Abstract
In this essay, we propose that embryos express a metabolic phenotype necessarily different from that of differentiated somatic cells and more like that of rapidly proliferating cancer cells. This metabolic adaptation, known as the Warburg Effect, supports rapid cell proliferation. One of the hallmarks of the Warburg Effect is that pyruvate is directed away from the tri-carboxylic acid cycle and metabolized to lactate, resulting in a buildup of glycolytic intermediates. Although this is a comparatively inefficient way to generate ATP, this adaptation allows the cell to meet other critical metabolic requirements, including biomass production and redox regulation. Thus, utilization of WE gives proliferating cells a selective growth advantage. This model represents a completely new understanding of embryo metabolism in the context of a broad, interconnected network of metabolic mechanisms that influence viability, versus the current dogma of carbohydrate metabolism via oxidative phosphorylation. A more complete understanding of embryo metabolism is critical to better support embryo viability in vitro, and to avoid forcing embryos to adapt to suboptimal culture conditions at a significant cost to future growth and development.
Keywords: metabolism, glucose, glycolysis, pentose phosphate pathway, TCA cycle, oxidative phosphorylation
Introduction
Although incremental improvements have been made in oocyte maturation and embryo culture in the last decade, significant progress in improving in vitro maturation, fertilization, and embryo culture technologies have remained elusive. Much remains to be understood about what the oocyte and embryo require in vitro to support successful development and production of healthy offspring. Adaptation to suboptimal culture conditions, resulting in altered embryo metabolism, not only results in reduced blastocyst formation and lower embryo viability, but also reduced maintenance of pregnancy, fetal growth, and offspring health. In other words, although the embryo has remarkable metabolic plasticity, this comes at a high cost. Therefore, to avoid adaptive stress associated with poor embryo quality, reduced pregnancy potential, and negative health implications in future offspring, it is essential that embryo culture conditions adequately reflect normal embryo physiology (Lane and Gardner 2007). Part of the challenge of developing optimal culture conditions is that preimplantation embryo metabolism is necessarily different from the generic, differentiated somatic cell.
Typically in cellular metabolism, glucose is metabolized through glycolysis to pyruvate, which then enters the tri-carboxylic acid (TCA) cycle and is oxidized to produce ATP (Figure 1). The study of embryo metabolism has historically focused on the fate of glucose and the carbohydrates derived from it, pyruvate and lactate. Dogma maintains that lactate and pyruvate are used early in development, while glycolysis increases at the time of blastocyst formation, when higher glucose uptake is a signature of more viable embryos (Gardner et al. 2001; Gardner and Leese 1987). This view is, however, somewhat biased by work in the mouse.
Figure 1.
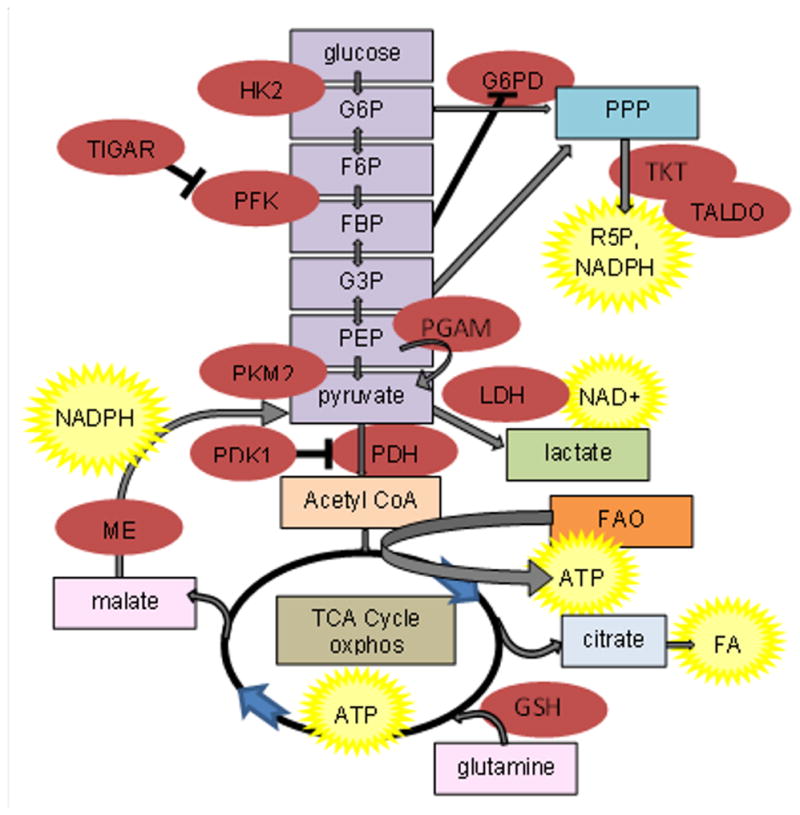
Glucose, fatty acid (FA) and glutamine metabolic pathways. Enzymes are in red ovals; end products are in yellow stars. Glycolysis is color-coded purple; the pentose phosphate pathway (PPP) is medium blue; the tricarboxylic acid cycle (TCA) cycle and oxidative phosphorylation (oxphos) are brown; fatty acid oxidation (FAO) is orange; and glutaminolysis is pink. G6P, glucose-6-phosphate; F6P, fructose-6-phosphate; FBP, fructose bis-phosphate; G3P, glucose-3- phosphate; phosphoenolpyruvate. Enzyme are as abbreviated as in the text.
Domestic species use more glucose in the cleavage stages, although utilization still increases during blastocyst formation. In the pig, glucose metabolism via glycolysis is active at the cleavage stages, particularly in in vivo-derived embryos, even though its use is 3–5x less than that of blastocysts (Swain et al. 2002, Flood and Weibold 1988). Glucose metabolism via glycolysis of in vitro-produced, pre-compaction bovine and ovine embryos is similar to that in the pig, although blastocyst glycolytic activity is higher in these ruminant species (Thompson and Tervit 1991; Rieger et al. 1992; Gardner et al. 1993; Swain et al. 2002). Pyruvate metabolism, however, is much lower in pig preimplantation embryos than sheep, cattle, or mouse embryos at a similar developmental stage, suggesting an increased reliance on glucose metabolism in pigs (Leese and Barton 1984; Rieger et al. 1992; Gardner et al. 1993; Swain et al. 2002). Ruminant blastocysts have a limited ability to oxidize glucose or pyruvate via the TCA cycle compared to mice, while pigs are intermediate in this ability (Gardner et al. 1993; Rieger et al. 1992; Swain et al. 2002). Lactate accumulation has traditionally been considered an adaptation to suboptimal culture conditions (Gardner and Leese 1990). In the last decade, a prevailing hypothesis has been that of the ‘quiet embryo’ (Baumann et al. 2007; Leese 2002; Leese et al. 2008; Leese et al. 2007). This hypothesis states that viable embryos, because they do not need to repair stress-induced damage, have lower oxidative phosphorylation (oxphos) activity and consume less oxygen. We suggest here that metabolism of the early mammalian embryo is very similar to other rapidly proliferating cells, such as tumor cells, and many components of the Warburg Effect (WE) are consistent with metabolism of the early embryo.
