Abstract
The T-box transcription factor Tbx3 plays multiple roles in normal development and disease. In order to function in different tissues and on different target genes, Tbx3 binds transcription factors or other cofactors specific to temporal or spatial locations. Examining the development of the mammary gland, limbs, and heart as well as the biology of stem cells and cancer provides insights into the diverse and common functions that Tbx3 can perform. By either repressing or activating transcription of target genes in a context-dependent manner, Tbx3 is able to modulate differentiation of immature progenitor cells, control the rate of cell proliferation, and mediate cellular signaling pathways. Because the direct regulators of these cellular processes are highly context-dependent, it is essential that Tbx3 has the flexibility to regulate transcription of a large group of targets, but only become active on a small cohort of them at any given time or place. Moreover, Tbx3 must be responsive to the variety of different upstream factors that are present in different tissues. Only by understanding the network of genes, proteins, and molecules with which Tbx3 interacts can we hope to understand the role that Tbx3 plays in normal development and how its aberrant expression can lead to disease. Because of its myriad functions in disparate developmental and disease contexts, Tbx3 is an ideal candidate for a systems-based approach to genetic function and interaction.
The T-box family of genes is an ancient and evolutionarily conserved group of transcription factor genes defined by their DNA-binding domain, known as the T-box. First discovered in mouse, the T-box family derives its name from the mesoderm-specification gene Brachyury (T) (1). Each T-box factor binds a specific core sequence, the T-half-site, found in the promoters of target genes, often in tandem or in different orientations. These T-half sites are accompanied by other transcription factor binding sites, giving them specificity (2). It is the interactions with these other transcription factors that allow T-box genes to play a variety of roles during disparate development processes (3).
The 17 members of the T-box gene family in mouse have been grouped into 5 subfamilies based on sequence similarity. Tbx3 is member of the Tbx2 subfamily, a group that also includes Tbx2, Tbx4, and Tbx5. This subfamily arose during a tandem duplication event followed by chromosomal duplication and dispersion. Tbx3 and Tbx2 are closely related members sharing 90% amino acid identity in the T-box and having many overlapping areas of expression (4). During normal mouse development, Tbx3 expression begins in the inner cell mass of the blastocyst, and then appears in the extraembryonic mesoderm during gastrulation. During organogenesis, Tbx3 is expressed in the nervous system, skeleton, eye, heart, kidney, lungs, pancreas, and mammary gland (5). There are two known isoforms of Tbx3 that result from differential splicing in the second intron, Tbx3 and Tbx3+2a, which includes 20 extra amino acids in the DNA binding domain of the protein (6). While both have been detected, there is no known unique role for one or the other specific isoform in development. A null allele of Tbx3 has been generated and homozygous mutant mice have defects in a number of structures such as the limbs, mammary glands, and heart. These mutants die by embryonic day (E) 16.5 with greater than 50% dead by E11.5, most likely due to yolk sac defects. A number of different organ-specific effector genes and transcription factors are aberrantly expressed in these mutants (7).
In humans, TBX3 mutations have been linked to ulnar-mammary syndrome (UMS, MIM 181450), a disease with variable penetrance characterized by shortened forelimbs, defective apocrine gland and genital development, and heart abnormalities (8-9). This phenotype is similar to that seen in Tbx3 mutant mice, although in humans the phenotype is seen in heterozygotes whereas in mice, only homozygotes have severe defects. The spectrum of affected organs in UMS is characteristic of diseases associated with T-box factors and is indicative of the complex transcriptional networks in which these genes participate during development (10). Tbx3 mutations have also recently been found to impact the pluripotency of embryonic stem cells and the invasiveness of cancer (11-15).
For Tbx3 to play a part in the development of so many different organs, it must interact with a network of genes and proteins specific to each spatial and temporal location of action. While Tbx3 most likely binds to its target promoters as a monomer, other factors are known to enhance Tbx3-mediated transcriptional activation or repression, hinting at a large network of factors that give specificity to Tbx3 activity (16-17). In addition, Tbx3 has been shown to have both activation and repression domains which may be modulated by other cofactors to ensure the proper function of the protein in each context (18). Only by understanding the function of Tbx3 by a systems approach in a variety of developmental contexts can we hope to unravel the network of genes of which Tbx3 is a part.
TBX3 IN DEVELOPMENT
Tbx3 in mammary gland development
The initiation and growth of the mammary gland is dependent on fibroblast growth factor (FGF) and WNT signaling and involves reciprocal interactions between the epidermis and the underlying mesenchyme in bilateral ‘milk lines’. Mesenchyme induces the formation of mammary placodes in five specialized areas along each flank of the embryo. The epidermal placode forms a mammary bud which in turn influences the surrounding mesenchyme to form the primary mammary mesenchyme. Tbx3 is initially expressed in the mesenchyme of the milk line prior to placode formation and then appears in the mammary placodes as one of the earliest markers of mammary epithelium. Expression in the mesenchyme gradually decreases while epithelial expression is maintained (5, 7, 19-20). During late gestation, Tbx3 is expressed in mammary mesenchyme surrounding the nipple (Fig. 1A) and in postnatal females it has been detected in virgin, pregnant, lactating and involuting mammary glands (21).
Figure 1.
Expression of Tbx3 (blue) in developing organ systems at different stages. (A) In mammary gland, Tbx3 is first expressed at E10.5 in the mesenchymal milk line and then appears as one of the earliest markers of the epithelial thickenings known as the mammary placodes. It continues to be expressed in the epithelium as the placode expands into the mammary bud and eventually forms the branching ductal system. Near term (E18.5), mesenchyme surrounding the nipple expresses Tbx3. (B) Tbx3 is first expressed in the posterior margin of the early limb buds and then in the posterior and anterior margins of both fore- and hindlimbs by E10.5. It is also expressed in the AER, continuously at first and then limited to the tips of the digits by E12.5. (C) Tbx3 is expressed in the AVC, SAN, OFT and atrioventricular bundle (AVB) starting around E10.5. It fully delineates the cardiac conduction system at E14.5 with expression in the SAN, AVN, AVB, and the bundle branches (BB).
