Abstract
Sepsis induced lymphocyte and dendritic cell apoptosis contributes to immunosuppression, which results in an inability to eradicate the primary infection as well as a propensity to acquire new, secondary infections. Another cellular process, autophagy, is also activated in immune cells and plays a protective role. In the present study, we demonstrate that interferon regulatory factor-1 (IRF-1) regulates both immune cell apoptosis and autophagy in a murine endotoxemia model. IRF-1 is activated at an early phase through a Toll-like receptor 4 (TLR4)-dependent, myeloid differentiation primary response gene 88 (MyD88)-independent manner in splenocytes. Further, IRF-1 knockout (KO) mice are protected from a lethal endotoxemia model. This protection is associated with decreased apoptosis and increased autophagy in splenocytes. IRF-1 KO mice experience decreased apoptotic cell loss, especially in CD4+ T lymphocytes and myeloid antigen presenting cells (APC). Meanwhile, IRF-1 KO mice demonstrate increased autophagy and improved mitochondrial integrity. This increased autophagy in KO mice is attributable, at least in part, to deactivationof mammalian target of rapamycin (mTOR)/P70S6 signaling - a main negative regulator of autophagy. Therefore, we propose a novel role for IRF-1 in regulating both apoptosis and autophagy in splenocytes in the setting of endotoxemia with IRF-1 promoting apoptosis and inhibiting autophagy.
Keywords: IRF-1, endotoxemia, apoptosis, autophagy, splenocyte
INTRODUCTION
Sepsis remains the leading cause of death of critically ill patients. In the United States, it accounts for an estimated 250,000 deaths per year. Mortality rates of up to 70% have been estimated. Moreover, its incidence is believed to be increasing at a rate of 1.5%/year (1). Lipopolysaccharide (LPS), a component of gram-negative bacteria cell walls, induces expression of many genes necessary for the execution of host defense function. Lethal endotoxemia is a widely used experimental model that mimics many features of septic shock, including elevated cytokine production and extensive leukocyte apoptosis (2).
Sepsis is characterized by an initial hyper-inflammatory response, followed by a period of immunosuppression that has been termed “immunoparalysis” (3, 4). Current data indicates that deregulated lymphocyte and dendritic cell apoptosis contributes to immunosuppression during sepsis (5, 6). Interventions aimed at reducing lymphocyte and dendritic cell apoptosis may improve survival in animal endotoxemia models (7–10).
Autophagy is also activated in sepsis and plays a protective role, by maintaining cellular homeostasis and limiting cell damage and death (11–13). In vivo, sepsis can induce autophagy in liver and kidney, which protects against apoptotic cell death (13, 14). In vitro studies suggest that LPS induces autophagy in both cardiomyocytes and macrophages (15, 16). Also, autophagyparticipates in a wide array of processes in the immune response with its immunologic functions spanning both innate and adaptive immunity (17–19).
Interestingly, recent studies indicate that crosstalk exists between apoptosis and autophagy. The inhibition of autophagy results in accelerated apoptotic cell death, while increased autophagy allows for cell survival by inhibiting apoptosis. Such a reciprocal regulation between apoptosis and autophagy has been found in some diseases (15, 20–22). Therefore, the “choice” between autophagy and apoptosis may be decisive for the fate of cells and thus important in the pathogenesis of a wide range of diseases.
The interferon regulatory factor-1 (IRF-1) was the first of 9 identified mammalian members of the IRF family. IRF-1 plays an important role in the initiation of innate immune responses and has been involved in multiple functions including cellular responses to inflammation and programmed cell death (23). IRF-1 promotes I/R injury induced HMGB1 release in hepatocytes (24) and also participates in mortality associated with disease models mediated by TNFα and IFNγ (25). Moreover, IRF-1 promotes apoptosis in breast and bladder cancer cells as well as hepatocytes (26–28). In immune cells, overexpression of IRF-1 enhances the sensitivity of DNA damage induced apoptosis in T lymphocytes (29); however, whether IRF-1 participates in apoptosis in immune cells in the setting of endotoxemia is still undetermined. Furthermore, whether IRF-1 can regulate autophagy in this setting is also unknown.
The present study investigates the activation and function of IRF-1 during endotoxemia. Our data suggests that IRF-1 is expressed in splenocytes at early time points following LPS administration. This activation is mediated through TLR4, specifically the MyD88 independent pathway. We confirm that IRF-1 KO mice are protected from LPS induced mortality and demonstrate for the first time that this protection is associated with decreased splenocyte apoptosis and increased autophagy. Therefore, we propose a novel, previously undescribed role for IRF-1 in regulating both apoptosis and autophagy in splenocytes in the setting of endotoxemia.
MATERIALS AND METHODS
Reagents and Antibodies
LPS from E coli 0111:B4 was obtained from Sigma-Aldrich (St. Louis, MO, USA). Rabbit polyclonal IRF-1 (M-20) Ab was from Santa Cruz (Santa Cruz, CA, USA). Rabbit cleaved caspase-3, -8, -9 Ab, Histone H3 Ab, mTOR/p-mTOR Ab, and P70S6/p-P70S6 Ab were all from Cell Signaling (Boston, MA, USA). Rabbit anti-LC3B Ab was from NOVUS (Littleton, CO, USA), β-actin Ab from Sigma-Aldrich (St. Louis, MO, USA).
Animals
Male B6.129S2-irf1tm1Mak/J (IRF1-KO) and matched C57BL/6J mice (8–10wks) were purchased from the Jackson Laboratory (Bar Harbor, ME). Male TLR4 KO, MyD88 KO, TRIF-KO mice, and WT counterparts were bred at the University of Pittsburgh (T. R. Billiar; originally provided from R. Medhzitov, Howard Hughes Medical Institute). Animals were maintained in a laminar flow housing apparatus. The Animal Care and Use Committee of the University of Pittsburgh approved animal protocols. Experiments were performed in adherence to the National Institutes of Health Guidelines for the Use of Laboratory Animals.
In vivo experimental design
Male IRF1-KO and matched C57BL/6 mice were injected intraperitoneally (i.p.) with a lethal dose of LPS (20 mg/kg). Control mice received injections of sterilized PBS. The mice were sacrificed at predetermined time points after LPS (20 mg/kg) i.p. injection for spleen samples.
