Abstract
8-OH-DPAT is a 5-HT1A/7 receptor agonist that enhances behavioral recovery after traumatic brain injury (TBI). This study is a first attempt to decipher whether the benefits induced by 8-OH-DPAT after TBI are mediated by 5-HT1A or 5-HT7 receptors. A single i.p. injection of 8-OH-DPAT (0.5 mg/kg) alone or co-administered with either the 5-HT1A or 5-HT7 receptor antagonists WAY 100635 (0.5 mg/kg) or SB 269970 HCl (2.0 mg/kg), respectively, or vehicle control (1.0 mL/kg) was given 15 min after cortical impact or sham injury. Function was assessed by established motor and cognitive tests. No difference in motor performance was observed among the TBI groups. Spatial acquisition was enhanced, relative to vehicle controls, by 8-OH-DPAT alone and when co-administered with WAY 100635, but not when combined with SB 269970 HCl. These data imply that 5-HT1A receptor antagonism does not abate the 8-OH-DPAT-induced cognitive benefits, but 5-HT7 receptor antagonism does, which suggests that the 8-OH-DPAT-induced benefits in this single administration paradigm may be mediated more by 5-HT7 versus 5-HT1A receptors. Evaluation of a specific 5-HT7 receptor agonist will further elucidate the contribution of 5-HT1A and 5-HT7 receptors on behavioral recovery conferred by acute 8-OH-DPAT treatment after TBI.
Keywords: controlled cortical impact, functional recovery, learning and memory, Morris water maze, neurobehavior, serotonin receptor agonists, traumatic brain injury
Introduction
8-hydroxy-2-(di-n-propylamino)tetralin (8-OH-DPAT) is a 5-HT1A/7 receptor agonist that has consistently been shown to attenuate histopathology and enhance spatial learning and memory after traumatic brain injury (TBI) produced with a well-established controlled cortical impact (CCI) injury model [3,4,13–15,17]. 8-OH-DPAT has also been shown to produce benefits in models of ischemia [7,8,25,27], and spinal cord injury [19]. In these studies the benefits have been primarily attributed to agonism at the 5-HT1A receptor, likely because of this receptors' high affinity to 8-OH-DPAT [1,20]. However, other studies evaluating behavior in CNS injury and non-injury models have reported 5-HT1A, 5-HT7, or 5-HT1A/7 mediated effects with 8-OH-DPAT.
In spinal cord-transected mice a single administration of 8-OH-DPAT (1.0 mg/kg; i.p.) produced hindlimb movement characteristic of normal locomotion (Landry et al., 2006). Furthermore, pretreatment with either the 5-HT1A or 5-HT7 receptor antagonists N-{2-[4-(2-methoxyphenyl)piperazin-1-yl]ethyl}-N-(pyridin-2-yl)cyclohexanecarboxamide (WAY 100635) or (2R)-1-[(3-Hydroxyphenyl)sulfonyl]-2-[2-(4-methyl-1-piperidinyl)ethyl] pyrrolidine (SB 269970 HCl), respectively, attenuated the 8-OH-DPAT-induced hindlimb improvement, suggesting that both 5-HT1A/7 receptor subtypes contributed to the benefit [18]. In a non-injury model, contextual learning was impaired in 5-HT7 receptor knockout mice [26], indicating the importance of this receptor, at least for this specific cognitive task. In an opposing view, Gasbarri and colleagues reported that reference memory, but not working memory, was improved following treatment with SB 269970 [10]. Further complicating the issue, Eriksson and colleagues reported that the 8-OH-DPAT-induced impairment of contextual learning was not attributed to, but rather counteracted by, stimulation of 5-HT7 receptors [9]. Thus, there is much ambiguity regarding the roles of the serotonin receptor subtypes 5-HT1A and 5-HT7 in the 8-OH-DPAT-induced cognitive effects seen in TBI as well as non-TBI models.
