Abstract
Although cancer tends to affect the elderly, most preclinical studies are performed in young subjects. In this study, we developed a melanoma-specific cancer immunotherapy that shows efficacy in aged but not young hosts by mitigating age-specific tumor-associated immune dysfunction. Both young and aged CD4+CD25hi regulatory T cells (Tregs) exhibited equivalent in vitro T cell suppression and tumor-associated augmentation in numbers. However denileukin diftitox (DT)-mediated Treg depletion improved tumor-specific immunity and was clinically effective only in young mice. DT-mediated Treg depletion significantly increased myeloid-derived suppressor cell (MDSC) numbers in aged but not young mice, and MDSC depletion improved tumor-specific immunity and reduced tumor growth in aged mice. Combining Treg depletion with anti-Gr-1 antibody was immunologically and clinically more efficacious than anti-Gr-1 antibody alone in aged B16-bearing mice, similar to Treg depletion alone in young mice. In contrast, DT increased MDSC in young and aged mice following MC-38 tumor challenge, although effects were greater in aged mice. Anti-Gr1 boosted DT effects in young but not aged mice. Aged anti-tumor immune effector cells are therefore competent to combat tumor when underlying tumor-associated immune dysfunction is appropriately mitigated, but this dysfunction varies with tumor, thus also varying responses to immunotherapy. By tailoring immunotherapy to account for age-related tumor-associated immune dysfunctions, cancer immunotherapy for aged patients with specific tumors can be remarkably improved.
Keywords: cancer, aging, immunotherapy, immune regulation, regulatory T cells, myeloid derived suppressor cells
Introduction
Among known factors associated with cancer development, advancing age remains the leading risk (1). Immune therapy for cancer is a scientifically sound approach, but clinical effects have generally been modest. Much of our understanding of tumor immunity comes from studies in young hosts. Few studies have examined the effects of age on response to immune therapy and on tumor-associated immune dysfunction.
Although anti-tumor immunity declines with age, as do other immune effector functions (2, 3), T cell functions in aged hosts can sometimes be improved (4). Thus, to the extent that underlying tumor-associated immune dysfunction can be reversed in aged hosts with cancer, clinically relevant anti-tumor immune responses could potentially be achieved.
Regulatory T cells (Tregs) are key mediators of tumor immune dysfunction, and reducing Treg function is a rational cancer immunotherapy strategy (5–8). Treg contributions to age-related decline in immune responses is contradictory, with some studies showing increases in Treg prevalence and/or function with age in humans and mice (9–12) whereas others show no changes or reduced Treg contributions (13, 14). Myeloid derived suppressor cells (MDSC) are regulatory cells that also increase in tumors (15–18) and suppress anti-tumor immunity (19). They are a heterogeneous population usually identified as CD11b+Gr-1+ cells in mice (17).
Although Treg depletion is an effective approach to improving anti-tumor immunity and responses to immunotherapy (6, 8), conflicting studies report the effect of Treg depletion as cancer immunotherapy in aged hosts (20, 21). MDSC depletion is another potentially effective approach to reverse cancer-associated immune dysfunction. Whereas MDSC contribute to immunopathology in aged hosts including in cancer (22, 23), the subject remains little studied, as does potential interactions between MDSC and Tregs.
We examined immune dysregulatory mechanisms in young versus aged hosts with cancer and assessed age-specific responses to Treg depletion, MDSC modulation or both. We showed that aged and young hosts with cancer responded differently to those immune therapies in a tumor-dependent fashion, but that, more importantly, clinically significant anti-tumor immune responses can be achieved even in aged tumor-bearing mice using age-specific immune therapies. These data suggest means to improve the efficacy of tumor immunotherapy in the population most at risk for cancers, and demonstrate that age and tumor interactions with the aged host are significant considerations in pre-clinical and clinical testing of immunotherapeutic strategies.
Materials and Methods
Mice
4–8 week old (NCI repository) and 17–19 month old (NIA repository) C57BL/6 (BL6) mice were purchased. Young FoxP3-IRES-red fluorescent protein (FIR) mice (24) provided by Richard Flavell and Foxp3DTR mice (25) from Alexander Rudensky were aged in our facility. “Young” mice are 2–6 months old. “Aged” mice are 22–26 months old. All animal studies were approved by the UTHSCSA Institutional Animal Care and Use Committee.
Tumor
B16F10 melanoma and MC-38 colon carcinoma were purchased from the ATCC. We engineered OVA-expressing B16F10 cells (26) and MC-38 cells (27), herein referred to as “B16” and “MC-38”, respectively, for simplicity. Tumor challenge was injection of 250,000 B16 or 1 × 106 MC-38 tumor cells subcutaneously as described (26). Tumor growth was measured using Vernier calipers and volume calculated as (length × width2)/2.
Treatments
Starting two days after tumor challenge, BL6 mice were injected intraperitoneally every other day with 5 µg denileukin diftitox (DT; Eisai, Research Triangle Park, NC ) or PBS control to deplete Tregs, and/or with 200 µg of anti-Gr-1 mAb (clone RB6.8C5-18) or isotype control to deplete Gr-1+ cells. Foxp3DTR mice were injected with 15 µg diphtheria toxin/kg or PBS once, and sacrificed 7–9 days later as indicated. B16-bearing mice were treated with 25 or 50 mg 5-fluorouracil/kg or PBS once, one week after tumor challenge, and sacrificed 7 days later.
Flow cytometry
We isolated and stained cells, and perform cell sorts as previously described (28), using LSR II and FACSAria flow cytometers and FACSDiva software (BD Bioscience, San Jose, CA). Anti-CD11b (M1/70), anti-interferon (IFN)-γ (XMG1.2), anti-CD25 (PC61), anti-CD69 (H1.2F3), anti-CD4 (GK1.5), anti-CD3 (500A2), anti-Gr-1 (RB6-8C5), Ly-6C (AL-21), and matched isotype control Abs were from BD Pharmingen (San Diego, CA). Anti-CD62L (MEL14), anti-CD44 (1M7), anti-interleukin (IL)-17A (17B7) and anti-Foxp3 (FJK-16a) were purchased from eBioscience (San Diego, CA), anti-CD8α (5H10) from Caltag Laboratories (Burlingame, CA), Ly6-G (1A8) and anti-CD44 (1M7) mAbs from Biolegend (San Diego, CA). CD8+ OVA-specific T cells were detected using OVA-specific pentamers (ProImmune, Oxford, UK).
Suppression assays
Treg suppression assays were done as previously described (26). Briefly, Tregs (CD4+CD25hi from BL6 mice or CD4+ Foxp3+CD25hi or CD4+ Foxp3+CD25lo from FIR mice) were sorted from spleens after CD4+ T cell enrichment (StemCell, Vancouver, BC). For MDSC suppression assays, CD11b+Gr-1hi cells were sorted from spleens. CFSE-labeled CD4+ responder T cells from naïve mice were incubated at 3 × 104 cell/well in 96-well plates with Tregs or MDSC, and CD3/CD28 T Cell Expander beads (Invitrogen). After 4 days incubation at 37°C, responder cell proliferation was measured by CFSE dilution by FACS.
Bone marrow cultures
At sacrifice, femurs were flushed, washed in PBS and bone marrow (BM) cells were cultured in 10% FCS/RPMI at 5 × 105 cells/well in 4ng/ml GM-CSF (PeProTech Inc, Rock Hill, NJ) at 37°C in 5% CO2. Cells were recovered for counting and FACS analysis on day 4.
Cytokine assays
Serum cytokines were assessed using a custom multiplex Bio-Plex Pro assay kit (Bio-Rad, Hercules, CA) according to the manufacturer’s protocol. Cytokines levels were measured using a Luminex 200 analyzer (Luminex Corp., Austin, TX).
Statistics
Statistical analyses were performed using Prizm software (GraphPad Inc, San Diego, CA). For tumor growth measurements, we used a one-way ANOVA comparing treatment arms. For all other single measurement assays, we used a two-tailed Mann-Whitney test. P values <0.05 were considered significant.
