Abstract
Objective
The caudate nucleus (CN) is a crucial component of the ventral striatum, which is part of a prefrontal-striatal-thalamic circuit that is modulated by limbic structures to subserve emotional processing. Bipolar disorder is thought to be underpinned by dysfunctional anterior limbic networks, although MRI studies examining the CN have shown equivocal results. As gross volumetric analyses may not detect subtle regional change, we aimed to clarify the role of the CN in bipolar disorder by undertaking shape analysis to detect regional reductions.
Methods
The CN was manually traced on MRI scans from 27 patients with bipolar-I disorder and 24 matched controls. A non-parametric spherical harmonic shape analysis was undertaken using the SPHARM toolkit.
Results
Whilst the left CN volume was consistently larger in the sample, there was no effect of group or gender or significant interactions between these variables. Volume did not correlate with illness duration or lithium dosage, but was larger in those with a history of psychosis at trend level. However, left caudate shape differed significantly between groups, with deflation in an area along the ventromedial surface (connecting to dorsolateral prefrontal regions) in bipolar patients. Psychotic patients showed increases in the dorsal head and body at trend level overall, in regions connecting to medial and orbitofrontal regions.
Conclusions
These findings suggest that subtle rather than gross structural changes occur in the CN, which may not be detectable by volumetric analysis alone, and reflect alterations in specific frontostriatal circuitry in the disorder.
Keywords: Bipolar affective disorder, frontostriatal, ventral striatum, caudate nucleus, limbic system
Introduction
The basal ganglia (BG) are a paired group of nuclei interconnected with the cerebral cortex, thalamus and brainstem. They have been implicated in a variety of processes, including associative, cognitive, mnemonic and motor functions (Slaght et al., 2002). More recently, understanding the complexity in structure, neurochemical interaction and anatomical interrelationships with the thalamus and cortex has led to an understanding of its central influence on executive function, emotion and cognitive behaviours (Alexander and Crutcher, 1990). Within this, the caudate nucleus (CN) is known for its role in higher-order motor control, and more recently learning and memory, particularly feedback processing (Packard and Knowlton, 2002). Mounting evidence suggests that emotional processing is linked to anatomically defined networks consisting of limbic structures that modulate iterative prefrontal-striatal-thalamic circuits (Szily and Keri, 2008). The ventral striatum, which includes the CN, is known to play a crucial role in this network (Lehericy et al., 2004).
Bipolar affective disorder (BPAD) is a DSM-IV axis-I mood disorder that confers considerable individual and societal morbidity and cost (Gardner et al., 2006). A significant body of evidence suggests that BPAD has a strong neurobiological basis that involves dysfunction of the anterior limbic brain networks, which include the BG (Strakowski et al., 2005). Specifically, metabolic (Baxter et al., 1985; Blumberg et al., 2000; O’Connell et al., 1995) and monoaminergic receptor (Pearlson et al., 1995) changes have been identified in the BG of BPAD patients, and medications routinely used to treat the illness, such as lithium and valproate, have been shown to alter the anatomy of the limbic system (Foland et al., 2007; Yucel et al., 2008). Antipsychotic medications, also used in the treatment of BPAD, block dopamine receptors in the BG (Miklowitz and Johnson, 2006), and are known to affect the size of structures within the BG (Chakos et al., 1994; Gur et al., 1998). However, the findings from these studies have been marked by a great degree of heterogeneity, as a result of small sample sizes, cross-sectional design and differing mood states in patients at the time of assessment (Langan and McDonald, 2009).