UMS in humans is characterized by variable abnormalities of the mammary gland ranging from normal to hypoplastic breasts, with missing or supernumerary nipples. A loss of function mutation of mouse Tbx3 results in the failure of mammary placode induction in homozygotes and aplasia or a decrease in the extent of branching of the ductal tree in heterozygous females. This effect on the developing mammary gland is independent of the repression of the Tbx3 target gene p19ARF (19). Although there is no evidence regarding the direct regulation of Tbx3 in mammary gland development or on its direct downstream targets, both WNT and FGF signaling feed into the Tbx3 regulatory network. Fgfr2b and Fgfr1/2c are upstream of Tbx3 expression, and Wnt10b, Lef1, and FGF signaling are all lost in the absence of Tbx3 (7, 20), indicating feed-forward and feed-back loops of regulation for the maintenance and/or induction of Tbx3 expression (Fig. 2). Similarly, Bmp4 overexpression inhibits Tbx3 expression in the mammary mesenchyme while, reciprocally, overexpression of Tbx3 represses Bmp4 (22). Tbx3, in combination with FGF signaling, may be upstream of Nrg3, a growth factor implicated in the initiation of mammary placodes, but the evidence is circumstantial (23-24).
Figure 2.
Diagram of known regulatory pathways and downstream targets of Tbx3 in the development of heart, mammary gland and limbs, as well as in embryonic and iPS stem cells. The variety of factors involved illustrates the context-dependent nature of Tbx3 interactions.
The closely related T-box gene, Tbx2, is expressed in the mesenchyme but not the epithelium during mammary development and although mutation of Tbx2 by itself does not result in a mammary gland phenotype, a genetic interaction with Tbx3 is evident in double heterozygotes by an exacerbation of mammary aplasia (19).
Tbx3 in limb development
In vertebrates, limbs develop as a set of lateral bulges from the lateral plate mesoderm on either side of body axis. The initial events in limb development involve proliferation of the lateral plate mesoderm and induction of the apical ectodermal ridge (AER)(25). Three signaling centers, the AER, the zone of polarizing activity (ZPA) and the nonridge ectoderm, are necessary for growth and patterning of limb buds, processes which involve complex signaling through the FGF and Sonic hedgehog (SHH) pathways (26). All four members of the Tbx2 subfamily are expressed during limb development. In mice, Tbx3 expression is first detected at the posterior margin of the early limb buds, and shortly thereafter in the anterior and posterior proximal mesenchyme and AER. As the limb bud elongates, Tbx3 anterior and posterior expression domains are expanded in the mesenchyme. By E13.5, AER expression is limited to the tips of the digits (5, 27)(Fig. 1B). A similar pattern is observed in the chick (28-31).
In UMS, posterior structures of the fore limb, e.g. the ulna and the fifth digit are missing (8). Mice homozygous for the Tbx3 null allele similarly exhibit missing or abnormal posterior fore limb elements, but unlike UMS also show severe hind limb abnormalities (7).
Little is known about the direct regulation of Tbx3 in limb development. Studies in the chick indicate that Tbx3 expression in the posterior of the limb buds is controlled via different mechanisms than the anterior. The posterior domain of Tbx3 expression depends on the ZPA signaling cascade and is regulated positively by Shh, but the anterior expression domain is negatively regulated by Shh and is dependent on continuous signaling by anteriorly produced BMPs, suggesting a potential role for Tbx3 in the antero-posterior patterning of the limb (31). A recent study places retinoic acid (RA) signaling upstream of Tbx3 in the limbs (32). In mice, Shh and Hand2 appear to be downstream targets of Tbx3 (7). Studies in chick have implicated Tbx3 in positioning the limb along the main body axis through a genetic interplay between Hand2 and Gli3, but the interrelationship of these genes is not clear (33). Inactivation of Dicer in mice results in a posterior shift and a delayed formation of hind limb bud which is accompanied by altered transcription of Tbx3, Hand2 and Gli3. This study showed that microRNA is also capable of inhibiting Tbx3 and Hand2 expression in vitro. Hence, Tbx3 and Hand2 might be downstream of Dicer-mediated regulation in limb bud positioning (34) (Fig. 2).
Tbx2 has a similar spatiotemporal expression pattern in limb buds in both chick and mice (27, 29-31) and is downregulated in Tbx3 mutants (7). Experiments in the chick have shown that Tbx3 and Tbx2 together specify the identity of posterior digits, acting through regulation of interdigital BMP signaling (35), possibly indicating a genetic interaction.
Tbx3 in heart development
The transformation from linear heart tube to the four-chambered heart is accomplished by the differential cell growth and distinct gene programs adopted by different regions in the heart. Starting at E9.5, the working myocardium cells undergo rapid and sustained proliferation to form the muscular chambers of the heart. The intervening regions of non-chamber myocardium, meanwhile, are held relatively mitotically inactive to form the constrictions between the chambers that will eventually become components of the cardiac conduction system (CCS).
Tbx3 expression is first detected in the heart at E8.5 and as the heart undergoes looping Tbx3 expression delineates the developing nodal conduction system with expression in the sinoatrial node (SAN) and atrioventricular node (AVN), as well as the endocardial cushions in the atrioventricular canal (AVC) and the mesenchyme of the outflow tract (OFT)(Fig. 1C). This expression pattern is almost identical to that of Tbx2 although no genetic interaction has been demonstrated in this tissue. Tbx3 is thought to have two distinct roles in the developing CCS: first, the restriction of cell division resulting in the constrictions between chambers, and secondly, the repression of a chamber-specific gene program and concomitant promotion of a conduction system-specific gene program. Despite the assumption that Tbx3 mutant embryos die at midgestation due to yolk sac deficiencies, their hearts have altered morphology including double outlet right ventricle, incomplete ventricular septation, and delayed aortic arch formation (36) due to increased cell division in the AVC and OFT leading to a lack of constriction (37). Mutant hearts also have ectopic expression of chamber myocardium genes, such as Cx40, Cx43, and Nppa, in the non-chamber AVC, a phenotype resembling that of Tbx2 mutants. Conversely, CCS-specific genes Hcn4 and Lbh are upregulated in regions where Tbx3 ectopic expression is induced, and functional conduction tissue develops (38) (Fig.2).