Results
Treg depletion is clinically efficacious in young but not in aged B16-bearing mice
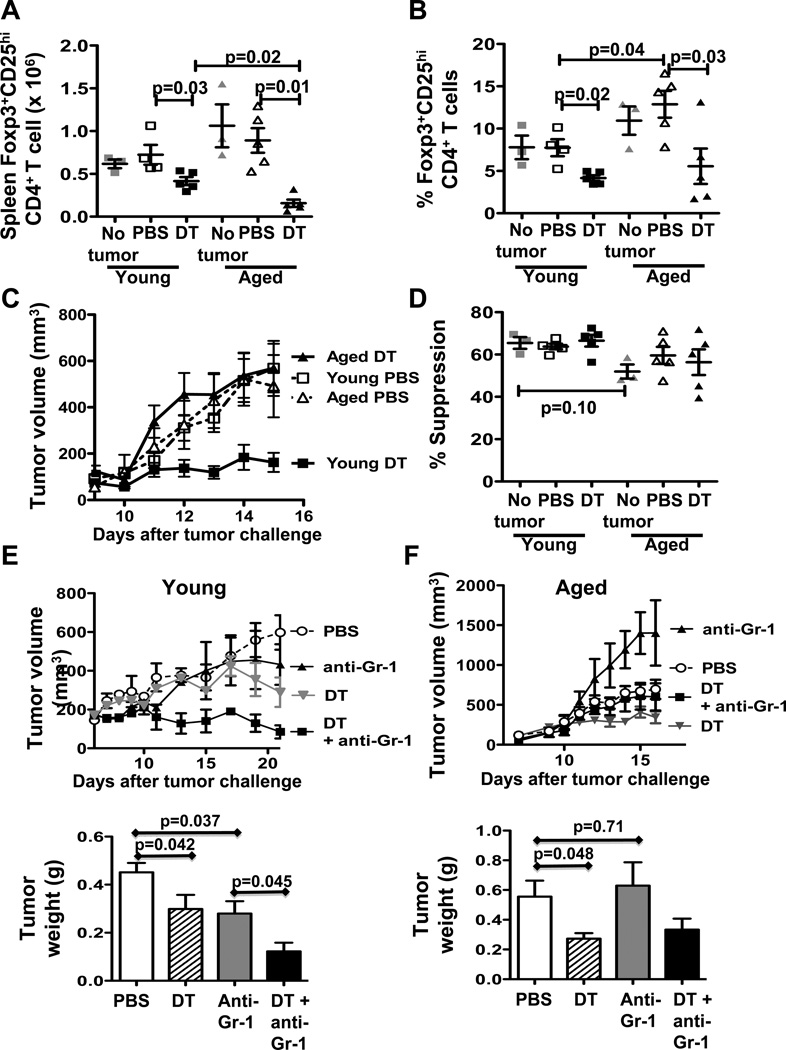
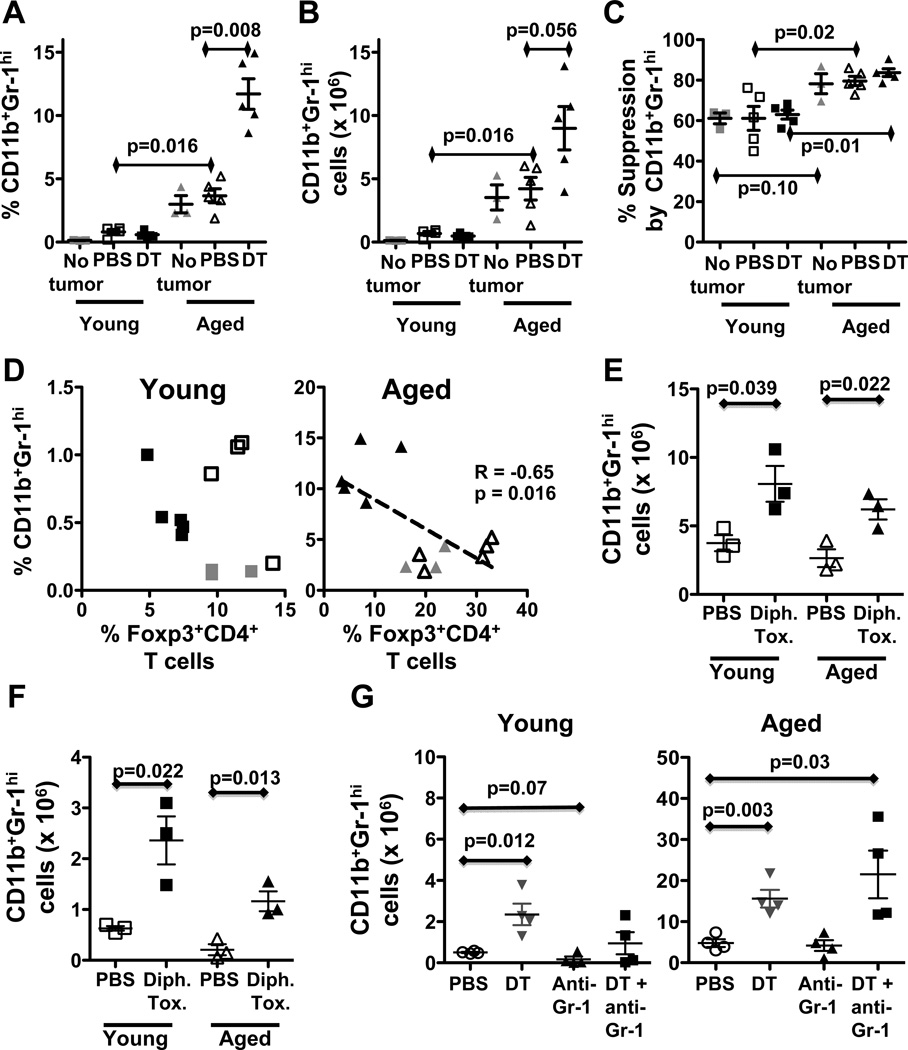
As CD4+CD25+Foxp3+ regulatory T cells contribute to immune dysfunction in cancer (6, 8), we first showed that naïve young and aged mice had comparable numbers of CD4+ Foxp3+CD25hi spleen phenotypic Tregs (P = 0.1, Fig. 1A). B16-challenged aged mice had a higher prevalence of spleen CD4+Foxp3+CD25hi T cells compared to young mice (Fig. 1B) but absolute numbers were similar (P = 0.5, Fig. 1A). Following challenge, B16 tumor grew similarly in young and aged mice in the absence of specific treatment (Fig. 1C). DT treatment, which depletes young mouse Treg efficiently (29), significantly reduced B16 growth in young mice as expected (P = 0.0001, young DT versus PBS), but strikingly had no effect in aged mice (P = 0.4, Fig. 1A). DT reduced numbers (Fig. 1A) and prevalence (Fig. 1B) of CD4+Foxp3+CD25hi T cells in spleens of young mice as expected and was even more effective in depleting Tregs in aged mice (Fig. 1A,B). Spleen CD4+CD25hi Tregs at baseline and after tumor challenge from young and aged mice were comparably suppressive in vitro, with no significant change after DT treatment (Fig. 1D).
Figure 1.
Treg depletion reduces B16 tumor growth in young but not aged mice. (A–D) Young and aged C57BL/6 mice challenged with B16 melanoma and treated with PBS or 5 µg denileukin diftitox (DT). A, Numbers and, B, prevalence of spleen Foxp3+CD25hi phenotypic Tregs in young versus aged naïve (No tumor, n=3) or tumor-bearing mice treated with PBS or DT (n=4 or 5). C, Tumor growth. D, Spleen CD4+CD25hi Treg suppressive activity in young and aged mice at 1:1 effector:Treg ratio. (E, F) Tumor growth (top panels) and final tumor weight (bottom panels) from young (E) or aged (F) C57BL/6 mice challenged with MC-38 carcinoma and treated with either PBS, DT, anti-Gr-1 antibody or DT+anti-Gr-1. Values are mean ± SEM.