Structural magnetic resonance imaging (MRI) studies have been increasingly utilised to characterise in vivo brain changes in bipolar patients (Malhi et al., 2002; Strakowski et al., 1999, 2005), albeit with similarly heterogeneous results (Arnone et al., 2009). The most robust findings have been enlargement of the lateral ventricles (Andreasen et al., 1990; Dewan et al., 1988; Nasrallah et al., 1982; Pearlson et al., 1984a, 1984b) and reduction in volume of the prefrontal cortex (Coffman et al., 1990; Lavretsky et al., 2004; Sax et al., 1999). In the limbic system, while some studies report enlarged amygdalae in BPAD (Altshuler et al., 2000; Frangou, 2005; Strakowski et al., 1999), some suggest overall volume reduction, or reduction in only one side (Blumberg et al., 2003; Chang et al., 2005b; Pearlson et al., 1997). Most, but not all, studies (Blumberg et al., 2003; Swayze et al., 1992) have failed to demonstrate volumetric change in the hippocampus (Altshuler et al., 2000; Blumberg et al., 2003; Chang et al., 2005b; Noga et al., 2001; Pearlson et al., 1997; Sax et al., 1999; Strakowski et al., 1999). The heterogeneity of neuroimaging findings in BPAD has largely been attributed to clinical and treatment, rather than imaging, variables (Arnone et al., 2009). Thus, in a recent meta-analysis of voxel-based morphometry studies, which identified reduced grey matter in anterior cingulate and fronto-insular regions, longer illness duration was associated with increased grey matter in the BG, subgenual anterior cingulate cortex and amygdala, and lithium treatment was associated with increased anterior cingulate cortex volume (Bora et al., 2010).
MRI studies of the BG have also been equivocal. While one early study suggested that lateral ventricular enlargement was a consequence of periventricular atrophy of structures such as the CN (Strakowski et al., 2002), most early studies failed to find a difference between BPAD patients and controls (Brambilla et al., 2001; Harvey et al., 1994; Strakowski et al., 1993; Swayze et al., 1992). Aylward et al. (1994) found bilaterally larger caudate volumes in male bipolar patients, but could not rule out the effects of comorbidity or medication. Strakowski et al. (1999) found striatal enlargement in BPAD patients compared to controls, with no association between medication exposure and enlargement. More recent studies have provided mixed results, with some suggesting CN size increases (DelBello et al., 2004; Noga et al., 2001; Strakowski et al., 2002), but others finding no differences between BPAD patients and controls (Beyer et al., 2004; Chang et al., 2005a; Sanches et al., 2005; Sax al., 1999). When juvenile samples haveet been examined, no volumetric changes have been demonstrated in CN and other BG nuclei (Ahn et al., 2007; Chang et al., 2005b; Sanches et al., 2005). The only study looking at older patients showed a reduction in right CN volume compared to controls after controlling for age and gender, suggesting an illness by age interaction at the level of the BG that may not be apparent in younger patients (Beyer et al., 2004). Few studies have controlled for medication when examining brain volumes in BPAD. Mood stabilisers such as lithium may demonstrate a neuroprotective effect (Rowe and Chuang, 2004; Young et al., 2002) and some region of interest (ROI) studies have found larger volumes in medicated subjects, or smaller volumes in drug-naïve patients (Foland et al., 2007; Frangou, 2005; Hwang et al., 2006; Wilke et al., 2004), including a differential effect of drug treatment on the shape of the CN in bipolar patients (Hwang et al., 2006). This was the only study to look at shape of the CN in BPAD and found that, whilst overall volume did not differ, significant shape changes were seen on anterior and ventral surfaces, particularly on the right side, and were only seen in drug-naïve bipolar patients (Hwang et al., 2006). This study suggested that examining shape in three dimensions, rather than measuring volume alone, may reveal subtle differences affecting structure and/or development of the CN in BPAD. A recent summary of functional and structural studies in BPAD noted that bipolar disorder is generally associated with striatal overactivity at a functional level, whereas structural findings have been mixed and that the most likely reason for this variability is the use of antipsychotic medication, which affect striatal volume (Marchand and Yurgelun-Todd, 2010).