The Warburg Effect
A hallmark of cancer cells is an alternative form of metabolism, first described by Warburg (Warburg 1956) and known as the WE, or aerobic glycolysis, in which pyruvate is directed away from the TCA cycle and is metabolized to lactate. Importantly, conversion of pyruvate to lactate produces NAD+, supporting further glycolytic activity. Although this is typically an anaerobic process, cancer cells utilize this pathway even when oxygen is abundant. Compared to oxphos, this is an inefficient way to generate ATP (2 moles of ATP per mole of glucose compared to 38 moles of ATP for mitochondrial respiration). Mitochondria do remain functional, and oxphos continues during the WE, potentially with alternative substrates, thus the effect is not due to inherent mitochondrial damage (Cairns et al. 2011; Locasale and Cantley 2010). In this scenario, oxphos catabolism is relatively independent of glucose metabolism (Locasale and Cantley 2010). Interestingly, this form of metabolism is not unique to cancer cells, but is observed in many other rapidly proliferating cell types (Lopez-Lazaro 2008).
The obvious question is, then: Why do proliferating cells utilize a less efficient method of generating ATP from glucose, even when oxygen levels are adequate? The answer may be that proliferating cells have more critical metabolic requirements beyond ATP production from glucose. The requirement for macromolecular synthesis to create new biomass, including DNA, proteins, and lipids, may be the most pressing cellular need — i.e., glucose-derived ATP may not be the primary driver of cellular metabolism in the proliferating cell. Instead, production of ribose-5-phosphate (R5P) for nucleic acid synthesis, fatty acids for lipid synthesis, and redox control may be the overarching metabolic goals of glucose metabolism (Cairns et al. 2011). This hypothesis is supported by a recent study using genome-scale metabolic modeling to investigate the cause of the WE (Shlomi et al. 2011). These processes use glucose as a carbon source, consume TCA cycle intermediates such that they must be replenished, and require NADPH as reductive power (Deberardinis et al. 2008). To produce R5P and NADPH, glucose is diverted into the pentose phosphate pathway (PPP, Figure 1). In fact, for cells to successfully proliferate, the bulk of carbon can’t be committed to oxphos catabolism for ATP generation, as that would be counterproductive to the biosynthetic needs of a proliferating cell (Vander Heiden et al. 2009). Utilization of the WE gives proliferating cells a selective growth advantage. Thus, adequate alternative sources, such as fatty acids or amino acids, must be available to support basal TCA activity and ATP production.
This selective growth advantage is achieved by a metabolic phenotype that results in a buildup of glycolytic intermediates. Cells achieve this phenotype by altering expression of metabolic enzymes and nutrient transporters. Key in this metabolic scenario is pyruvate kinase (PKM2), specifically the splice isoform M2. M2 is the fetal form of PKM2, and the dimeric form of this isozyme is present in most cancer cells (Deberardinis et al. 2008), enabling the switch to aerobic glycolysis and promoting proliferation. The dimeric form, promoted by phosphotyrosine binding, displays low pyruvate kinase activity (Christofk et al. 2008; Hitosugi et al. 2009; Vander Heiden et al. 2010). In actuality, M2 slows glycolysis, resulting in the accumulation of glycolytic metabolites upstream of its activity that can instead be channeled to upstream biosynthetic processes (Cairns et al. 2011; Christofk et al. 2008; DeBerardinis 2008). Specifically, the accumulation of the glycolytic intermediate fructose-bis-phosphate suppresses glucose-6-phosphate dehydrogenase (G6PD), an enzyme in the oxidative arm of the PPP, thereby favoring metabolism of glycolytic intermediates via the non-oxidative arm of the PPP to produce R5P. Phosphoenol pyruvate (PEP), another glycolytic intermediate, acts as a phosphate donor to phosphoglycerate mutase (PGAM), which is able to produce pyruvate in the absence of PK activity and thus uncouples ATP production from PEP mediated phosphotransfer (Vander Heiden et al. 2010). The slowing of glycolysis by M2 also promotes activity of the oxidative arm of the PPP, and thus NADPH production. In fact, a recent study demonstrates that in cancer cells, reactive oxygen species directly inhibit PKM2, diverting glucose flux into the PPP to generate reducing potential that allows these cells to sustain proliferation while maintaining redox homeostasis (Anastasiou et al. 2011). This may be critical for cellular protection from reactive oxygen species, as NADPH provides reducing power to both the glutathione (GSH) and thioredoxin (TRX) systems. Accumulation of glucose metabolites also results in activation of hypoxia inducible factor (HIF1), and PKM2 acts as a translational coactivator with HIF1 (Luo and Semenza 2011). HIF1 mediates the switch to aerobic glycolysis by activating transcription of glucose transporters and glycolytic enzymes as well as by controlling expression of lactate dehydrogenase (LDH), which converts pyruvate to lactate, and of phosphate dehydrogenase kinase (PDK1), which inhibits pyruvate dehydrogenase kinase (PDH) to reduce entry of pyruvate into the TCA cycle (Luo and Semenza 2011). Although HIF1 supports aerobic glycolysis and represses oxphos, hypoxia itself may not play a major role as HIF1 can be activated in normoxic conditions by phosphoinositide-3-kinase (PI3K) (Cairns et al. 2011).
In addition to an increased use of glucose, cancer cells also take up and metabolize high levels of glutamine. Glutamine is used to replenish TCA cycle intermediates removed for fatty acid synthesis, to produce NADPH via malic enzyme (ME), a process called glutaminolysis, and for GSH synthesis (Deberardinis et al. 2008; Vousden and Ryan 2009). The glutaminase (GLS) enzyme has two forms: GLS1 favors conversion to alpha ketoglutarate, entrance into the TCA cycle, and anabolic outcomes, and is typical of proliferating cells while GLS2 favors synthesis of GSH and ATP production, is stimulated by p53, and is typical of quiescent cells (Cairns et al. 2011; Deberardinis et al. 2008; Maddocks and Vousden 2011; Suzuki et al. 2010).
A critical cellular signaling pathway utilized in the WE is the Akt/PI3K cascade. Akt (protein kinase B) promotes glucose transporter (GLUT) activity and activates glycolytic enzymes. cMYC also activates enzymes of glycolysis, favors expression of PKM2 M2, and increases glutamine transporter, although it increases GLS2 expression to support GSH synthesis, which is more typical of quiescent cells (Cairns et al. 2011; Dang 2010). mTOR (mammalian target of rapimycyin) increases the surface expression of GLUTs and activates HIF1, and is itself activated by Akt/PI3K (DeBerardinis 2008). P53 antagonizes the WE by stimulating TCA cycle activity, reducing expression of GLUTs, inhibiting PGAM activity, and decreasing glycolytic flux via stimulation of TIGAR (aka C12orf5 (chromosome 12, open reading frame 5)) expression (Maddocks and Vousden 2011; Vousden and Ryan 2009).