Treg depletion improves tumor antigen-specific immunity in young but not aged hosts with B16
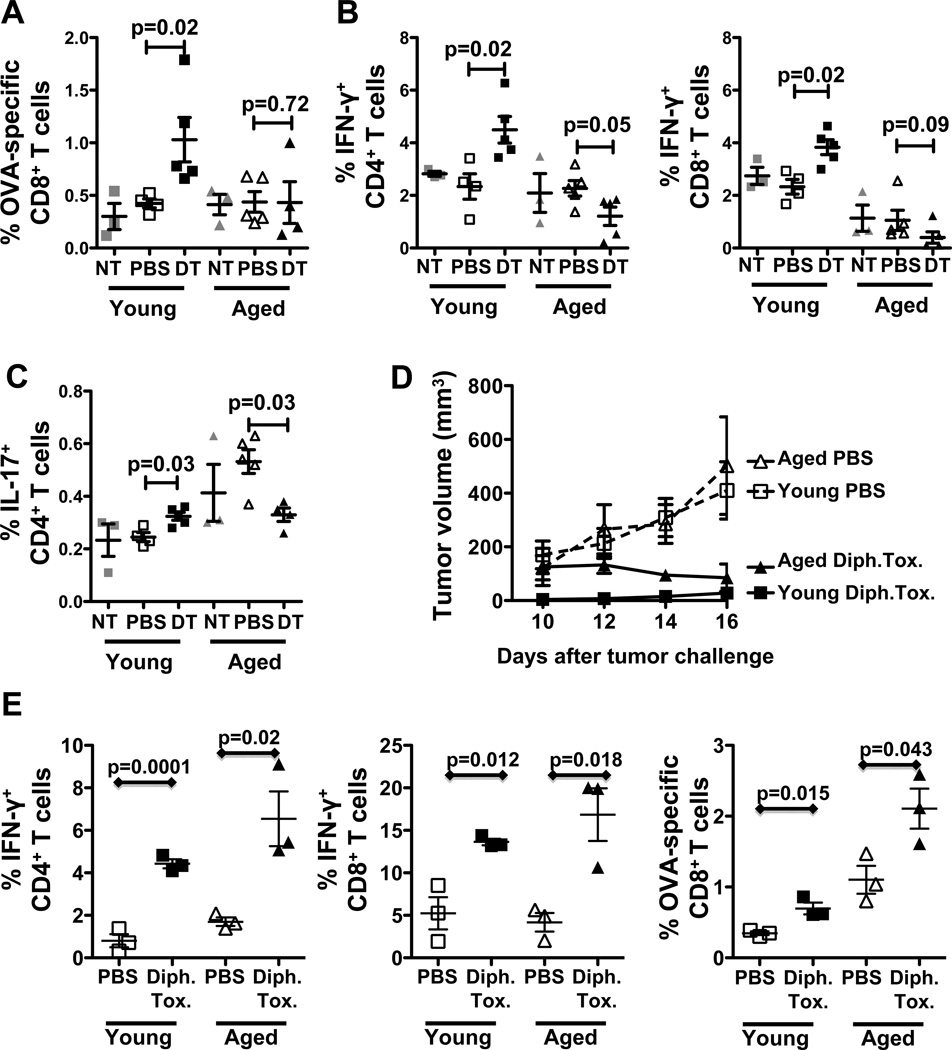
Untreated, aged B16-bearing mice have a higher prevalence of IL-17+ (Fig. 2A, Supplementary Fig. 1) and activated T cells (Supplementary Fig. 2) compared to young mice, consistent with greater inflammation in age (27, 30). DT increased tumor-antigen specific CD8+ T cells (Fig. 2A) in spleens of young but not aged B16-bearing mice. DT increased the prevalence and numbers of IFN-γ- and IL-17-producing CD4+ and CD8+ spleen T cells in young B16-bearing mice while reducing these cells in aged mice (Fig. 2B,C; Supplementary Fig. 1), suggesting differential age-related regulatory control of these cytokines producing T cells in B16-bearing mice. Finally, DT increased the prevalence of CD44−CD62L+ effector memory CD8+ T cells in young but not aged mice and their activation as measured by CD69 expression (Supplementary Fig. 2A,B). DT increased the prevalence and activation of naïve CD62L+CD4+ T cells in young mice but reduced CD69 expression in aged mice (Supplementary Fig. 2B,D). Together, these data are consistent with better immune improvement in DT-treated young versus aged hosts with B16 melanoma.
Figure 2.
DT-mediated Treg depletion improves anti-tumor immunity in young but not aged tumor-bearing mice. (A–C) Young and aged C57BL/6 mice challenged with OVA expressing B16 melanoma and treated as in Fig. 1. A, Spleen OVA-specific CD8+ T cells prevalence. B, Spleen IFN-γ+ CD4+ and CD8+ T cells prevalence. (NT = no tumor, naïve controls). (D, E) Young and aged Foxp3DTR mice challenged with B16 and treated with 15 µg diphtheria toxin (Dipht. Tox)/kg or PBS. D, Tumor growth in young and aged Foxp3DTR mice. E, Prevalence of IFN-γ+ CD4+ and CD8+ T cells and OVA-specific CD8+ T cells. Values are mean ± SEM.
Lack of efficacy of DT in aged mice could result from: i) a defect in aged Treg in hampering anti-tumor immunity, ii) a defect in aged effector cells to produce significant anti-tumor immunity following Treg depletion, or iii) additional age-dependent regulatory mechanisms preventing effective anti-tumor immunity after Treg depletion.
Functional Foxp3+CD25lo Tregs increase with age
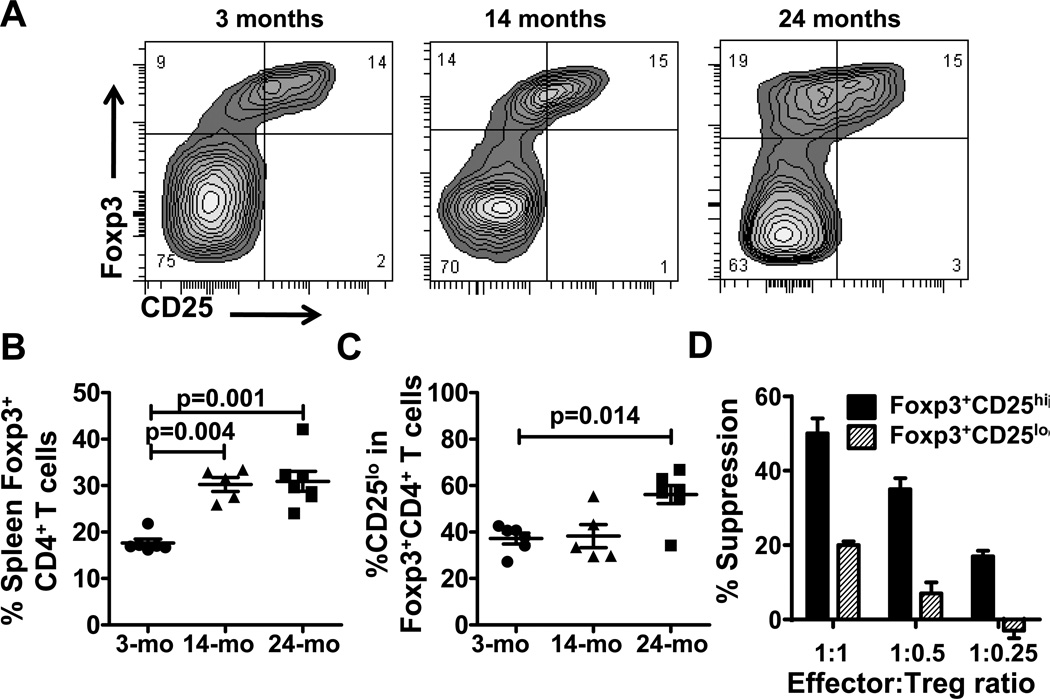
To test for potential additional Treg populations, we used FIR mice in which Tregs are identified as red fluorescent protein (RFP) +Foxp3+ cells (24). Foxp3+CD4+ T cell prevalence increased with age (Fig. 3A,B) and in particular of Foxp3+CD4+ T cells expressing low levels of CD25 (CD4+FoxP3+CD25lo) (Fig. 3C), previously described as being equally suppressive as conventional CD4+Foxp3+CD25hi Tregs in young naïve mice (31). CD4+Foxp3+CD25lo Tregs from aged naïve mice were functional but less suppressive than conventional CD4+Foxp3+CD25hi Tregs in vitro (Fig. 3D).