Splenocytes isolation
Splenic tissue was harvested from euthanized mice and washed with cold PBS. Splenocytes were prepared into a single-cell suspension by squeezing the spleen in a culture plate and then fragmenting the red blood cells with RBC lysing buffer from Sigma-Aldrich (St. Louis, MO, USA). Cell suspensions were prepared at a concentration of 5×106 cells/ml.
Cellular and nuclear protein extraction
Cells collected were centrifugated at 5000 rpm for 5 min. These pellets were then resuspended on ice with lysis buffer (20 mM Tris, 137 mM NaCl, 2 mM EDTA, 10% glycerol, 1% Triton X-100, 1 μM sodium orthovanadate, 100 μM DTT, 200 μM PMSF, 10 μg/ml leupeptin, 0.15 U/ml aprotinin, 50 mM sodium fluoride, 10 mM sodium pyrophosphate, 2.5 μg/ml pepstatin A, 1 mM benzamidine, and 40 mM α-glycerophosphate) for 45 min, then centrifuged at 13000 rpm for 15 min at 4°C. Protein concentration was quantitated with bicinchoninic acid protein assay reagent (Pierce Chemical Co). For nuclear protein isolation, the cell pellet was lysed with NE-PER nuclear and cytoplasmic extraction reagent (Thermo Scientific).
Western blot analysis
Protein obtained from cultured cells or tissue was electrophoresed in 8–15% SDS-PAGE gels and then transferred to Trans-Blot nitrocellulose membranes (Bio-Rad Laboratories) which were blocked for 1 h at room temperature with 5% nonfat milk and then incubated in a HRP-conjugated secondary Ab against the primary Ab at room temperature for 1 h. Membranes were developed with the Super Signal West Pico chemiluminescent kit (Pierce Chemical Co.) and exposed to film (Eastman Kodak). Densitometry was performed by Image J software from the National Institutes of Health (Bethesda, MD).
Apoptosis assessment by tissue sections TUNEL and cleaved caspase-3 immunohistochemistry staining
The spleen were excised and fixed in 10% buffered formaldehyde, and paraffin embedded tissue sections at 6 μm was prepared. ApopTag Peroxidase In Situ Apoptosis Detection Kit (S7100) from Chemicon (Temecula, CA, USA) was used for TUNEL staining. The experiment was performed according to manufacturer’s protocol. Cleaved caspase-3 was detected by immunohistochemistry staining as described below. Briefly, tissue sections were deparaffinized in xylene, rehydrated and incubated in 0.05% saponin. Then sections were incubated in 1% hydrogen peroxide to quench endogenous peroxidase activity. After incubation in 1.5% normal blocking serum, tissue sections were incubated with rabbit anti-cleaved caspase-3 (1:100) overnight at 4°C. After washing, sections were incubated with biotin-conjugated goat anti-rabbit secondary antibody. Followed by incubation with avidin biotin enzyme, tissue sections were incubated in peroxidase substrate, washed, and counter-stained in hematoxylin. The slides were observed by light microscopy for evidence of apoptosis by blinded observers. Quantification of the images was processed and analyzed as described previously. Positive staining was presented as percentage of stained area over total area (% area stained) (30). Each experiment was repeated a minimal of three times.
Flow cytometry analysis
Splenic tissue was aseptically harvested from IRF-1 KO and WT mice at 16 h after LPS administration and prepared as a single cell suspension. Cells harvested were used for apoptosis analysis and mitochondrial mass measurement directly. Apoptotic cells were detected by using the FITC-Annexin V/7-AAD apoptosis detection kit according to the manufacture protocol (BD Biosciences). Immune cells were identified using two panels. Panel 1: CD4+ T cells were identified as CD3+ (Biolegend, 145-2C11, PE) and CD4+ (Biolegend, GK1.5, Pacific Blue). CD8+ T cells were CD3+ and CD8+ (Biolegend, 53–6.7, PE-Cy7). Panel 2: Mac/DC were identified as CD11b+ (Biolegend, M1/70, Pacific Blue) and NK1.1- (eBioscience, PK136, PE). NK cells were CD11b+NK1.1+, and B cells were B220+ (Biolegend, RA3-6B2, PE-Cy7). For measurement of mitochondrial mass, splenocytes were stained for 45 min at 37°C with 200 nM of MitoTracker Green FM and MitoTracker Deep Red FM (Invitrogen)(11). Data were acquired with a BD FACS LSR Fortessa flow cytometer (BD Biosciences) and analyzed with FlowJo analytical software (Treestar). Each experiment was repeated a minimal of three times.
Transmission electron microscopy (TEM)
Splenic tissues were harvested 16 h following LPS administration. The specimens were fixed in cold 2.5% glutaraldehyde in 0.1 M PBS. The specimens were rinsed in PBS, post-fixed in 1% Osmium Tetroxide with 0.1% potassium ferricyanide, rinsed in PBS, dehydrated through a graded series of ethanol and embedded in Epon. Semi-thin (300 nm) sections were stained with 0.5% Toluidine Blue and examined under the light microscope. Ultrathin sections (75 nm) were stained with uranyl acetate and Reynold’s lead citrate. Sections were viewed by JEM-1011 electron microscope (Pittsburgh, PA, USA). Apoptosis is characterized by cell shrinkage, chromatin condensation and apoptotic body formation. Percentage of apoptotic cells among total cells was used for apoptosis quantification. Autophagy is characterized by the presence of phospholipids bilayers or cytoplasmic material within lysosomes. 8–10 randomly selected fields (approximately 3000 μm2) were examined in blinded manner for evidence of autophagy. Determining the number of autophagic vacuoles per cross- sectioned cell was used for autophagy quantification (31).
Statistical analysis
Quantitative data were presented as mean ± SD and analyzed by the Student’s t test or ANOVA by SPSS16.0. P value of <0.05 was considered statistically significant. Qualitative data were representative of three experiments.
