Abstract
Introduction
Since, most pathogens enter through the mucosa it is important to develop vaccines that induce mucosal immunity. To this end, we generated a novel adenovirus (Ad) vaccine that displays the sigma1 protein from reovirus to target junctional adhesion molecule 1 (JAM1) and sialic acid.
Materials/Methods
Replication-defective Ad5 vectors were modified by replacement of the Ad fiber protein with Sigma 1 (T3Dσ1) protein of reovirus T3D in previous work. Ad5 and Ad5-Sigma1 were compared in mouse models for gene delivery and vaccination to monitor cytokine, antibody, and T cell responses. The viruses were also tested for the ability to transduce and mature dendritic cells.
Results/Conclusions
Ad5-Sigma1 was 40-fold less efficient at gene delivery in vivo, yet was capable of inducing equal or greater cellular immune responses and systemic IFN-γ levels than Ad5 after intranasal administration. Despite weaker gross transduction, intranasal administration of Ad5-Sigma1 produced more GFP-positive MHCII cells in the draining lymph nodes, less GFP+/MHCII+ cells in the lungs and mediated modestly better maturation of dendritic cells in vitro. These data suggest that targeting gene-based vaccination via the Sigma1 protein may enhance the T cell immune response perhaps by skewing immune responses to encoded antigens.
Introduction
Most pathogens enter the body at mucosal surfaces. It has been estimated that as much as 90% of HIV-1 infections occur by sexual transmission. In these cases, infection is thought to occur at vaginal, rectal, and urethral mucosal surfaces (reviewed in 1). Given that the mucosal surface is the predominant entry route for pathogens, there has been increasing interest in the development of vaccines that can generate robust antibody and cellular responses at mucosal surfaces (reviewed in 2).
When vaccine strategies have been applied for mucosal immunization, they have in most cases taken advantage of the potential to expose one mucosal site to antigens and evoke responses at other mucosal sites. This unique biology of the mucosal immune system allows one to deliver vaccines to less invasive sites (e.g. nasally, orally, etc.) in order to elicit responses at sites that are less accessible, but more relevant for protection (e.g. for HIV, antibody and cellular immune responses in the vagina or rectum 3). While many vectors could in theory be used for mucosal immunization, not all can deliver genes into the nasal-associated lymphoid tissue (NALT) and gut-associated lymphoid tissue (GALT) as efficiently as they do when applied for systemic immunization.
Adenoviruses (Ads) are potent gene delivery vectors that elicit both systemic and mucosal responses as gene-based vaccines. Relevant to mucosal vaccination, the most commonly used adenovirus serotype, Ad5, is a respiratory virus that would be expected to elicit potent mucosal immune responses. Ad5 uses a well-characterized pathway for internalization in vitro. Ad5 binds to the Coxsackie and Adenovirus Receptor (CAR) at the knob domain of the fiber 4. After fiber-mediated attachment of the virus, further binding of the penton base to cellular alpha V integrins mediates internalization 5. Surprisingly, Ad5's primary receptor, CAR, is actually sequestered on the basolateral surface of airway epithelial cells 6 making Ad5 less efficient at infecting the nasal passages and the lungs 6, 7. Therefore, Ad5 may not be optimal for gene delivery to mucosal epithelial cells to produce antigens for cross-priming of mucosal immune responses. An alternate strategy for mucosal vaccination is to deliver antigens directly into mucosal dendritic cells. Unfortunately, dendritic cells do not express CAR and are poorly transduced by Ad5 virus 8.
Reoviruses are non-enveloped RNA viruses that infect mammals at respiratory or gastric mucosa 9. Initial binding and infection are mediated by the Sigma1 protein of T3D reovirus that binds to α–linked sialic acid and junctional adhesion molecule 1 (JAM1) 9. In contrast to the Ad5 receptor CAR, sialic acid is expressed ubiquitously on all cells. Moreover, JAM1 is expressed on mucosal surfaces including Peyer's patches and is also highly expressed on dendritic cells 8.
Both Ad5 and reovirus have evolved fiber and Sigma1 to bind their receptors. Surprisingly, these distinctly different viruses have separately evolved their receptor binding proteins with strikingly similar structures with shafts containing β-spiral repeats 10. The two proteins are both trimers with shaft and knob (or head) type structures. Ad5 fiber proteins have varied shaft lengths due to different numbers of β-spiral repeats fused to a knob domain that binds the receptor. Sigma1 has a shorter β-spiral repeat domain fused to an α-helical coiled-coil domain on its N-terminus and its head or knob domain on its C-terminus. This head domain binds to JAM1 whereas the shaft binds to sialic acid.
Given Sigma1's structural homology to fiber and its tropism for mucosa, we previously engineered a chimeric adenovirus that displays the Sigma1 protein, beginning at amino acid 18 of reovirus fused to the N-terminal 44 amino acids of fiber (8 and Fig. 1). This original Ad-sigma1 chimeric virus (Ad5-T3Dσ1) was shown in vitro to retarget to both sialic acid and JAM1 and to no longer target CAR 8. Notably this virus was able to more efficiently transduce dendritic cells than Ad5 given that DCs express JAM1 and sialic acid rather than CAR. In this work, we have characterized the in vivo transduction and immunization activity of Ad5-Sigma1 in mice.
Figure 1. Ad genomes expressing wildtype and chimeric viral protein structures.
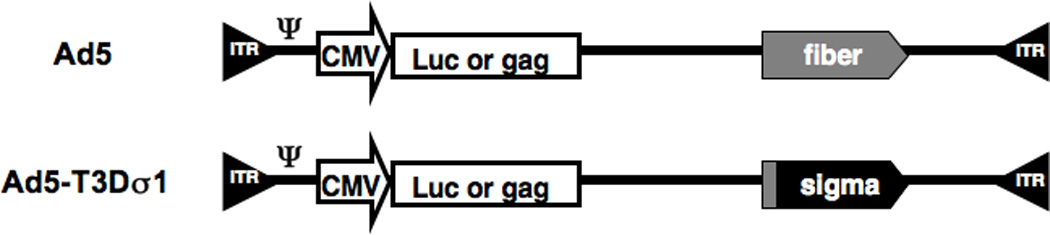
The portion of fiber, the tail, responsible for docking into the penton base of the Ad capsid is shown as a gray box. The Ad5 and Ad5-Sigma1 genomes expressing either Luciferase or HIV-1 HXB2 p55 gag genes are shown.
Results
In Vivo Transduction by Ad-Sigma1
Mice were injected with 1 × 1010 virus particles (v.p.) of Ad5 and Ad5-T3Dσ1 viruses expressing luciferase-IRES-hrGFP. Mice were injected intramuscularly (i.m.) to represent vaccination into the systemic compartment. Mice were inoculated intranasally (i.n.) to represent a mucosal vaccination route. Under standard in vivo imaging conditions for luciferase activity, Ad5 transduction was readily observed. In contrast, Ad5-Sigma1 was not (Figure 2A). Quantitation of in vivo luminescence revealed Ad5-Sigma1 expression was 10-fold lower by the i.m. route and 40-fold lower by the i.n. route (p < 0.01 and < 0.001, respectively, Figure 2B).
Figure 2. In Vivo Transduction.
