CSF in helper T cells, which is essential for the effector phase of autoimmune neuroinflammation. Nat Immunol. 2011;12:560–567. doi: 10.1038/ni.2027. [DOI] [PubMed] [Google Scholar]
- Collison LW, Chaturvedi V, Henderson AL, Giacomin PR, Guy C, Bankoti J, Finkelstein D, Forbes K, Workman CJ, Brown SA, Rehg JE, Jones ML, Ni HT, Artis D, Turk MJ, Vignali DA. IL-35-mediated induction of a potent regulatory T cell population. Nat Immunol. 2010;11:1093–1101. doi: 10.1038/ni.1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cua DJ, Sherlock J, Chen Y, Murphy CA, Joyce B, Seymour B, Lucian L, To W, Kwan S, Churakova T, Zurawski S, Wiekowski M, Lira SA, Gorman D, Kastelein RA, Sedqwick JD. Interleukin-23 rather than interleukin-12 is the crucial cytokine for autoimmune inflammation of the brain. Nature. 2003;421:744–748. doi: 10.1038/nature01355. [DOI] [PubMed] [Google Scholar]
- Duong TT, Finkelman FD, Singh B, Strejan GH. Effect of anti- interferon-gamma monoclonal antibody treatment on the development of experimental allergic encephalomyelitis in resistant mouse strains. J Neuroimmunol. 1994;53:101–107. doi: 10.1016/0165-5728(94)90069-8. [DOI] [PubMed] [Google Scholar]
- El-Behi M, Ciric B, Dai H, Yan Y, Cullimore M, Safavi F, Zhang GX, Dittel BN, Rostami A. The encephalitogenicity of TH17 cells is dependent on IL-1- and IL-23-induced production of the cytokine GM-CSF. Nat Immunol. 2011;12:568–575. doi: 10.1038/ni.2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falcone M, Bloom BR. A T helper cell 2 (Th2) immune responses against non-self antigens modifies the cytokine profile of autoimmune T cells and protects against experimental allergic encephalomyelitis. J Exp Med. 1997;185:901–907. doi: 10.1084/jem.185.5.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falcone M, Rajan AJ, Bloom BR, Brosnan CF. A critical role for IL-4 in regulating disease severity in experimental allergic encephalomyelitis as demonstrated in IL-4-deficient C57BL/6 mice and BALB/c mice. J Immunol. 1998;160:4822–4830. [PubMed] [Google Scholar]
- Faria AM, Maron R, Ficker SM, Slavin AJ, Spahn T, Weiner HL. Oral tolerance induced by continuous feeding: enhanced up-regulation of transforming growth factor–β/interleukin-10 and suppression of experimental autoimmune encephalomyelitis. J Autoimmun. 2003;20:135–145. doi: 10.1016/s0896-8411(02)00112-9. [DOI] [PubMed] [Google Scholar]
- Faria AM, Weiner HL. Oral tolerance. Immunol Rev. 2005;206:232–259. doi: 10.1111/j.0105-2896.2005.00280.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferber IA, Brocke S, Taylor-Edwards C, Ridgway W, Dinisco C, Steinman L, Dalton D, Fathman CG. Mice with a disrupted IFN-γ gene are susceptible to the induction of experimental autoimmune encephalomyelitis (EAE) J Immunol. 1996;156:5–7. [PubMed] [Google Scholar]
- Fife BT, Huffnagle GB, Kuziel WA, Karpus WJ. CC chemokine receptor 2 is critical for induction of experimental autoimmune encephalomyelitis. J Exp Med. 2000;192:899–905. doi: 10.1084/jem.192.6.899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiori P, Ristori G, Cacciani A, Buttinelli C, Falcone M, di Giovanni S, Montesperelli C, Pozzilli C, Salvetti M. Down-regulation of cell-surface CD4 co-receptor expression and modulation of experimental allergic encephalomyelitis. Int Immunol. 1997;9:541–545. doi: 10.1093/intimm/9.4.541. [DOI] [PubMed] [Google Scholar]
- Gabryšová L, Christensen JR, Wu X, Kissenpfennig A, Malissen B, O’Garra A. Integrated T-cell receptor and costimulatory signals determine TGF-β-dependent differentiation and maintenance of Foxp3+ regulatory T cells. Eur J Immunol. 2011;41:1242–1248. doi: 10.1002/eji.201041073. [DOI] [PubMed] [Google Scholar]