To determine whether the 8-OH-DPAT-induced benefits are mediated by 5-HT1A or 5-HT7 receptors, the current study was designed to block 5-HT1A and 5-HT7 receptor activity by co-administering their respective selective antagonists, WAY 100635 and SB 269970 HCl with 8-OH-DPAT and then testing for motor and cognitive performance after CCI injury.
Sixty-four male Harlan Sprague-Dawley rats weighing 300–325 g on the day of surgery were housed in standard steel-wire mesh cages in a temperature (21 ± 1°C) and light (on 7:00 a.m. to 7:00 p.m.) controlled environment with continued access to food and water. After one week of acclimatization, the rats were prepared for surgery as previously described [3,4,13–15,17]. Briefly, anesthesia was induced and maintained with inspired concentrations of 4% and 2% isoflurane, respectively, in 2:1 N2O:O2 in a vented anesthesia chamber. After endotracheal intubation the rats were placed in a stereotaxic frame and ventilated mechanically. All rats were maintained at 37 ± 0.5°C during surgery with a heating blanket. Utilizing aseptic techniques a craniectomy was made in the right hemisphere with a hand held trephine and the TBI was produced by impacting the exposed cortex 2.8 mm at 4 m/sec. After the impact, anesthesia was discontinued, the incision was promptly sutured, and the rats were extubated and placed in a temporary Plexiglas holding cage where core temperature was measured with a rectal probe at the time of treatment, which was 15 min after TBI or sham injury, and every 15 min thereafter for 105 min. After the last temperature reading the rats were returned to their home cages. Sham rats underwent similar surgical procedures, but were not subjected to the cortical impact.
Following surgery, the rats were randomly assigned to four TBI (n=10–12 per group) and four sham (n=5 per group) groups. The dose (0.5 mg/kg), timing (15 min post-injury), and administration route (i.p.) of 8-OH-DPAT was selected based on previous studies from our laboratory showing this regimen to be neuroprotective and to promote behavioral recovery after TBI [3,4,13–15,17]. The doses for the 5-HT1A and 5-HT7 receptor antagonists WAY 100635 (0.5 mg/kg) or SB 269970 HCl (2.0 mg/kg) were selected based on preliminary data from our laboratory and were administered immediately before injury (i.e., 15 min before 8-OH-DPAT).
All experimental procedures were approved by the Animal Care and Use Committee at the University of Pittsburgh and were conducted in accordance with the recommendations provided in the Guide for the Care and Use of Laboratory Animals (National Academy Press, 2011). Every attempt was made to limit the number of subjects used and to minimize their discomfort.
Motor function was assessed with well-established beam tasks [3,4,13–15,17]. Beam-balance consisted of placing the rat on an elevated narrow beam (1.5 cm wide) and recording the time it remained on for a maximum of 60 sec. The beam-walk consisted of recording the elapsed time to traverse the beam (2.5 cm wide × 100 cm long). Testing was conducted immediately before surgery (to establish a baseline measure) as well as on post-operative days 1–5, and consisted of three trials (60 sec allotted time with an inter-trial interval of 30 sec) per day on each task. The average daily scores for each subject were used in the statistical analyses.
Spatial learning was assessed in a Morris water maze (MWM) task demonstrated to be sensitive to cognitive function/dysfunction after TBI [3,4,11,13–15,17]. Briefly, the maze consisted of a plastic pool (180 cm diameter; 60 cm high) filled with tap water (26 ± 1°C) to a depth of 28 cm and was situated in a room with salient visual cues that remained constant throughout the study. The platform was a clear Plexiglas stand (10 cm diameter, 26 cm high) that was positioned 26 cm from the maze wall in the southwest quadrant and held constant for each rat. Spatial learning acquisition began on post-operative day 14 and consisted of providing a block of four daily trials (4-min inter-trial interval) for five consecutive days (14–18) to locate the platform when it was submerged 2 cm below the water surface (i.e., invisible to the rat). For each daily block of trials the rats were placed in the pool facing the wall at each of the four possible start locations (north, east, south, and west) in a randomized manner. Each trial lasted until the rat climbed onto the platform or until 120 sec had elapsed, whichever occurred first. Rats that failed to locate the goal within the allotted time were manually guided to it. All rats remained on the platform for 30 sec before being placed in a heated incubator between trials. The times of the 4 daily trials for each rat were averaged and used in the statistical analyses. The data were obtained using a spontaneous motor activity recording & tracking (SMART) system (San Diego Instruments, San Diego, CA).