We aimed to expand on this dataset, particularly the findings of Hwang et al. (2006), by examining a group of established BPAD-I patients who were scanned when euthymic, antipsychotic-free for at least one year, using an established caudate tracing protocol, and examining shape using a sophisticated non-parametric shape analysis. We hypothesised that shape differences would exist when comparing bipolar patients to controls, regionalised pathology in the ventral CN, and that patients not treated with lithium would show reductions in this area compared to lithium-treated patients.
Methods
Subjects
Twenty-seven patients (Table 1) with BPAD-I were recruited from a specialist Mood Disorders Clinic in Sydney, Australia. Diagnoses were made by a research psychiatrist using the DSM-IV SCID-P (First et al., 1998), supplemented by case note review.
Table 1.
Demographic data for patient groups, displayed as means/standard deviations, or numbers of subgroups.
| Controls (n = 24) | Bipolar (n = 27) | Variable | p | |
|---|---|---|---|---|
| Age at scan (years) | 38.67 ± 11.07 | 38.38 ± 10.87 | t = 0.091 | 0.93 |
| Gender (M/F) | 7/17 | 8/19 | χ2 = 0.001 | 0.97 |
| Current NART-IQ | 115.08 ± 9.59 | 113.80 ± 7.13 | t = 0.53 | 0.60 |
| Education level (years) | 14.58 ± 2.10 | 14.64 ± 2.83 | t = −0.080 | 0.94 |
| Age at illness onset (years) | 24.88 ± 8.37 | |||
| Illness duration (years) | 13.50 ± 10.13 | |||
| Lifetime manic episodes | 8.85 ± 10.17 | |||
| Lifetime depressive episodes | 11.12 ± 10.77 | |||
| History of psychosis (Y/N) | 16/11 | |||
| Family history (BP/UP/BUP/N/U) | 3/5/2/11/5 | |||
| Medication at scan time(LI/VAL/CBZ/LIVAL/VALCBZ/MF/U) | 8/7/1/4/5/1 |
NART: National Adult Reading Test; IQ: intelligence quotient.
For family history, BP: family history of bipolar disorder; UP: unipolar depression; BUP: both bipolar disorder and unipolar depression; N: no family history; U: unknown.
For medication at scan time, LI: lithium; VAL: valproate; CBZ: carbamazepine; LIVAL: both lithium and valproate; VALCBZ: both valproate and carbamazepine; MF: medication free; U: unknown.
The family history of bipolar and unipolar affective disorder is summarised in Table 1. Medication history is also described in Table 1; the patient whose medication status was unknown was excluded from medication analysis. All other patients were previously exposed to antipsychotic medication, although none within 12 months of entering the study.
Twenty-four controls were recruited via advertisement and matched for age and education. They were screened for a personal and family history of psychiatric or neurological disorder using the SCIP-NP. Participants were excluded if they had a history of ongoing substance misuse, neurological disease or, in patients, a comorbid axis I or II DSM-IV diagnosis requiring treatment. All participants were right-handed. All participants provided written, informed consent prior to participating and the study had Prince of Wales Hospital and University of New South Wales ethics committee permission.
MRI acquisition and pre-processing
Scans were acquired on a 1.5 T GE Signa scanner located at the Royal Prince Alfred Hospital, Sydney, Australia. Imaging parameters were: echo time (TE), 5.3 ms; repetition time (TR), 12.2 ms; field of view, 24.9 cm; voxel dimensions, 0.977 × 0.977 × 1.6 mm thick coronal slices. All MRI data were transferred from CD to a Linux Debian 3.1 workstation and coded to ensure participant confidentiality and blinded rating.
Each participant’s image was stripped of extracerebral tissue (Smith, 2002) and aligned to the N27 template (Holmes et al., 1998) via a six-degree rigid-body transformation (Jenkinson and Smith, 2001) using tools contained in the FSL software package (www.fmrib.ox.ac.uk/fsl). No re-scaling or warping was performed, but the images were resampled to 1 mm3 voxels in the process.