RESULTS
IRF-1 expression in splenocytes occurs at early time points following LPS administration and is mediated through TLR4-TRIF dependent signaling
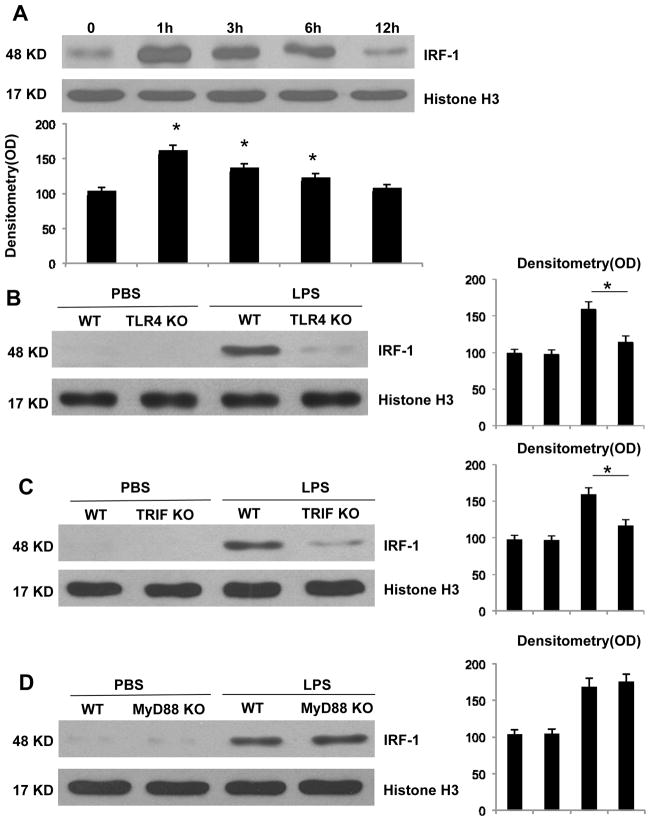
IRF-1 is an important immune regulator and plays a role in the progression of endotoxemia. As previously described (25), IRF-1 KO mice experienced significantly improved survival outcomes following LPS administration compared to their WT counterparts at 96 h (data not shown). Spleen is a large lymphatic organ, so we further verified the expression of IRF-1 in splenocytes. Since IRF-1 is an immediate-early transcription factor, nuclear IRF-1 is thought to be its active, functional form. Thus, we measured by Western blot the expression of IRF-1 in splenocyte nuclei as representative of active IRF-1. After LPS administration, splenocytes were harvested at predetermined time points. LPS induced IRF-1 expression in splenocytes with peak levels occurring at 1 h (Fig. 1A). We further investigated the mechanism for increased IRF-1 expression in splenocytes in response to LPS. One hr following either PBS or LPS administration, splenocytes were isolated from WT, TLR4 KO, TRIF, and MyD88KO mice, respectively. Splenocytes from TLR4 KO mice failed to demonstrate increased IRF-1 expression in response to LPS stimulation (Fig. 1B). Similarly, LPS administration failed to induce IRF-1 in splenocytes obtained from TRIF deficient mice (Fig. 1C); however, those obtained from MyD88 KO mice exhibited normal LPS induced IRF-1 (Fig. 1D). These results demonstrate that IRF-1 is expressed at early time points in splenocytes in response to LPS administration, and that this process occurs through a TLR4-TRIF dependent, MyD88-independent manner.
Figure 1. IRF-1 activation at early phase and is mediated through the TLR4-TRIF dependent manner in response to LPS stimulation.
(A) After LPS injection at predetermined time points, splenocytes were isolated from WT mice. Nuclear proteins were isolated and IRF-1 activation was analyzed by Western blot. WT and TLR4 KO (B), TRIF KO (C), MyD88 KO (D) mice were injected with PBS or LPS for 1 h and splenocytes were isolated. Nuclear IRF-1 was analyzed by Western blot. *, P<0.05; results are representative of 3 separate independent experiments.
IRF-1 KO mice have decreased apoptosis in splenocytes following LPS exposure
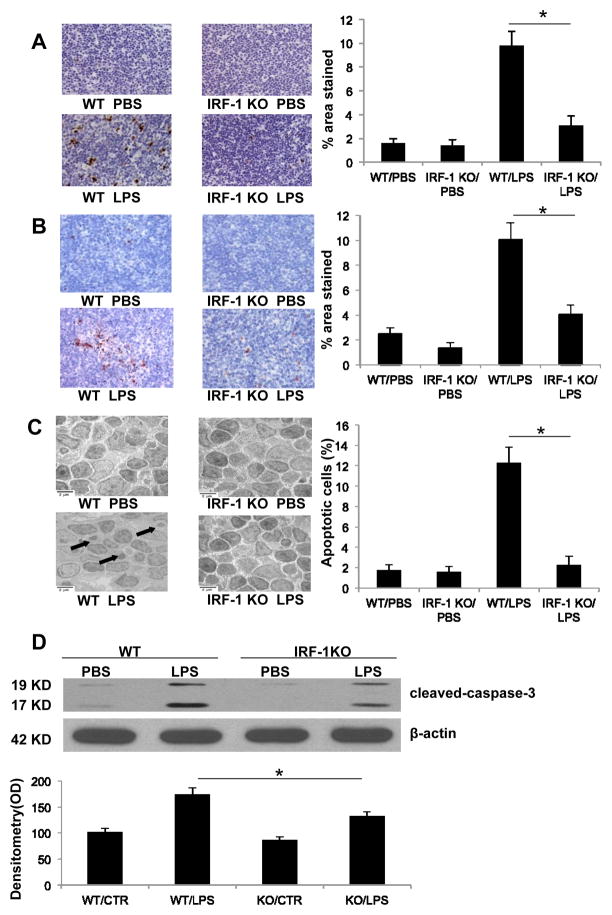
Apoptosis is one of the best-characterized mechanisms that contribute to sepsis induced end organ damage, immunosuppression, and death (5, 6). To analyze the incidence of apoptosis in splenocytes following LPS administration, splenic tissue was harvested from LPS injected IRF-1 WT and KO mice and subjected to TUNEL staining, cleaved caspase-3 staining, and TEM. Splenic tissue obtained from IRF-1 KO mice exhibited less TUNEL positive cells (Fig. 2A), decreased cleaved caspase-3 staining (Fig. 2B), and decreased apoptotic body formation (Fig. 2C) compared to those obtained from WT mice 16 h after LPS injection. Western blot for caspase-3 cleavage confirmed similar results, with splenocytes from IRF-1 KO mice demonstrating decreased caspase-3 cleavage in response to LPS stimulation compared to those obtained from their WT counterparts (Fig. 2D). These results suggest that IRF-1 participates in the induction of apoptosis in splenocytes following endotoxin exposure in vivo.