Statistical analyses were performed on data collected by observers blinded to treatment conditions using Statview 5.0.1 software (Abacus Concepts, Inc., Berkeley, CA). The motor and cognitive data were analyzed by repeated-measures analysis of variance (ANOVA). When the overall ANOVA revealed a significant effect, the data were further analyzed with the Bonferroni/Dunn post-hoc test to determine specific group differences. The data are presented as the mean ± standard error of the mean (S.E.M.) and are considered significant when corresponding p values are ≤ 0.005 as determined by the Bonferroni/Dunn post-hoc statistic after adjusting for multiple comparisons.
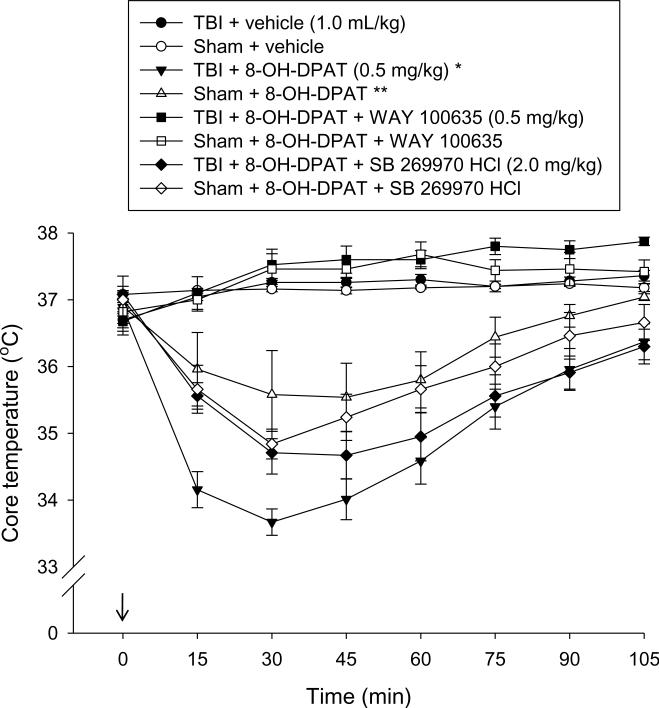
All groups exhibited normothermic core temperatures prior to treatment (Fig. 1). Post-treatment analysis of temperature revealed a significant group effect [p < 0.0001], which was due to a marked hypothermic effect in the TBI and sham groups receiving 8-OH-DPAT and 8-OH-DPAT + SB 269970 HCl relative to the vehicle-treated groups [p < 0.0001]. As depicted in Fig. 1 hypothermia was evident within 15 min of administration, reached a peak response by 30 min, and then steadily subsided over time. The 8-OH-DPAT-induced hypothermic effect was prevented by the co-administration of WAY 100635 [p < 0.0001 vs. TBI + 8-OH-DPAT]. The hypothermia effect induced by 8-OH-DPAT was more pronounced in the TBI vs. Sham group [p < 0.0001].
Fig. 1.
Mean (± S.E.M.) core temperatures (°C). All groups were normothermic prior to administration of vehicle or 8-OH-DPAT (time point 0, 15 min after cortical impact or sham injury). The 5-HT1A and 5-HT7 selective antagonists, WAY 100635 and SB 269770 HCl, respectively, were administered immediately before injury. Both 8-OH-DPAT alone and 8-OH-DPAT + SB 269970 HCl produced a rapid, mild, and transient hypothermic response in both the TBI and sham groups. * p < 0.0001 vs. all groups, except TBI + 8-OH-DPAT + SB 269970 HCl. ** p < 0.0001 vs. all groups, except Sham + 8-OH-DPAT + SB 269970 HCl.