Embryos and The Warburg Effect
Although proliferating cells utilize the WE, the control of glucose metabolism is more complex than was previously appreciated (rather than simply lactate production) underlying the final metabolic result that ultimately contributes to biomass synthesis. Embryos, as proliferating cells, have some aspects of the WE in common with cancer cells. Yet, there are important differences between these two types of proliferating cells that require metabolic modifications. Both cell types require the biosynthesis of nucleic acids for cell proliferation, although the embryo requires less lipid and protein than cancer cells because, at the preimplantation stages, there is no true cell growth. Thus, mechanisms critical to fatty acid and amino acid synthesis in cancer cells may play a reduced role in embryos, although mechanisms relevant to R5P and NADPH production for nucleotide synthesis and redox control, respectively, may be quite similar. We propose that embryos do utilize aerobic glycolysis to generate glycolytic intermediates for R5P and NADPH production, as well as glutaminolysis for NADPH generation. Pyruvate may be converted to lactate to maintain NAD+ levels that support elevated glycolysis. The TCA cycle and oxphos are still active and functional, relying on fatty acid oxidation (FAO) to generate ATP as well as to replenish cycle intermediates. This model represents a completely new understanding of embryo metabolism in the context of a broad, interconnected network of metabolic mechanisms that influence viability, versus the current dogma of carbohydrate metabolism via oxidative phosphorylation.
Although the quiet embryo hypothesis may initially seem to be at odds with the WE, these two theories may, in fact, not be very different. We propose that oxphos is functional, but primarily utilizes fatty acids rather than glucose to provide ATP. Although glucose uptake is high, control mechanisms such as dimeric PKM2 actually slow glycolysis to increase intermediates, which are then shuttled to other metabolic fates. In both scenarios, energy (ATP) demands are thought to be relatively low. The difference, we hypothesize, is that other cellular demands, such as R5P and NADPH, in fact keep alternative metabolic pathways (for example, the PPP and glutaminolysis) quite active. Several independent publications support the existence of the WE in embryos. Inhibition of TCA cycle activity at the time of embryo compaction improves pig and cow embryo development (Machaty et al. 2001; Rieger et al. 2002; Thompson et al. 2000), potentially by reducing ATP production from glucose and forcing glucose metabolism, via the PPP, to establish a favorable redox state within the embryo (Thompson et al. 2000). Stimulating PPP activity in 8–16 cell bovine embryos increases glucose metabolism without affecting lactate production, and reduces lipid accumulation (De La Torre-Sanchez et al. 2006), which is consistent with the WE.
A recent report demonstrates that hyperglycemia in the early cleavage-stage bovine embryo results in decreased development to the blastocyst stage, and dysregulation of gene expression in those blastocysts (Cagnone et al. 2012). These authors hypothesize that increased expression of metabolic genes suggests an upregulation in metabolism, resulting in an ‘un-quiet’ embryo that is less viable. The observed gene expression suggests NADPH levels are reduced, sorbitol is increased via the polyol pathway, glucose metabolism is shifted from glycolysis to the hexosamine pathway, oxidative phosphorylation is decreased, and lipid accumulation increased — typical metabolic perturbations associated with diabetes (Cagnone et al. 2012). Interestingly, this study also noted an upregulation of genes associated with the WE: TKTL1, HIF1A, and LDHA. It is not clear if the WE is related to hyperglycemia and viability of the resultant impaired embryo. It is possible that WE genes are upregulated in embryos exposed to the hyperglycemic conditions used (5 mM glucose) as a result of the very low level of glucose in the control media used for comparison (0.2 mM), such that the control embryos may not have enough glucose to support optimal metabolism. Glucose is present in the bovine oviduct on day 3 of the estrous cycle at a concentration of ~ 2.5 mM (Hugentobler et al. 2008; Hugentobler et al. 2010). The presence of 1.5 mM glucose in bovine embryo culture media is beneficial when compared to the absence of glucose (Kwun et al. 2003). A widely used bovine embryo culture medium, SOF (synthetic oviductal fluid) (Tervit et al. 1972), contains 1.5 mM glucose in the first step of bovine preimplantation embryo culture (Gandhi et al. 2000; Steeves and Gardner 1999). Interestingly, the addition of ethylenediaminetetraacetic acid (EDTA) in the initial step of embryo culture with glucose is commonly used to inhibit glycolysis and to increase embryo development in cattle and mice (Lane and Gardner 2001). The result of this practice may be that glycolytic intermediates build up and enter the PPP, supporting the WE. Yet, there are culture media for both cow (CR1aa (Rosenkrans and First 1994); mSOF (Takahashi and First 1992)) and pig (PZM (porcine zygote media) (Yoshioka et al. 2002)) preimplantation embryos that successfully support embryonic development without glucose. Similar to the situation in the cow, the pig oviduct contains glucose (0.25 mM in the post ovulatory, mated ampulla (Nichol et al. 1992)), and there are also successful pig embryo culture media that contain glucose (NCSU23, (Petters et al. 1990; Petters and Wells 1993)). A recent report demonstrates that the addition of glucose to PZM during the final stages of culture increases blastocyst survival and ATP content, and in combination with glycine, increases blastocyst hatching (Mito et al. 2012). In the hamster, where glucose at greater than 1–2 mM is inhibitory to blastocyst development, low levels of glucose (0.5 mM) are beneficial for implantation and fetal development compared to no glucose at all (Ludwig et al. 2001). Because the cow and pig embryo are able to develop in vitro from the 1-cell stage to blastocyst without glucose, it may be possible that in glucose-free media, pyruvate is converted to PEP by mitochondrial enzymes, an energy consuming process. Then, PEP may participate in the reversible reactions of glycolysis to the point that intermediates are produced, which then enter the PPP (Fig. 1).