Figure 2. IRF-1 KO mice have decreased apoptosis in splenocytes following LPS exposure.
Permanent blocks of splenic tissue obtained from WT and IRF-1 KO mice 16 h following PBS or LPS (20 mg/kg) injection were sectioned and (A) TUNEL staining or (B) cleaved caspase-3 immunohistochemistry staining was performed (magnitude X 200). Positive staining was presented as percentage of stained area over total area (% area stained). (C) Splenic tissue obtained from WT and IRF-1 KO mice 16 h following PBS or LPS (20 mg/kg) injection was imaged by transmission electron microscope (magnitude X 5000). Arrow points to apoptotic bodies. Percentage of apoptotic cells among total cells was used for apoptosis quantification. (D) WT and IRF-1 KO mice were injected with PBS or LPS (20 mg/kg) for 16 h and splenocytes were isolated. Caspase-3 cleavage was analyzed by Western blot. *, P<0.05; results are representative of 3 separate independent experiments.
Apoptosis is reduced predominantly in splenic CD4+ T lymphocytes and myeloid antigen presenting cells (APCs) in IRF-1 KO mice following LPS exposure
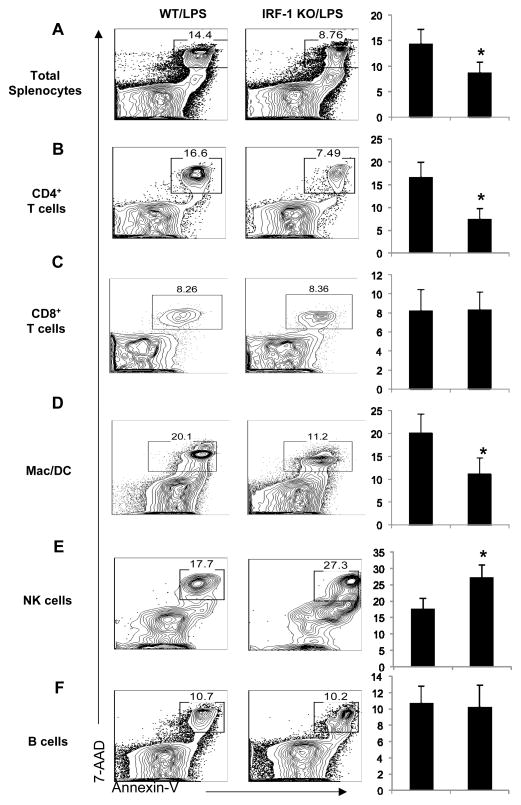
As demonstrated above, IRF-1 promotes splenocyte apoptosis in endotoxemia. However, splenocytes consist of different cell populations, such as CD4+ and CD8+ T lymphocytes, B-lymphocytes, myeloid APC (dendritic cells and macrophages), and NK cells, with different cell populations having different immune functions. We next explored which cell population(s) may be more sensitive to IRF-1 induced apoptosis in endotoxemia. Flow cytometry analysis with quantitative evaluation of apoptotic cells in homogenized splenic tissues demonstrated that LPS challenge causes a significant increase in total numbers of apoptotic splenocytes (Fig. 3A), especially CD4+ T lymphocytes (Fig. 3B) and myeloid APC (Fig. 3D) in WT compared to KO mice at the 16 h time point. CD8+ T lymphocyte (Fig. 3C) and B lymphocyte apoptosis were not affected by IRF-1 at this time point (Fig. 3F). Interestingly, IRF-1 may inhibit NK cells apoptosis, as KO mice demonstrated increased NK cell apoptosis compared to their WT counterparts (Fig. 3E).
Figure 3. Apoptosis is selectively reduced in splenic CD4+ T lymphocytes and myeloid APC in IRF-1 KO mice following LPS exposure.
WT and IRF1-KO mice were challenged with LPS (20 mg/kg) as described and total splenocytes were harvested. Apoptotic cells (Annexin V+7-AAD+) were detected by flow cytometry. (A) Total splenocytes, (B) CD3+CD4+ T cells, (C) CD3+CD8+ T cells, (D) CD11b+NK1.1- myeloid APC including dendritic cells and macrophages, (E) CD11b+NK1.1+ NK cells, and (F) B220+ B cells were analyzed. The percent of apoptotic cells was quantified for each cell population. *, P<0.05; results are representative of 3 separate independent experiments.