Despite the statistical differences among the sham treatment groups in temperature regulation, there were no differences revealed in any behavioral measure and thus the data were pooled and analyzed as one group (denoted as SHAM).
No pre-surgical differences were observed among groups in either the beam-balance or beam-walk tasks as all rats were able to balance for the allotted 60 sec and traverse the beam within the 5 sec training criterion. However, following the CCI injury significant impairments were detected in all TBI groups vs. SHAM controls on both tests as expected. Both beam-balance and beam-walk performance improved gradually in all TBI groups over time with no significant group differences revealed [p's > 0.05].
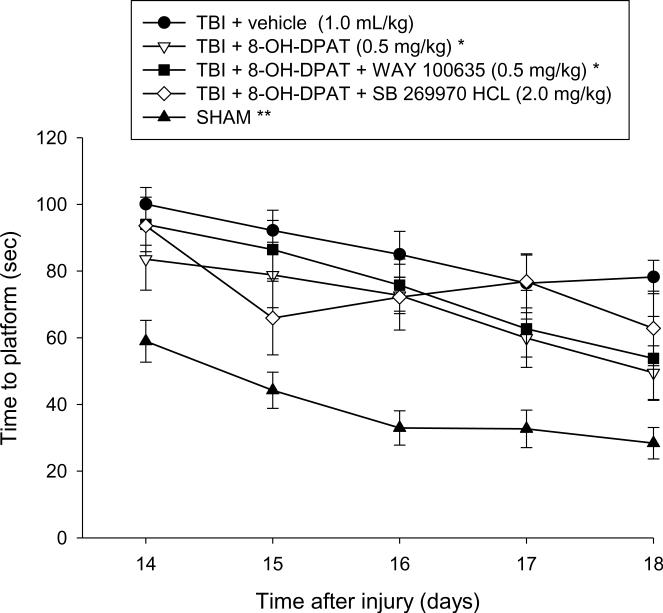
Analysis of the spatial acquisition data revealed significant Group [F4,59 = 19.718, p < 0.0001] and Day [F4,236 = 15.030, p < 0.0001] differences. The post-hoc analysis revealed that the TBI + 8-OH-DPAT and TBI + 8-OH-DPAT + WAY 100635 groups were able to locate the escape platform significantly quicker over time vs. the TBI + vehicle control [p = 0.0005 and p = 0.0024, respectively; Fig. 2]. Although trending to perform better than the TBI + vehicle group, the TBI + 8-OH-DPAT + SB 269970 HCl did not reach the p ≤ 0.005 level of significance required by the Bonferonni/Dunn statistic [p = 0.012]. However, the 8-OH-DPAT + SB 269970 HCl group also did not differ from any of the TBI groups, regardless of treatment [p ≥ 0.32]. The SHAM group was significantly better than all TBI groups [p < 0.0001].
Fig. 2.
Mean (± S.E.M.) time (sec) to locate a hidden (submerged) platform in a water maze. *p ≤ 0.0024 vs. TBI + vehicle control. **p < 0.0001 vs. all TBI groups. No other comparisons were significant.
Analysis of the probe (memory retention) data revealed that the both the TBI + 8-OH-DPAT and SHAM groups spent a greater percentage of the 30 sec allotted time in the target quadrant vs. the TBI + vehicle group (35.1 ± 1.1 % and 35.8 ± 1.7 %, respectively, vs. 24.6 ± 2.6 %) [p = 0.0033, and p = 0.0003, respectively], but did not differ from one another [p = 0.81], which is indicative of intact memory comparable to non-injured controls (Fig. 3). No other probe comparisons were significant. Additionally, no significant difference in swim speed (range = 28.6 ± 1.0 cm/sec to 33.2 ± 1.1 cm/sec) was observed among the groups [p > 0.05].