Expression of metabolic genes in embryos appears to be similar to that in cancer cells. We have searched our existing databases (microarray for mouse in vivo versus in vitro matured oocytes (unpublished data, RLK)) and pig adult versus prepubertal oocytes (Paczkowski et al. 2011b), and deep sequencing of pig blastocysts produced in high versus low oxygen (unpublished data, RSP) for expression of genes key to the WE and the related metabolic control scenario. Our existing databases support our hypothesis: oocytes and embryos do express genes necessary, and in some cases critical, to anaerobic glycolysis mechanisms (Table 1). Higher GLUT1 expression is associated with better quality mouse oocytes (Table 1, unpublished data, RLK). HK2 is the predominant form of hexokinase (HK) in both mouse oocytes (unpublished data, RLK) and pig blastocysts (Redel et al., 2012). PKM2 M2 is present in mouse oocytes and pig blastocysts (Table 1, unpublished data, RLK, RSP), whereas the M1 variant, via RT-PCR, could either not be detected or was at very low levels in the pig (Redel et al. 2012). The M1 variant can be detected in the mouse oocyte, but expression levels do not change between maturation in vivo or in vitro, with high or low oxygen (unpublished data, RLK). PGAM1 is present in mouse oocytes (Table 1, unpublished data, RLK) and pig blastocysts (Redel et al., 2012). Mouse and pig oocytes (unpublished data, RLK; Paczkowski et al. 2011b) and pig embryos (Redel et al. 2012) express PDK1, PDHA1, and LDHA, B and C; PDK1 expression is higher in pig blastocysts grown in 5% oxygen versus 95% air (~20% oxygen). Non-oxidative PPP enzymes transketolase/transketolase-like 1 (TKT/TKTL1) and transaldolase 1 (TALDO1) are present in these three tissues, and TALDO1 expression is higher in pig blastocysts grown in low oxygen compared to an air environment (Redel et al., 2012). G6PD, of the oxidative PPP arm, is present in pig blastocysts (Redel et al., 2012). ME, capable of converting malate from glutamine into pyruvate, is expressed; elevated ME expression is associated with higher quality mouse oocytes (unpublished data, RLK); and both ME1 and ME2 are present in pig blastocysts (Redel et al. 2012). GLS is expressed in pig blastocysts (Redel et al., 2012), while GLS2 is present in pig oocytes (Paczkowski et al., 2011b). HIF1A is present in pig and mouse oocytes (Paczkowski et al., 2011b; unpublished data, RLK) and pig blastocysts (Redel et al. 2012). Akt and PI3K are expressed in all three tissues: Akt expression was higher in good quality oocytes (unpublished data, RLK), and both Akt1 and PI3K expression were elevated in blastocysts grown in low oxygen as compared to an air environment (unpublished data, RSP). Tumor protein p53 (TP53) was not detected in pig oocytes or blastocysts (Paczkowski et al., 2011b; Redel et al., 2012). TIGAR is present in mouse oocytes (unpublished data, RLK) and pig blastocysts (Redel et al. 2012), and its expression is higher in lower quality oocytes (Table 1). Ras (RASA1 and RASA2 in pigs) and mTOR are present in mouse oocytes (unpublished data, RLK) and pig blastocysts (Redel et al., 2012). Finally, the machinery for FAO (acyl-coA dehydrogenase members (ACADL, ACAD9), acyl-coA synthetase long-chain family member 3 (ACSL3), and carnitine palmitoyltransferase (CPT)) is present in mouse and pig oocytes (unpublished data, RLK; Paczkowski et al., 2011b) and pig blastocysts (with the exception of ACSL3; Redel et al., 2012). Interestingly, ACAD9 is higher in blastocysts grown in low oxygen (unpublished data, RSP). Both CPT1a and CPT2 are present in pig blastocysts, but only CPT2 is present in pig oocytes (unpublished data, RSP). We found that CPT2 is present in the denuded mouse oocyte while CPT1b is not (unpublished data, RLK); another report identifies CPT1b in the mouse cumulus oocyte complex following the initiation of oocyte maturation (Dunning et al., 2010). On the other hand, fatty acid synthesis machinery (acetyl-coA carboxylase alpha (ACACA), ATP citrate lyase (ACLY), fatty acid synthase (FASN)) is partially lacking (unpublished data, RSP). Furthermore, expression patterns demonstrate that genes associated with the WE, including PKM2 M2 and PDKs, are expressed in the early pig embryo, and suggest that metabolism is shifted to anaerobic glycolysis with concomitantly low TCA cycle activity (Redel et al. 2012). Significantly, conditions supporting embryogenesis, namely low oxygen, increase the expression of WE genes, suggesting that modification of metabolism to support increasing biosynthetic needs is beneficial to embryo development (Redel et al. 2012). Similarly, maturation in a low (5%) oxygen environment improves bovine oocyte quality and subsequent blastocyst development, an effect apparently mediated by an upregulation of genes involved in glycolysis, including GLUT1, GAPDH, and LDHA (Bermejo-Alvarez et al. 2010).
Table 1.
Effect of oocyte quality and blastocyst culture condition on expression of genes critical to WE phenotype. The number of reads generated from deep sequencing projects for each condition are listed below.
| Gene | Specie/stage | A† | B† | P value |
|---|---|---|---|---|
| GLUT1 | Mouse oocyte | 228.9 | 213.6 | 0.03 |
| HK2 | Pig blastocyst | 23.9 | 12.2 | 0.07 |
| PKM2 | Mouse oocyte | 44.3 | 12.9 | 0.15 |
| Pig blastocyst | 39.1 | 49.2 | 0.36 | |
| PGAM1 | Mouse oocyte | 120.1 | 75.0 | 0.19 |
| Pig blastocyst | 137.5 | 116.1 | 0.23 | |
| PDK1 | Mouse oocyte | 19.7 | 27.2 | <0.01 |
| TKT | Pig blastocyst | 318 | 249 | 0.02 |
| ME | Mouse oocyte | 472.3 | 39.4 | <0.01 |
| Mouse oocyte | 7.7 | 1.2 | 0.09 | |
| AKT | Pig oocyte | 241.7 | 157.9 | 0.04 |
| Pig blastocyst | 24.0 | 17.5 | 0.06 | |
| PI3K | Pig blastocyst | 20.0 | 6.4 | 0.06 |
| TIGAR | Mouse oocyte | 14.3 | 21.1 | 0.01 |
| ACAD9 | Pig blastocyst | 37 | 24 | 0.02 |
Comparison between A and B for mouse oocytes is in vivo versus in vitro matured (unpublished data, RLK), for pig oocytes adult versus prepubertal derived (Paczkowski et al., 2011), and for pig blastocysts cultured in 5% O2 versus 95% air (unpublished data,RSP), respectively.
Embryos, the Warburg Effect, and Fatty Acids
We hypothesize that the preimplantation embryo relies on fatty acid β-oxidation to provide the required ATP such that glucose metabolism can be shifted towards the WE. In cancer cells, fatty acid synthesis is critical for the production of lipids for new membranes (Deberardinis et al. 2008). Fatty acids are synthesized in the cytosol from citrate removed from the TCA cycle, via ACLY, ACAC, and FASN. The carbons used are glucose-derived, further suggesting that the TCA cycle is functional during aerobic glycolysis (DeBerardinis 2008). Although the preimplantation embryo is certainly a rapidly proliferating cell, it has less demand for membrane production than do cancer cells, as the embryo does not immediately produce new biomass. Thus, fatty acid synthesis may not be as critical to preimplantation embryogenesis. Therefore, in contrast to cancer cells, embryos may favor FAO as a source of ATP and to maintain TCA cycle activity when glucose-derived carbon is being diverted to the PPP for synthesis of R5P and NADPH. Maintaining mitochondrial activity with fatty acid-derived carbons could easily be accomplished, as glucose metabolism is relatively uncoupled from oxphox in aerobic glycolysis (Samudio et al. 2009).
Although the metabolism of oocytes and embryos has been studied extensively, the contribution of fatty acid β-oxidation has been relatively ignored. Several species, including cow, pig, and cat, have large stores of intracellular lipids, while the mouse has very little. Recent evidence suggests that lipid metabolism may play a role in energy production in the oocyte and embryo, even in species with variable amounts of intracellular lipid (Downs et al. 2009; Dunning et al. 2010). Indirect evidence suggests that pig and cow oocytes and embryos metabolize fatty acids (Sturmey et al. 2009). In pig and cow oocytes, inhibition of FAO reduces oocyte competence (Ferguson and Leese 2006; Sturmey and Leese 2003). Mouse embryo development and cell number was decreased when FAO was blocked during culture (Hewitson et al. 1996). Interestingly, although glucose uptake did not change in this experiment, lactate production was decreased, suggesting a different fate for glucose when ATP is generated from lipid metabolism. Although development was not reduced when FAO is inhibited in pig embryos, glucose metabolism is upregulated (Sturmey and Leese 2008), again suggesting an adjustment of glucose metabolism, potentially towards oxphos to compensate for a loss of optimal ATP production via FAO. Cow embryos behave similarly when FAO is inhibited, although development is compromised (Ferguson and Leese 2006). The addition of fatty acids or carnitine, to stimulate FAO, to oocyte and embryo culture medium has primarily shown positive effects on development, although results are variable due, in part, to differences in fatty acids and concentrations used (Dunning et al. 2010; Leroy et al. 2005; Marei et al. 2010; Somfai et al. 2011; Spindler et al. 2000; Van Hoeck et al. 2011; Wu et al. 2011).