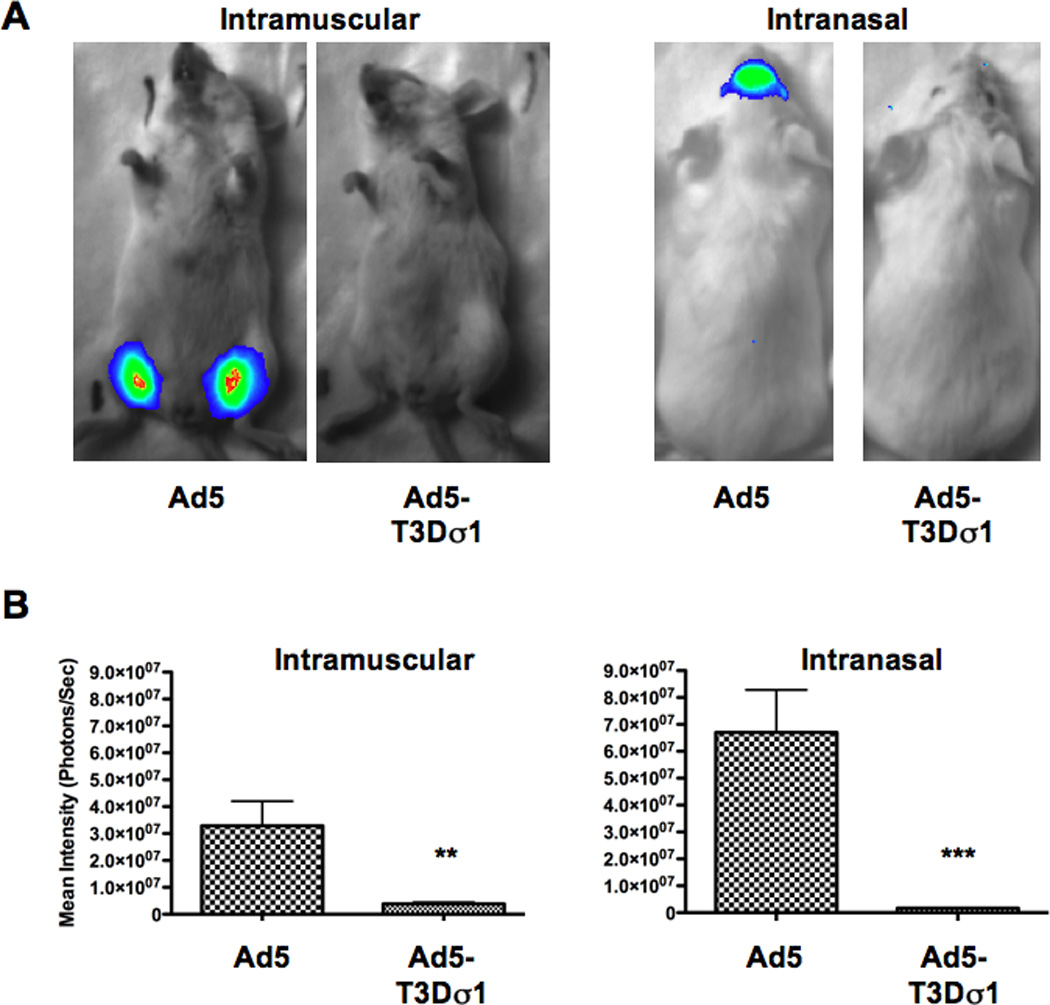
Mice were immunized with Ad5 or Ad5-Sigma1 expressing luciferase intramuscularly or intranasally and imaged 24 hours later (A). Quantitation of emitted luminescence showed statistically significant differences in overall transduction and protein expression between Ad5 and Ad5-Sigma1 i.m. (p < 0.01) and i.n. (p < 0.001) immunized mice (B). Groups of 5 mice were used and error bars indicate standard error.
Antibody Responses Generated by Ad5 and Ad5-Sigma1
Groups of 10 female BALB/c mice were inoculated by the i.m. and i.n routes with 1 × 1010 virus particles (v.p.) of Ad5 and Ad5-Sigma1 expressing HIV-1 HXB2 p55 gag to evaluate cellular and humoral immune responses (Figure 3). These data largely mimicked differences observed by luciferase imaging. By both routes, Ad5 generated markedly stronger IgG and IgA levels in the serum than Ad5-Sigma1. Of note for mucosal vaccination, only the intranasal route of Ad5 inoculation generated detectable vaginal IgA and IgG antibodies against HIV-1 gag Fig. 3B and D).
Figure 3. Humoral Immune Responses.
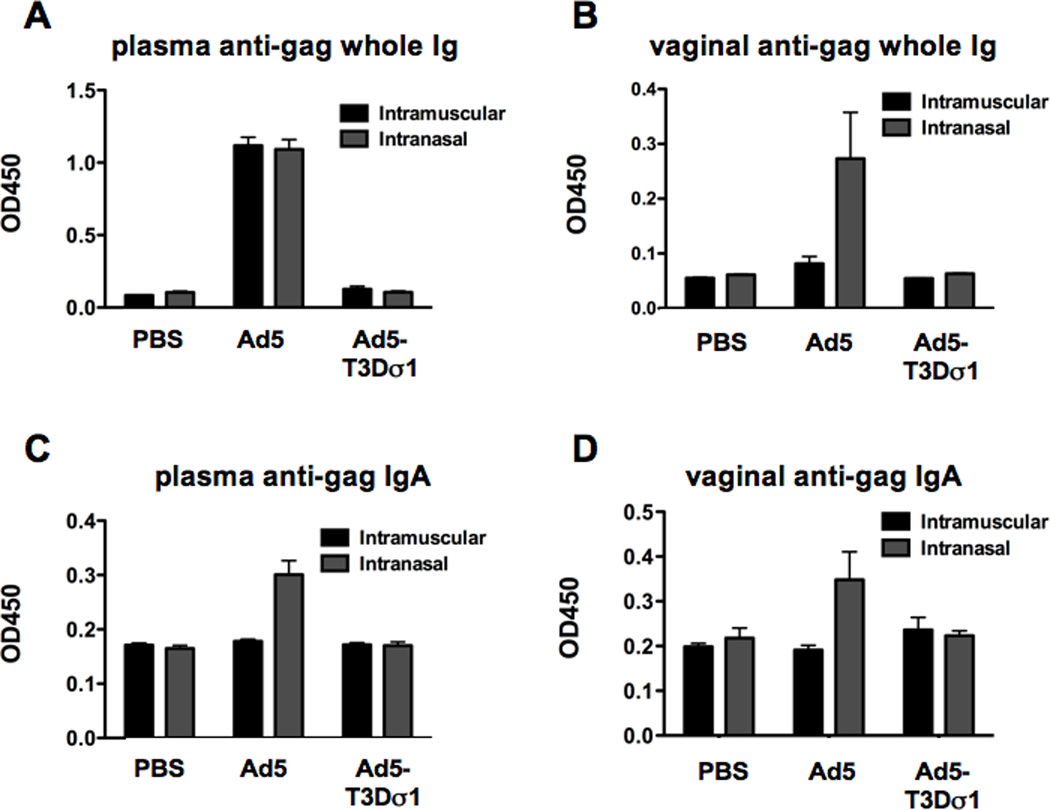
Mice were immunized intramuscularly and intranasally with Ad5 or Ad5-Sigma1 expressing HIV-1 HXB2 p55 gag. Two weeks after immunization sera and vaginal washes were obtained. Anti-gag humoral immune responses were determined by ELISA. Plasma (A) and vaginal (B) anti-gag whole immune responses were determined. Mucosal anti-gag IgA immune responses in plasma (C) and vaginal (D) washes were determined. Groups of 10 mice were used and error bars indicate standard error.