Fig. 3.
Mean (± S.E.M.) percentage of time spent in the target quadrant (i.e., where platform was previously located) following a single probe trial 19 days after TBI or sham injury. *p = 0.003 vs. TBI + vehicle. **p < 0.0003 vs. TBI + vehicle. No other comparisons were significant. The dotted line represents performance at the chance level (25%).
The primary goal of the study was to elucidate the role of 5-HT1A or 5-HT7 receptors on cognitive recovery conferred by a single systemic administration of 8-OH-DPAT after experimental TBI. The data showed that 8-OH-DPAT facilitated the acquisition of spatial learning as has been shown in previous studies [3,4,13–15,17]. Furthermore, co-administering 8-OH-DPAT and WAY 100635, a selective 5-HT1A receptor agonist, also lead to statistical improvements in cognitive performance relative to the vehicle-treated TBI group. This finding indicates that blocking 5-HT1A receptors does not affect learning in this paradigm and suggests they may not be critically involved in the 8-OH-DPAT mediated benefits. In contrast, co-administration of 8-OH-DPAT and SB 269970 HCl, a selective 5-HT7 receptor antagonist, did not result in the typical 8-OH-DPAT-induced benefit, which indicates that blocking 5-HT7 receptors attenuates behavioral recovery and implies that this receptor subtype may play more of a role in the pro-cognitive effect of 8-OH-DPAT.
However, while the results tend to support a more pronounced role for 5-HT7 versus 5-HT1A receptors in the 8-OH-DPAT-induced benefits in this paradigm, important caveats regarding data interpretation should be kept in mind until further studies yield similar findings. First, we have previously shown that pharmacotherapies that have a high affinity for 5-HT1A receptors and little to no effect on 5-HT7 receptors, such as repinotan HCL and buspirone, produce cognitive enhancement similar to 8-OH-DPAT in this model of TBI [16,24]. Second, the administration of repinotan HCL after either permanent or transient middle cerebral artery occlusion results in significant neuroprotection, as evidenced by reductions in infarct size. Moreover, the repinotan-induced neuroprotection is abolished by WAY 100635, indicating that the benefits are mediated through the 5-HT1A receptor [2]. Thus, while the findings point to a more involved role for 5-HT7 versus 5-HT1A receptors on 8-OH-DPAT mediated improvement, evaluation of a specific 5-HT7 receptor agonist is needed to further elucidate the contribution of 5-HT1A and 5-HT7 receptors on behavioral recovery by acute 8-OH-DPAT treatment after TBI. Such studies are currently on-going.
While we have previously shown that the therapeutic efficacy conferred by 8-OHDPAT is not mediated by concomitant hypothermia [13], we chose to measure temperature in the current study because the 8-OH-DPAT-induced hypothermic response can be considered a robust marker for 5-HT1A [6,22] and perhaps 5-HT7 activity [12,23], which would support the physiological relevance of the 5-HT antagonist doses selected. As previously shown [13,17], 8-OH-DPAT produced a hypothermic response that began within 15 min of administration, peaked at 30 min, and slowly subsided over time. Moreover, the data add to the literature implicating 5-HT1A receptor stimulation in mediating hypothermia after 8-OH-DPAT as no hypothermic response was observed when WAY 100635 was co-administered. In contrast, co-administration of SB 269970 HCl still resulted in a hypothermic response, albeit moderate relative to 8-OH-DPAT alone. These findings suggests that the dose of WAY 100635 used was physiologically relevant, which strengthens the behavioral data and that 5-HT7 receptors play little or no role in the 8-OH-DPAT-induced hypothermia effect after TBI. Interestingly, as in previous studies from our laboratory, hypothermia was more pronounced in the TBI group vs. sham controls, which may be due to hypothalamic dysfunction after injury [5].