Volumetric measures
Intracranial volume
Intracranial volume (ICV) was measured by manual tracing using the dura mater as the major anatomical boundary. Where the dura mater was not visible, the cerebral contour was outlined. Other landmarks were the under-surfaces of the frontal lobe, the dorsum sellae, clivus, and the attachment of the dura to the posterior cutting across to the anterior arch of CI at the cranio-vertebral junction. Both inter- and intrarater correlation coefficient measurements were 0.99 for 10 randomly selected images (Eritaia et al., 2000).
CN volume
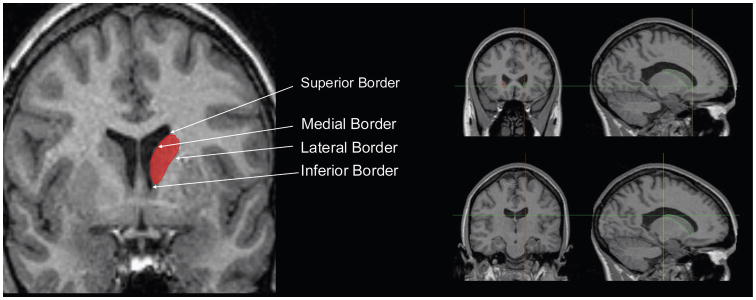
The methodology for caudate volume used in this study has been previously described (Hannan et al., 2010). Briefly, whole brain images were first aligned using a 6-parameter transformation to the Montreal Neurological Institute (MNI) image using the automated FLIRT (FMRIB’s Linear Image Registration Tool) from the FSL toolbox (Jenkinson and Smith, 2001; Jenkinson et al., 2002). Images were re-sliced isotropically (1.0 mm3). Manual tracing of CN volumes was then done using ANALYZE 7.2 (MAYO Clinic, USA) software. Left and right caudate perimeters were traced manually on each contiguous 1.0 mm thick coronal slice. The slices were aligned with the plane perpendicular to the line between the anterior and posterior commissure. The lateral ventricle was used to define the anterior, posterior, medial and superior boundaries of the CN (Figure 1). Dorsally, the caudate is easily demarcated from the white matter that surrounds it – as such, the traces continued posteriorly and anteriorly until the caudate was no longer visible in the slice. Inferiorly, the caudate shares borders with the striaterminalis and the nucleus accumbens. The caudate has been shown to perform distinct functional roles and have different neuroconnectivity from the nucleus accumbens (Parent and Hazrati, 1995) so it was important to exclude it in the traces. For tracing purposes, the nucleus accumbens was demarcated by a line connecting the inferior margin of the lateral ventricle with the inferior border of the internal capsule, bordering with the caudate from the slice most anterior where the putamen appeared as a distinct entity to the slice most posterior where the internal capsule extends past the inferior point of the lateral ventricle. There are cell bridges between the putamen and caudate; these were traced to their midpoint when the voxel resolution allowed. Seven randomly selected images were measured twice to establish inter- and intrarater reliability, which were calculated using an intraclass correlation coefficient (ICC; model 3, two-way mixed). Interrater ICC reliabilities were 0.919 (right) and 0.803 (left). Intrarater ICC reliabilities were 0.907 (right) and 0.945 (left).
Figure 1.
Example of manually traced boundaries of caudate nucleus on coronal slice of MRI scan on left; on right, anterior (top right) and posterior (bottom right) margins of the caudate nucleus..
CN shape
Shape analysis was undertaken in a semi-automated fashion using the University of North Carolina shape analysis toolkit (Styner et al., 2006) (www.nitrc.org/projects/spharm-pdm). Segmented 3D label maps were initially processed to ensure interior holes were filled, followed by morphological closing and minimal smoothing. These were then subjected to spherical harmonic shape description (SPHARM-PDM), with reconstructed boundary surfaces of all segmentations mapped onto the unit sphere and described via a set of coefficients weighting spherical harmonic basis functions. The correspondence between surfaces was established by a parameter-space rotation based on the first-order expansion of the spherical harmonics. All surfaces were then uniformly sampled into sets of 1002 surface points each. These surfaces were then aligned to a study-averaged template for each structure (left and right caudate and putamen) using rigid-body Procrustes alignment (Bookstein, 1997), with normalisation for head size using ICV with a scaling factor, fi, where fi = (mean(ICV)/ICVi)1/3.