- Getts DR, Shankar S, Chastain EM, Martin A, Getts MT, Wood K, Miller SD. Current landscape for T-cell targeting in autoimmunity and transplantation. J Immunother. 2011;3:853–780. doi: 10.2217/imt.11.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorelik L, Constant S, Flavell RA. Mechanism of transforming growth factor β-induced inhibition of T helper type 1 differentiation. J Exp Med. 2002;195:1499–1505. doi: 10.1084/jem.20012076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gran B, Zhang GX, Yu S, Li J, Chen XH, Ventura ES, Kamoun M, Rostami A. IL-12p35-deficient mice are susceptible to experimental autoimmune encephalomyelitis: evidence for redundancy in the IL-12 system in the induction of central nervous system autoimmune demyelination. J Immunol. 2002;169:7104–7110. doi: 10.4049/jimmunol.169.12.7104. [DOI] [PubMed] [Google Scholar]
- Gyülvészi G, Haak S, Becher B. IL-23-driven encephalo-tropism and Th17 polarization during CNS-inflammation in vivo. Eur J Immunol. 2009;39:1864–1869. doi: 10.1002/eji.200939305. [DOI] [PubMed] [Google Scholar]
- Hofstetter HH, Ibrahim SM, Koczan D, Kruse N, Weishaupt A, Toyka KV, Gold R. Therapeutic efficacy of IL-17 neutralization in murine experimental autoimmune encephalomyelitis. Cell Immunol. 2005;237:123–130. doi: 10.1016/j.cellimm.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Inobe J, Slavin AJ, Komagata Y, Chen Y, Liu L, Weiner HL. IL-4 is a differentiation factor for transforming growth factor-β secreting Th3 cells and oral administration of IL-4 enhances oral tolerance in experimental allergic encephalomyelitis. Eur J Immunol. 1998;28:2780–2790. doi: 10.1002/(SICI)1521-4141(199809)28:09<2780::AID-IMMU2780>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Juedes AE, Hjelmstrom P, Bergman CM, Neild AL, Ruddle NH. Kinetics and cellular origin of cytokines in the central nervous system: insight into mechanisms of myelin oligodendrocyte glycoprotein-induced experimental autoimmune encephalomyelitis. J Immunol. 2000;164:419–426. doi: 10.4049/jimmunol.164.1.419. [DOI] [PubMed] [Google Scholar]
- Jun S, Gilmore W, Callis G, Rynda A, Haddad A, Pascual DW. A live diarrheal vaccine imprints a Th2 cell bias and acts as an anti-inflammatory vaccine. J Immunol. 2005;175:6733–6740. doi: 10.4049/jimmunol.175.10.6733. [DOI] [PubMed] [Google Scholar]
- Karpus WJ, Kennedy KJ, Kunkel SL, Lukacs NW. Monocyte chemotactic protein 1 regulates oral tolerance induction by inhibition of T helper cell 1-related cytokines. J Exp Med. 1998;187:733–741. doi: 10.1084/jem.187.5.733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura A, Naka T, Kishimoto T. IL-6-dependent and -independent pathways in the development of interleukin 17-producing T helper cells. Proc Natl Acad Sci USA. 2007;104:12099–12104. doi: 10.1073/pnas.0705268104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochetkova I, Trunkle T, Callis G, Pascual DW. Vaccination without auto-antigen protects against collagen II-induced arthritis via immune deviation and regulatory T cells. J Immunol. 2008;181:2741–2452. doi: 10.4049/jimmunol.181.4.2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochetkova I, Golden S, Crist K, Callis G, Pascual DW. IL-35 stimulation of CD39+ regulatory T cells confers protection against collagen II-induced arthritis via the production of IL-10. J Immunol. 2010;184:7144–7153. doi: 10.4049/jimmunol.0902739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochetkova I, Crist K, Callis G, Pascual DW. Segregated regulatory CD39+ CD4+ T cell function: TGF-β-producing Foxp3− and IL-10-producing Foxp3+ cells are interdependent for protection against collagen-induced arthritis. J Immunol. 2011;187:4654–4666. doi: 10.4049/jimmunol.1100530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komiyama Y, Nakae S, Matsuki T, Nambu A, Ishigame H, Kakuta S, Sudo K, Iwakura Y. IL-17 plays an important role in the development of experimental autoimmune encephalomyelitis. J Immunol. 2006;177:566–573. doi: 10.4049/jimmunol.177.1.566. [DOI] [PubMed] [Google Scholar]