In conclusion, while the current data provide a snapshot suggesting that 5-HT7 receptors may be more involved than 5-HT1A receptors in the 8-OH-DPAT mediated cognitive improvement after CCI injury, the interpretation should be tempered by the findings of previous studies showing that highly selective 5-HT1A receptor agonists, such as repinotan HCl produce significant behavioral benefits, which can be reversed by specific antagonists, such as WAY 100635. Further research is necessary to definitively determine which serotonin receptor subtype is more important in the current treatment approach versus a delayed and chronic administration paradigm of 8-OH-DPAT that is more clinically relevant [4,14]. It is likely that both receptors are relevant.
Highlights
-
➢
8-OH-DPAT induces cognitive benefits after experimental TBI
-
➢
5-HT1A receptor antagonism does not abate the 8-OH-DPAT-induced cognitive benefits
-
➢
5-HT7 receptor antagonism attenuates the 8-OH-DPAT-induced cognitive benefits
-
➢
The 8-OH-DPAT-induce cognitive benefits after TBI may be mediated more by 5-HT7 vs. 5-HT1A receptors
-
➢
8-OH-DPAT-induced hypothermia is blocked by 5-HT1A, but not 5-HT7, receptor antagonism
Acknowledgements
Supported, in part, by NIH grants HD043851, HD046700, and NS060005 (AEK)
Abbreviations
- 5-HT
serotonin
- 8-OH-DPAT
8-hydroxy-2-(di-n-propylamino)tetralin
- WAY 100635
N-{2-[4-(2-methoxyphenyl)piperazin-1-yl]ethyl}-N-(pyridin-2-yl)cyclohexanecarboxamide
- SB 269970 HCl
(2R)-1-[(3-Hydroxyphenyl)sulfonyl]-2-[2-(4-methyl-1-piperidinyl)ethyl] pyrrolidine
- TBI
traumatic brain injury
- CCI
controlled cortical impact
- i.p.
intraperitoneal
- ANOVA
repeated-measures analysis of variance
- S.E.M.
standard error of the mean
- MWM
Morris water maze
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
There are no conflicts of interest to report
References
- [1].Barnes NM, Sharp T. A review of central 5-HT receptors and their function. Neuropharmacology. 1999;38:1083–1152. doi: 10.1016/s0028-3908(99)00010-6. [DOI] [PubMed] [Google Scholar]
- [2].Berends AC, Luiten GM, Nyakas C. A review of the neuroprotective properties of the 5-HT1A receptor agonist repinotan HCl (BAY × 3702) in ischemic stroke. CNS Drug Rev. 2005;11:379–402. doi: 10.1111/j.1527-3458.2005.tb00055.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Cheng JP, Aslam HA, Hoffman AN, Zafonte RD, Kline AE. The neurobehavioral benefit conferred by a single systemic administration of 8-OH-DPAT after brain trauma is confined to a narrow therapeutic window. Neurosci. Lett. 2007;416:165–168. doi: 10.1016/j.neulet.2007.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Cheng JP, Hoffman AN, Zafonte RD, Kline AE. A delayed and chronic treatment regimen with the 5-HT1A receptor agonist 8-OH-DPAT after cortical impact injury facilitates motor recovery and acquisition of spatial learning. Behav. Brain Res. 2008;194:79–85. doi: 10.1016/j.bbr.2008.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Csuka E, Hans VHJ, Ammann E, Trentz O, Kossmann T, Morganti-Kossmann MC. Cell activation and inflammatory response following traumatic axonal injury in the rat. NeuroReport. 2000;11:2587–2590. doi: 10.1097/00001756-200008030-00047. [DOI] [PubMed] [Google Scholar]