Cellular Immune Responses Generated by Vectors Expressing HIV-1 gag
The mice that were inoculated above were sacrificed two weeks after immunization and their splenocytes and cervical lymph nodes were analyzed for T cell responses by ELISPOT (Figure 4). An MHC I-restricted gag peptide was used to evaluate CD8 T (CTL) cell responses. A three-peptide pool was used to evaluate MHC II-restricted T helper (Th) cell responses. Under these conditions, Ad5-Sigma1 generated surprisingly robust CTL and Th responses in the spleens of the mice by both routes of inoculation. By the i.m. route, Ad5-Sigma1 generated equal CTL and Th cell numbers as Ad5 in the spleen (Figure 4A) despite the fact that both lucferase and gag antibody responses were 10-fold lower than those by Ad5 (Figures 2 and 3). This effect was even stronger by the mucosal i.n. route where Ad5-Sigma1 actually generated stronger Th cell responses than Ad5 (p = <0.0001) (Figure 4C) under conditions of 40-fold weaker transduction (Figure 2). Ad5 induced stronger CTL and Th responses in the lymph nodes as compared to Ad5-Sigma1 when delivered i.m. (Figure 4B). However, Ad5 and Ad5-Sigma1 induced equivalent CTL and Th cellular responses in the lymph nodes of mice immunized intranasally (Figure 4D).
Figure 4. Cellular Immune Responses.
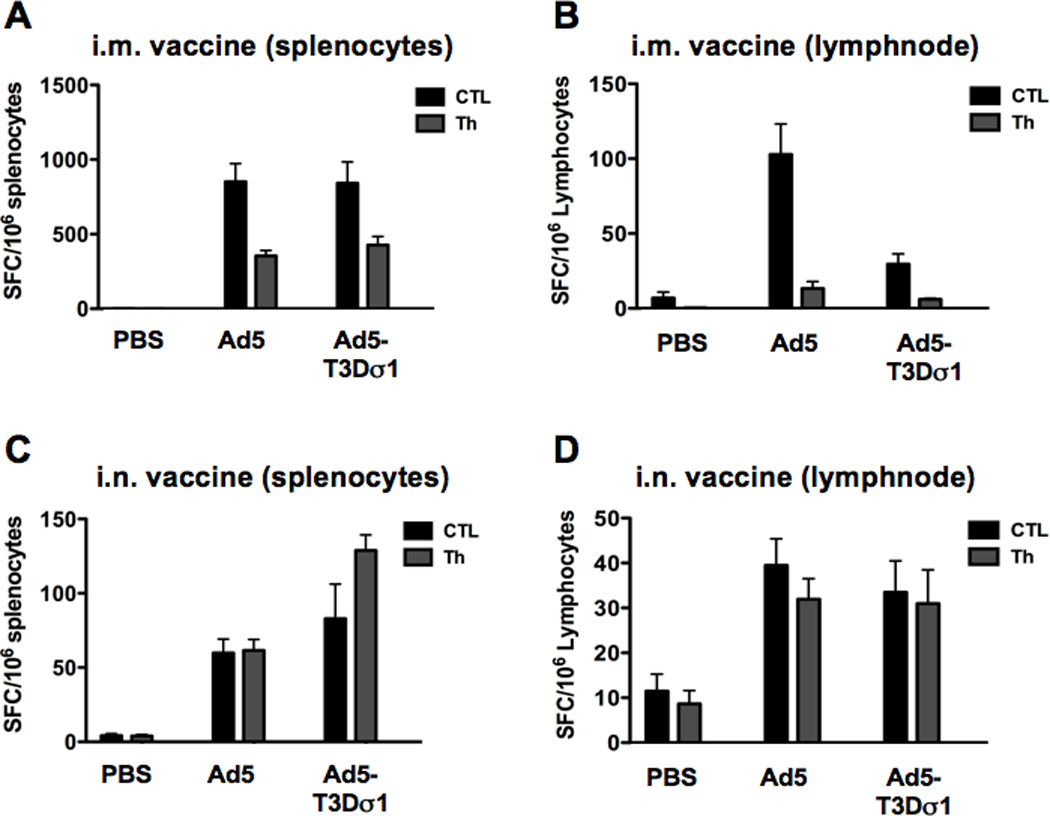
Mice were immunized intramuscularly and intranasally with Ad5 or Ad5-Sigma1 expressing HIV-1 HXB2 p55 gag. Two weeks after immunization splenocytes and lymphocytes from the cervical lymph nodes were obtained. Cellular immune responses were detected in the spleens (A) and lymph nodes (B) of mice immunized intramuscularly. CTL responses were significantly lower in mice immunized with Ad5-Sigma1 (p < 0.01). Cellular immune responses were detected in the spleens (C) and lymph nodes (D) of mice immunized intranasally. Mice immunized with Ad5-Sigma1 had significantly higher Th responses (p < 0.0001). Groups of 10 mice were used and error bars indicate standard error.
Immune Responses are Amplified When Ad5 and Ad5-Sigma1 Are Combined
These data indicated that Ad5-Sigma1 was 10 to 40-fold less efficient at transduction. This lower bulk gene delivery correlated well with reduced antibody responses generated by Ad5-Sigma1 and was consistent with humoral responses being tied to raw antigen production. While Ad5-Sigma1 appeared weak at transduction, it generated surprisingly stronger Th responses under conditions of drastically reduced gene delivery.
Given their differing activities with Ad5 generating antibody responses and Ad5-Sigma1 generating stronger Th responses, we hypothesized that Ad5-Sigma1 might be able to boost anti-gag antibody responses from Ad5 by using the increased Th responses induced by Ad5-Sigma1. To test this, groups of ten mice were immunized mucosally by the intranasal route with Ad5 and Ad5-Sigma1 in different combinations. Mice were immunized either with Ad5, Ad5-Sigma1, or a combination of both. If two viruses were used in combination 2.5 × 109 v.p. of each were mixed and delivered i.n. to each mouse. The single vector control for this combination was delivery of 2.5 × 109 v.p. of the single virus supplemented with 2.5 × 109 v.p. with Ad5 control virus (Ad5 Cx) that did not express HIV-1 HXB2 p55 gag. To compare the combination, groups of mice were also inoculated with 5 × 109 v.p. of virus to equalize the maximum total virus delivered in the combination group (Figure 5).
Figure 5. Combined Effects of Ad5 and Ad5-Sigma1 on Immune Responses.