- Kroenke MA, Carlson TJ, Andjelkovic AV, Segal BM. IL-12- and IL-23-modulated T cells induce distinct types of EAE based on histology, CNS chemokine profile, and response to cytokine inhibition. J Exp Med. 2008;205:1535–1541. doi: 10.1084/jem.20080159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuchroo VK, Das MP, Brown JA, Ranger AM, Zamvil SS, Sobel RA, Weiner HL, Nabavi N, Glimcher LH. B7-1 and B7-2 costimulatory molecules activate differentially the Th1/Th2 developmental pathways: application to autoimmune disease therapy. Cell. 1995;80:707–718. doi: 10.1016/0092-8674(95)90349-6. [DOI] [PubMed] [Google Scholar]
- Lafaille JJ, Keere F, Hsu AL, Baron JL, Haas W, Raine CS, Tonegawa S. Myelin basic protein-specific T helper 2 (Th2) cells cause experimental autoimmune encephalomyelitis in immunodeficient hosts rather than protect them from the disease. J Exp Med. 1997;186:307–312. doi: 10.1084/jem.186.2.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langrish CL, McKenzie BS, Wilson NJ, de Waal Malefyt R, Kastelein RA, Cua DJ. IL-12 and IL-23: master regulators of innate and adaptive immunity. Immunol Rev. 2004;202:96–105. doi: 10.1111/j.0105-2896.2004.00214.x. [DOI] [PubMed] [Google Scholar]
- Langrish CL, Chen Y, Blumenschein WM, Mattson J, Basham B, Sedgwick JD, McClanahan T, Kastelein RA, Cua DJ. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J Exp Med. 2005;201:233–240. doi: 10.1084/jem.20041257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Nourbakhsh B, Safavi F, Li K, Xu H, Cullimore M, Zhou F, Zhang G, Rostami A. Kit (W-sh) mice develop earlier and more severe experimental autoimmune encephalomyelitis due to absence of immune suppression. J Immunol. 2011;187:274–282. doi: 10.4049/jimmunol.1003603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linington C, Berger T, Perry L, Weerth S, Hinze-Selch D, Zhang Y, Lu HC, Lassmann H, Wekerle H. T cells specific for the myelin oligodendrocyte glycoprotein mediate an unusual autoimmune inflammatory response in the central nervous system. Eur J Immunol. 1993;23:1364–1372. doi: 10.1002/eji.1830230627. [DOI] [PubMed] [Google Scholar]
- Liu Y, Zhang P, Li J, Kulkarni AB, Perruche S, Chen W. A critical function for TGF-β signaling in the development of natural CD4+CD25+Foxp3+ regulatory T cells. Nat Immunol. 2008;9:632–640. doi: 10.1038/ni.1607. [DOI] [PubMed] [Google Scholar]
- Lu L, Ma J, Wang X, Wang J, Zhang F, Yu J, He G, Xu B, Brand DD, Horwitz DA, Shi W, Zheng SG. Synergistic effect of TGF-β superfamily members on the induction of Foxp3+ Treg. Eur J Immunol. 2010;40:142–152. doi: 10.1002/eji.200939618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maloy KJ, Powrie F. Regulatory T cells in the control of immune pathology. Nat Immunol. 2001;2:816–822. doi: 10.1038/ni0901-816. [DOI] [PubMed] [Google Scholar]
- Marshall E. Drug trials. Violent reaction to monoclonal antibody therapy remains a mystery. Science. 2006;311:1688–1689. doi: 10.1126/science.311.5768.1688. [DOI] [PubMed] [Google Scholar]