We have conducted experiments to evaluate the importance of fatty acids during oocyte maturation in the cow, pig, and mouse, and found that FAO is essential to oocyte nuclear maturation in all three species (Paczkowski et al. 2011a). Pig oocytes were the most sensitive to inhibition of FAO, while cow oocytes were intermediate, and mouse oocytes were the least sensitive. The sensitivity of oocyte nuclear maturation to inhibition of FAO parallels the amount of fatty acids in the cytoplasm of these three species, suggesting that the amount of intracellular lipids may indicate the relative importance of fatty acid metabolism to oocyte nuclear maturation and possibly the level of reliance of the oocytes and embryos of each species on the WE.
Mitochondria
Mitochondria have a unique morphology during embryogenesis. Mitochondria in the oocyte are spherical with few cristae. They begin to elongate at the 4-cell to morula stages, and by the blastocyst stage the trophectoderm cells contain elongated mitochondria while the inner cell mass cells contain spherical mitochondria (Houghton 2006). A similar situation occurs in cardiac myocytes: immature cardiomyocytes display few cristae and lower membrane potential, and closure of the mitochondrial permeability transition pore drives maturation of the mitochondria during differentiation (Hom et al. 2011). Interestingly, glucose affects the structure, function, and distribution of mitochondria in mouse embryos (Han et al. 2008), supporting our hypothesis of a complex and interrelated metabolic mechanism. An increase in both cristae and mitochondrial number are associated with increased respiration. Thus, an increase in embryo respiration and oxygen consumption occurs simultaneously with a change in mitochondrial morphology in mouse embryos (Stern et al. 1971). In the trophectoderm, ATP is used for the Na+-K+ ATPase pump to create and maintain the blastocoel cavity. Cells of the inner cell mass are less metabolically active, with less oxygen consumed, less ATP produced, and lower amino acid turnover. Interestingly, female mice that carry pathogenic mitochondrial mutations are fertile (Inoue et al. 2000). Their oocytes and embryos survive despite severe mitochondrial dysfunction and reduced oxidative phosphorylation, suggesting that other metabolic mechanisms, rather than simply the ability to generate large amounts of ATP, may be critical for oocyte and embryo competence; this also supports a role for the WE in embryogenesis.
Other rapidly proliferating cell types, such as embryonic stem cells, induced pluripotent cells, and yeast cells, have similarities to early embryos, including a non-somatic type mitochondrial morphology and reliance on glycolysis to meet energy demands (Varum et al. 2011). Lower mitochondrial activity, described in spermatogonia and embryonic stem cells, may be an important feature of pluripotency (Ramalho-Santos et al. 2009). Human embryonic stem cells have increased glycolysis, and maintain anaerobic glycolysis as a key metabolic pathway even when oxygen is present (Varum et al. 2011). Embryonic stem cells have mitochondria that are globular in shape with few cristae and with low membrane potential, in contrast to differentiated cell lines, which have higher concentrations of ATP and lower concentrations of lactate. Correspondingly, embryonic stem cells rely less on oxidative phosphorylation than differentiated cells. In addition, embryonic stem cells overexpress HK2 and TKT and have lower levels of PDH (Varum et al. 2011), similar to what is observed in the WE. Interestingly, PKM2 interacts with OCT-4 in stem cells to support maintenance of the undifferentiated state (Lee et al. 2008).
In contrast to these other rapidly proliferating cells, early mammalian embryos produce very little, if any, RNA during the first few cleavage divisions, instead relying on maternally derived messages and protein. At a species-specific cleavage stage, the embryo begins significant RNA synthesis, and at that point begins to control its own development. How this lack of RNA synthesis fits into the overall picture of glucose metabolism is yet to be determined.
Conclusions
We suggest that the WE plays a critical role in embryo metabolism, and that culture conditions supporting in vitro development of viable embryos will lead to an up regulation of this mechanism. We propose that the same basic metabolic processes exist in oocytes and embryos of all mammalian species, but their interaction and regulation are related to the speed of cell cleavage, the rate to the blastocyst stage, and their reliance on intracellular stores of fatty acids for ATP generation. In general, all of these embryos must produce ATP for cellular energy, handle challenges from reactive oxygen species, and create building blocks for growth and development. These basic biochemical processes interact, leading to a complex physiological interplay that impacts oocyte and embryo quality and viability. To better understand the role of the WE in embryo development, several hypotheses could be proposed and tested. For example, do culture conditions that promote embryo viability also induce expression of genes characteristic of the WE? Does species-specific regulation of these genes reflect the speed of embryo growth and implantation timing, with species that have greater cell proliferation prior to implantation/attachment relying more heavily on WE mechanisms? Does manipulation of WE mechanisms alter embryo developmental competence? Does closure of the mitochondrial permeability transition pore in the early embryo drive mitochondrial maturation, and thus alter development as with stem cells? Investigation of these hypotheses, among others, will help to clarify the metabolic mechanisms controlling developmental competence in preimplantation embryos. If, as we propose, the WE does play a role in embryo metabolism, we would predict that specific alterations to the in vitro culture environment, including supplementation with specific growth factors that support and promote WE mechanisms (possibly in a species specific manner), would result in improved in vitro embryo development and viability compared to current culture media systems.
Acknowledgments
The authors would like to acknowledge support from the National Foundation for Fertility Research (RLK), the National Institutes of Health (U42 RR018877, RSP; R01 RR013438, RSP) and Food for the 21st Century (RSP).