Splenocytes from IRF-1 KO mice demonstrate increased autophagy compared to WT mice following LPS exposure
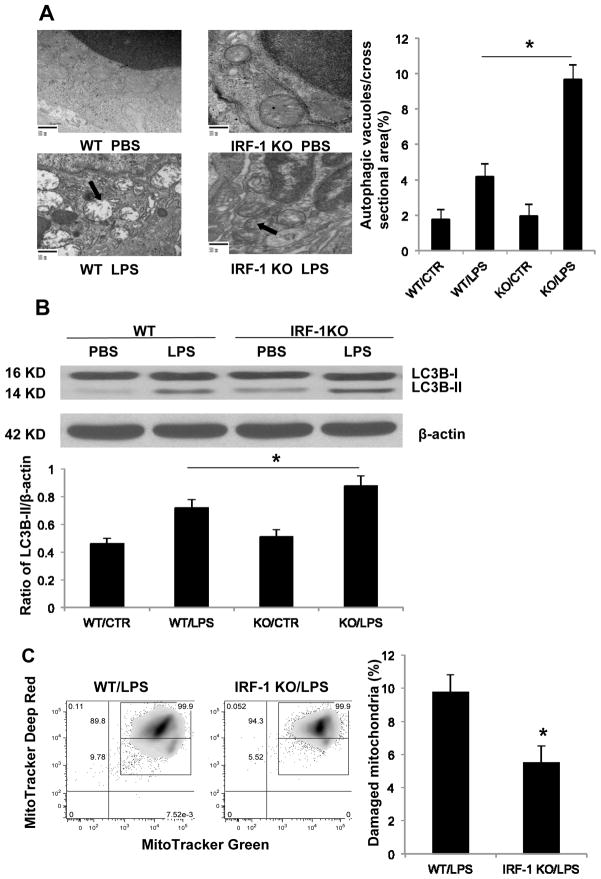
Autophagy is also activated in sepsis and plays a protective role (11–14). During autophagy, a cytosolic LC3-I is conjugated to phosphatidylethanolamine (PE) to form LC3-II, which is frequently used as a biomarker for autophagy. To determine if IRF-1 affects splenocyte autophagy following LPS administration, western blot for LC3B-II and TEM were performed. Splenic tissue obtained from LPS injected IRF-1 KO mice demonstrated increased evidence of autophagy and improved mitochondrial integrity compared to tissue obtained from WT mice, which demonstrated evidence of extensive mitochondrial damage (Fig. 4A). Additionally, splenocytes from IRF-1 KO mice revealed an increased autophagic response after LPS stimulation, as demonstrated by increased cytoplasmic LC3B-II activation (Fig. 4B). Autophagy controls the clearance of damaged mitochondrial. Disruption of autophagy generates a defect in mitochondrial homeostasis, which results in the accumulation of damaged mitochondrial and increased ROS production (11). We further assessed the functional mitochondrial pool by using MitoTracker Deep Red and MitoTracker Green staining. The percentage of damaged mitochondria in IRF-1 WT splenocytes was 9.78% while it was only 5.52% in IRF-1 KO splenocytes (Fig. 4C), demonstrating more mitochondrial damage in presence of IRF-1 following LPS administration. Together, these data indicate that mice lacking IRF-1 experienced increased splenocyte autophagy following endotoxemia in vivo, which may play a role in mitochondrial homeostasis maintenance.
Figure 4. Splenocytes from IRF-1 KO mice demonstrate increased autophagy compared to WT mice following LPS exposure.
(A) Splenic tissue obtained from WT and IRF-1 KO mice 16 h following PBS or LPS (20 mg/kg) injection imaged by transmission electron microscope (magnitude X 20000). Arrows point to damaged mitochondria and autophagy. Determining the number of autophagic vacuoles per cross-sectioned cell was used for autophagy quantification. (B) WT and IRF-1 KO mice were injected with PBS or LPS (20 mg/kg) for 16 h and splenocytes were isolated. LC3B-II was analyzed by Western blot. (C) WT and IRF-1 KO mice were injected with LPS (20 mg/kg) for 16 h and splenocytes were isolated and stained with 200 nM MitoTracker Deep Red and MitoTracker Green for 45 min at 37°C. Representative flow cytometry plots are represented. *, P<0.05; results are representative of 3 separate independent experiments.
LPS induced IRF-1 activation in splenocytes promotes both intrinsic and extrinsic apoptotic pathways and inhibits autophagy by modulating mTOR/p70S6 activity
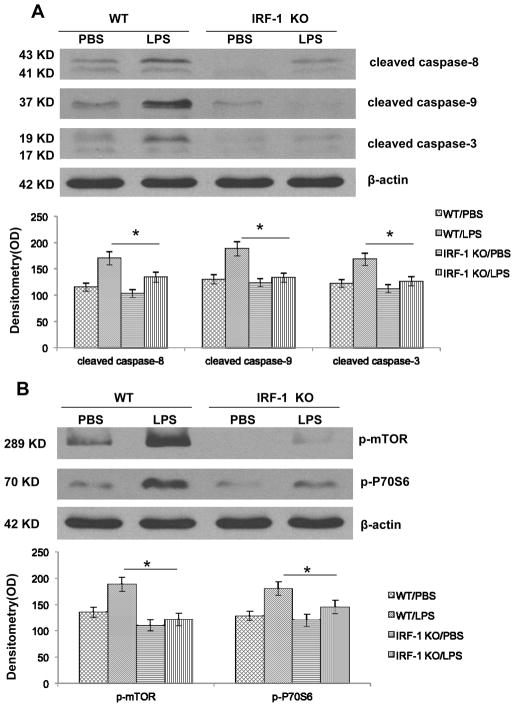
Apoptosis can proceed by two mechanistically distinct pathways, a receptor-mediated “extrinsic” pathway that proceeds by activation of caspase-8 or a mitochondrial mediated “intrinsic” pathway that proceeds through caspase-9. During sepsis, lymphocytes undergo both extrinsic and intrinsic apoptosis (32). Among the many well-described regulators of autophagy, the mammalian target of rapamycin (mTOR) is the best-characterized negative regulator. To further explore the mechanism of how IRF-1 modulates autophagy and apoptosis, splenocytes were isolated from both WT and KO mice 16 h after LPS injection. IRF-1 KO splenocytes showed decreased cleavage of caspase-8, -9 and -3 in endotoxemia (Fig. 5A). Meanwhile, WT splenocytes demonstrated the activation of mTOR and its downstream effector P70S6 after LPS injection; and, absence of IRF-1 abolished this activation (Fig. 5B). Thus, our present data suggest that IRF-1 participates in both extrinsic and intrinsic apoptotic pathways. Deactivation of mTOR and its downstream P70S6 may account for the increased autophagic flux in IRF-1 KO mice.
Figure 5. IRF-1 activation promotes both intrinsic and extrinsic apoptotic pathway and inhibits autophagy by modulating mTOR/P70S6 activity.
(A) WT and IRF-1 KO mice were injected with PBS or LPS (20 mg/kg) for 16 h and splenocytes were isolated. Caspase-3, -8, -9 cleavage was analyzed by Western blot. (B) WT and IRF-1 KO mice were injected with PBS or LPS (20 mg/kg) for 16 h and splenocytes were isolated. mTOR and P70S6 activation were analyzed by Western blot. *, P<0.05; results are representative of 3 separate independent experiments.
DISCUSSION
Despite major advances in critical care management and antibiotic therapies, sepsis remains a challenging clinical problem. More recent research has shown that although sepsis may initially be characterized by excessive systemic inflammation, there may be a shift towards an anti-inflammatory, immunosuppressed state in the later stages of the disease. During this phase, patients may have difficulty eradicating invasive pathogens and are susceptible to life-threatening secondary hospital-acquired infections (33). Sepsis induced immune dysfunction occurs at a later stage, making it an attractive therapeutic target.