Statistical analysis
No data needed to be excluded for any reason. Data was analysed using SPSS 15.0 for Windows. Alpha level was set to 0.05 for all tests. Comparison was made between the bipolar-I group (BPAD-I) and the healthy controls (HC). Another comparison was made between the BPAD-I, this time separated on the basis of history of psychosis, and HC, in the same manner. Demographic data were compared using χ2 analyses for categorical data (gender, family history), independent-samples t-tests for age, current IQ, education level in years, intracranial and caudate volumes, with Levene’s test for equality of variance being applied to determine the appropriate t-test. Repeated-measures analysis of variance was used to test for interaction effects by hemisphere within subjects and gender and diagnosis between subjects. Pearson’s correlation was used to investigate correlations between volume and duration of illness, lithium dosage and valproate dosage.
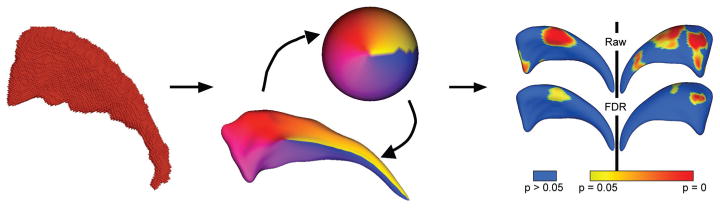
To compare structural shape between BPAD-I patients and controls, we computed the local Hotelling T2 two-sample mean difference, and corrected for multiple comparisons using false discovery rate (FDR) (Genovese et al., 2002). We generated mean difference magnitude displacement maps and significance maps of the local p-values in raw format, and corrected for multiple comparisons (Figure 2). We also computed an overall, global shape difference via averaging the local group differences across the whole surface. In addition, we performed a shape-based correlation analysis. This was done by first computing local surface normals (perpendicular vector to the surface) on the average surface across all datasets. Then the local difference vector from each individual surface to that average was projected onto the normal, thus deriving a single scalar value representing the shape difference of the individual shape to the average surface along the normal (Styner et al., 2006). These values were then correlated with illness variables based on Spearman’s rank-order correlation. Raw and corrected p-values were calculated as per group differences, and displacement and significance maps were generated (Figure 2).
Figure 2.
The SPHARM process and analysis. On left, binarised image is pre-processed and smoothed. Centre, image is converted to surface meshes, and a spherical parameterisation is computed for the surface mesh, and meshes are aligned to establish correspondence across all surfaces. Right, group differences are computed using Hotelling T2 sample metric, and significance maps computed for each analysis, both raw uncorrected p-value maps and FDR-corrected maps.
Results
Table 1 shows the demographic features between groups. There were no differences in age, gender balance, premor-bid IQ or level of education.
The raw volumetric data of the ICV, right and left caudate nucleus volume (CNV) are shown in Table 2. The ICV was well matched between groups. There was no statistically significant overall difference between left and right CNV between groups.
Table 2.