Abbreviations
- FAO
fatty acid oxidation
- NAD[PH]
nicotinamide adenine dinucleotide [phosphate]
- oxphos
oxidative phosphorylation
- PPP
pentose phosphate pathway
- R5P
ribose 5-phosphate
- TCA
tri-carboxylic acid [cycle]
- WE
Warburg Effect
Genes or proteins
- ACACA
acetyl-coA carboxylase alpha
- ACLY
ATP citrate lyase
- Akt
protein kinase B
- FASN
fatty acid synthase
- G6PD
glucose-6-phosphate dehydrogenase
- GLUT
glucose transporter
- GLS
glutaminase
- GSH
glutathione
- HIF1
hypoxia inducible factor
- HK
hexokinase
- LDH
lactate dehydrogenase
- M2
fetal form of PKM2
- ME
malic enzyme
- mTOR
mammalian target of rapimycin
- PDH
pyruvate dehydrogenase kinase
- PDK
phosphate dehydrogenase kinase
- PEP
phosphoenolpyruvate
- PFK
phosphofructokinase
- PGAM
phosphoglycerate mutase
- PK[M2]
pyruvate kinase [isoform M2]
- PI3K
phosphoinositide-3-kinase
- TALDO
transaldolase
- TIGAR
chromosome 12, open reading frame 5
- TKT
transketolase
References
- Anastasiou D, Poulogiannis G, Asara JM, Boxer MB, Jiang J-K, Shen M, Bellinger G, Sasaki AT, Locasale JW, Auld DS, Thomas CJ, Vander Heiden MG, Cantley LC. Inhibition of pyruvate kinase M2 by reactive oxygen species contributes to cellular antioxidant response. Science. 2011;334:1278–1283. doi: 10.1126/science.1211485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann CG, Morris DG, Sreenan JM, Leese HJ. The quiet embryo hypothesis: molecular characteristics favoring viability. Mol Reprod Dev. 2007;74:1345–1353. doi: 10.1002/mrd.20604. [DOI] [PubMed] [Google Scholar]
- Bermejo-Alvarez P, Lonergan P, Rizos D, Gutierrez-Adan A. Low oxygen tension during IVM improves bovine oocyte competence and enhances anaerobic glycolysis. RBM Online. 2010;20:341–349. doi: 10.1016/j.rbmo.2009.12.006. [DOI] [PubMed] [Google Scholar]
- Cagnone GLM, Dufort I, Vigneault C, Sirard M-A. Differential Gene Expression Profile in Bovine Blastocysts Resulting from Hyperglycemia Exposure During Early Cleavage Stages. Biol Reprod. 2012 doi: 10.1095/biolreprod.111.094391. [DOI] [PubMed] [Google Scholar]
- Cairns RA, Harris IS, Mak TW. Regulation of cancer cell metabolism. Nat Rev Cancer. 2011;11:85–95. doi: 10.1038/nrc2981. [DOI] [PubMed] [Google Scholar]
- Christofk HR, Vander Heiden MG, Wu N, Asara JM, Cantley LC. Pyruvate kinase M2 is a phosphotyrosine-binding protein. Nature. 2008;452:181–186. doi: 10.1038/nature06667. [DOI] [PubMed] [Google Scholar]
- Dang CV. Rethinking the Warburg effect with Myc micromanaging glutamine metabolism. Cancer Res. 2010;70:859–862. doi: 10.1158/0008-5472.CAN-09-3556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De La Torre-Sanchez JF, Gardner DK, Preis K, Gibbons J, Seidel GE., Jr Metabolic regulation of in vitro-produced bovine embryos. II. Effects of phenazine ethosulfate, sodium azide and 2,4-dinitrophenol during post-compaction development on glucose metabolism and lipid accumulation. Reprod Fertil Dev. 2006;18:597–607. doi: 10.1071/rd05064. [DOI] [PubMed] [Google Scholar]
- DeBerardinis RJ. Is cancer a disease of abnormal cellular metabolism? New angles on an old idea. Genet Med. 2008;10:767–777. doi: 10.1097/GIM.0b013e31818b0d9b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deberardinis RJ, Sayed N, Ditsworth D, Thompson CB. Brick by brick: metabolism and tumor cell growth. Curr Op Genet Dev. 2008;18:54–61. doi: 10.1016/j.gde.2008.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downs SM, Mosey JL, Klinger J. Fatty acid oxidation and meiotic resumption in mouse oocytes. Mol Reprod Dev. 2009;76:844–853. doi: 10.1002/mrd.21047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunning KR, Cashman K, Russell DL, Thompson JG, Norman RJ, Robker RL. Beta-Oxidation Is Essential for Mouse Oocyte Developmental Competence and Early Embryo Development. Biol Reprod. 2010;83:909–918. doi: 10.1095/biolreprod.110.084145. [DOI] [PubMed] [Google Scholar]
- Ferguson EM, Leese HJ. A potential role for triglyceride as an energy source during bovine oocyte maturation and early embryo development. Mol Reprod Dev. 2006;73:1195–1201. doi: 10.1002/mrd.20494. [DOI] [PubMed] [Google Scholar]
- Flood MR, Weibold JL. Glucose metabolism by preimplantation pig embryos. J Reprod Fertil. 1988;84:7–12. doi: 10.1530/jrf.0.0840007. [DOI] [PubMed] [Google Scholar]
- Gandhi AP, Lane M, Gardner DK, Krisher RL. A single medium supports development of bovine embryos throughout maturation, fertilization and culture. Hum Reprod. 2000;15:395–401. doi: 10.1093/humrep/15.2.395. [DOI] [PubMed] [Google Scholar]
- Gardner DK, Lane M, Batt P. Uptake and metabolism and pyruvate and glucose by individual sheep preattachment embryos developed in vivo. Mol Reprod Dev. 1993;36:313–319. doi: 10.1002/mrd.1080360305. [DOI] [PubMed] [Google Scholar]
- Gardner DK, Lane M, Stevens J, Schoolcraft WB. Noninvasive assessment of human embryo nutrient consumption as a measure of developmental potential. Fertil Steril. 2001;76:1175–1180. doi: 10.1016/s0015-0282(01)02888-6. [DOI] [PubMed] [Google Scholar]
- Gardner DK, Leese HJ. Assessment of embryo viability prior to transfer by the noninvasive measurement of glucose uptake. J Exp Zool. 1987;242:103–105. doi: 10.1002/jez.1402420115. [DOI] [PubMed] [Google Scholar]
- Gardner DK, Leese HJ. Concentrations of nutrients in mouse oviduct fluid and their effects on embryo development and metabolism in vitro. J Reprod Fertil. 1990;88:361–368. doi: 10.1530/jrf.0.0880361. [DOI] [PubMed] [Google Scholar]
- Han Z, Vassena R, Chi MMY, Potireddy S, Sutovsky M, Moley KH, Sutovsky P, Latham KE. Role of glucose in cloned mouse embryo development. Am J Physiol Endocrinol Metab. 2008;295:E798–809. doi: 10.1152/ajpendo.00683.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewitson LC, Martin KL, Leese HJ. Effects of metabolic inhibitors on mouse preimplantation embryo development and the energy metabolism of isolated inner cell masses. Mol Reprod Dev. 1996;43:323–330. doi: 10.1002/(SICI)1098-2795(199603)43:3<323::AID-MRD6>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Hitosugi T, Kang S, Vander Heiden MG, Chung T-W, Elf S, Lythgoe K, Dong S, Lonial S, Wang X, Chen GZ, Xie J, Gu T-L, Polakiewicz RD, Roesel JL, Boggon TJ, Khuri FR, Gilliland DG, Cantley LC, Kaufman J, Chen J. Tyrosine phosphorylation inhibits PKM2 to promote the Warburg effect and tumor growth. Sci Signal. 2009;2:ra73. doi: 10.1126/scisignal.2000431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hom JR, RAQ, Hoffman DL, de Mesy Bentley KL, Molkentin JD, Sheu S-S, Porter J, GA The permeability transition pore controls cardiac mitochondrial maturation and myocyte differentiation. Developmental Cell. 2011;21:469–478. doi: 10.1016/j.devcel.2011.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houghton FD. Energy metabolism of the inner cell mass and trophectoderm of the mouse blastocyst. Differentiation. 2006;74:11–18. doi: 10.1111/j.1432-0436.2006.00052.x. [DOI] [PubMed] [Google Scholar]