- [6].Cryan JF, Kelliher P, Kelly JP, Leonard BE. Comparative effects of serotonergic agonists with varying efficacy at the 5-HT1A receptor on core body temperature: modification by the selective 5-HT1A receptor antagonist WAY 100635. J. Psychopharmacol. 1999;13:278–283. doi: 10.1177/026988119901300310. [DOI] [PubMed] [Google Scholar]
- [7].De Vry J, Dietrich H, Glaser T, Heine H-G, E., Jork R, Maertins T, Mauler F, Opitz W, Scherling D, Schohe-Loop R, Schwarz T. BAY × 3702. Drugs of the Future. 1997;22:341–349. [Google Scholar]
- [8].De Vry J, Schohe-Loop R, Heine H-G, Greuel JM, Mauler F, Schmidt B, Sommermeyer H, Glaser T. Characterization of the aminomethylchroman derivative BAY × 3702 as a highly potent 5-hydroxytryptamine 1A receptor agonist. J. Pharmacol. Exp. Ther. 1998;284:1082–1094. [PubMed] [Google Scholar]
- [9].Eriksson TM, Golkar A, Ekström JC, Svenningsson P, Ögren SO. 5-HT7 receptor stimulation by 8-OH-DPAT counteracts the impairing effect of 5-HT1A receptor stimulation on contextual learning in mice. Eur. J. Pharmacol. 2008;596:107–110. doi: 10.1016/j.ejphar.2008.08.026. [DOI] [PubMed] [Google Scholar]
- [10].Gasbarri A, Cifariello A, Meneses A. Effect of 5-HT7 antagonist SB-269970 in the modulation of working memory and reference memory in the rat. Behav. Brain Res. 2008;195:164–170. doi: 10.1016/j.bbr.2007.12.020. [DOI] [PubMed] [Google Scholar]
- [11].Hamm RJ, Dixon CE, Gbadebo DM, Singha AK, Jenkins LW, Lyeth BG, Hayes RL. Cognitive deficits following traumatic brain injury produced by controlled cortical impact. J. Neurotrauma. 1992;9:11–20. doi: 10.1089/neu.1992.9.11. [DOI] [PubMed] [Google Scholar]
- [12].Hedlund PB, Kelly L, Mazur C, Lovenberg T, Sutcliffe JG, Bonaventure P. 8-OH-DPAT acts on both 5-HT1A and 5-HT7 receptors to induce hypothermia in rodents. Eur. J. Pharmacol. 2004;487:125–132. doi: 10.1016/j.ejphar.2004.01.031. [DOI] [PubMed] [Google Scholar]
- [13].Kline AE, Massucci JL, Dixon CE, Zafonte RD, Bolinger BD. The therapeutic efficacy conferred by the 5-HT1A receptor agonist 8-hydroxy-2-(di-n-propylamino)tetralin (8-OH-DPAT) after experimental traumatic brain injury is not mediated by concomitant hypothermia. J. Neurotrauma. 2004;21:175–185. doi: 10.1089/089771504322778631. [DOI] [PubMed] [Google Scholar]
- [14].Kline AE, McAloon RL, Henderson KA, Bansal UK, Ganti BM, Ahmed RH, Gibbs RB, Sozda CN. Evaluation of a combined therapeutic regimen of 8-OH-DPAT and environmental enrichment after experimental traumatic brain injury. J. Neurotrauma. 2010;27:2021–2032. doi: 10.1089/neu.2010.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Kline AE, Wagner AK, Westergom BP, Malena RR, Zafonte RD, Olsen AS, Sozda CN, Luthra P, Panda M, Cheng JP, Aslam HA. Acute treatment with the 5-HT1A receptor agonist 8-OH-DPAT and chronic environmental enrichment confer neurobehavioral benefit after experimental brain trauma. Behav. Brain Res. 2007;177:186–194. doi: 10.1016/j.bbr.2006.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Kline AE, Yu J, Horváth E, Marion DW, Dixon CE. The selective 5-HT1A receptor agonist repinotan HCl attenuates histopathology and spatial learning deficits following traumatic brain injury in rats. Neuroscience. 2001;106:547–555. doi: 10.1016/s0306-4522(01)00300-1. [DOI] [PubMed] [Google Scholar]