On a protein level, it appears that Tbx3 regulates its targets by cooperatively binding their promoters along with other transcription factors. For example, Tbx3 has been shown to bind cooperatively with Msx1 and Msx2 in the repression of Cx43 (16). Similarly, Tbx2 has been shown to bind to Nkx2.5 and repress Nppa, a known Tbx3 target, but in the absence of Tbx2, Tbx5 binds to Nkx2.5 and activates Nppa (39)(Fig. 2). This suggests a regulatory mechanism whereby binding competition with a network of transcription factors determines which gene program will be expressed in a given tissue.
Tbx3 mutant heart abnormalities result from increased cell division in the regions of Tbx3 expression implicating Tbx3 in the regulation of cell dynamics in the process of heart looping and growth. Conversely, despite its role in the regulation of the gene expression profile of the CCS, Tbx3 mutant hearts have normal conduction velocity and several of the conductive structures are present. This discrepancy is likely due to the functional overlap of Tbx3 with Tbx2, which has been shown to bind to and regulate many of the same targets. Nonetheless, some patients with UMS show conduction defects in line with abnormal development of conduction structures (9). These defects are similar to those in mice mutant for Tbx2, highlighting the potential functional overlap with Tbx3 in the development of the CCS (40).
TBX3 IN STEM CELL BIOLOGY
In addition to its key roles in development, Tbx3 also plays a role in both the establishment and maintenance of pluripotency in embryonic stem (ES) cells and induced pluripotent stem (iPS) cells. ES cells are derived from the inner cell mass (ICM) of preimplantation blastocysts and rely on the LIF/STAT3 pathway to maintain pluripotency. In the embryo, Tbx3 is first expressed in the ICM (7) and this expression is recapitulated in ES cells. Tbx3 expression is highest when ES cells are undifferentiated and decreases as cells differentiate into embryoid bodies, suggesting its importance in the maintenance of pluripotency (41).
In ES cells, Oct4 and Nanog, two recognized markers of pluripotency, act as repressors of differentiation towards a trophectoderm and endodermal fate, respectively. Similarly, Tbx3 is able to block differentiation into mesoderm, ectoderm, trophectoderm, and neural crest cell fates (41-42). ES cells treated with shRNA against Tbx3 downregulate both Oct4 and Nanog, and show differentiated morphology and reduced alkaline phosphatase activity. To function as a mediator of pluripotency, Tbx3 is able to act with Klf4 to regulate the expression of Nanog specifically, lying at the center of a LIF-independent pluripotency pathway in ES cells (43). In addition to blocking differentiation, Tbx3 also appears to play a role in the differentiation of ES cells into extraembryonic endoderm (ExEn) as overexpression of Tbx3 in ES cells induces differentiation into cells with ExEn morphology as well as expression of ExEn markers such as Gata6 (41). This dual functionality suggests that Tbx3 takes part in a complex regulatory network where it is able to function both as a repressor of specific cell fates and an activator of others. In this way, ES cells are poised to differentiate into a given cell type quickly when the proper signals are received: the relief of one repression module allows the activation of another. The complexity of Tbx3 in the pluripotency network is evident as the promoter of Tbx3 itself is bound by a number transcription factors at the core of the genetic regulation circuit of pluripotency (44) (Fig. 2). Mechanistically, Tbx3 is able to regulate transcription at the level of DNA, but also on an epigenetic level: Tbx3 binding to the Gata6 promoter is necessary to activate transcription but Tbx3 is also able to mediate the histone methylation of H3K27me3 at the Gata6 promoter (41).
In addition to the maintenance of pluripotency, Tbx3 may also play a role in the establishment of pluripotency in iPS cells. Fibroblasts with induced expression of Tbx3 in combination with the reprogramming factors Sox2, Oct4, and Klf4 express pluripotency markers more rapidly than fibroblasts without. Moreover, iPS cells with induced Tbx3 expression contributed to enhanced germ line contribution and transmission (45).
TBX3 IN CANCER
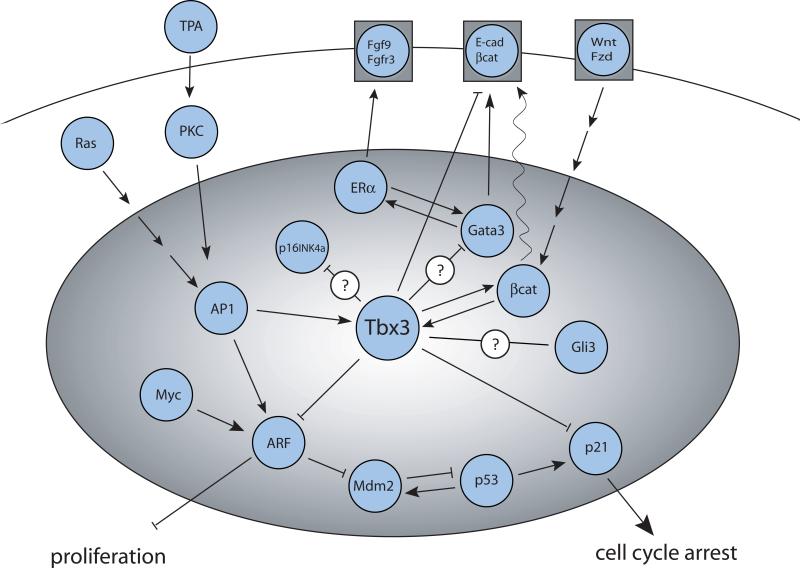
Tbx3 is amplified and/or overexpressed in many tumors (46-59) (Table 1). Accumulating evidence suggests that Tbx3 contributes to tumorigenesis through interaction with components of several major oncogenic pathways (Fig. 3), some with which Tbx3 is known to interact in other contexts. Activation of the canonical Wnt-β-catenin pathway has been linked to many types of cancer. Beta catenin plays dual roles depending on intracellular localization: in the nucleus it acts as the main effector of WNT signaling and at the plasma membrane as a component of adherens junctions where it links E-cadherin with the actin cytoskeleton (60). Tbx3 is a downstream target of the Wnt-β-catenin pathway in liver tumorigenesis, and recent evidence suggests that there is a feedback loop by which Tbx3 can upregulate β-catenin (50). Thus, Tbx3 could be a critical mediator of cellular responses to proliferative and anti-apoptotic signals delivered by β-catenin. Interestingly, Tbx3 represses E-cadherin (51), which has been implicated in metastasis of invasive epithelial tumors (61). Together these findings suggest that Tbx3 can enhance tumor invasiveness through both E-cadherin repression and β-catenin upregulation. Additionally, phorbol ester 12-O-tetradecanoylphorbol-13-acetate (TPA) treatment leads to downregulation of E-cadherin, and as TPA activates TBX3 in a PKC-dependent manner (62), it is possible that upregulation of TBX3 is mediating this process.