Apoptosis is an important mechanism for cell death in sepsis and occurs in many end organs. Accelerated apoptosis induced loss of immune cells may be a key trigger of the immunosuppressive state during sepsis. In experimental sepsis models, with the exception of the thymus, most lymphoid tissues do not show marked evidence of apoptosis until a late time point (>12 h) after initiation of infection. Deregulated apoptotic cell death is proposed to contribute to the increase in morbidity and mortality that is seen in this hypo-responsive phase in sepsis (6).
Direct apoptotic depletion of critical immune cells, such as B-lymphocytes, CD4+ T lymphocytes, and dendritic cells, among others, may lead to subsequent failure of the adaptive and innate immune systems (34, 35). The potential importance of apoptosis has been illustrated by studies showing that prevention of lymphocyte apoptosis improves the survival in experimental animal model of sepsis. In transgenic mouse experiments, over-expression of the anti-apoptotic, pro-survival protein Bcl-2 in T lymphocytes has demonstrated obvious protection against sepsis induced death (36). Furthermore, specific caspase-3 inhibitors prevent sepsis induced lymphocyte apoptosis, decrease systemic bacterial counts, and improve outcomes; all of which were associated with matured lymphocytes (37, 38).
To gain insight into the potential impact of splenocyte apoptosis in sepsis, it is essential to determine the extent of loss and type of splenocytes that are affected in the disorder. Loss of CD4+ T lymphocytes limits macrophage activation and impairs the proper inflammatory response to the invading organism. Loss of dendritic cells in sepsis may significantly impair B and T cell function. The death of dendritic cells may also be an important mechanism of immunosuppression in sepsis (35). Conversely, NK cell depletion by apoptosis may lead to attenuated neutrophils infiltration in liver and lung, decreased lymphocyte apoptosis in spleen, and an overall improvement in outcome (39). We find, in the present study, that IRF-1 promotes CD4+ T lymphocyte and dendritic cell apoptosis. Interestingly, we confirm IRF-1 inhibits NK cell apoptosis, which may accelerate T lymphocyte and dendritic cell apoptosis.
Although lymphocyte apoptosis has been well demonstrated in sepsis, the role of another cellular pathway, autophagy, remains to be elucidated. Autophagy can be stimulated by multiple cellular stresses, such as reactive oxygen species (ROS), protein aggregates, damaged organelles, and intracellular pathogens. All of these cellular stresses occur during the progression of sepsis. Also, apoptosis and autophagy share common components and can regulate and modify the activity of each other. The coordinated regulation of autophagy and apoptosis is also essential for maintenance of lymphocytes homeostasis. Autophagy deficiency leads to apoptosis in lymphocytes. In the absence of the autophagy protein Atg5, both CD4+ and CD8+ T lymphocytes fail to undergo efficient proliferation and rapidly undergo apoptosis in the periphery lymphoid organs (40). Our experiments demonstrate that IRF-1 can influence both apoptosis and autophagy in splenocytes in the setting of endotoxemia with IRF-1 potentially serving to regulate the balance between apoptosis and autophagy. IRF-1 is involved in a variety cellular homoeostatic processes, including apoptosis, cell cycle survival, and immune modulation. Despite the fact that it is a well-characterized pro-apoptotic regulator, IRF-1 is shown here for the first time to promote apoptosis and inhibit autophagy in splenocytes in endotoxemia.
Once we established a link between IRF-1 activation in splenocytes and the regulation of both apoptosis and autophagy, we went on to investigate the downstream mechanisms by which IRF-1 might regulate these two cellular processes. We found that IRF-1 overexpression leads to increased mTOR activation and consequently decreased autophagy. These results suggest that IRF-1 activation can inhibit autophagy, in part, through the modulation of mTOR/P70S6 activity. Also, IRF-1 leads to caspase-8 and -9 cleavages, which are hallmarks of extrinsic and intrinsic apoptotic pathways.
In this study, we also provide evidence that IRF-1 activation is indeed dependent on intact TLR4 signaling, as TLR4 KO mice did not demonstrate increased IRF-1 activation in response to LPS. The fact that TRIF KO mice do not respond to LPS with increased IRF-1 activation, while MyD88 KO mice do, provides evidence that the TLR4 dependent activation of IRF-1 precedes through the TRIF signaling pathway. However, our observation that IRF-1 activation leads to decreased autophagy is discordant with the results of Xu et al., who previously have shown that LPS induced autophagy is TRIF dependent (16). These discordant results suggest that apart from IRF-1, there may be another downstream mediator of TLR4/TRIF, which can induce autophagy.
In summary, we define a novel role for the transcription factor IRF-1 in endotoxemia. IRF-1 activation promotes apoptosis in splenocytes and conversely inhibits autophagy. Therefore, IRF-1 may prove to be a therapeutic target in managing the persistently difficult clinical problem of sepsis.
Acknowledgments
Financial Support: Howard Hughes Medical Institute Physician-Scientist Award American College of Surgeons Faculty Research Fellowship
ABBREVIATION
- LPS
Lipopolysaccharide
- i.p
intraperitoneal
- TLR4
Toll-like receptor 4
- MyD88
Myeloid differentiation primary response gene 88
- TIR
Toll-IL-1-resistance
- TRIF
domain-containing adaptor inducing IFN-B
- IFNγ
interferon gamma
- IRF-1
interferon regulatory factor 1
- LC3
microtubule-associated protein 1 light chain 3
- mTOR
mammalian target of rapamycin
- WT
wild type
- KO
knockout
- TEM
transmission electron microscope
- APC
Antigen presenting cells
Contributor Information
Lemeng Zhang, Email: zhangl4@upmc.edu.
Jon S Cardinal, Email: cardinaljs@upmc.edu.
Pinhua Pan, Email: panp@upmc.edu.
Brian R. Rosborough, Email: rosborough.brian@medstudent.pitt.edu.
Ying Chang, Email: changy4@upmc.edu.
Wei Yan, Email: yanw@upmc.edu.
Hai Huang, Email: huangh2@upmc.edu.
Timothy R Billiar, Email: billiartr@upmc.edu.
Matthew R Rosengart, Email: rosengartmr@upmc.edu.