- McGeachy MJ, Stephens LA, Anderton SM. Natural recovery and protection from autoimmune encephalomyelitis: contribution of CD4+CD25+ regulatory cells within the central nervous system. J Immunol. 2005;175:3025–3032. doi: 10.4049/jimmunol.175.5.3025. [DOI] [PubMed] [Google Scholar]
- Miller SD, Vanderlugt CL, Lenschow DJ, Pope JG, Karandikar NJ, Del Canto MC, Bluestone JA. Blockade of CD28/B7-1 interaction prevents epitope spreading and clinical relapses of murine EAE. Immunity. 1995;3:739–745. doi: 10.1016/1074-7613(95)90063-2. [DOI] [PubMed] [Google Scholar]
- Ochoa-Repáraz J, Riccardi C, Rynda A, Jun S, Callis G, Pascual DW. Regulatory T cell vaccination without autoantigen protects against experimental autoimmune encephalomyelitis. J Immunol. 2007;178:1791–1799. doi: 10.4049/jimmunol.178.3.1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochoa-Repáraz J, Rynda A, Ascón MA, Yang X, Kochetkova I, Riccardi C, Callis G, Trunkle T, Pascual DW. IL-13 production by regulatory T cells protects against experimental autoimmune encephalomyelitis independently of autoantigen. J Immunol. 2008;181:954–968. doi: 10.4049/jimmunol.181.2.954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Offner H, Subramanian S, Wang C, Afentoulis M, Vandenbark AA, Huan J, Burrows GG. Treatment of passive experimental autoimmune encephalomyelitis in SJL mice with a recombinant TCR ligand induces IL-13 and prevents axonal injury. J Immunol. 2005;175:4103–4111. doi: 10.4049/jimmunol.175.6.4103. [DOI] [PubMed] [Google Scholar]
- Okamoto K, Iwai Y, Oh-Hora M, Yamamoto M, Morio T, Aoki K, Ohya K, Jetten AM, Akira S, Muta T, Takayanagi H. IκBζ regulates TH17 development by cooperating with ROR nuclear receptors. Nature. 2010;464:1381–1385. doi: 10.1038/nature08922. [DOI] [PubMed] [Google Scholar]
- Olsson T. Cytokine-producing cells in experimental autoimmune encephalomyelitis and multiple sclerosis. Neurology. 1995;45:S11–S15. doi: 10.1212/wnl.45.6_suppl_6.s11. [DOI] [PubMed] [Google Scholar]
- Pascual DW, Hone DM, Hall S, van Ginkel FW, Yamamoto M, Walters N, Fujihashi K, Powell RJ, Wu S, Vancott JL, Kiyono H, McGhee JR. Expression of recombinant enterotoxigenic Escherichia coli colonization factor antigen I by Salmonella typhimurium elicits a biphasic T helper cell response. Infect Immun. 1999;67:6249–6256. doi: 10.1128/iai.67.12.6249-6256.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual DW, Trunkle T, Sura J. Fimbriated Salmonella enterica serovar Typhimurium abates initial inflammatory responses by macrophages. Infect Immun. 2002;70:4273–4281. doi: 10.1128/IAI.70.8.4273-4281.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren X, Ye F, Jiang Z, Chu Y, Xiong S, Wang Y. Involvement of cellular death in TRAIL/DR5-dependent suppression induced by CD4+CD25+ regulatory T cells. Cell Death Differ. 2007;14:2076–2084. doi: 10.1038/sj.cdd.4402220. [DOI] [PubMed] [Google Scholar]
- Rynda A, Maddaloni M, Mierzejewska D, Ochoa-Repáraz J, Maślanka T, Crist K, Riccardi C, Barszczewska B, Fujihashi K, McGhee JR, Pascual DW. Low-dose tolerance is mediated by microfold cell ligand, reovirus protein σ1. J Immunol. 2008;180:5187–5200. doi: 10.4049/jimmunol.180.8.5187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rynda A, Maddaloni M, Ochoa-Repáraz J, Callis G, Pascual DW. IL-28 supplants requirement for Treg cells in protein σ1-mediated protection against murine experimental autoimmune encephalomyelitis (EAE) PloS-ONE. 2010;5:e8720. doi: 10.1371/journal.pone.0008720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rynda-Apple A, Huarte E, Maddaloni M, Callis G, Skyberg JA, Pascual DW. Active immunization using a single dose immunotherapeutic abates established EAE via IL-10 and regulatory T cells. Eur J Immunol. 2011;41:313–323. doi: 10.1002/eji.201041104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaguchi S, Miyara M, Costantino CM, Hafler DA. FOXP3+ regulatory T cells in the human immune system. Nat Rev Immunol. 2010;10:490–500. doi: 10.1038/nri2785. [DOI] [PubMed] [Google Scholar]