Figure 3.
CD25loFoxp3+ Tregs are increase in aged naïve mice. A, Foxp3 versus CD25 expression in CD4+ T cells from spleen of one representative 3-, 14- and 24-month old naïve FIR mice. B, Percentage of Foxp3+CD4+ T cells in 3-, 14- and 24-month old naïve FIR mice (n = 5–6 mice/group). C, Percentage of CD25lo cells among Foxp3+CD4+ T cells. D, Foxp3+CD25hi versus Foxp3+CD25lo Treg suppressive activity from 24-month old mice.
DT depletes both CD25hi and CD25lo Foxp3+ Tregs in tumor-bearing aged mice
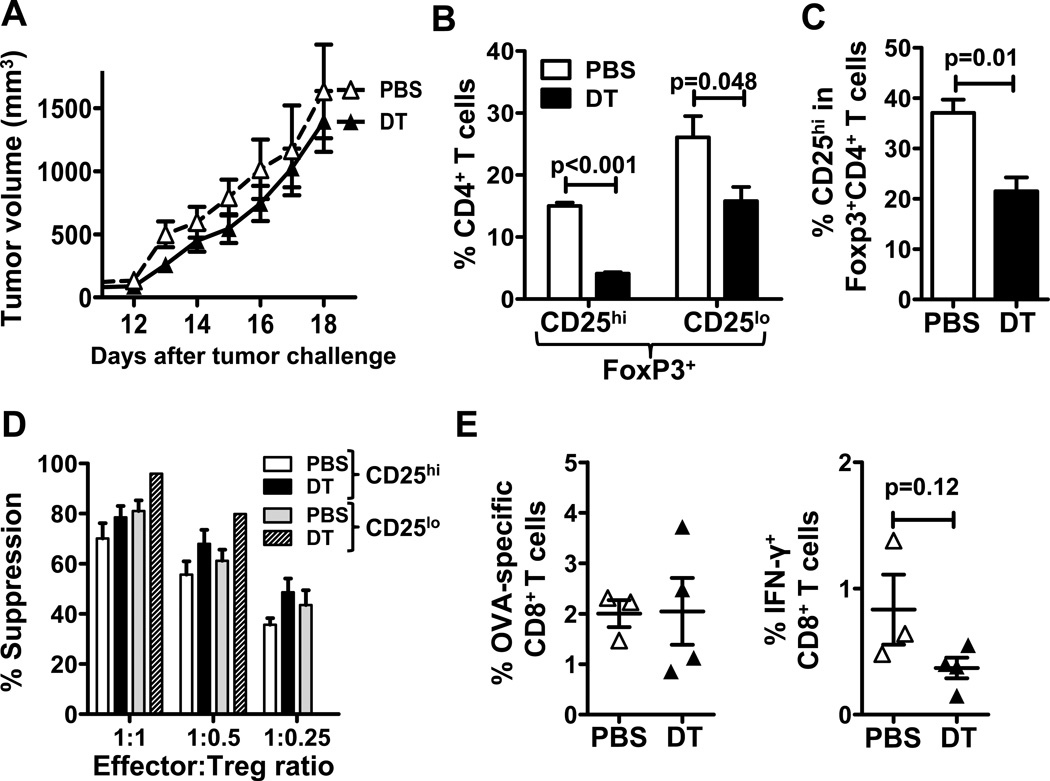
Because DT targets IL-2 receptor-expressing cells and not Tregs specifically (6), it was possible that DT failed to deplete this Foxp3+CD25lo Treg subpopulation prominent in aged tumor-bearing mice. Aged FIR mice were challenged with B16 and treated with DT. Tumor growth in aged FIR mice was comparable to that in WT mice, and DT treatment was again clinically ineffective (Fig. 4A). Two days after the last DT injection, both CD25hi and CD25loFoxp3+CD4+ spleen T cells were reduced, although conventional Foxp3+CD25hi Tregs were preferentially depleted (Fig. 4B). The proportion of CD25hi Tregs among total Foxp3+ cells was significantly reduced by DT treatment (P = 0.01, Fig. 4C).
Figure 4.
DT depletes CD25hi and CD25lo Foxp3+ Tregs in tumor-bearing aged mice. 22-month old FIR mice were challenged with B16 tumor cells and treated with either PBS or DT. A, B16 tumor growth in PBS (n = 3) versus DT (n = 4) treated mice. B, Percentage of Foxp3+CD25hi and Foxp3+CD25lo Tregs in spleen CD4+ T cells at day 18. C, Prevalence of CD25hi T cells among Foxp3+CD4+ T cells. D, Foxp3+CD25hi and Foxp3+CD25lo Treg suppressive activity from mice treated with PBS or DT. E, OVA-specific and IFN-γ+ CD8+ spleen T cells prevalence.
CD25hi and CD25lo Foxp3+CD4+ T cells are equally suppressive in aged B16-bearing mice
We tested the suppressive function of CD25hi and CD25lo Foxp3+CD4+ populations and found that both Treg subsets were equally suppressive in aged B16-bearing mice (Fig. 4D) in contrast to their differential suppression capacity in aged naïve mice (Fig. 3D), suggesting that tumors can alter the function of a specific Treg subset, in this case making it more suppressive. Nonetheless, DT treatment did not affect the suppressive function of remaining Tregs in aged mice (Fig. 4D). As in WT mice, DT treatment did not significantly increased tumor-specific or IFN-γ+CD8+ T cells in spleens of DT-treated B16-bearing FIR mice (Fig. 4E). Thus, lack of clinical efficacy of DT treatment in aged B16-bearing mice is not due to lack of depletion of a specific Treg subpopulation.
Treg depletion increases MDSC numbers in B16-bearing aged mice
As differential Treg depletion seemed unlikely to explain differential treatment effects, we tested DT treatment effects on MDSC as they are reportedly increased in aged mice and contribute to immune dysfunction in cancer (32), including in aged hosts (22). Consistent with prior reports (22, 33), aged mice had a higher prevalence (Fig. 5A) and numbers (Fig. 5B) of spleen CD11b+Gr-1hi MDSC at baseline and after tumor challenge. DT treatment slightly reduced the numbers and prevalence of CD11b+Gr-1hi MDSC in young tumor-bearing mice (Fig 5A,B). By striking contrast, DT significantly increased the prevalence and numbers of CD11b+Gr-1hi MDSC in aged B16-bearing mice (Fig 5A,B). MDSC were also significantly more suppressive in aged versus young mice, both at baseline and after B16 challenge, which was not affected by DT treatment (Fig. 5C).
Figure 5.
DT-mediated Treg depletion increased CD11b+Gr-1hi MDSC in B16-bearing and naïve aged mice. (A–D) Young and aged mice challenged with B16 cells and treated as in Fig. 1. A, Prevalence and, B, numbers of spleen CD11b+Gr-1hi phenotypic MDSC in young versus aged naïve (No tumor) or tumor-bearing mice treated with PBS or DT. C, Spleen CD11b+Gr-1hi MDSC suppression at 1:1 Effector:MDSC ratio. D, Negative correlation between splenic CD11+Gr-1hi MDSCs and Foxp3+CD4+ splenic Tregs in aged but not in young tumor-bearing mice. E, Spleen CD11b+Gr-1hi cell numbers in young and aged B16-bearing Foxp3DTR mice treated with PBS or diphtheria toxin (Dipht. Tox). F, Spleen CD11b+Gr-1hi cell numbers in young and aged naïve Foxp3DTR mice treated with PBS or diphtheria toxin (Dipht. Tox). G, Spleen CD11b+Gr-1hi cell numbers in young or aged C57BL/6 mice challenged with MC-38 carcinoma and treated with PBS, DT, anti-Gr-1 or DT+anti-Gr-1.