Allan Tsung, Email: tsunga@upmc.edu.
References
- 1.Aneja R, Fink MP. Promising therapeutic agents for sepsis. Trends Microbiol. 2007;15(1):31–7. doi: 10.1016/j.tim.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 2.Park BS, Song DH, Kim HM, Choi BS, Lee H, Lee JO. The structural basis of lipopolysaccharide recognition by the TLR4-MD-2 complex. Nature. 2009;458(7242):1191–5. doi: 10.1038/nature07830. [DOI] [PubMed] [Google Scholar]
- 3.Chopra M, Sharma AC. Distinct cardiodynamic and molecular characteristics during early and late stages of sepsis-induced myocardial dysfunction. Life Sci. 2007;81(4):306–16. doi: 10.1016/j.lfs.2007.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xiao H, Siddiqui J, Remick DG. Mechanisms of mortality in early and late sepsis. Infect Immun. 2006;74(9):5227–35. doi: 10.1128/IAI.01220-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hotchkiss RS, Swanson PE, Freeman BD, Tinsley KW, Cobb JP, Matuschak GM, Buchman TG, Karl IE. Apoptotic cell death in patients with sepsis, shock, and multiple organ dysfunction. Crit Care Med. 1999;27(7):1230–51. doi: 10.1097/00003246-199907000-00002. [DOI] [PubMed] [Google Scholar]
- 6.Wesche DE, Lomas-Neira JL, Perl M, Chung CS, Ayala A. Leukocyte apoptosis and its significance in sepsis and shock. J Leukoc Biol. 2005;78(2):325–37. doi: 10.1189/jlb.0105017. [DOI] [PubMed] [Google Scholar]
- 7.Wesche-Soldato DE, Swan RZ, Chung CS, Ayala A. The apoptotic pathway as a therapeutic target in sepsis. Curr Drug Targets. 2007;8(4):493–500. doi: 10.2174/138945007780362764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chung CS, Song GY, Lomas J, Simms HH, Chaudry IH, Ayala A. Inhibition of Fas/Fas ligand signaling improves septic survival: differential effects on macrophage apoptotic and functional capacity. J Leukoc Biol. 2003;74(3):344–51. doi: 10.1189/jlb.0102006. [DOI] [PubMed] [Google Scholar]
- 9.Hotchkiss RS, Nicholson DW. Apoptosis and caspases regulate death and inflammation in sepsis. Nat Rev Immunol. 2006;6(11):813–22. doi: 10.1038/nri1943. [DOI] [PubMed] [Google Scholar]
- 10.Schwulst SJ, Muenzer JT, Peck-Palmer OM, Chang KC, Davis CG, McDonough JS, Osborne DF, Walton AH, Unsinger J, McDunn JE, Hotchkiss RS. Bim siRNA decreases lymphocyte apoptosis and improves survival in sepsis. Shock. 2008;30(2):127–34. doi: 10.1097/shk.0b013e318162cf17. [DOI] [PubMed] [Google Scholar]
- 11.Nakahira K, Haspel JA, Rathinam VA, Lee SJ, Dolinay T, Lam HC, Englert JA, Rabinovitch M, Cernadas M, Kim HP, Fitzgerald KA, Ryter SW, Choi AM. Autophagy proteins regulate innate immune responses by inhibiting the release of mitochondrial DNA mediated by the NALP3 inflammasome. Nat Immunol. 2011;12(3):222–30. doi: 10.1038/ni.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Levine B, Yuan J. Autophagy in cell death: an innocent convict? J Clin Invest. 2005;115(10):2679–88. doi: 10.1172/JCI26390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carchman EH, Rao J, Loughran PA, Rosengart MR, Zuckerbraun BS. Heme oxygenase-1-mediated autophagy protects against hepatocyte cell death and hepatic injury from infection/sepsis in mice. Hepatology. 2011;53(6):2053–62. doi: 10.1002/hep.24324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hsiao HW, Tsai KL, Wang LF, Chen YH, Chiang PC, Chuang SM, Hsu C. The Decline of Autophagy Contributes to Proximal Tubular Dysfunction During Sepsis. Shock. 2011 doi: 10.1097/SHK.0b013e318240b52a. [DOI] [PubMed] [Google Scholar]
- 15.Hamacher-Brady A, Brady NR, Gottlieb RA. Enhancing macroautophagy protects against ischemia/reperfusion injury in cardiac myocytes. J Biol Chem. 2006;281(40):29776–87. doi: 10.1074/jbc.M603783200. [DOI] [PubMed] [Google Scholar]
- 16.Xu Y, Jagannath C, Liu XD, Sharafkhaneh A, Kolodziejska KE, Eissa NT. Toll-like receptor 4 is a sensor for autophagy associated with innate immunity. Immunity. 2007;27(1):135–44. doi: 10.1016/j.immuni.2007.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Deretic V. Autophagy in immunity and cell-autonomous defense against intracellular microbes. Immunol Rev. 2011;240(1):92–104. doi: 10.1111/j.1600-065X.2010.00995.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schmid D, Munz C. Innate and adaptive immunity through autophagy. Immunity. 2007;27(1):11–21. doi: 10.1016/j.immuni.2007.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xu Y, Liu XD, Gong X, Eissa NT. Signaling pathway of autophagy associated with innate immunity. Autophagy. 2008;4(1):110–2. doi: 10.4161/auto.5225. [DOI] [PubMed] [Google Scholar]
- 20.Mizushima N, Levine B, Cuervo AM, Klionsky DJ. Autophagy fights disease through cellular self-digestion. Nature. 2008;451(7182):1069–75. doi: 10.1038/nature06639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Levine B, Deretic V. Unveiling the roles of autophagy in innate and adaptive immunity. Nat Rev Immunol. 2007;7(10):767–77. doi: 10.1038/nri2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Levine B. Eating oneself and uninvited guests: autophagy-related pathways in cellular defense. Cell. 2005;120(2):159–62. doi: 10.1016/j.cell.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 23.Kroger A, Koster M, Schroeder K, Hauser H, Mueller PP. Activities of IRF-1. J Interferon Cytokine Res. 2002;22(1):5–14. doi: 10.1089/107999002753452610. [DOI] [PubMed] [Google Scholar]