- Hugentobler SA, Humpherson PG, Leese HJ, Sreenan JM, Morris DG. Energy substrates in bovine oviduct and uterine fluid and blood plasma during the oestrous cycle. Mol Reprod Dev. 2008;75:496–503. doi: 10.1002/mrd.20760. [DOI] [PubMed] [Google Scholar]
- Hugentobler SA, Sreenan JM, Humpherson PG, Leese HJ, Diskin MG, Morris DG. Effects of changes in the concentration of systemic progesterone on ions, amino acids and energy substrates in cattle oviduct and uterine fluid and blood. Reprod Fertil Dev. 2010;22:684–694. doi: 10.1071/RD09129. [DOI] [PubMed] [Google Scholar]
- Inoue K, Nakada K, Ogura A, Isobe K, Goto Y, Nonaka I, Hayashi JI. Generation of mice with mitochondrial dysfunction by introducing mouse mtDNA carrying a deletion into zygotes. Nature Genetics. 2000;26:176–181. doi: 10.1038/82826. [DOI] [PubMed] [Google Scholar]
- Kwun J, Chang K, Lim J, Lee E, Lee B, Kang S, Hwang W. Effects of exogenous hexoses on bovine in vitro fertilized and cloned embryo development: Improved blastocyst formation after glucose replacement with fructose in a serum-free culture medium. Mol Reprod Dev. 2003;65:167–174. doi: 10.1002/mrd.10309. [DOI] [PubMed] [Google Scholar]
- Lane M, Gardner DK. Inhibiting 3-phosphoglycerate kinase by EDTA stimulates the development of the cleavage stage mouse embryo. Mol Reprod Dev. 2001;60:233–240. doi: 10.1002/mrd.1083. [DOI] [PubMed] [Google Scholar]
- Lane M, Gardner DK. Embryo culture medium: which is the best? Best Pract Res Clin Obstet Gynaecol. 2007;21:83–100. doi: 10.1016/j.bpobgyn.2006.09.009. [DOI] [PubMed] [Google Scholar]
- Lee J, Kim HK, Han Y-M, Kim J. Pyruvate kinase isozyme type M2 (PKM2) interacts and cooperates with Oct-4 in regulating transcription. Int J Biochem Cell Biol. 2008;40:1043–1054. doi: 10.1016/j.biocel.2007.11.009. [DOI] [PubMed] [Google Scholar]
- Leese HJ. Quiet please, do not disturb: a hypothesis of embryo metabolism and viability. Bioessays. 2002;24:845–849. doi: 10.1002/bies.10137. [DOI] [PubMed] [Google Scholar]
- Leese HJ, Barton AM. Pyruvate and glucose uptake by mouse ova and preimplantation embryos. J Reprod Fertil. 1984;32:9–13. doi: 10.1530/jrf.0.0720009. [DOI] [PubMed] [Google Scholar]
- Leese HJ, Baumann CG, Brison DR, McEvoy TG, Sturmey RG. Metabolism of the viable mammalian embryo: quietness revisited. Mol Hum Reprod. 2008;14:667–672. doi: 10.1093/molehr/gan065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leese HJ, Sturmey RG, Baumann CG, McEvoy TG. Embryo viability and metabolism: obeying the quiet rules. Hum Reprod. 2007;22:3047–3050. doi: 10.1093/humrep/dem253. [DOI] [PubMed] [Google Scholar]
- Leroy JLMR, Vanholder T, Mateusen B, Christophe A, Opsomer G, de Kruif A, Genicot G, Van Soom A. Non-esterified fatty acids in follicular fluid of dairy cows and their effect on developmental capacity of bovine oocytes in vitro. Reproduction. 2005;130:485–495. doi: 10.1530/rep.1.00735. [DOI] [PubMed] [Google Scholar]
- Locasale JW, Cantley LC. Altered metabolism in cancer. Bmc Biology. 2010;8:88. doi: 10.1186/1741-7007-8-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Lazaro M. The warburg effect: why and how do cancer cells activate glycolysis in the presence of oxygen? Anticancer Agents Med Chem. 2008;8:305–312. doi: 10.2174/187152008783961932. [DOI] [PubMed] [Google Scholar]
- Ludwig TE, Lane M, Bavister BD. Differential effect of hexoses on hamster embryo development in culture. Biol Reprod. 2001;64:1366–1374. doi: 10.1095/biolreprod64.5.1366. [DOI] [PubMed] [Google Scholar]
- Luo W, Semenza GL. Pyruvate kinase M2 regulates glucose metabolism by functioning as a coactivator for hypoxia-indicible factor 1 in cancer cells. Oncotarget. 2011;2:551–556. doi: 10.18632/oncotarget.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machaty Z, Thompson JG, Abeydeera LR, Day BN, Prather RS. Inhibitors of mitochondrial ATP production at the time of compaction improve development of in vitro produced porcine embryos. Mol Reprod Dev. 2001;58:39–44. doi: 10.1002/1098-2795(200101)58:1<39::AID-MRD6>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Maddocks ODK, Vousden KH. Metabolic regulation by p53. Journal of Molecular Medicine. 2011;89:237–245. doi: 10.1007/s00109-011-0735-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marei WF, Wathes DC, Fouladi-Nashta AA. Impact of linoleic acid on bovine oocyte maturation and embryo development. Reproduction. 2010;139:979–988. doi: 10.1530/REP-09-0503. [DOI] [PubMed] [Google Scholar]
- Mito T, Yoshioka K, Yamashita S, Suzuki C, Noguchi M, Hoshi H. Glucose and glycine synergistically enhance the in vitro development of porcine blastocysts in a chemically defined medium. Reprod Fertil Dev. 2012 doi: 10.1071/RD11197. dx.doi.org/10.1071/RD11197. [DOI] [PubMed] [Google Scholar]
- Nichol R, Hunter RH, Gardner DK, Leese HJ, Cooke GM. Concentrations of energy substrates in oviductal fluid and blood plasma of pigs during the peri-ovulatory period. J Reprod Fertil. 1992;96:699–707. doi: 10.1530/jrf.0.0960699. [DOI] [PubMed] [Google Scholar]
- Paczkowski M, Silva E, Schoolcraft WB, Krisher RL. Comparative importance of fatty acid oxidation to oocyte nuclear maturation in murine, bovine and porcine species. SSR Annual Meeting; Portland, OR, USA. 2011a. p. 598. [Google Scholar]
- Paczkowski M, Yuan Y, Fleming-Waddell J, Bidwell CA, Spurlock D, Krisher RL. Alterations in the transcriptome of porcine oocytes derived from prepubertal and cyclic females is associated with developmental potential. J Animal Sci. 2011b;89:3561–3571. doi: 10.2527/jas.2011-4193. [DOI] [PubMed] [Google Scholar]
- Petters RM, Johnson BH, Reed ML, Archibong AE. Glucose, glutamine and inorganic phosphate in early development of the pig embryo in vitro. J Reprod Fertil. 1990;89:269–275. doi: 10.1530/jrf.0.0890269. [DOI] [PubMed] [Google Scholar]
- Petters RM, Wells KD. Culture of pig embryos. J Reprod Fertil-Suppl. 1993;48:61–73. [PubMed] [Google Scholar]
- Ramalho-Santos J, Varum S, Amaral S, Mota PC, Sousa AP, Amaral A. Mitochondrial functionality in reproduction: from gonads and gametes to embryos and embryonic stem cells. Hum Reprod Update. 2009;15:553–572. doi: 10.1093/humupd/dmp016. [DOI] [PubMed] [Google Scholar]