MDSC prevalence was inversely correlated with Treg prevalence only in aged B16-bearing mice (Fig. 5D) suggesting that Tregs could regulate MDSC numbers in aged B16-bearing mice and that Treg depletion in aged mice leads to an undesired increase in MDSC, thus negating the beneficial anti-tumor effects of DT-mediated Treg depletion observed in young mice. Increased spleen MDSC following DT treatment and their highly suppressive character was confirmed in aged B16-bearing FIR mice (Supplementary Fig. 3A–C). Because MDSC subsets could have differential function, we then showed that DT had similar effects on CD11b+Gr-1med MDSC (Supplementary Fig. 3D).
MDSC depletion is clinically efficacious in aged but not in young B16-bearing mice
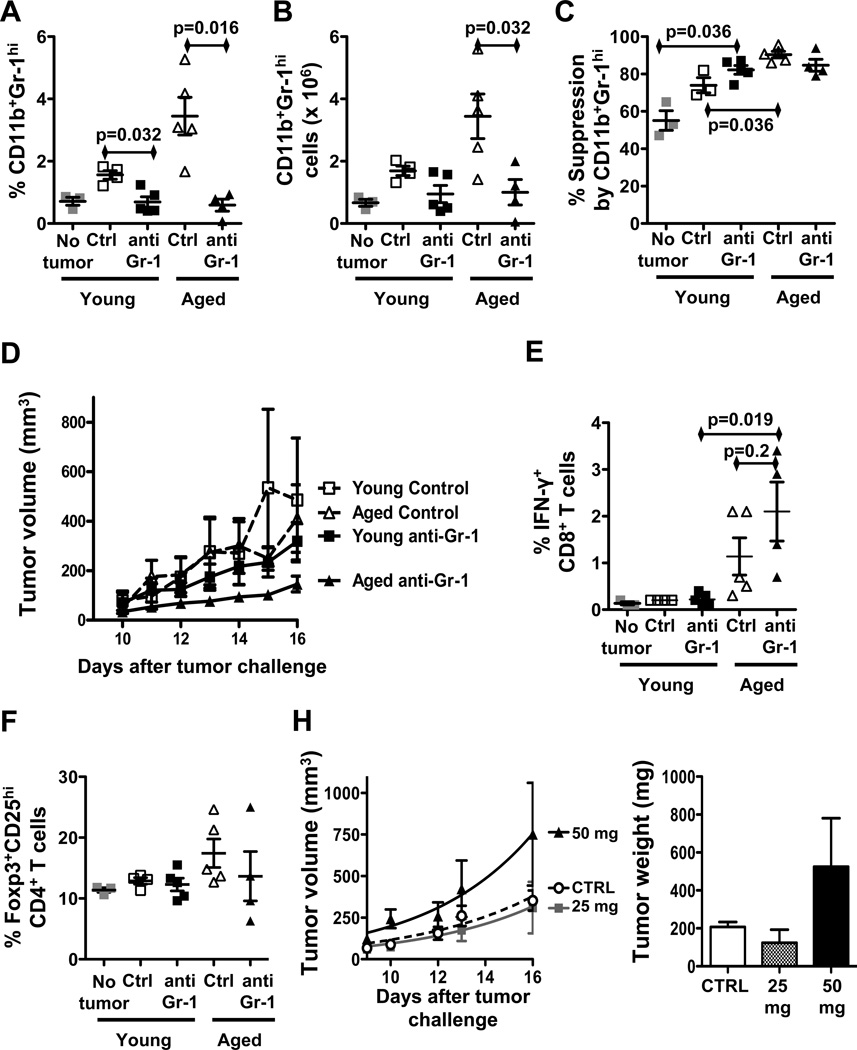
We tested effects of MDSC depletion given their deleterious effects. Anti-Gr-1 antibody depleted CD11b+Gr1hi MDSC in spleens of young and aged B16-bearing mice (Fig. 6A,B). Similar to DT treatment, anti-Gr-1 antibody did not significantly affect the suppressive function of remaining MDSC in young or aged mice (Fig. 6C). However, by contrast to DT-mediated Treg depletion, anti-Gr1-mediated depletion of MDSC resulted in significantly slower tumor growth in aged but not young B16-bearing mice (P = 0.007 for aged, Fig. 6D). MDSC depletion yielded significantly more IFN-γ+CD8+ T cells in aged versus young mice (Fig. 6E) suggesting improved anti-tumor immunity as a mechanism for treatment efficacy, without significant effect on the prevalence of Foxp3+CD25hi Tregs (Fig. 6F) or Treg function (Supplementary Fig. 4A) in both young and aged mice. Surprisingly, anti-Gr-1 antibody increased CD11b+Gr1med MDSC in young but not aged hosts (Supplementary Fig. 4B).
Figure 6.
MDSC depletion improves anti-tumor immunity in aged mice. (A–F) Young and aged mice challenged with B16 and treated with isotype control or anti-Gr-1 antibody. A, Prevalence and B, numbers of CD11b+Gr-1hi spleen MDSC in tumor-bearing mice. C, Spleen MDSC suppressive function at 1:1 Effector:MDSC ratio. D, Tumor growth. E, IFN-γ+CD8+T cells prevalence. F, Foxp3+CD25hiCD4+ Tregs prevalence. H, Tumor growth and final tumor weight in young C57BL/6 mice challenged with B16 and treated with a single dose of 25 mg or 50 mg/kg 5-fluorouracil or with PBS (CTRL).
Combining Treg depletion with MDSC depletion increases clinical response in aged B16-bearing mice
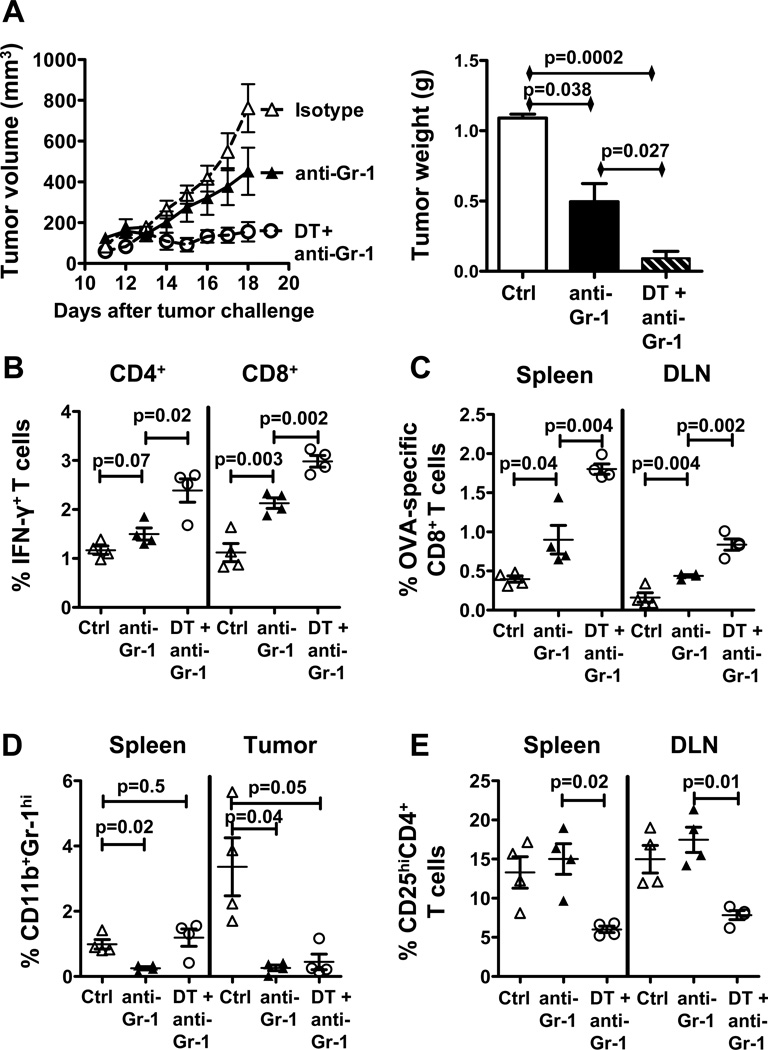
As MDSC appear to contribute more to cancer-related immune dysfunction in aged versus young mice with B16, and because DT-mediated Treg depletion increased these dysfunctional MDSC in aged B16-bearing mice, we hypothesized that combining both Treg and MDSC depletion would significantly boost anti-tumor immunity and clinical efficacy in aged mice. Thus, we challenged aged BL6 mice with B16 and treated with anti-Gr-1 antibody alone or combined with DT. Combination therapy significantly reduced tumor growth and weight in aged mice better than anti-Gr-1 treatment alone (Fig. 7A). Reduced tumor growth was associated with increased IFN-γ+CD4+and IFN-γ+CD8+ T cell prevalence (Fig. 7B), and increased tumor-specific CD8+ T cell prevalence in spleen and tumor draining lymph nodes (Fig. 7C). Anti-Gr-1 treatment again reduced MDSC in spleen and tumor (Fig. 7D). Combination therapy reduced CD25hiCD4+ phenotypic Tregs similar to DT treatment alone (Fig. 7E). In confirmation, combination therapy significantly reduced tumor growth in aged FIR mice over anti-Gr-1 antibody alone (Supplementary Fig. 4C,D).