Means for intracranial volume and left/right caudate volume for control and bipolar groups.
| Controls (n = 24) | Bipolar (n = 27) | t | p | |
|---|---|---|---|---|
| Intracranial volume (× 106 mm3) | 1.461 ± 0.148 | 1.475 ± 0.123 | −0.364 | 0.72 |
| Right caudate volume (mm3) | 3779 ± 476 | 3836 ± 522 | −0.410 | 0.68 |
| Left caudate volume (mm3) | 3876 ± 431 | 3926 ± 506 | −0.377 | 0.71 |
Repeated-measures analysis of variance was undertaken to examine the effect of gender and laterality on CNV in these groups, with hemisphere as the within-subject factor and gender and diagnosis as between-subject factors. There was a significant effect of hemisphere (left > right; F(1,47) = 13.36, p = 0.001). However, there was no significant effect of group (F(1,47) = 0.221, p = 0.803), and no significant interactions (hemisphere by gender, F(1,47) = 2.615, p = 0.113; hemisphere by diagnosis, F(1,47) = 0.244, p = 0.624; hemisphere by diagnosis by gender, F(1,47) = 0.100, p = 0.753).
There was no correlation with illness duration (pright = 0.147, pleft = 0.227), lithium dosage (n = 12, pright = 0.509; pleft = 0.456) or valproate dosage (n = 12; pright = 0.911, pleft = 0.928). Left and right CNV correlated strongly with each other (r = 0.908, p < 0.001), and the strength of this correlation did not differ between the groups (z = 0.826, p = 0.409).
There was a negative correlation between CNV and age that reached trend levels in the sample as a whole (rright = −0.264, pright = 0.056; rleft = −0.272, pleft = 0.064), although when the BPAD and HC groups were compared, correlation coefficients remained similar, but analyses became non-significant. These correlation coefficients did not differ however between the two groups, suggesting that this loss of significant correlation may have related to sample size (zleft = 0.182, pleft = 0.855; zleft = 0.206; pleft = 0.837).
Comparisons were also made between the HC group, patients with a history of psychosis (BPAD-P) and those without (BPAD-NP). A repeated-measures analysis was conducted to investigate the effect of a history of psychotic symptoms, using left and right CNV as within-subject factors and group and gender as between-subject factors. There was an overall trend for a between-group effect for a larger caudate in those with a psychotic history (F(1,47) = 2.56, p = 0.09); there was no significant effect of gender, or other interaction effects [group by gender (F(1,47) = 0.73, p = 0.49), hemisphere, hemisphere by gender, hemisphere by group (F(1,47) = 0.92, p = 0.59), or hemisphere by group by gender (F(1,47) = 1.02, p = 0.32) effects.