- [17].Kline AE, Yu J, Massucci JL, Zafonte RD, Dixon CE. Protective effects of the 5-HT1A receptor agonist 8-hydroxy-2-(di-n-propylamino)tetralin (8-OH-DPAT) against traumatic brain injury-induced cognitive deficits and neuropathology in adult male rats. Neurosci. Lett. 2002;333:179–182. doi: 10.1016/s0304-3940(02)01101-1. [DOI] [PubMed] [Google Scholar]
- [18].Landry ES, Lapointe NP, Rouillard C, Levesque D, Hedlund PB, Guertin PA. Contribution of spinal 5-HT1A and 5-HT7 receptors to locomotor-like movement induced by 8-OH-DPAT in spinal cord-transected mice. Eur. J. Pharmacol. 2006;24:535–546. doi: 10.1111/j.1460-9568.2006.04917.x. [DOI] [PubMed] [Google Scholar]
- [19].Lapointe NP, Guertin PA. Synergistic effects of D⅕ and 5-HT1A/7 receptor agonists on locomotor movement induction in complete spinal cord-transected mice. J. Neurophysiol. 2008;100:160–168. doi: 10.1152/jn.90339.2008. [DOI] [PubMed] [Google Scholar]
- [20].Meneses A. 5-HT system and cognition. Neurosci. Biobehav. Rev. 1999;23:1111–1125. doi: 10.1016/s0149-7634(99)00067-6. [DOI] [PubMed] [Google Scholar]
- [21].Meneses A, Perez-Garcia G. 5-HT1A receptors and memory. Neurosci. Biobehav. Rev. 2007;31:705–727. doi: 10.1016/j.neubiorev.2007.02.001. [DOI] [PubMed] [Google Scholar]
- [22].Millan MJ, Rivet JM, Canton H, Le Marouille-Girardon S, Gobert A. Induction of hypothermia as a model of 5-hydroxytryptamine1A receptor-mediated activity in the rat: a pharmacological characterization of the actions of novel agonists and antagonists. J. Pharmacol. Exp. Ther. 1993;264:1364–1376. [PubMed] [Google Scholar]
- [23].Naumenko VS, Kondaurova EM, Popova NK. On the role of brain 5-HT7 receptor in the mechanism of hypothermia: comparision with hypothermia mediated via 5-HT1A and 5-HT3 receptor. Neuropharmacology. 2011;61:1320–1365. doi: 10.1016/j.neuropharm.2011.08.022. [DOI] [PubMed] [Google Scholar]
- [24].Olsen AS, Sozda CN, Cheng JP, Hoffman AN, Kline AE. Traumatic brain injury-induced cognitive and histological deficits are attenuated by delayed and chronic treatment with the 5-HT1A receptor agonist buspirone. J. Neurotrauma. doi: 10.1089/neu.2012.2358. unpublished results. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Prehn JH, Backhauss C, Karkoutly C, Nuglisch J, Peruche B, Rossberg C, Krieglstein J. Neuroprotective properties of 5-HT1A receptor agonists in rodent models of focal and global cerebral ischemia. Eur. J. Pharmacol. 1991;203:213–222. doi: 10.1016/0014-2999(91)90717-5. [DOI] [PubMed] [Google Scholar]
- [26].Roberts AJ, Krucker T, Levy CL, Slanina KA, Sutcliffe JG, Hedlund PB. Mice lacking 5-HT receptors show specific impairments in contextual learning. Eur. J. Neurosci. 2004;19:1913–1922. doi: 10.1111/j.1460-9568.2004.03288.x. [DOI] [PubMed] [Google Scholar]
- [27].Semkova I, Wolz P, Krieglstein J. Neuroprotective effect of 5-HT1A receptor agonist, Bay x 3702, demonstrated in vitro and in vivo. Eur. J. Pharmacol. 1998;359:251–260. doi: 10.1016/s0014-2999(98)00634-7. [DOI] [PubMed] [Google Scholar]