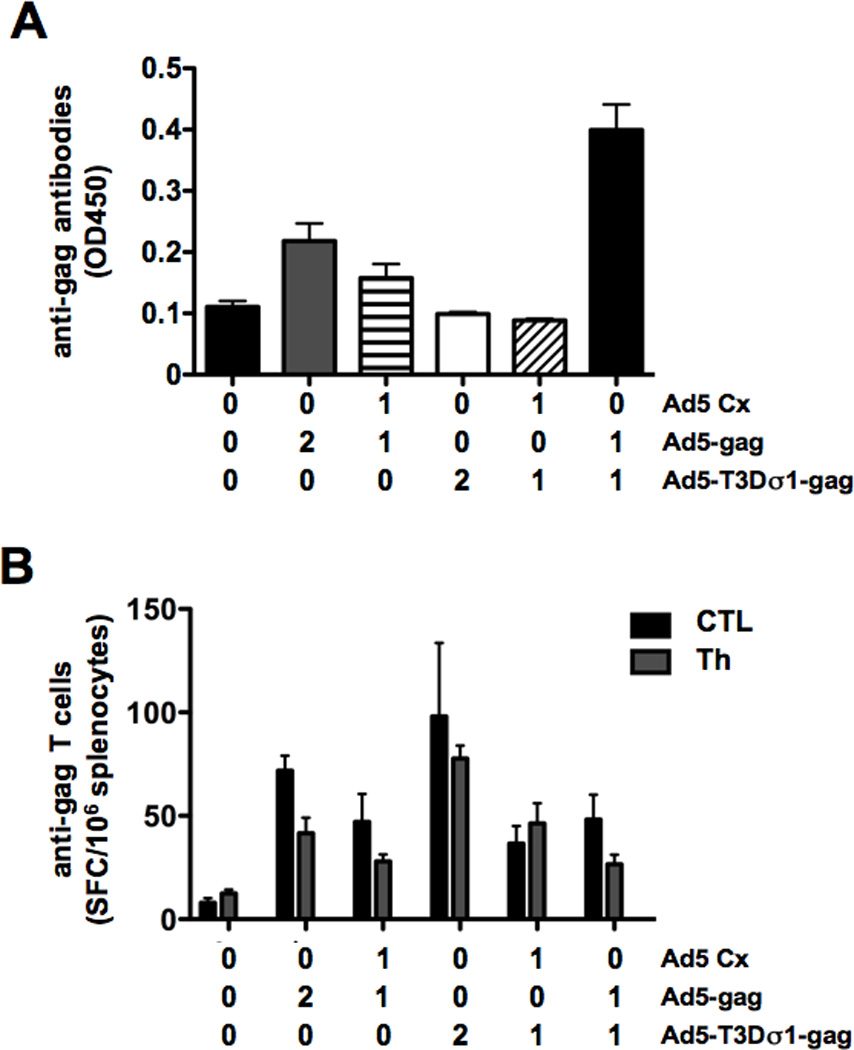
Mice were immunized intranasally with Ad5 and Ad5-Sigma1 expressing HIV-1 HXB2 p55 gag individually or in combination. Values on the X-axis indicate the concentration of virus used to immunize the mice (0 = none, 1 = 2.5 × 109 vp, and 2 = 5 × 109 vp). Ad5 Cx does not express HIV-1 HXB2 p55 gag and is a control virus. Ad5 in combination with Ad5-Sigma1 induces a synergistic humoral immune response in which greater than additive effects are produced (A). Cellular immune responses were not shown to improve when Ad5 and Ad5-Sigma1 were used in combination (B). Groups of 5 mice were used and error bars indicate standard error.
As seen previously, the Ad5-Sigma1 virus was unable to induce anti-gag humoral immune responses as compared to the Ad5 virus (Figure 5A). When Ad5 Cx virus was used in combination with Ad5 or Ad5-Sigma1 there was no additive effect. However, when Ad5 and Ad5-Sigma1 both expressing gag were mixed together, there was a synergistic effect on antibody responses resulting in greater anti-gag humoral immunity than the sum of the viruses when used alone (p = 0.001). This synergistic effect was not observed for cellular immune responses (Figure 5B). However, again Ad5-Sigma1 generated stronger T helper responses than Ad5 (p = 0.05). In fact, the combination of Ad5 and Ad5-Sigma1 virus had an antagonistic effect on Th cellular responses when compared to Ad5 or Ad5-Sigma1 alone (p = 0.001) (Figure 5B).
Ad5-Sigma1 Generates Stronger Systemic IFN-γ levels than Ad5
The vaccination data above suggested that although Ad5-Sigma1 was weaker at raw gene transfer, it generated stronger T cell responses with weak antibody responses. To test if this effect might be related to changes in T helper responses, groups of 10 BALB/c mice were immunized intranasally with 1 × 1010 vp of Ad5 or Ad5-T3Dσ1-gag virus. DPBS was used as a negative control. Sera was collected 7 days after immunization during T cell expansion and were analyzed for IL-4 and IFN-γ levels by ELISA (Fig. 6A). Under these conditions, increased IL-4 and IFN-γ levels were observed in the Ad-immunized mice, but not in buffer treated animals. IL-4 levels were not statistically different between the Ad5 and Ad5-Sigma1 groups (p = 0.5). In contrast, IFN-γ levels were significantly higher in the Ad5-Sigma1 group than in the Ad5 group (p = 0.02 by two-tailed T test). These data indicated that Ad5-Sigma1 induced stronger systemic IFN-γ responses after intranasal administration than Ad5.
Figure 6. Th1/Th2/Th17 Responses.
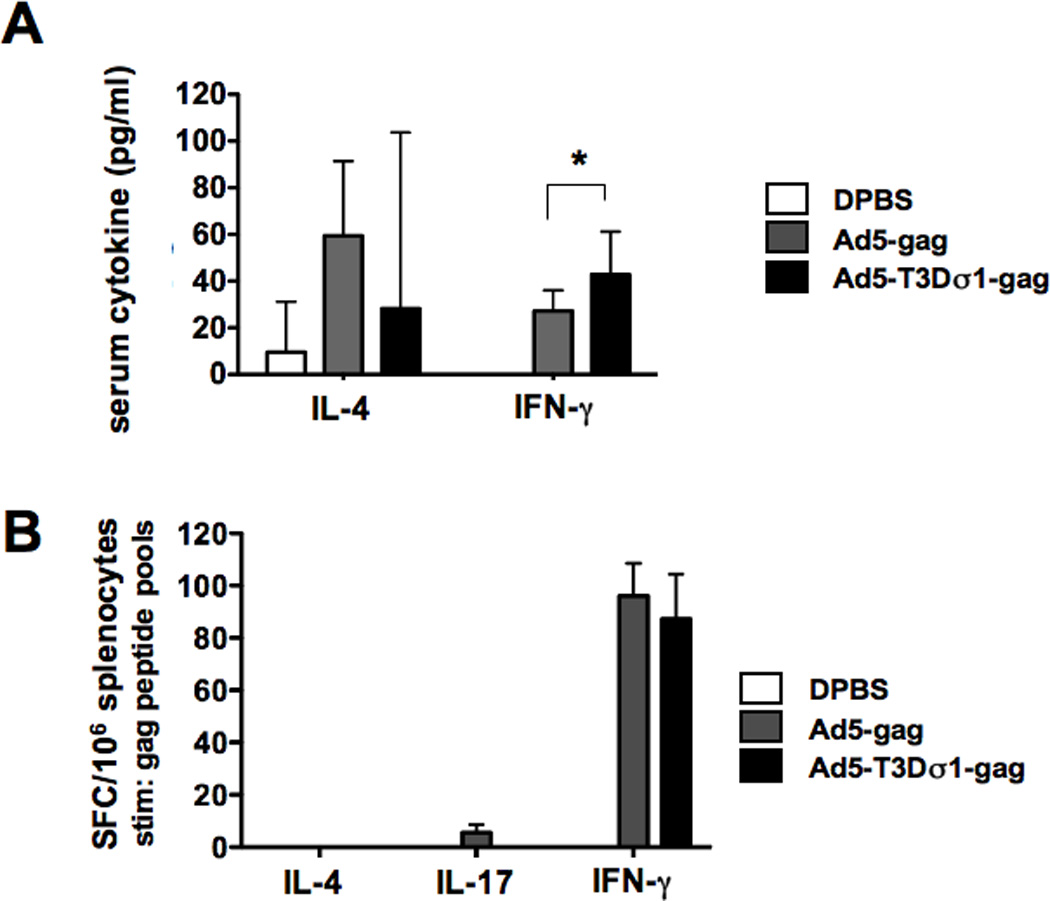
Groups of 10 mice were immunized intranasally with 1 × 1010 vp Ad5 or Ad5-T3Dσ1-gag viruses. A. Sera was collected and assayed for cytokine expression 7 days post-immunization. Interleukin-4 (IL-4) and interferon-γ (IFN-γ) cytokine were measure by ELISA. B. Groups of 5 mice were immunized with 1 × 1010 vp Ad5 or Ad5-T3Dσ1-gag viruses. Two weeks after immunization splenocytes were obtained. The splenocytes were stimulated with the B consensus gag peptides (NIH AIDS Research and Reference Reagent Program Catalog Number: 8117) in two separate pools. IFN-γ, IL-4, and IL-17 cellular immune responses were detected by ELISPOT. Error bars show standard error and the asterisk indicates p = <0.05 by two-tailed T-TEST.