Table I.
Incidence of TBX3 expression in human cancers and corresponding normal tissue in the mouse. Cancers listed are those in which TBX3 has been shown to be amplified and/or overexpressed.
| Cancer | No. (%) of specimens with expression TBX3 | Method of detection | Corresponding normal expression of Tbx3 | References |
|---|---|---|---|---|
| Breast | 48/50 (96) | WB and real time PCR | Mammary epithelium and mesenchyme of developing gland | (46, 48, 55) |
| Melanoma1 | 7/12 (58) | WB | Melanocytes* | (51) |
| Pancreatic | 7 | Microarray | Developing pancreas | (57, 59, 72) |
| Cervical | 48 | Microarray | Unknown | (49) |
| Ovarian | 21/29 (70) | MALDI-Tof-MS | Not detected (unpublished) | (48) |
| Prostate | ND | GWAS | Adult prostate | (46-47, 53) |
| Colorectal | 1 | RT-PCR | Adult colon | (46, 56) |
| Liver | (70-87) | Microarray and WB | Hepatoblasts | (50, 73) |
| Gastric | 1 | Microarray | Developing stomach | (54, 72) |
| Glioblastoma | ND | Microarray | Developing CNS | (58) |
| Pheochromocytoma | ND | Microarray | Adult adrenal gland | (46, 52) |
melanoma cell lines in vitro
Also present in human melanocyte cell lines
GWAS, genome-wide association study; MALDI-Tof-MS, matrix-assisted laser desorption/ionization time of flight mass spectrometry; RT-PCR, reverse transcriptase–polymerase chain reaction; WB, western blot; ND, not determined
Figure 3.
The Tbx3 interactome in cancer. Known and hypothetical molecular interactions between Tbx3 and components of several signaling pathways important in oncogenesis are drawn from a variety of contexts.
As in normal mammary gland development, FGF signaling is upstream of Tbx3 expression in breast cancer (63). Moreover, estrogen can upregulate TBX3 levels in breast cancer via paracrine FGF9-FGFR3 signaling and the upregulation of TBX3 expands the pool of functional estrogen receptor (ER) negative cancer stem-like cells. This implies that resistance to anti-estrogen therapy which is common in breast cancer might be accompanied by an increase in FGF-TBX3 signaling and a consequent increase in the proportion of cancer stem-like cells. Thus, targeting of FGF-TBX3 pathway could be a useful strategy for refractory breast cancers. Moreover, TBX3 can affect the equilibrium of cell type differentiation within breast epithelial cancers, which is context dependent for a given cancer cell population (64). Together these studies suggest that TBX3 could play important roles in cell plasticity within breast cancer.
Upregulation of Tbx3 suppresses the expression of ARF (p19ARF in mouse and p14ARF in humans) and possibly p16INK4a, and promotes the bypass of senescence through inactivation of p53 via ARF-MDM2-p53 tumor suppressor pathway (15, 23, 46, 65-66). Tbx3 can also directly repress the p21Cip1/WAF1 promoter (6) and bypass senescence independently of p53. The knockdown of TBX3 in both melanoma and breast cancer cell lines leads to reduction in anchorage-independent growth, migration and tumor formation, and a decrease in pro-senescence factors that results in increased proliferation (14). It was previously suggested that Tbx3 and its splice variant Tbx3 + 2a, are functionally distinct in inhibition of senescence (46). However, a subsequent study convincingly demonstrated that both isoforms function as anti-senescence factors, bind the same T-half-site and target the same genes (6). Also, Tbx3 can promote Ras and c-Myc associated transformation (15, 67). These findings together imply that Tbx3 cooperates with oncogenic Ras and c-Myc by suppressing ARF activity. A recent study identified GATA3 and GLI3 as putative TBX3 downstream targets in breast cancer (68). Although chromatin immunoprecipitation analysis confirmed direct binding of TBX3 to both of these targets, the functional significance of these findings is not known. Interestingly, GATA3 was shown to inhibit breast cancer metastasis by directly upregulating E-cadherin levels (69). It is tempting to speculate that TBX3 could be repressing GATA3 or alternatively affecting E-cadherin levels by binding to both GATA3 and E-cadherin. Gli3 belongs to the hedgehog (Hh) signaling network and is required for normal mammary bud formation (70). Since deregulation of Hh pathway is implicated in a wide variety of aggressive and metastatic cancer, the predicted Tbx3-Gli3 interaction warrants further investigation.
Conclusion
The Tbx3 transcriptional network is highly context dependent. This flexibility allows the protein to assume different functions that are specialized for the time and place of expression. Nonetheless, there are common themes that run through the network that hint at more general functions for the gene. In the heart and ES cells, Tbx3 blocks the differentiation of multipotent tissues. This inhibition of differentiation may play a role in cancers when Tbx3 is overexpressed or amplified: induction of an undifferentiated “stem-like” cancer cell by Tbx3 may initiate the process of tumor formation and cell migration. This repressive function is evident in in vitro assays where a transcriptional repression module has been noted (71). Conversely, Tbx3 can induce diffentiation in different contexts. In ES cells, for example, Tbx3 promotes differentiation into ExEn. In the mammary gland as well, Tbx3 induces differentiation of the mammary placodes. Indeed, by binding to tissue-specific transcription factors, Tbx3 may be able to either repress or activate the differentiation of multipotent progenitors in a context-dependent manner.