- 24.Dhupar R, Klune JR, Evankovich J, Cardinal J, Zhang M, Ross M, Murase N, Geller DA, Billiar TR, Tsung A. Interferon regulatory factor 1 mediates acetylation and release of high mobility group box 1 from hepatocytes during murine liver ischemia-reperfusion injury. Shock. 2010;35(3):293–301. doi: 10.1097/SHK.0b013e3181f6aab0. [DOI] [PubMed] [Google Scholar]
- 25.Senaldi G, Shaklee CL, Guo J, Martin L, Boone T, Mak TW, Ulich TR. Protection against the mortality associated with disease models mediated by TNF and IFN-gamma in mice lacking IFN regulatory factor-1. J Immunol. 1999;163(12):6820–6. [PubMed] [Google Scholar]
- 26.Stang MT, Armstrong MJ, Watson GA, Sung KY, Liu Y, Ren B, Yim JH. Interferon regulatory factor-1-induced apoptosis mediated by a ligand-independent fas-associated death domain pathway in breast cancer cells. Oncogene. 2007;26(44):6420–30. doi: 10.1038/sj.onc.1210470. [DOI] [PubMed] [Google Scholar]
- 27.Papageorgiou A, Dinney CP, McConkey DJ. Interferon-alpha induces TRAIL expression and cell death via an IRF-1-dependent mechanism in human bladder cancer cells. Cancer Biol Ther. 2007;6(6):872–9. doi: 10.4161/cbt.6.6.4088. [DOI] [PubMed] [Google Scholar]
- 28.Kano A, Haruyama T, Akaike T, Watanabe Y. IRF-1 is an essential mediator in IFN-gamma-induced cell cycle arrest and apoptosis of primary cultured hepatocytes. Biochem Biophys Res Commun. 1999;257(3):672–7. doi: 10.1006/bbrc.1999.0276. [DOI] [PubMed] [Google Scholar]
- 29.Tamura T, Ishihara M, Lamphier MS, Tanaka N, Oishi I, Aizawa S, Matsuyama T, Mak TW, Taki S, Taniguchi T. An IRF-1-dependent pathway of DNA damage-induced apoptosis in mitogen-activated T lymphocytes. Nature. 1995;376(6541):596–9. doi: 10.1038/376596a0. [DOI] [PubMed] [Google Scholar]
- 30.Chung CS, Venet F, Chen Y, Jones LN, Wilson DC, Ayala CA, Ayala A. Deficiency of Bid protein reduces sepsis-induced apoptosis and inflammation, while improving septic survival. Shock. 2010;34(2):150–61. doi: 10.1097/SHK.0b013e3181cf70fb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Watanabe E, Muenzer JT, Hawkins WG, Davis CG, Dixon DJ, McDunn JE, Brackett DJ, Lerner MR, Swanson PE, Hotchkiss RS. Sepsis induces extensive autophagic vacuolization in hepatocytes: a clinical and laboratory-based study. Lab Invest. 2009;89(5):549–61. doi: 10.1038/labinvest.2009.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hotchkiss RS, Osmon SB, Chang KC, Wagner TH, Coopersmith CM, Karl IE. Accelerated lymphocyte death in sepsis occurs by both the death receptor and mitochondrial pathways. J Immunol. 2005;174(8):5110–8. doi: 10.4049/jimmunol.174.8.5110. [DOI] [PubMed] [Google Scholar]
- 33.Hotchkiss RS, Karl IE. The pathophysiology and treatment of sepsis. N Engl J Med. 2003;348(2):138–50. doi: 10.1056/NEJMra021333. [DOI] [PubMed] [Google Scholar]
- 34.Hotchkiss RS, Tinsley KW, Swanson PE, Schmieg RE, Jr, Hui JJ, Chang KC, Osborne DF, Freeman BD, Cobb JP, Buchman TG, Karl IE. Sepsis-induced apoptosis causes progressive profound depletion of B and CD4+ T lymphocytes in humans. J Immunol. 2001;166(11):6952–63. doi: 10.4049/jimmunol.166.11.6952. [DOI] [PubMed] [Google Scholar]
- 35.Hotchkiss RS, Tinsley KW, Swanson PE, Grayson MH, Osborne DF, Wagner TH, Cobb JP, Coopersmith C, Karl IE. Depletion of dendritic cells, but not macrophages, in patients with sepsis. J Immunol. 2002;168(5):2493–500. doi: 10.4049/jimmunol.168.5.2493. [DOI] [PubMed] [Google Scholar]
- 36.Peck-Palmer OM, Unsinger J, Chang KC, McDonough JS, Perlman H, McDunn JE, Hotchkiss RS. Modulation of the Bcl-2 family blocks sepsis-induced depletion of dendritic cells and macrophages. Shock. 2009;31(4):359–66. doi: 10.1097/SHK.0b013e31818ba2a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hotchkiss RS, Chang KC, Swanson PE, Tinsley KW, Hui JJ, Klender P, Xanthoudakis S, Roy S, Black C, Grimm E, Aspiotis R, Han Y, Nicholson DW, Karl IE. Caspase inhibitors improve survival in sepsis: a critical role of the lymphocyte. Nat Immunol. 2000;1(6):496–501. doi: 10.1038/82741. [DOI] [PubMed] [Google Scholar]
- 38.Hotchkiss RS, Tinsley KW, Swanson PE, Chang KC, Cobb JP, Buchman TG, Korsmeyer SJ, Karl IE. Prevention of lymphocyte cell death in sepsis improves survival in mice. Proc Natl Acad Sci U S A. 1999;96(25):14541–6. doi: 10.1073/pnas.96.25.14541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Barkhausen T, Frerker C, Putz C, Pape HC, Krettek C, van Griensven M. Depletion of NK cells in a murine polytrauma model is associated with improved outcome and a modulation of the inflammatory response. Shock. 2008;30(4):401–10. doi: 10.1097/SHK.0b013e31816e2cda. [DOI] [PubMed] [Google Scholar]
- 40.Pua HH, Dzhagalov I, Chuck M, Mizushima N, He YW. A critical role for the autophagy gene Atg5 in T cell survival and proliferation. J Exp Med. 2007;204(1):25–31. doi: 10.1084/jem.20061303. [DOI] [PMC free article] [PubMed] [Google Scholar]