Ad5 and Ad5-Sigma1 Generate Similar Th1, Th2, and Th17 Responses
The data above suggested differences in T helper cell responses after intranasal immunization with the two vectors. To test if differences in Th1, Th2, or Th17 responses were being provoked by the vectors, 10 BALB/c mice were immunized intranasally with 1 × 1010 vp of Ad5 or Ad5-T3Dσ1-gag virus and splenocytes were analyzed for T cell responses by ELISPOT 14 days later (Figure 6B). Two pools of conserved overlapping 15-mer peptides spanning gag were used to ensure that all T cell responses might be detected. Under these conditions, both Ad5 and Ad5-Sigma1 generated no IL-4 and similar IFN-γ responses after stimulation by the peptide pools (Figure 6B). This contrasted with stronger responses generated by Ad-sigma1 by the single peptides used at higher concentrations (Figure 4). Interestingly, Ad5 generated low IL-17 responses that were somewhat higher than was generated by Ad-sigma1 (p = 0.08 by one tailed T test).
Maturation of Immature Human Dendritic Cells by Ad5 and Ad5-Sigma1
The data above suggested that Ad5-Sigma1 might interact with the immune system differently than Ad5. Dendritic cells express JAM1 but not CAR, translating into more efficient transduction of human DCs by Ad-sigma1 than by Ad5 8. Given this, we tested if Ad5-Sigma1 affects the maturation of immature human dendritic cells. CD14+ cells were isolated from human blood and these were incubated with Ad5 or Ad5-Sigma1. 72 hours later, treated and untreated cells were stained for the maturation marker CD83 and were analyzed by flow cytometry (Fig. 7A). CD83 levels on untreated cells remained low (mean fluorescence intensity-MFI = 7.99). In contrast, positive control cells treated with TNF∝ and IPGE2 produced 20-fold increases in CD83 levels (MFI = 188.0). Ad5 induced intermediate increases in CD83 (MFI = 34.33). Ad5-Sigma1 induced slightly higher CD83 levels with an MFI of 38.80.
Figure 7. Interactions with Antigen-presenting Cells.
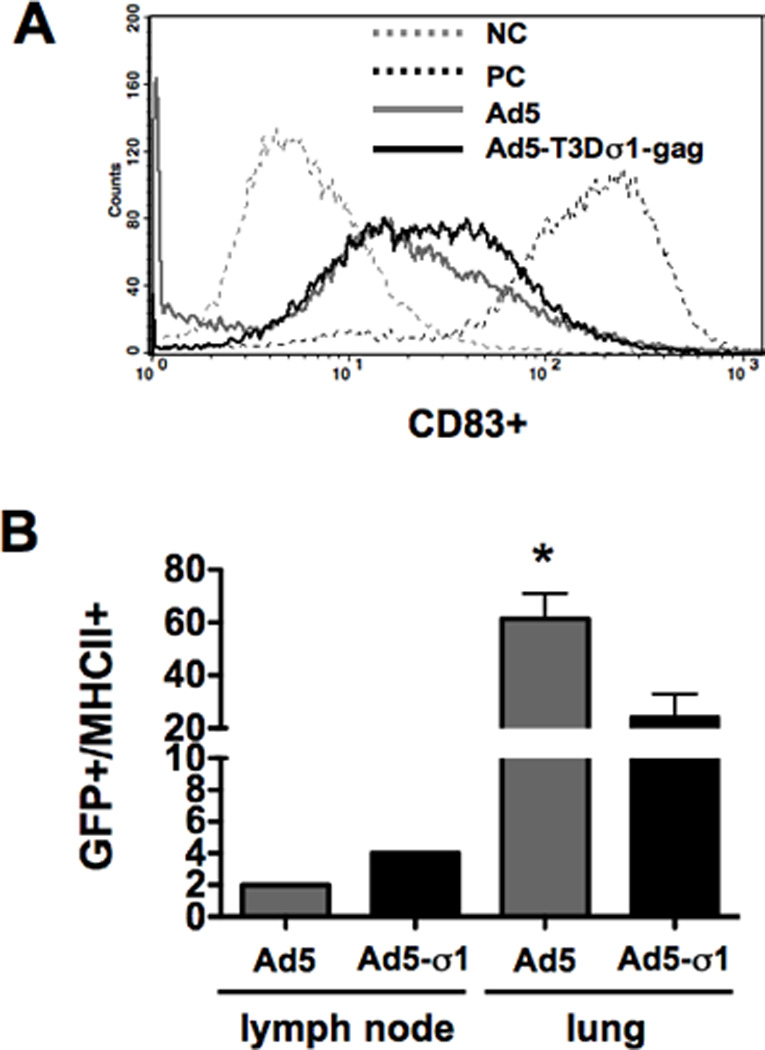
A. Dendritic cell maturation induced by the viruses was assayed. Fresh human dendritic cells were isolated using anti-CD14 magnetic beads and incubated for 72 hours. The cells were then stimulated with Ad5 or Ad5-T3Dσ1-gag viruses. A maturation cocktail containing TNF-∝ and IPGE2 was used as a positive control. The hDCs were analyzed by flow cytometry using anti-CD83-PE antibody. B. In vivo transduction of lymph node (LN) and lung MHCII+ cells by Ad5 and Ad5-Sigma1. Mice were immunized intranasally with 1 × 1010 vp Ad5 or Ad5-T3Dσ1 virus expressing luciferase-IRES-hrGFP. The lungs and cervical lymph nodes were analyzed for transduction (GFP) and MHC-II using PE labeled anti-mouse I-A/I-E antibody.