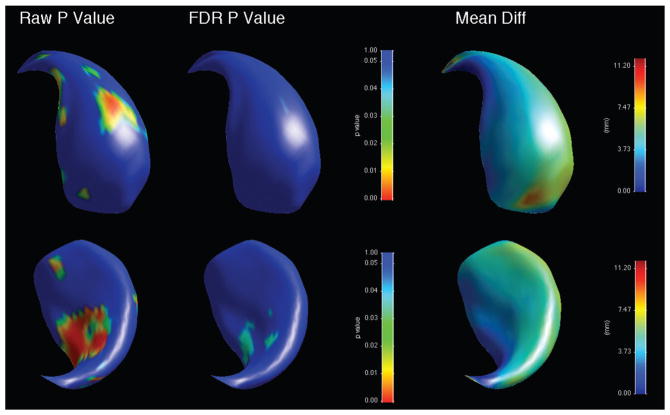
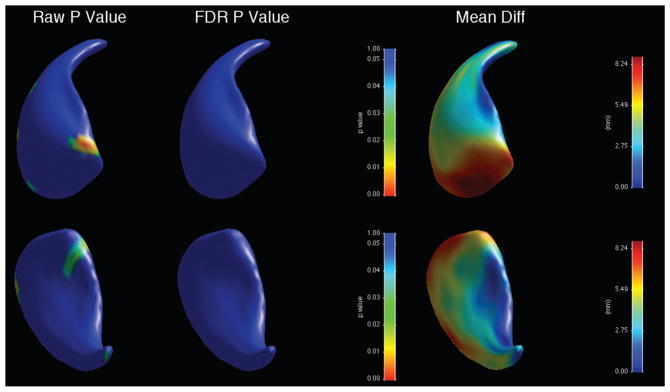
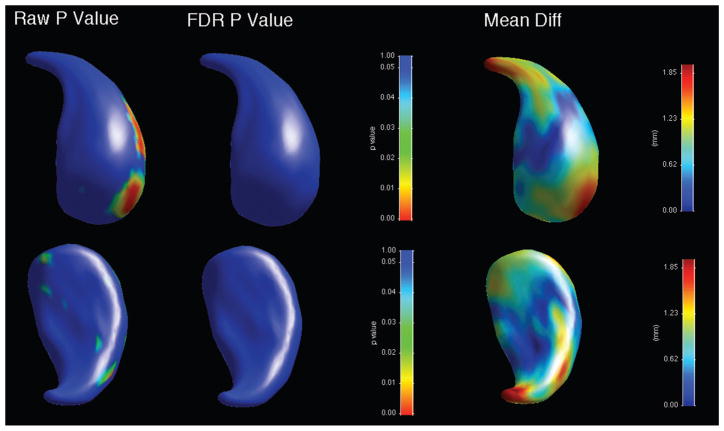
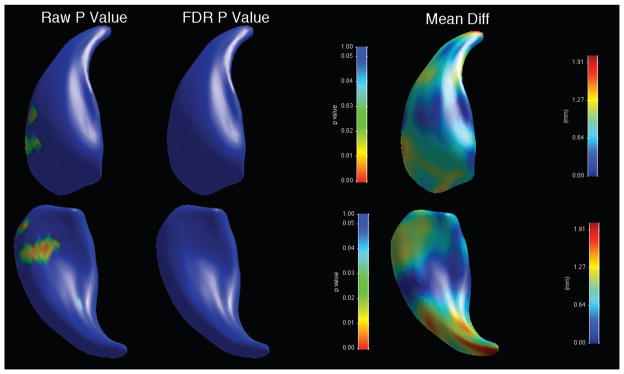
When shape was analysed, there was an overall significant shape difference in the left caudate (bipolar patients showing reductions compared to controls, average p-value across surface = 0.028), with regions of significant shape difference that survived FDR correction localised along the ventromedial surface (Figure 3, Table 3). There was no significant overall shape difference on the right (Figure 4). When psychotic and non-psychotic groups were compared, there was a trend towards an overall significant shape difference in the left caudate (non-psychotic deflated compared to psychotic; average p-value across surface = 0.096, Figure 5), with areas on the dorsal body and head that did not survive FDR correction; there was no overall significant shape difference in the right caudate (Figure 6). When the lithium-treated bipolar patients were compared to those not on lithium treatment, there were no significant shape difference in the left or right caudate nuceli (Table 3).
Figure 3.
Significance maps for comparison between bipolar patients and controls for left caudate, with areas in blue indicating regions where p > 0.05. Top row shows dorsomedial aspect, bottom row ventrolateral. On left, raw uncorrected p-value maps; middle, FDR-corrected p-value maps; right, mean difference maps in mm.
Table 3.
Significance (expressed as p-values) for overall group surface comparisons.
| p | ||
|---|---|---|
| Left caudate | Right caudate | |
| Bipolar vs controls | 0.028* | 0.193 |
| Psychotic vs non-psychotic bipolar | 0.096** | 0.211 |
| Lithium-treated vs non-lithium bipolar | 0.873 | 0.537 |
p < 0.05
p < 0.10.
Figure 4.
Significance maps for comparison between bipolar patients and controls for right caudate. Top row shows dorsomedial aspect, bottom row ventrolateral. On left, raw uncorrected p-value maps; middle, FDR-corrected p-value maps; right, mean difference maps in mm.
Figure 5.
Significance maps for comparison between psychotic and non-psychotic bipolar patients for left caudate. Top row shows dorsomedial aspect, bottom row ventrolateral. On left, raw uncorrected p-value maps; middle, FDR-corrected p-value maps; right, mean difference maps in mm.