Tbx3 also appears to play a role in cell proliferation in a number of different contexts: in the heart, Tbx3 depletion leads to an excess of cell proliferation in the structures where it is normally expressed. Cell proliferation might also be altered in the limb in the absence of Tbx3 as mice deficient for the gene have shortened fore- and hind- limbs, a phenotype that is largely recapitulated in human UMS. This role is highlighted in cancers where Tbx3 is overexpressed or amplified as it results in the bypass of senescence through inactivation of the p53 pathway, while the knockdown of TBX3 leads to an increase in proliferation.
Finally, Tbx3 appears to play a role as a mediator of cellular signaling by modulating a number of signaling pathways. Tbx3 can control WNT signaling in the mammary gland and limb buds, as well as in various cancer models. FGF and SHH signaling are also modulated by Tbx3 in various contexts. As with cell proliferation, Tbx3 may be able to regulate these pathways generally, but rely on specific signals to impart specificity to this function.
In order for it to assume such distinct functions, Tbx3 interacts with other factors to give a regional and temporal specificity to its action. Given the evidence of Tbx3 functioning in protein complexes with transcription factors of myriad different families and as a competitor for binding to transcriptional targets, it is reasonable to conclude that Tbx3 is able to mediate a specific set of activities, but that available cofactors determine how it will act in specific contexts. The necessity of these cofactors in determining what function Tbx3 will have makes it an important target for studying with a systems-based approach.
Contributor Information
Andrew J. Washkowitz, Columbia University Medical Center
Svetlana Gavrilov, Columbia University Medical Center.
Salma Begum, Columbia University Medical Center.
Virginia E. Papaioannou, Columbia University Medical Center, vep1@columbia.edu
References
- 1.Herrmann BG, Labeit S, Poustka A, King TR, Lehrach H. Cloning of the T gene required in mesoderm formation in the mouse. Nature. 1990;343(6259):617–622. doi: 10.1038/343617a0. [DOI] [PubMed] [Google Scholar]
- 2.Tada M, Smith JC. T-targets: clues to understanding the functions of T-box proteins. Dev Growth Differ. 2001;43(1):1–11. doi: 10.1046/j.1440-169x.2001.00556.x. [DOI] [PubMed] [Google Scholar]
- 3.Papaioannou VE, Silver LM. The T-box gene family. Bioessays. 1998;20(1):9–19. doi: 10.1002/(SICI)1521-1878(199801)20:1<9::AID-BIES4>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 4.Agulnik SI, Garvey N, Hancock S, Ruvinsky I, Chapman DL, et al. Evolution of mouse T-box genes by tandem duplication and cluster dispersion. Genetics. 1996;144(1):249–254. doi: 10.1093/genetics/144.1.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chapman DL, Garvey N, Hancock S, Alexiou M, Agulnik SI, et al. Expression of the T-box family genes, Tbx1-Tbx5, during early mouse development. Dev Dyn. 1996;206(4):379–390. doi: 10.1002/(SICI)1097-0177(199608)206:4<379::AID-AJA4>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 6.Hoogaars WM, Barnett P, Rodriguez M, Clout DE, Moorman AF, et al. TBX3 and its splice variant TBX3 + exon 2a are functionally similar. Pigment Cell Melanoma Res. 2008;21(3):379–387. doi: 10.1111/j.1755-148X.2008.00461.x. [DOI] [PubMed] [Google Scholar]
- 7.Davenport TG, Jerome-Majewska LA, Papaioannou VE. Mammary gland, limb and yolk sac defects in mice lacking Tbx3, the gene mutated in human ulnar mammary syndrome. Development. 2003;130(10):2263–2273. doi: 10.1242/dev.00431. [DOI] [PubMed] [Google Scholar]
- 8.Bamshad M, Lin RC, Law DJ, Watkins WC, Krakowiak PA, et al. Mutations in human TBX3 alter limb, apocrine and genital development in ulnar-mammary syndrome. Nat Genet. 1997;16(3):311–315. doi: 10.1038/ng0797-311. [DOI] [PubMed] [Google Scholar]
- 9.Linden H, Williams R, King J, Blair E, Kini U. Ulnar Mammary syndrome and TBX3: expanding the phenotype. Am J Med Genet A. 2009;149A(12):2809–2812. doi: 10.1002/ajmg.a.33096. [DOI] [PubMed] [Google Scholar]
- 10.Packham EA, Brook JD. T-box genes in human disorders. Hum Mol Genet. 2003;12:R37–44. doi: 10.1093/hmg/ddg077. Spec No 1. [DOI] [PubMed] [Google Scholar]
- 11.Ivanova N, Dobrin R, Lu R, Kotenko I, Levorse J, et al. Dissecting self-renewal in stem cells with RNA interference. Nature. 2006;442(7102):533–538. doi: 10.1038/nature04915. [DOI] [PubMed] [Google Scholar]
- 12.Kim J, Chu J, Shen X, Wang J, Orkin SH. An extended transcriptional network for pluripotency of embryonic stem cells. Cell. 2008;132(6):1049–1061. doi: 10.1016/j.cell.2008.02.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lu R, Yang A, Jin Y. Dual functions of T-box 3 (Tbx3) in the control of self-renewal and extraembryonic endoderm differentiation in mouse embryonic stem cells. J Biol Chem. 2011;286(10):8425–8436. doi: 10.1074/jbc.M110.202150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peres J, Davis E, Mowla S, Bennett DC, Li JA, et al. The Highly Homologous T-Box Transcription Factors, TBX2 and TBX3, Have Distinct Roles in the Oncogenic Process. Genes Cancer. 2010;1(3):272–282. doi: 10.1177/1947601910365160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rowley M, Grothey E, Couch FJ. The role of Tbx2 and Tbx3 in mammary development and tumorigenesis. J Mammary Gland Biol Neoplasia. 2004;9(2):109–118. doi: 10.1023/B:JOMG.0000037156.64331.3f. [DOI] [PubMed] [Google Scholar]