In Vivo Transduction of MHCII-positive cells by Ad5 and Ad5-Sigma1
Ad-Sigma1 transduces human DCs more efficiently than Ad5 8 and produces modest increases in DC maturation. To determine if virus interactions in vivo might also play a role, groups of three BALB/c mice were inoculated intranasally with Ad5 or Ad5-Sigma1 expressing the luciferase IRES hrGFP cassette and cervical lymph nodes (LN) and lung cells were harvested 24 hours later. Single cell suspensions were stained for MHC II and transduction of these cells was assessed by flow cytometry for hrGFP expression (Fig. 7B). Due to limited cell numbers, the lymph nodes from the three mice were pooled from each group whereas the lung samples from each were treated separately. In contrast to the much stronger bulk transduction observed by Ad5 (Fig. 2), Ad5-Sigma1 generated approximately 2-times as many GFP+/MHCII+ cells in the draining lymph nodes of the mice than Ad5. In the lungs, the two vectors generated 5 to 30-times more GFP+/MHCII+ cells than in the lymph nodes. However in this case, Ad5 produced 2.5-fold higher lung GFP+/MHCII+ cells than Ad5-Sigma1 (p < 0.05). Therefore, in contrast to the 40-fold higher gross transduction of nasal and lung tissues by Ad5, transduction of MHCII+ cells by the two vectors were similar with Ad5-sigma1 mediating equal or better transduction of antigen-presenting cells in the draining lymph nodes.
Discussion
Mucosal immunity is of great importance for the induction of prophylactic immune responses against infectious diseases by vaccination. This has been high lighted by the recent failure of the HIV-1 STEP trial. The mucosal barrier is composed of highly specialized innate and adaptive immune complexes. It is at this barrier that infectious agents must be stopped. It is thought that stimulation of immune responses at the mucosal membrane will result in greater mucosal immunity. The most commonly used viral vector for vaccine studies, Ad5, generates robust systemic immune responses. However, Ad5 seems to be restricted to lung epithelial cells and not to antigen presenting cells.
In this study we re-engineered the Ad5 vector to express the reovirus Sigma1 gene to increase transduction of mucosal cells and dendritic cells. At first glance, using in vivo imaging, Ad5-Sigma1 appeared to be nonfunctional or to have markedly reduced activity. The absence of luciferase activity indicated that relatively few bulk cells were transduced by the chimeric Ad5-Sigma1 virus as compared to the wildtype Ad5. However, when we studied the ability of Ad5-Sigma1 to induce systemic and mucosal immunity, we were surprised to find that it was more effective than Ad5 at inducing cellular immunity. Therefore, although it appeared that the retargeted virus had only low bulk tissue transduction, the small amount of transduction that did occur induced potent systemic and mucosal immune responses that were equivalent to or greater than that of wildtype Ad5 virus. The amount of antigen produced and the resulting immune responses did not seem to correlate in this case suggesting that the chimeric Ad5-Sigma1 and the wildtype Ad5 viruses were stimulating cellular immune responses via separate mechanisms.
Another striking difference between Ad5-Sigma1 and Ad5 was observed in their abilities to drive humoral immune responses. Unlike the cellular immune responses, Ad5-Sigma1 failed to induce anti-gag systemic or mucosal antibodies. One would expect that humoral immune responses would at least somewhat resemble that of the cellular immune responses as was seen for the Ad5 virus. Again, these results reiterate that Ad5-Sigma1 is stimulating immune responses in a manner very different from that of wildtype Ad5 virus. If low levels of transduction are resulting in greater cellular immune responses than expected it’s possible that Ad5-Sigma1 is targeting cells capable of inducing strong or altered immune responses as compared to Ad5. Indeed, the observations that Ad5-Sigma1 drives increased systemic IFN-γ responses is consistent with this hypothesis.
It stands to reason that the different mechanisms of immunity induced by chimeric and wildtype viruses would not be mutually exclusive and that by stimulating immunity via both mechanisms there would at least be an additive effect. Therefore, we immunized animals with wildtype and chimeric viruses individually and in combination. Indeed, this was the case for humoral immunity. In fact, when used in combination, the chimeric and wildtype viruses induced a synergistic effect on the humoral immune response. To rule out any non-specific additive effects of wildtype Ad5 we included the use of a control Ad5 expressing an irrelevant gene. No non-specific additive responses were seen for either wildtype or chimeric viruses. Ironically, there was no additive effect seen in the cellular immune responses. In fact, the combination of the viruses resulted in an antagonistic response and ablated the enhanced Th responses. However, when used alone Ad5-Sigma1 again induced significantly stronger Th responses than Ad5 alone. The results indicate that the mechanism by which the chimeric and wildtype Ad5 induce cellular immunity may actually be mutually exclusive. Perhaps this would explain the antagonistic response.
As mucosal immunity is paramount to vaccine development it is essential that we explore vaccine vectors that stimulate strong mucosal immune responses. Here we show a retargeted chimeric vector that appears significantly impaired when studied using in vivo imaging. It was surprising to discover that this chimeric virus was capable of inducing cellular immune responses that were equivalent to or greater than that of wildtype Ad5. The mechanism by which Ad5-Sigma1 could induce significantly greater Th responses in the absence of humoral responses is as yet unclear. It is well-known that antibody responses after genetic immunization are proportional to the level of transduction by the vector. Therefore, it is not surprising that Ad-Sigma1 that mediates less efficient bulk transduction also mediates weak antibody responses by itself.
In contrast, the bettter transduction and maturation of DCs and MHCII+ cells by Ad5-Sigma1 may stimulate T helper cells better than Ad5. This could explain the synergistic induction of humoral immunity when Ad5 and Ad5-Sigma1 are used in combination since Ad5 was shown to induce more protein expression as measured by in vivo luciferase activity. The overall lack of Ad5-Sigma1’s ability to transduce bulk mucosal epithelial cells and lower overall protein expression may be the effect of structural constraint induced by the fusion of the two genes. Small changes in the chimeric fusion gene may alleviate these constraints and repair Ad5-Sigma1’s overall transduction levels. Alternately, the Sigma 1 protein may be acting as a protein adjuvant for immune responses. The relative roles of receptor targeting and adjuvant effects by Sigma 1 is under investigation.
Here we show the characterization of a chimeric Ad5 virus, Ad5-Sigma1, to induce systemic and mucosal immune responses. While overall virus transduction and protein expression levels are significantly decreased the cellular immune responses were equivalent to or greater than wildtype Ad5. Combining the two viruses reversed the lack of humoral immune responses and resulted in a synergistic effect. This effect indicates that the two viruses may be acting through different mechanisms to stimulate the immune system. It is possible that further manipulation of the chimeric fusion protein could potentially reverse the lack of overall transduction and would result in a superior viral vector for inducing both humoral and cellular immunity.
Materials and Methods
Generation of Chimeric Fiber-Sigma 1 T3D proteins
The chimeric Fibtail-Sigma 1 protein from T3D reovirus was created as previously described 8. Briefly, the N-terminal 44 amino acids of Ad5 fiber (Fibtail) involved in docking into the penton base were fused to amino acid 18 of the T3D Sigma 1 protein (Figure 1). To detect the chimeric proteins two c-Myc tags and one hexahistidine tag were added to the C-terminus of the chimera. The chimeric proteins were cloned into the plasmid peTPL bearing a CMV promoter, the Ad tripartite repeat from pDV55 11, and a bovine growth hormone polyadenylation sequence for maximal expression as well as the E4 domain and an intervening zeocin-resistance gene for homologous recombination in bacteria 12.