- Skapenko A, Kalden JR, Lipsky PE, Schulze-Koops H. The IL-4 receptor α-chain-binding cytokines, IL-4 and IL-13, induce forkhead box P3-expressing CD25+CD4+ regulatory T cells from CD25− CD4+ precursors. J Immunol. 2005;175:6107–6116. doi: 10.4049/jimmunol.175.9.6107. [DOI] [PubMed] [Google Scholar]
- Sospedra M, Martin R. Immunology of multiple sclerosis. Annu Rev Immunol. 2005;23:683–747. doi: 10.1146/annurev.immunol.23.021704.115707. [DOI] [PubMed] [Google Scholar]
- Stephens LA, Malpass KH, Anderton SM. Curing CNS autoimmune disease with myelin-reactive Foxp3+ Treg. Eur J Immunol. 2009;39:1108–1117. doi: 10.1002/eji.200839073. [DOI] [PubMed] [Google Scholar]
- Sutton C, Brereton C, Keogh B, Mills KH, Lavelle EC. A crucial role for interleukin 1 in the induction of IL-17-producing T cells that mediate autoimmune encephalomyelitis. J Exp Med. 2006;203:1685–1691. doi: 10.1084/jem.20060285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor PA, Lees CJ, Blazar BR. The infusion of ex vivo activated and expanded CD4+ CD25+ immune regulatory cells inhibits graft - versus - host disease lethality. Blood. 2002;99:3493–3499. doi: 10.1182/blood.v99.10.3493. [DOI] [PubMed] [Google Scholar]
- Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM, Stockinger B. TGFβ in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 2006;24:179–189. doi: 10.1016/j.immuni.2006.01.001. [DOI] [PubMed] [Google Scholar]
- Vieira PL, Christensen JR, Minaee S, O’Neill EJ, Barrat FJ, Boonstra A, Barthlott T, Stockinger B, Wraith DC, O’Garra A. IL-10-secreting regulatory T cells do not express Foxp3 but have comparable regulatory function to naturally occurring CD4+CD25+ regulatory T cells. J Immunol. 2004;172:5986–5993. doi: 10.4049/jimmunol.172.10.5986. [DOI] [PubMed] [Google Scholar]
- Walters N, Sura M, Trunkle T, Pascual DW. Enhanced immunoglobulin A response and protection against Salmonella enterica serovar Typhimurium in the absence of the substance P receptor. Infect Immun. 2005;73:317–324. doi: 10.1128/IAI.73.1.317-324.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams JL, Kithcart AP, Smith KM, Shawler T, Cox GM, Whitacre CC. Memory cells specific for myelin oligodendrocyte glycoprotein (MOG) govern the transfer of experimental autoimmune encephalomyelitis. J Neuroimmunol. 2011;234:84–92. doi: 10.1016/j.jneuroim.2011.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willenborg DO, Fordham S, Bernard CC, Cowden WB, Ramshaw IA. IFN-γplays a critical down-regulatory role in the induction and effector phase of myelin oligodendrocyte glycoprotein-induced autoimmune encephalomyelitis. J Immunol. 1996;157:3323–3327. [PubMed] [Google Scholar]
- Young DA, Lowe LD, Booth SS, Whitters MJ, Nicholson L, Kuchroo VK, Collins M. IL-4, IL-10, IL-13, and TGF-β from an altered peptide ligand-specific Th2 cell clone down-regulate adoptive transfer of experimental autoimmune encephalomyelitis. J Immunol. 2000;164:3563–3572. doi: 10.4049/jimmunol.164.7.3563. [DOI] [PubMed] [Google Scholar]
- Zheng S, Wang J, Horwitz DA. Cutting edge: FoxP3+CD4+CD25+ regulatory T cells induced by IL-2 and TGF-β are resistant to Th17 conversion by IL-6. J Immunol. 2008;180:7112–7116. doi: 10.4049/jimmunol.180.11.7112. [DOI] [PubMed] [Google Scholar]