- Redel BK, Brown AN, Spate LD, Whitworth KM, Green JA, Prather RS. Glycolysis in preimplantation development is partially controlled by the Warburg Effect. Mol Reprod Dev. 2012 doi: 10.1002/mrd.22017. [DOI] [PubMed] [Google Scholar]
- Rieger D, Loskutoff NM, Betteridge KJ. Developmentally related changes in the uptake and metabolism of glucose, glutamine and pyruvate by cattle embryos produced in vitro. Reprod Fertil Dev. 1992;4:547–557. doi: 10.1071/rd9920547. [DOI] [PubMed] [Google Scholar]
- Rieger D, McGowan LT, Cox SF, Pugh PA, Thompson JG. Effect of 2,4-dinitrophenol on the energy metabolism of cattle embryos produced by in vitro fertilization and culture. Reprod Fertil Dev. 2002;14:339–343. doi: 10.1071/rd02038. [DOI] [PubMed] [Google Scholar]
- Rosenkrans CF, Jr, First NL. Effect of free amino acids and vitamins on cleavage and developmental rate of bovine zygotes in vitro. J Anim Sci. 1994;72:434–437. doi: 10.2527/1994.722434x. [DOI] [PubMed] [Google Scholar]
- Samudio I, Fiegl M, Andreeff M. Mitochondrial uncoupling and the Warburg effect: molecular basis for the reprogramming of cancer cell metabolism. Cancer Res. 2009;69:2163–2166. doi: 10.1158/0008-5472.CAN-08-3722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shlomi T, Benyamini T, Gottlieb E, Sharan R, Ruppin E. Genome-scale metabolic modeling elucidates the role of proliferative adaptation in causing the Warburg effect. PLoS Comput Biol. 2011;7:e1002018. doi: 10.1371/journal.pcbi.1002018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somfai T, Kaneda M, Akagi S, Watanabe S, Haraguchi S, Mizutani E, Dang-Nguyen TQ, Geshi M, Kikuchi K, Nagai T. Enhancement of lipid metabolism with L-carnitine during in vitro maturation improves nuclear maturation and cleavage ability of follicular porcine oocytes. Reprod Fertil Dev. 2011;23:912–920. doi: 10.1071/RD10339. [DOI] [PubMed] [Google Scholar]
- Spindler RE, Pukazhenthi BS, Wildt DE. Oocyte metabolism predicts the development of cat embryos to blastocyst in vitro. Mol Reprod Dev. 2000;56:163–171. doi: 10.1002/(SICI)1098-2795(200006)56:2<163::AID-MRD7>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Steeves TE, Gardner DK. Temporal and differential effects of amino acids on bovine embryo development in culture. Biol Reprod. 1999;61:731–740. doi: 10.1095/biolreprod61.3.731. [DOI] [PubMed] [Google Scholar]
- Stern S, Biggers JD, Anderson E. Mitochondria and early development of the mouse. J Exp Zool. 1971;176:179–191. doi: 10.1002/jez.1401760206. [DOI] [PubMed] [Google Scholar]
- Sturmey RG, Leese HJ. Energy metabolism in pig oocytes and early embryos. Reproduction. 2003;126:197–204. doi: 10.1530/rep.0.1260197. [DOI] [PubMed] [Google Scholar]
- Sturmey RG, Leese HJ. Role of glucose and fatty acid metabolism in porcine early embryo development. Reprod Fertil Dev. 2008;20:149. [Google Scholar]
- Sturmey RG, Reis A, Leese HJ, McEvoy TG. Role of fatty acids in energy provision during oocyte maturation and early embryo development. Reprod Dom Anim. 2009;44(Suppl 3):50–58. doi: 10.1111/j.1439-0531.2009.01402.x. [DOI] [PubMed] [Google Scholar]
- Suzuki S, Tanaka T, Poyurovsky MV, Nagano H, Mayama T, Ohkubo S, Lokshin M, Hosokawa H, Nakayama T, Suzuki Y, Sugano S, Sato E, Nagao T, Yokote K, Tatsuno I, Prives C. Phosphate-activated glutaminase (GLS2), a p53-inducible regulator of glutamine metabolism and reactive oxygen species. PNAS, USA. 2010;107:7461–7466. doi: 10.1073/pnas.1002459107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swain JE, Bormann CL, Clark SG, Walters EM, Wheeler MB, Krisher RL. Use of energy substrates by various stage preimplantation pig embryos produced in vivo and in vitro. Reproduction. 2002;123:253–260. [PubMed] [Google Scholar]
- Takahashi Y, First NL. In vitro development of bovine one cell embryos: influence of glucose, lactate, pyruvate, amino acids and vitamins. Theriogenology. 1992;37:963–978. doi: 10.1016/0093-691x(92)90096-a. [DOI] [PubMed] [Google Scholar]
- Tervit HR, Whittingham DG, Rowson LEA. Successful culture in vitro of sheep and cattle ova. J Reprod Fertil. 1972;30:493–497. doi: 10.1530/jrf.0.0300493. [DOI] [PubMed] [Google Scholar]
- Thompson JG, McNaughton C, Gasparrini B, McGowan LT, Tervit HR. Effect of inhibitors and uncouplers of oxidative phosphorylation during compaction and blastulation of bovine embryos cultured in vitro. J Reprod Fertil. 2000;118(1):47–55. [PubMed] [Google Scholar]
- Thompson JG, Simpson AC, Pugh PA, Wright RW, Jr, Tervit HR. Glucose utilization by sheep embryos derived in vivo and in vitro. Reprod Fertil Dev. 1991;3:571–576. doi: 10.1071/rd9910571. [DOI] [PubMed] [Google Scholar]
- Van Hoeck V, Sturmey RG, Bermejo-Alvarez P, Rizos D, Gutierrez-Adan A, Leese HJ, Bols PEJ, Leroy J. Elevated non-esterified fatty acid concentrations during bovine oocyte maturation compromise early embryo physiology. PLoS ONE. 2011;6:e23183. doi: 10.1371/journal.pone.0023183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Scence. 2009;324:1029–1033. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vander Heiden MG, Locasale JW, Swanson KD, Sharfi H, Heffron GJ, Amador-Noguez D, Christofk HR, Wagner G, Rabinowitz JD, Asara JM, Cantley LC. Evidence for an alternative glycolytic pathway in rapidly proliferating cells. Science. 2010;329:1492–1499. doi: 10.1126/science.1188015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varum S, Rodrigues AS, Moura MB, Momcilovic O, Easley CAT, Ramalho-Santos J, Van Houten B, Schatten G. Energy metabolism in human pluripotent stem cells and their differentiated counterparts. PLoS ONE. 2011;6:e20914. doi: 10.1371/journal.pone.0020914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vousden KH, Ryan KM. p53 and metabolism. Nat Rev Cancer. 2009;9(10):691–700. doi: 10.1038/nrc2715. [DOI] [PubMed] [Google Scholar]
- Warburg O. On the origin of cancer cells. Scence. 1956;123:309–314. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]
- Wu GQ, Jia BY, Li JJ, Fu XW, Zhou GB, Hou YP, Zhu SE. L-carnitine enhances oocyte maturation and development of parthenogenetic embryos in pigs. Theriogenology. 2011;76:785–793. doi: 10.1016/j.theriogenology.2011.04.011. [DOI] [PubMed] [Google Scholar]
- Yoshioka K, Suzuki C, Tanaka A, Anas IMK, Iwamura S. Birth of piglets derived from porcine zygotes cultured in a chemically defined medium. Biol Reprod. 2002;66:112–119. doi: 10.1095/biolreprod66.1.112. [DOI] [PubMed] [Google Scholar]