Figure 7.
Combination of Treg and MDSC depletion slows tumor growth and improves anti-tumor immunity in aged mice. Aged mice challenged with B16 melanoma and treated with isotype control, anti-Gr-1 antibody alone or in combination with DT (n = 4 per group). A, Tumor growth (left) and final tumor weight (right). B, Spleen IFN-γ+CD4+and IFN-γ+CD8+ T cells prevalence. C, Percentage of OVA-specific CD8+ T cells in spleen and tumor-draining lymph nodes (DLN). D, Percentage of CD11b+Gr-1hi MDSC in spleen and tumor. E, Percentage of CD25+CD4+ Tregs in spleen and DLN.
Tregs restrain MDSC numbers in young and aged naïve and B16-bearing hosts
DT clearly depletes Tregs, but is not Treg-specific (6). To test specific Tregs contributions to MDSC effects we challenged syngeneic Foxp3DTR mice with diphtheria toxin, which depletes only Tregs (34). Following a single injection of diphtheria toxin, young and aged B16-bearing mice experienced similar MDSC increases 9 days later (Fig. 5E) confirming Treg-mediated MDSC control in B16 tumor, but not just in aged hosts. To test if effects depended on tumor, we depleted Tregs from young and aged naïve Foxp3DTR mice and found similar MDSC increases (Fig. 5F). Thus, DT has an age- and tumor-specific effect on MDSC control, but this effect might not be exclusively from Treg depletion.
High-order Treg depletion improves immune and clinical responses to B16 in aged hosts
Treg depletion is essentially complete in Foxp3DTR mice treated with diphtheria toxin (34)(and data not shown), contrasting to lesser Treg depletion with DT (Fig. 1A,B). This high-order Treg depletion in aged Foxp3DTR mice improved clinical tumor control (Fig. 2D), IFN-γ production and anti-tumor immunity (Fig. 2E). Thus, with sufficient Treg reduction alone, aged hosts can mount clinically effective anti-tumor immunity (Fig. 2D,E).
Treatment effects are tumor-dependent
Because DT treatment effects could be tumor- as well as age-dependent, we assessed treatment effects in MC-38 colon carcinoma. Unlike results in B16, DT-mediated Treg depletion was beneficial to tumor growth (Fig. 1E,F) and anti-tumor immunity (Supplementary Fig. 5A–F) in young and aged mice. Anti-Gr-1 antibody further boosted clinical effects in young but not aged hosts (Fig. 1E,F) although DT increased CD11b+Gr-1hi MDSC in both (Fig. 5G). A significant increase in CD11b+Gr-1med was also observed only in young MC-38-bearing mice treated with DT (Supplementary Fig. 3E). Tumor weights (Fig. 1F) were similar in aged B16-bearing mice treated with PBS and anti-Gr-1-treated mice despite volumetric differences.
DT and Treg depletion affect myelopoiesis in vitro
To test if Treg depletion effects on MDSC related to altered myelopoiesis, we cultured BM from B16-bearing Foxp3DTR mice and MC-38-bearing WT mice with 4 ng/ml GM-CSF for 4 days. High-order Treg depletion or DT treatment skewed BM differentiation towards the Ly-6ChiLy-6G− subset in B16-bearing Foxp3DTR mice (Supplementary Fig. 6A), and MC-38-bearing C57/BL6 mice (Supplementary Fig. 7A,B). These data establish that Tregs and DT treatment can alter myelopoiesis in tumor-bearing mice, but additional work is required to establish the in vivo significance. GM-CSF, VEGF, M-CSF, IL-6 (among others) can drive MDSC generation in vivo (32), but we found similar serum levels of these cytokines in B16 (Supplementary Fig. 6B) or MC-38 (Supplementary Fig. 7C) bearing young or aged mice following high-order Treg depletion or DT treatment, and equivalent increases in GM-CSF+ T lymphocytes in B16-bearing (Supplementary Fig. 6C,D) and MC-38-bearing mice (Supplementary Fig. 7D). Further, DT did not alter GM-CSF, VEGF, M-CSF (not shown) or IL-6 in young or aged B16-bearing mice (Supplementary Fig. 8). These data establish that Tregs control factors related to MDSC generation in tumor-bearing mice.
5-fluorouracil does not treat B16 melanoma effectively
Finally, we tested an agent contemplated for MDSC depletion in humans, 5-fluorouracil. In striking contrast to a published report (35), 5-fluorouracil did not deplete MDSC in B16 melanoma and worsened clinical tumor response (Fig. 6H) with no effect on non-Treg T cells, B cells or non-MDSC myeloid cell subsets (not shown), and no alteration of MDSC or Treg function in vitro (Supplementary Fig. 9).
Discussion
Although increasing age is the biggest risk factor for cancer, surprisingly few studies have addressed the specific consequences of age on anti-tumor immunity, and fewer still have examined age effects on tumor immunotherapy. Age-dependent immune alterations including the generally decreased performance of effector T cells could reduce anti-tumor immunity. Naive T cells from older individuals show functional defects such as impaired ability to proliferate, produce relevant cytokines, and reduced differentiation into effector T cells (12–13). Thus, many immunotherapies effective in young hosts are less so in aged hosts. For example, tumor rejection mediated through OX40 signals decreases with age, as does effector T cell differentiation (36). However, effective cancer immunotherapy for aged individuals is a realistic goal, as some age-associated immune defects are reversible. As examples, tumor immunity in aged mice can be rescued with sufficient co-signaling (37), and reduced T cell priming boosted by the immune co-signaling CD137 (41BB) pathway (38).
A complementary approach to boosting effector arms of immunity in cancer immunotherapy is to reduce tumor-associated immune dysfunction (5, 6). As we and others have previously demonstrated the utility of depleting Tregs as a means to reverse tumor-associated immune dysfunction to treat cancer in (young) mouse models (6), we tested the concept in aged hosts.
In our B16 melanoma model, Treg depletion with DT was immunologically and clinically effective in young mice as expected. Disappointingly, though, depleting Tregs with DT in aged hosts did not improve anti-tumor immunity or immune-mediated tumor rejection. For humane reasons, we studied tumor growth, not survival, but tumor growth and weights are good surrogates for survival in B16 melanoma. We showed that Treg numbers and in vitro function of young and aged Tregs were comparable, and that DT equally depleted young and aged Tregs. Thus, poor clinical efficacy of Treg depletion in aged hosts could be attributed to a defect in aged anti-tumor effector cells, other immunosuppressive mechanisms, or to tumor-specific factors (21). To assess for additional regulatory populations, we found an age-associated increase in CD25loFoxp3+ Tregs as reported, and showed that they were more suppressive in the tumor environment than in naïve mice. It is likely that Tregs contribute differentially to immunopathology in a tumor-dependent fashion as seen in both young and aged hosts (6, 20, 21), but effects of distinct Treg subsets remain to be defined. We further found an age-associated increase in MDSC that were more suppressive in vitro compared to young counterparts as reported (22). Surprisingly, DT-mediated Treg depletion increased MDSC numbers in aged but not young hosts with B16 melanoma, suggesting a mechanism for DT treatment failure, and suggesting that MDSC depletion combined with Treg depletion could be useful cancer immunotherapy in aged hosts in this model.