Adenoviruses
First generation replication defective (E1/E3 deleted) Ad5 vectors were constructed using the Ad-Easy system in 293A cells as described in 13. Ad5 viruses with wildtype fiber expressing a firefly luciferase IRES hrGFP cassette and the HIV-1 p55 gag codon-optimized gene of strain HXB2 cassette from the CMV promoter were used as controls. The peTPL plasmids bearing the fiber tail Sigma 1 chimera was linearized and recombined into these adenovirus plasmids using homologous recombination at fiber and E4 regions with zeocin selection as in 8. Briefly, the linearized chimeric genomes were transfected into fiber expressing 633 cells 11 in the presence of 0.3 µM dexamethasone and 4 µg/ml of polybrene. The chimeric viruses were amplified by serial passage in 633 cells until the last infection. Virus was purified from 20 100-mm dishes by CsCl gradient centrifugation to remove residual wildtype fiber and these were used to infect a cell factory of 293 cells to produce virions displaying only the virally-encoded Sigma 1 chimeric protein. Recombinant adenoviruses expressing wild-type fiber were purified twice by CsCl gradient centrifugation and quantitated by OD260.
Animals
Female BALB/c mice (6–8 weeks old) were purchased from Charles River Laboratories (Wilmington, Massachusetts, USA) and housed in the Mayo Clinic Animal Facility under the Association for Assessment and Accreditation of Laboratory Animal Care (AALAC) guidelines with animal use protocols approved by the Mayo Clinic Animal Use and Care Committee. All animal experiments were carried out according to the provisions of the Animal Welfare Act, PHS Animal Welfare Policy, the principles of the NIH Guide for the Care and Use of Laboratory Animals, and the policies and procedures of Mayo Clinic.
Mice were immunized intramuscularly (i.m.) or intranasally (i.n.). Mice immunized by the i.m. route received 1 × 1010 vp/mouse in two twenty-five µl injections into each mouse's quadriceps muscles. Mice immunized by the i.n. route received 1 × 1010 vp/mouse in 20 µl total volume (10 µl per nare). Two weeks after immunization, the mice were bled, euthanized and spleens and lymph nodes were collected.
In Vivo Luciferase Imaging
The luciferase-expressing viruses were administered as indicated in the text and the mice were imaged at varied times on a Lumazone Imaging System (Roper Scientific) as in 16. Mice were anesthetized with isoflurane, injected intraperitonealy (i.p.) with d-luciferin at a concentration of 20 mg/ml in PBS in a volume of 200 µl and the mice were immediately placed into the Lumazone Imager and images were captured. All images were taken with a 10-minute exposure and 2×2 binning using no filters and no photo-multiplication. Data analysis was performed on each image using background subtracted mean intensities detected by the Lumazone Imaging Software at each time point and graphed using Prism Graphing Software.
Enzyme Linked Immunosorbent Assay (ELISA)
To measure humoral immune responses to transgenes ELISAs were performed on mouse sera as previously described 17. Briefly, Immulon 4 HBX plates (Thermo, Milford, MA) were coated with 100 µl of HIV-1 gag protein, SF162 gp120 (NIH AIDS Reagent and Repository) or FireFly luciferase (Roche, Switzerland) at 1 µg/ml in PBS for 2 hours at room temperature (RT). The plates were blocked for 1 h with BSA at 2 mg/ml for 1 hour. Sera were diluted 1:100 in PBS with BSA (1 mg/ml) and added to the plate for 1 h at RT. The plates were washed with 5 times PBS and 100 µl of Goat anti-mouse HRP conjugated antibody (Pierce, Rockford, IL) diluted 1:2000 in PBS with BSA (1 mg/ml) was added to the plate for 1 h at RT. The plates were washed 5 times with PBS and 100 µl of 1 Step Ultra TMB-ELISA substrate (Pierce, Rockford, IL) was added for 1 h at RT. The reaction was stopped with 50 µl of 2 M sulfuric acid and analyzed at 450 nm using a Beckman Coulter DTX 880 Multimode Detector.
Enzyme-linked immune spot (ELISpot) assay
To measure cellular responses to Gag, splenocytes were incubated in the presence of peptides at a concentration of 5 µg/ml. Gag CTL and T helper responses were determined using the VGGHQAAMQMLKDTINEEAA peptide (containing the H-2Kd-restricted peptide AMQMLKDTI) and a peptide pool (ATLEEMM TACQGVGGPSHKA, TSNPPIPVGDIYKRWIILGL, and FKTLRAEQATQEVKNWMTDT), respectively. The spleens from individual mice were minced and then forced through a 40 µm Nylon cell strainer (BD Labware, Franklin Lakes, NJ). Mesenteric and cervical lymph nodes were dissected from individual mice and processed as described for splenocytes. Single-cell suspensions of splenocytes or lymphocytes were plated in 96-well polyvinylidene difluoride-backed plates (MultiScreen-IP, Millipore, Billerica, MA) coated with 50 µl of anti-mouse IFN-γ mAb AN18 (5 µg/ml; Mabtech, Stockholm, Sweden) overnight at 4°C. The plates were blocked with HEPES-buffered complete RPMI medium at 37°C for 2 hr. Equal volumes (50 µl) of each peptide pool and splenocytes (107 cells/ml) were added to the wells in duplicate. Plates were incubated overnight (14 to 16 hr) at 37°C with 5% CO2. After the plates were washed 6 times with PBS, 50 µl of 1:1000-diluted biotinylated anti-mouse IFN-γ mAb (Mabtech, Stockholm, Sweden) was added to each well. Plates were incubated at RT for 2 hr and then washed 3 times with PBS. Fifty µl of streptavidin-alkaline phosphatase conjugate (1:1000 dilution; Mabtech, Stockholm, Sweden) were added to each well. After incubation at RT for 1 hr, the plates were washed 5 times with PBST. Finally, 100 µl of BCIP/NBT (Plus) alkaline phosphatase substrate (Moss, Pasadena, MD) were added to each well. The plates were incubated at RT for 10 min. After washing with water, plates were air-dried. Spots were counted using an automated ELISpot plate reader (Immunospot counting system, CTL Analyzers, Cleveland, OH) and expressed as spot-forming cells (SFC) per 106 splenocytes.
Transduction of Immature Dendritic Cells
Peripheral blood mononuclear cells (PBMCs) were collected under a Baylor College of Medicine institutional review board (IRB)-approved protocol and informed consent was obtained from all donors. PBMCs were used to generate human monocyte-derived immature dendritic cells (DCs) by the “adherence method” or by CD14 isolation as in 14, 15. Adherent or CD14-selected monocytes were then cultured in Cell Genix/GlutaMAX-I media with 800 U/mL granulocyte-macrophage colony-stimulating factor (GM-CSF) (Sargramostim Leukine, Immunex, Seattle, WA) and 1000 U/mL interleukin-4 (IL-4) (R&D Systems, Minneapolis, MN) for 5 days with IL-4 and GM-CSF replenishment on days 2 and 4. On day 5, they were transduced with the indicated vectors.
Human Dendritic Cell Maturation Assay
Human PBMCs were collected from using a Trima cone. Lymphocytes were purified on a Histopaque 1077 density gradient (Sigma Aldrich). DC) were purified using anti-CD14 magnetic beads and cultured in CellGenix DC media supplemented with 2800 U/ml of GM-CSF and 1000U/ml of IL-4. The cells were incubated for 72 hours and then stimulated with Ad5 or Ad5-T3Dσ1-gag viruses. A positive control maturation cocktail containing TNF-∝ and IPGE2 was used as a positive control. The DCs were then analyzed by flow cytometry on a FACSCalibur (BD Biosciences, Mountain View, CA) using anti-CD83-PE antibody (Immunotech IM2218, Marseille Cedex 9, France).