- 16.Boogerd KJ, Wong LY, Christoffels VM, Klarenbeek M, Ruijter JM, et al. Msx1 and Msx2 are functional interacting partners of T-box factors in the regulation of Connexin43. Cardiovasc Res. 2008;78(3):485–493. doi: 10.1093/cvr/cvn049. [DOI] [PubMed] [Google Scholar]
- 17.Coll M, Seidman JG, Muller CW. Structure of the DNA-bound T-box domain of human TBX3, a transcription factor responsible for ulnar-mammary syndrome. Structure. 2002;10(3):343–356. doi: 10.1016/s0969-2126(02)00722-0. [DOI] [PubMed] [Google Scholar]
- 18.Carlson H, Ota S, Campbell CE, Hurlin PJ. A dominant repression domain in Tbx3 mediates transcriptional repression and cell immortalization: relevance to mutations in Tbx3 that cause ulnarmammary syndrome. Hum Mol Genet. 2001;10(21):2403–2413. doi: 10.1093/hmg/10.21.2403. [DOI] [PubMed] [Google Scholar]
- 19.Jerome-Majewska LA, Jenkins GP, Ernstoff E, Zindy F, Sherr CJ, Papaioannou VE. Tbx3, the ulnarmammary syndrome gene, and Tbx2 interact in mammary gland development through a p19Arf/p53-independent pathway. Dev Dyn. 2005;234(4):922–933. doi: 10.1002/dvdy.20575. [DOI] [PubMed] [Google Scholar]
- 20.Eblaghie MC, Song SJ, Kim JY, Akita K, Tickle C, Jung HS. Interactions between FGF and Wnt signals and Tbx3 gene expression in mammary gland initiation in mouse embryos. J Anat. 2004;205(1):1–13. doi: 10.1111/j.0021-8782.2004.00309.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Platonova N, Scotti M, Babich P, Bertoli G, Mento E, et al. TBX3, the gene mutated in ulnarmammary syndrome, promotes growth of mammary epithelial cells via repression of p19ARF, independently of p53. Cell Tissue Res. 2007;328(2):301–316. doi: 10.1007/s00441-006-0364-4. [DOI] [PubMed] [Google Scholar]
- 22.Cho KW, Kim JY, Song SJ, Farrell E, Eblaghie MC, et al. Molecular interactions between Tbx3 and Bmp4 and a model for dorsoventral positioning of mammary gland development. Proc Natl Acad Sci U S A. 2006;103(45):16788–16793. doi: 10.1073/pnas.0604645103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Howard B, Ashworth A. Signalling pathways implicated in early mammary gland morphogenesis and breast cancer. PLoS Genet. 2006;2(8):e112. doi: 10.1371/journal.pgen.0020112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Howard B, Panchal H, McCarthy A, Ashworth A. Identification of the scaramanga gene implicates Neuregulin3 in mammary gland specification. Genes Dev. 2005;19(17):2078–2090. doi: 10.1101/gad.338505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.King M, Arnold JS, Shanske A, Morrow BE. T-genes and limb bud development. Am J Med Genet A. 2006;140(13):1407–1413. doi: 10.1002/ajmg.a.31250. [DOI] [PubMed] [Google Scholar]
- 26.Martin GR. The roles of FGFs in the early development of vertebrate limbs. Genes Dev. 1998;12(11):1571–1586. doi: 10.1101/gad.12.11.1571. [DOI] [PubMed] [Google Scholar]
- 27.Gibson-Brown JJ, Agulnik SI, Chapman DL, Alexiou M, Garvey N, et al. Evidence of a role for T-box genes in the evolution of limb morphogenesis and the specification of forelimb/hindlimb identity. Mech Dev. 1996;56(1-2):93–101. doi: 10.1016/0925-4773(96)00514-x. [DOI] [PubMed] [Google Scholar]
- 28.Gibson-Brown JJ, Agulnik SI, Silver LM, Niswander L, Papaioannou VE. Involvement of T-box genes Tbx2-Tbx5 in vertebrate limb specification and development. Development. 1998;125(13):2499–2509. doi: 10.1242/dev.125.13.2499. [DOI] [PubMed] [Google Scholar]
- 29.Gibson-Brown JJ, Agulnik SI, Silver LM, Papaioannou VE. Expression of T-box genes Tbx2-Tbx5 during chick organogenesis. Mech Dev. 1998;74(1-2):165–169. doi: 10.1016/s0925-4773(98)00056-2. [DOI] [PubMed] [Google Scholar]
- 30.Logan M, Simon HG, Tabin C. Differential regulation of T-box and homeobox transcription factors suggests roles in controlling chick limb-type identity. Development. 1998;125(15):2825–2835. doi: 10.1242/dev.125.15.2825. [DOI] [PubMed] [Google Scholar]
- 31.Tümpel S, Sanz-Ezquerro JJ, Isaac A, Eblaghie MC, Dobson J, Tickle C. Regulation of Tbx3 expression by anteroposterior signalling in vertebrate limb development. Dev Biol. 2002;250(2):251–262. [PubMed] [Google Scholar]
- 32.Ballim RD, Mendelsohn C, Papaioannou VE, Prince S. The ulnar-mammary syndrome gene, Tbx3, is a direct target of retinoic acid signalling pathway, which regulates its expression during mouse limb development. Molecular Biology of the Cell. doi: 10.1091/mbc.E11-09-0790. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rallis C, Buono JD, Logan MPO. Tbx3 can alter limb position along the rostrocaudal axis of the developing embryo. Development. 2005;132:1961–1970. doi: 10.1242/dev.01787. [DOI] [PubMed] [Google Scholar]