In support, anti-Gr-1 antibody depleted MDSC effectively in young and aged hosts, but improved anti-tumor immunity and was clinically effective only in aged hosts with B16. Consistent with increased MDSC reducing DT efficacy is aged hosts, addition of anti-Gr-1 antibody improved Treg depletion efficacy in aged, but not young hosts in this model. These data confirm that aged effector cells remain competent and can mediate important effector functions provided that the impeding immune dysfunction is reduced. As anti-Gr-1 antibody is not specific for MDSC, dysfunctional contributions from other Gr-1+ cells, such as plasmacytoid dendritic cells, are not entirely excluded and require further investigation.
As DT only incompletely depletes Tregs, and targets IL-2 receptor-expressing cells, not Tregs specifically, we used Foxp3DTR mice in which specific, high-order Treg depletion is possible. High-order Treg depletion in young and aged naïve or B16-bearing hosts equally boosted MDSC. These data confirm that Treg constrain tumor MDSC, but demonstrate that DT-mediated Treg depletion differs from high-order Treg depletion in MDSC effects, which could be due to better Treg depletion in Foxp3DTR mice, lack of depletion of a specific Treg subset by DT, DT effects on another IL-2 receptor expressing cell, or reduced Treg function from the greater inflammation in Foxp3DTR versus DT-treated mice. Understanding DT-mediated effects on MDSC restraint helps define mechanisms of treatment responses, important to clinical translation, as DT is FDA-approved for some cancers, and depletes Tregs in humans (6). Understanding mechanisms of MDSC restraint by Tregs in cancer furthers basic knowledge of cancer immunopathology. Both approaches are important to developing optimal, translatable cancer immunotherapies.
MDSC are myeloid cells generated through cooperation of numerous factors (32). We found that DT and high-order Treg depletion had similar effects on in vitro myelopoiesis, including skewing to potentially more immunosuppressive Ly-6C+ MDSC, and increasing in vivo T cell GM-CSF production but without clear effects on global GM-CSF, VEGF, M-CSF or IL-6. Thus, Tregs and DT each can affect myelopoiesis, which might affect immunotherapy outcomes. DT effects on MDSC deserve additional attention to help translate concepts, as selective high-order Treg depletion in humans is currently unachievable, and could pose autoimmune risks in any regard.
High-order Treg depletion alone also improved immune and clinical responses to B16 in aged hosts, further demonstrating the underlying immune competence of aged anti-tumor effector cells, despite the difficulty in translating this approach clinically. In contrast to DT effects, though, high-order Treg depletion was clinically and immunologically efficacious despite increases in MDSC. This result could be due to Treg-specific effects in Foxp3DTR mice, to alterations in the inflammatory environment from high-order Treg depletion, or differential MDSC functions after DT versus high-order Treg depletion, among other factors. If clinical effects are specifically from high-order Treg depletion, as opposed to from effects on some IL-2-expressing cell population, that suggests that IL-2 receptor targeting agents for human Treg depletion such as denileukin diftitox or daclizumab might be ultimately inferior to agents that specifically target human Tregs (which do not yet exist). Understanding this point could greatly improve Treg management strategies.
Strikingly, in another tumor, MC-38 colon carcinoma, MDSC depletion was effective in young hosts and further boosted utility of DT-mediated Treg depletion. By contrast, and distinct from B16 results, DT alone was useful in aged hosts but not further augmented by MDSC depletion. Reminiscent of Foxp3DTR results, DT improved immunity in young and aged hosts, despite increasing MDSC in both. Increased anti-tumor immunity correlated with clinical outcomes supporting the concept that immune dysfunction was improved with the distinct approaches. These data suggest that immune dysfunction is not only age-dependent but also tumor-dependent, affecting both Tregs and MDSC. Our preliminary ex vivo studies did not disclose a noticeable difference in myelopoiesis in bone marrow of mice bearing MC-38 or B16, or in cytokines affecting MDSC generation following Treg depletion in each tumor. Additional work is required to understand specifics of MDSC generation and immunopathogenesis in individual tumors as a function of age and as a consequence of distinct treatment modalities.
Because MDSC depletion alone and in combination with Treg depletion was effective in distinct tumors and hosts, we assessed a translational approach. 5-fluorouracil, is FDA-approved and thus relatively easily tested as human cancer immunotherapy. Our surprising finding that 5-fluorouracil does not deplete MDSC in B16 and worsens the clinical course suggests that MDSC depletion agents will also have variable effects depending on the tumor and host. Thus, unlike DT, which depletes Tregs in humans analogous to animal models, MDSC depletion studies in pre-clinical models might not predict human clinical effects as well.
Together, our data demonstrate that the function of aged anti-tumor effector T cells can be improved by reducing age-related immune dysfunction, with positive clinical benefits. Strategies to reduce immune dysfunction must be tailored to account for age-related and tumor-related immune dysfunctions for optimal utility. Thus, improved and efficacious cancer immune therapy for aged hosts, who are at the greatest risk for cancer, is a realistic goal that can be met with a better understanding of the specific effects of age on basal and tumor-related immune dysfunction. Strategies we propose are clinically translatable as FDA-approved agents such as denileukin diftitox, cyclophosphamide and daclizumab deplete Tregs (6), and FDA-approved agents such as 5-fluorouracil kill MDSC in some models (35).
Supplementary Material
Acknowledgements
Thanks to Kristina M. Church, Xiuhua Sun, and Pei Yi Lin, for technical help.
Grant Support
Studies were funded by the Voelcker Foundation, The Holly Beach Public Library Association, 1RC2 AG036613, 5P30CA54174, Texas STARS and the Owens Foundation.
Footnotes
Conflict of interest: The authors have declared that no conflict of interest exists.