Cytokine ELISA
The TH1/Th2 cytokine assays were performed using the eBioscience Th1/Th2 ELISA Ready-SET-Go kit. Groups of 10 mice were immunized intranasally with 1 × 1010 vp of Ad5 or Ad5-T3Dσ1-gag virus. DPBS was used as a negative control. Sera was collected 7 days post-immunization, diluted 1:4 in DPBS and assayed for interleukin-4 and interferon-γ cytokine expression as described by the manufacturer (eBioscience, San Diego, CA, USA).
In Vivo Transduction of Antigen Presenting Cells (APC)
Groups of 3 mice were immunized intranasally with 1 × 1010 vp of Ad5 or Ad5-T3Dσ1 virus expressing luciferase-IRES-hrGFP. Lungs and cervical lymph nodes were harvested 24 hrs. after immunization. The lungs from individual mice were washed twice with PBS and resuspended in 2 ml of PBS. The lungs were minced, vortexed and then filtered through a 40 µm Nylon cell strainer (BD Labware, Franklin Lakes, NJ). In order to increase the total cell numbers for analysis, all of the lymph nodes were combined and processed as previously described. The lymph node and lung single-cell suspensions were then stained for MHC-II using PE labeled anti-mouse I-A/I-E for 1 h at 4°C. The cells were washed with PBS and fixed in 1% formalin overnight. The cells were then analyzed by flow cytometry for GFP and PE signals.
Statistical Analyses
Data was evaluated using GraphPad Prism 4 software. Unpaired, two-tailed Tests and One Way ANOVA with Bonferroni post test were used to determine statistical significance. P values ≤ 0.05 were considered statistically significant.
Supplementary Material
Acknowledgements
We would like to thank Mary Barry and Shannon May for their excellent technical assistance. We would like to particularly than Michael Strausbauch for help with flow cytometry. We would also like to thank Dr. Allan Dietz and Peggy Bulur for purification of dendritic cells from normal donors. This work was supported by NIH/NIAID grant R01 AI065304.
References
- 1.Lehner T, Anton PA. Mucosal immunity and vaccination against HIV. Aids. 2002;16(Suppl 4):S125–S132. doi: 10.1097/00002030-200216004-00017. [DOI] [PubMed] [Google Scholar]
- 2.Simecka JW. Mucosal immunity of the gastrointestinal tract and oral tolerance. Adv Drug Deliv Rev. 1998;34(2–3):235–259. doi: 10.1016/s0169-409x(98)00042-8. [DOI] [PubMed] [Google Scholar]
- 3.Kaneko H, Bednarek I, Wierzbicki A, Kiszka I, Dmochowski M, Wasik TJ, et al. Oral DNA vaccination promotes mucosal and systemic immune responses to HIV envelope glycoprotein. Virology. 2000;267(1):8–16. doi: 10.1006/viro.1999.0093. [DOI] [PubMed] [Google Scholar]
- 4.Bergelson JM, Cunningham JA, Droguett G, Kurt-Jones EA, Krithivas A, Hong JS, et al. Isolation of a common receptor for Coxsackie B viruses and adenoviruses 2 and 5. Science (New York, NY. 1997;275(5304):1320–1323. doi: 10.1126/science.275.5304.1320. [DOI] [PubMed] [Google Scholar]
- 5.Wickham TJ, Mathias P, Cheresh DA, Nemerow GR. Integrins avb3 or avb5 promote adenovirus internalization but not virus attachment. Cell. 1993;73:309–319. doi: 10.1016/0092-8674(93)90231-e. [DOI] [PubMed] [Google Scholar]
- 6.Zabner J, Freimuth P, Puga A, Fabrega A, Welsh MJ. Lack of high affinity fiber receptor activity explains the resistance of ciliated airway epithelia to adenovirus infection. J Clin Invest. 1997;100(5):1144–1149. doi: 10.1172/JCI119625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grubb BR, Pickles RJ, Ye H, Yankaskas JR, Vick RN, Engelhardt JF, et al. Inefficient gene transfer by adenovirus vector to cystic fibrosis airway epithelia of mice and humans. Nature. 1994;371:802–806. doi: 10.1038/371802a0. [DOI] [PubMed] [Google Scholar]
- 8.Mercier GT, Campbell JA, Chappell JD, Stehle T, Dermody TS, Barry MA. A chimeric adenovirus vector encoding reovirus attachment protein sigma1 targets cells expressing junctional adhesion molecule 1. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(16):6188–6193. doi: 10.1073/pnas.0400542101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chappell JD, Gunn VL, Wetzel JD, Baer GS, Dermody TS. Mutations in type 3 reovirus that determine binding to sialic acid are contained in the fibrous tail domain of viral attachment protein sigma1. J Virol. 1997;71(3):1834–1841. doi: 10.1128/jvi.71.3.1834-1841.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Forrest JC, Campbell JA, Schelling P, Stehle T, Dermody TS. Structure-function analysis of reovirus binding to junctional adhesion molecule 1: Implications for the mechanism of reovirus attachment. The Journal of biological chemistry. 2003 doi: 10.1074/jbc.M305649200. [DOI] [PubMed] [Google Scholar]
- 11.Von Seggern DJ, Huang S, Fleck SK, Stevenson SC, Nemerow GR. Adenovirus vector pseudotyping in fiber-expressing cell lines: improved transduction of Epstein-Barr virus-transformed B cells. J Virol. 2000;74(1):354–362. doi: 10.1128/jvi.74.1.354-362.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Campos SK, Barry MA. Rapid construction of capsid-modified adenoviral vectors through bacteriophage lambda red recombination. Human Gene Therapy. 2004;15(11):1125–1130. doi: 10.1089/hum.2004.15.1125. [DOI] [PubMed] [Google Scholar]
- 13.Mok H, Palmer DJ, Ng P, Barry MA. Evaluation of polyethylene glycol modification of first-generation and helper-dependent adenoviral vectors to reduce innate immune responses. Mol Ther. 2005;11(1):66–79. doi: 10.1016/j.ymthe.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 14.Gottschalk S, Edwards OL, Sili U, Huls MH, Goltsova T, Davis AR, et al. Generating CTLs against the subdominant Epstein-Barr virus LMP1 antigen for the adoptive immunotherapy of EBV-associated malignancies. Blood. 2003;101(5):1905–1912. doi: 10.1182/blood-2002-05-1514. [DOI] [PubMed] [Google Scholar]
- 15.Leen AM, Sili U, Savoldo B, Jewell AM, Piedra PA, Brenner MK, et al. Fiber-modified adenoviruses generate subgroup cross-reactive, adenovirus-specific cytotoxic T lymphocytes for therapeutic applications. Blood. 2004;103(3):1011–1019. doi: 10.1182/blood-2003-07-2449. [DOI] [PubMed] [Google Scholar]
- 16.Hofherr SE, Shashkova EV, Weaver EA, Khare R, Barry MA. Modification of adenoviral vectors with polyethylene glycol modulates in vivo tissue tropism and gene expression. Mol Ther. 2008;16(7):1276–1282. doi: 10.1038/mt.2008.86. [DOI] [PubMed] [Google Scholar]
- 17.Mercier GT, Nehete PN, Passeri MF, Nehete BN, Weaver EA, Templeton NS, et al. Oral immunization of rhesus macaques with adenoviral HIV vaccines using enteric-coated capsules. Vaccine. 2007;25(52):8687–8701. doi: 10.1016/j.vaccine.2007.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