- 34.Zhang Z, O'Rourke JR, McManus MT, Lewandoski M, Harfe BD, Sun X. The microRNA-processing enzyme Dicer is dispensable for somite segmentation but essential for limb bud positioning. Developmental Biology. 2011;351:254–265. doi: 10.1016/j.ydbio.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Suzuki T, Takeuchi J, Koshiba-Takeuchi K, Ogura T. Tbx genes specify posterior digit identity through Shh and BMP signaling. Developmental Cell. 2004;6:43–53. doi: 10.1016/s1534-5807(03)00401-5. [DOI] [PubMed] [Google Scholar]
- 36.Mesbah K, Harrelson Z, Theveniau-Ruissy M, Papaioannou VE, Kelly RG. Tbx3 is required for outflow tract development. Circ Res. 2008;103(7):743–750. doi: 10.1161/CIRCRESAHA.108.172858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ribeiro I, Kawakami Y, Buscher D, Raya A, Rodriguez-Leon J, et al. Tbx2 and Tbx3 regulate the dynamics of cell proliferation during heart remodeling. PLoS One. 2007;2(4):e398. doi: 10.1371/journal.pone.0000398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hoogaars WM, Engel A, Brons JF, Verkerk AO, de Lange FJ, et al. Tbx3 controls the sinoatrial node gene program and imposes pacemaker function on the atria. Genes Dev. 2007;21(9):1098–1112. doi: 10.1101/gad.416007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Habets PEMH. Cooperative action of Tbx2 and Nkx2.5 inhibits ANF expression in the atrioventricular canal: implications for cardiac chamber formation. Genes & Development. 2002;16(10):1234–1246. doi: 10.1101/gad.222902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Aanhaanen WTJ, Boukens BJD, Sizarov A, Wakker V, de Gier-de Vries C, et al. Defective Tbx2-dependent patterning of the atrioventricular canal myocardium causes accessory pathway formation in mice. The Journal of Clinical Investigation. 2011;121(2):534–544. doi: 10.1172/JCI44350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lu R, Yang A, Jin Y. Dual Functions of T-Box 3 (Tbx3) in the Control of Self-renewal and Extraembryonic Endoderm Differentiation in Mouse Embryonic Stem Cells. Journal of Biological Chemistry. 2011;286(10):8425–8436. doi: 10.1074/jbc.M110.202150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ivanova N, Dobrin R, Lu R, Kotenko I, Levorse J, et al. Dissecting self-renewal in stem cells with RNA interference. Nature. 2006;442(7102):533–538. doi: 10.1038/nature04915. [DOI] [PubMed] [Google Scholar]
- 43.Niwa H, Ogawa K, Shimosato D, Adachi K. A parallel circuit of LIF signalling pathways maintains pluripotency of mouse ES cells. Nature. 2009;460(7251):118–122. doi: 10.1038/nature08113. [DOI] [PubMed] [Google Scholar]
- 44.Kim J, Chu J, Shen X, Wang J, Orkin SH. An Extended Transcriptional Network for Pluripotency of Embryonic Stem Cells. Cell. 2008;132(6):1049–1061. doi: 10.1016/j.cell.2008.02.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Han J, Yuan P, Yang H, Zhang J, Soh BS, et al. Tbx3 improves the germ-line competency of induced pluripotent stem cells. Nature. 463(7284):1096–1100. doi: 10.1038/nature08735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fan W, Huang X, Chen C, Gray J, Huang T. TBX3 and its isoform TBX3+2a are functionally distinctive in inhibition of senescence and are overexpressed in a subset of breast cancer cell lines. Cancer Res. 2004;64(15):5132–5139. doi: 10.1158/0008-5472.CAN-04-0615. [DOI] [PubMed] [Google Scholar]
- 47.Gudmundsson J, Besenbacher S, Sulem P, Gudbjartsson DF, Olafsson I, et al. Genetic correction of PSA values using sequence variants associated with PSA levels. Sci Transl Med. 2010;2(62):62ra92. doi: 10.1126/scitranslmed.3001513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lomnytska M, Dubrovska A, Hellman U, Volodko N, Souchelnytskyi S. Increased expression of cSHMT, Tbx3 and utrophin in plasma of ovarian and breast cancer patients. Int J Cancer. 2006;118(2):412–421. doi: 10.1002/ijc.21332. [DOI] [PubMed] [Google Scholar]
- 49.Lyng H, Brovig RS, Svendsrud DH, Holm R, Kaalhus O, et al. Gene expressions and copy numbers associated with metastatic phenotypes of uterine cervical cancer. BMC Genomics. 2006;7:268. doi: 10.1186/1471-2164-7-268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Renard CA, Labalette C, Armengol C, Cougot D, Wei Y, et al. Tbx3 is a downstream target of the Wnt/beta-catenin pathway and a critical mediator of beta-catenin survival functions in liver cancer. Cancer Res. 2007;67(3):901–910. doi: 10.1158/0008-5472.CAN-06-2344. [DOI] [PubMed] [Google Scholar]
- 51.Rodriguez M, Aladowicz E, Lanfrancone L, Goding CR. Tbx3 represses E-cadherin expression and enhances melanoma invasiveness. Cancer Res. 2008;68(19):7872–7881. doi: 10.1158/0008-5472.CAN-08-0301. [DOI] [PubMed] [Google Scholar]
- 52.Suh I, Shibru D, Eisenhofer G, Pacak K, Duh QY, et al. Candidate genes associated with malignant pheochromocytomas by genome-wide expression profiling. Ann Surg. 2009;250(6):983–990. doi: 10.1097/SLA.0b013e3181b248bb. [DOI] [PubMed] [Google Scholar]
- 53.Witte JS. Personalized prostate cancer screening: improving PSA tests with genomic information. Sci Transl Med. 2010;2(62):62ps55. doi: 10.1126/scitranslmed.3001861. [DOI] [PubMed] [Google Scholar]