Literature cited
- 1.Feng Z, Hu W, Rajagopal G, Levine AJ. The tumor suppressor p53: cancer and aging. Cell Cycle. 2008;7:842–847. doi: 10.4161/cc.7.7.5657. [DOI] [PubMed] [Google Scholar]
- 2.Fulop T, Larbi A, Kotb R, de Angelis F, Pawelec G. Aging, immunity, and cancer. Discov Med. 2011;11:537–550. [PubMed] [Google Scholar]
- 3.Vallejo AN. Immunological hurdles of ageing: indispensable research of the human model. Ageing Res Rev. 2011;10:315–318. doi: 10.1016/j.arr.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Haynes L, Eaton SM, Burns EM, Rincon M, Swain SL. Inflammatory cytokines overcome age-related defects in CD4 T cell responses in vivo. J Immunol. 2004;172:5194–5199. doi: 10.4049/jimmunol.172.9.5194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Curiel TJ. Tregs and rethinking cancer immunotherapy. J Clin Invest. 2007;117:1167–1174. doi: 10.1172/JCI31202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Curiel TJ. Regulatory T cells and treatment of cancer. Curr Opin Immunol. 2008;20:241–246. doi: 10.1016/j.coi.2008.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P, et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10:942–949. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- 8.Zou W. Regulatory T cells, tumour immunity and immunotherapy. Nat Rev Immunol. 2006;6:295–307. doi: 10.1038/nri1806. [DOI] [PubMed] [Google Scholar]
- 9.Zhang H, Podojil JR, Luo X, Miller SD. Intrinsic and induced regulation of the age-associated onset of spontaneous experimental autoimmune encephalomyelitis. J Immunol. 2008;181:4638–4647. doi: 10.4049/jimmunol.181.7.4638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rosenkranz D, Weyer S, Tolosa E, Gaenslen A, Berg D, Leyhe T, et al. Higher frequency of regulatory T cells in the elderly and increased suppressive activity in neurodegeneration. J Neuroimmunol. 2007;188:117–127. doi: 10.1016/j.jneuroim.2007.05.011. [DOI] [PubMed] [Google Scholar]
- 11.Zhao L, Sun L, Wang H, Ma H, Liu G, Zhao Y. Changes of CD4+CD25+Foxp3+ regulatory T cells in aged Balb/c mice. J Leukoc Biol. 2007;81:1386–1394. doi: 10.1189/jlb.0506364. [DOI] [PubMed] [Google Scholar]
- 12.Kryczek I, Liu R, Wang G, Wu K, Shu X, Szeliga W, et al. FOXP3 defines regulatory T cells in human tumor and autoimmune disease. Cancer Research. 2009;69:3995–4000. doi: 10.1158/0008-5472.CAN-08-3804. [DOI] [PubMed] [Google Scholar]
- 13.Kozlowska E, Biernacka M, Ciechomska M, Drela N. Age-related changes in the occurrence and characteristics of thymic CD4(+) CD25(+) T cells in mice. Immunology. 2007;122:445–453. doi: 10.1111/j.1365-2567.2007.02667.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thomas DC, Mellanby RJ, Phillips JM, Cooke A. An early age-related increase in the frequency of CD4+ Foxp3+ cells in BDC2.5NOD mice. Immunology. 2007;121:565–576. doi: 10.1111/j.1365-2567.2007.02604.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ostrand-Rosenberg S, Sinha P. Myeloid-derived suppressor cells: linking inflammation and cancer. J Immunol. 2009;182:4499–4506. doi: 10.4049/jimmunol.0802740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li H, Han Y, Guo Q, Zhang M, Cao X. Cancer-expanded myeloid-derived suppressor cells induce anergy of NK cells through membrane-bound TGF-beta 1. J Immunol. 2009;182:240–249. doi: 10.4049/jimmunol.182.1.240. [DOI] [PubMed] [Google Scholar]
- 17.Youn JI, Nagaraj S, Collazo M, Gabrilovich DI. Subsets of myeloid-derived suppressor cells in tumor-bearing mice. J Immunol. 2008;181:5791–5802. doi: 10.4049/jimmunol.181.8.5791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marigo I, Dolcetti L, Serafini P, Zanovello P, Bronte V. Tumor-induced tolerance and immune suppression by myeloid derived suppressor cells. Immunol Rev. 2008;222:162–179. doi: 10.1111/j.1600-065X.2008.00602.x. [DOI] [PubMed] [Google Scholar]
- 19.Huang B, Pan PY, Li Q, Sato AI, Levy DE, Bromberg J, et al. Gr-1+CD115+ immature myeloid suppressor cells mediate the development of tumor-induced T regulatory cells and T-cell anergy in tumor-bearing host. Cancer Res. 2006;66:1123–1131. doi: 10.1158/0008-5472.CAN-05-1299. [DOI] [PubMed] [Google Scholar]
- 20.Dominguez AL, Lustgarten J. Implications of aging and self-tolerance on the generation of immune and antitumor immune responses. Cancer Res. 2008;68:5423–5431. doi: 10.1158/0008-5472.CAN-07-6436. [DOI] [PubMed] [Google Scholar]
- 21.Sharma S, Dominguez AL, Lustgarten J. High accumulation of T regulatory cells prevents the activation of immune responses in aged animals. J Immunol. 2006;177:8348–8355. doi: 10.4049/jimmunol.177.12.8348. [DOI] [PubMed] [Google Scholar]
- 22.Grizzle WE, Xu X, Zhang S, Stockard CR, Liu C, Yu S, et al. Age-related increase of tumor susceptibility is associated with myeloid-derived suppressor cell mediated suppression of T cell cytotoxicity in recombinant inbred BXD12 mice. Mech Ageing Dev. 2007;128:672–680. doi: 10.1016/j.mad.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 23.Enioutina EY, Bareyan D, Daynes RA. A role for immature myeloid cells in immune senescence. J Immunol. 2011;186:697–707. doi: 10.4049/jimmunol.1002987. [DOI] [PubMed] [Google Scholar]
- 24.Wan YY, Flavell RA. Regulatory T-cell functions are subverted and converted owing to attenuated Foxp3 expression. Nature. 2007;445:766–770. doi: 10.1038/nature05479. [DOI] [PubMed] [Google Scholar]
- 25.Kim JM, Rasmussen JP, Rudensky AY. Regulatory T cells prevent catastrophic autoimmunity throughout the lifespan of mice. Nat Immunol. 2007;8:191–197. doi: 10.1038/ni1428. [DOI] [PubMed] [Google Scholar]
- 26.Lin PY, Sun L, Thibodeaux SR, Ludwig SM, Vadlamudi RK, Hurez VJ, et al. B7-H1-Dependent Sex-Related Differences in Tumor Immunity and Immunotherapy Responses. J Immunol. 2010;185:2747–2753. doi: 10.4049/jimmunol.1000496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tomihara K, Shin T, Hurez VJ, Yagita H, Pardoll DM, Zhang B, et al. Aging-associated B7-DC(+) B cells enhance anti-tumor immunity via Th1 and Th17 induction. Aging Cell. 2012;11:128–138. doi: 10.1111/j.1474-9726.2011.00764.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Curiel TJ, Wei S, Dong H, Alvarez X, Cheng P, Mottram P, et al. Blockade of B7-H1 improves myeloid dendritic cell-mediated antitumor immunity. Nat Med. 2003;9:562–567. doi: 10.1038/nm863. [DOI] [PubMed] [Google Scholar]
- 29.Litzinger MT, Fernando R, Curiel TJ, Grosenbach DW, Schlom J, Palena C. IL-2 immunotoxin denileukin diftitox reduces regulatory T cells and enhances vaccine-mediated T-cell immunity. Blood. 2007;110:3192–3201. doi: 10.1182/blood-2007-06-094615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ouyang X, Yang Z, Zhang R, Arnaboldi P, Lu G, Li Q, et al. Potentiation of Th17 cytokines in aging process contributes to the development of colitis. Cell Immunol. 2011;266:208–217. doi: 10.1016/j.cellimm.2010.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nishioka T, Shimizu J, Iida R, Yamazaki S, Sakaguchi S. CD4+CD25+Foxp3+ T cells and CD4+CD25-Foxp3+ T cells in aged mice. J Immunol. 2006;176:6586–6593. doi: 10.4049/jimmunol.176.11.6586. [DOI] [PubMed] [Google Scholar]
- 32.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9:162–174. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lustgarten J. Cancer, aging and immunotherapy: lessons learned from animal models. Cancer Immunol Immunother. 2009;58:1979–1989. doi: 10.1007/s00262-009-0677-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim J, Lahl K, Hori S, Loddenkemper C, Chaudhry A, deRoos P, et al. Cutting edge: depletion of Foxp3+ cells leads to induction of autoimmunity by specific ablation of regulatory T cells in genetically targeted mice. J Immunol. 2009;183:7631–7634. doi: 10.4049/jimmunol.0804308. [DOI] [PubMed] [Google Scholar]
- 35.Vincent J, Mignot G, Chalmin F, Ladoire S, Bruchard M, Chevriaux A, et al. 5-Fluorouracil selectively kills tumor-associated myeloid-derived suppressor cells resulting in enhanced T cell-dependent antitumor immunity. Cancer Res. 2010;70:3052–3061. doi: 10.1158/0008-5472.CAN-09-3690. [DOI] [PubMed] [Google Scholar]
- 36.Ruby CE, Weinberg AD. OX40-enhanced tumor rejection and effector T cell differentiation decreases with age. J Immunol. 2009;182:1481–1489. doi: 10.4049/jimmunol.182.3.1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lustgarten J, Dominguez AL, Thoman M. Aged mice develop protective antitumor immune responses with appropriate costimulation. J Immunol. 2004;173:4510–4515. doi: 10.4049/jimmunol.173.7.4510. [DOI] [PubMed] [Google Scholar]
- 38.Bansal-Pakala P, Croft M. Defective T cell priming associated with aging can be rescued by signaling through 4-1BB (CD137) J Immunol. 2002;169:5005–5009. doi: 10.4049/jimmunol.169.9.5005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.