- 54.Yamashita S, Tsujino Y, Moriguchi K, Tatematsu M, Ushijima T. Chemical genomic screening for methylation-silenced genes in gastric cancer cell lines using 5-aza-2'-deoxycytidine treatment and oligonucleotide microarray. Cancer Sci. 2006;97(1):64–71. doi: 10.1111/j.1349-7006.2006.00136.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yarosh W, Barrientos T, Esmailpour T, Lin L, Carpenter PM, et al. TBX3 is overexpressed in breast cancer and represses p14 ARF by interacting with histone deacetylases. Cancer Res. 2008;68(3):693–699. doi: 10.1158/0008-5472.CAN-07-5012. [DOI] [PubMed] [Google Scholar]
- 56.Zhang JF, He ML, Qi D, Xie WD, Chen YC, et al. Aqueous extracts of Fructus Ligustri Lucidi enhance the sensitivity of human colorectal carcinoma DLD-1 cells to doxorubicin-induced apoptosis via Tbx3 suppression. Integr Cancer Ther. 2011;10(1):85–91. doi: 10.1177/1534735410373921. [DOI] [PubMed] [Google Scholar]
- 57.Cavard C, Audebourg A, Letourneur F, Audard V, Beuvon F, et al. Gene expression profiling provides insights into the pathways involved in solid pseudopapillary neoplasm of the pancreas. J Pathol. 2009;218(2):201–209. doi: 10.1002/path.2524. [DOI] [PubMed] [Google Scholar]
- 58.Etcheverry A, Aubry M, de Tayrac M, Vauleon E, Boniface R, et al. DNA methylation in glioblastoma: impact on gene expression and clinical outcome. BMC Genomics. 2010;11:701. doi: 10.1186/1471-2164-11-701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hansel DE, Rahman A, House M, Ashfaq R, Berg K, et al. Met proto-oncogene and insulin-like growth factor binding protein 3 overexpression correlates with metastatic ability in well-differentiated pancreatic endocrine neoplasms. Clin Cancer Res. 2004;10(18 Pt 1):6152–6158. doi: 10.1158/1078-0432.CCR-04-0285. [DOI] [PubMed] [Google Scholar]
- 60.Schmalhofer O, Brabletz S, Brabletz T. E-cadherin, beta-catenin, and ZEB1 in malignant progression of cancer. Cancer Metastasis Rev. 2009;28(1-2):151–166. doi: 10.1007/s10555-008-9179-y. [DOI] [PubMed] [Google Scholar]
- 61.Kowalski PJ, Rubin MA, Kleer CG. E-cadherin expression in primary carcinomas of the breast and its distant metastases. Breast Cancer Res. 2003;5(6):R217–222. doi: 10.1186/bcr651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mowla S, Pinnock R, Leaner VD, Goding CR, Prince S. PMA-induced up-regulation of TBX3 is mediated by AP-1 and contributes to breast cancer cell migration. Biochem J. 2010;433(1):145–153. doi: 10.1042/BJ20100886. [DOI] [PubMed] [Google Scholar]
- 63.Fillmore CM, Gupta PB, Rudnick JA, Caballero S, Keller PJ, et al. Estrogen expands breast cancer stem-like cells through paracrine FGF/Tbx3 signaling. Proc Natl Acad Sci U S A. 2010;107(50):21737–21742. doi: 10.1073/pnas.1007863107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gupta PB, Fillmore CM, Jiang G, Shapira SD, Tao K, et al. Stochastic state transitions give rise to phenotypic equilibrium in populations of cancer cells. Cell. 2011;146(4):633–644. doi: 10.1016/j.cell.2011.07.026. [DOI] [PubMed] [Google Scholar]
- 65.Lingbeek ME, Jacobs JJ, van Lohuizen M. The T-box repressors TBX2 and TBX3 specifically regulate the tumor suppressor gene p14ARF via a variant T-site in the initiator. J Biol Chem. 2002;277(29):26120–26127. doi: 10.1074/jbc.M200403200. [DOI] [PubMed] [Google Scholar]
- 66.Brummelkamp TR, Kortlever RM, Lingbeek M, Trettel F, MacDonald ME, et al. TBX-3, the gene mutated in Ulnar-Mammary Syndrome, is a negative regulator of p19ARF and inhibits senescence. J Biol Chem. 2002;277(8):6567–6572. doi: 10.1074/jbc.M110492200. [DOI] [PubMed] [Google Scholar]
- 67.Carlson H, Ota S, Song Y, Chen Y, Hurlin PJ. Tbx3 impinges on the p53 pathway to suppress apoptosis, facilitate cell transformation and block myogenic differentiation. Oncogene. 2002;21(24):3827–3835. doi: 10.1038/sj.onc.1205476. [DOI] [PubMed] [Google Scholar]
- 68.Mosca E, Bertoli G, Piscitelli E, Vilardo L, Reinbold RA, et al. Identification of functionally related genes using data mining and data integration: a breast cancer case study. BMC Bioinformatics. 2009;10(Suppl 12):S8. doi: 10.1186/1471-2105-10-S12-S8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yan W, Cao QJ, Arenas RB, Bentley B, Shao R. GATA3 inhibits breast cancer metastasis through the reversal of epithelial-mesenchymal transition. J Biol Chem. 2010;285(18):14042–14051. doi: 10.1074/jbc.M110.105262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hatsell SJ, Cowin P. Gli3-mediated repression of Hedgehog targets is required for normal mammary development. Development. 2006;133(18):3661–3670. doi: 10.1242/dev.02542. [DOI] [PubMed] [Google Scholar]
- 71.He M, Wen L, Campbell CE, Wu JY, Rao Y. Transcription repression by Xenopus ET and its human ortholog TBX3, a gene involved in ulnar-mammary syndrome. Proc Natl Acad Sci U S A. 1999;96(18):10212–10217. doi: 10.1073/pnas.96.18.10212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Begum S, Papaioannou VE. Dynamic expression of Tbx2 and Tbx3 in developing mouse pancreas. Gene Expr Patterns. 2011 doi: 10.1016/j.gep.2011.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Suzuki A, Sekiya S, Buscher D, Izpisua Belmonte JC, Taniguchi H. Tbx3 controls the fate of hepatic progenitor cells in liver development by suppressing p19ARF expression. Development. 2008;135(9):1589–1595. doi: 10.1242/dev.016634. [DOI] [PubMed] [Google Scholar]