Figure 6.
Significance maps for comparison between psychotic and non-psychotic bipolar patients for right caudate. Top row shows dorsomedial aspect, bottom row ventrolateral. On left, raw uncorrected p-value maps; middle, FDR-corrected p-value maps; right, mean difference maps in mm.
Discussion
In this paper we used a manual tracing protocol to compare the volumes and shapes of CN in patients with BPAD-I and controls matched for age, sex and education. We found that while there was no significant difference in volume between these groups, there was a small regional shape difference in the bipolar group, with a reduction in the ventral region of the body of the left caudate. This region has been demonstrated to connect to dorsolateral and medial prefrontal cortex and orbitofrontal cortex via primate tracing studies (Haber et al., 2000) and diffusion-tensor imaging tractography studies in humans (Draganski et al., 2008; Leh et al., 2007). Cortical connections from ventral caudate overlap considerably with those from the dorsal region (Draganski et al., 2008). The frontal-subcortical loops that pass through the ventral caudate include the medial prefrontal loop that connects ventral caudate to the anterior cingulate, and state-related hyperactivity in this circuit has been described in bipolar mania (Blumberg et al., 2000).
CN volumes tended to decrease with age as has been described in healthy controls (Passe et al., 1997), and there were no significant differences in this effect between controls and the BPAD-I group, suggesting no age-by-illness interaction. There were no statistically significant correlations with caudate measures and clinical variables, including age of onset, family history, number of manic or depressive episodes, or medication dosage. However, there was a trend to smaller caudate nuclei in BPAD-I patients with no history of psychosis compared to BPAD-I patients with a history of psychosis, with the trend being stronger for the right caudate than the left. Shape analysis also suggested a trend towards a difference in the left caudate in psychotic patients, who showed an increase in the dorsal head and body of the caudate in regions connecting orbital and medial prefrontal cortex (Haber et al., 2000). No changes were seen in the right caudate in either analysis, and there were no relationships between duration of illness and lithium or valproate treatment and caudate shape.
The regional reductions in the left CN in bipolar patients may result from alterations to neuronal structure at a microscopic level, which are detectable neuroradiologically at a macroscopic level. Reductions in both glial and neuronal density have been reported in DLPFC in BPAD (Rajkowska et al., 2001), and of GABAergic neurons in entorhinal and anterior cingulate cortex (Pantazopoulos et al., 2007; Woo et al., 2004). It is possible that direct striatal neuronal loss (such as the GABAergic medium spiny neuron (MSN) population), such as that seen in Huntington’s disease, may result in surface deflation. However, it is also possible that atrophy of the MSN dendritic arbor as a loss of reduced dopaminergic input – such as that occurring in Parkinson’s disease (McNeill et al., 1988) – could result in subtle volumetric differences without direct cellular loss. Thus, in the absence of an understanding of the relationship between CN changes and those in other brain regions, it is difficult to determine if these changes are primary pathology in the caudate, or secondary to changes elsewhere in frontal-subcortical loops (Marchand and Yurgelun-Todd, 2010).
The trend towards a significant expansion in the left caudate head in psychotic bipolar patients compared to non-psychotic patients raises questions as to how psychosis may result in grey matter increases. Psychosis is described as causing both increases (Fornito et al., 2009) and decreases (Koo et al., 2008) in grey matter volume in BPAD patients, and this has been linked to possible overactivity of the hypothalamic-pituitary-amygdala (HPA) axis in first-presentation psychosis (Fornito et al., 2009). However, these patients were euthymic, non-psychotic and not actively treated for psychosis, suggesting that any HPA-linked changes in early psychosis were unlikely to be causing these changes. Another possibility is that patients with a previous history of psychosis may have had greater cumulative exposure to antipsychotic medication, and in spite of the dataset being antipsychotic-free for at least 12 months prior to scanning, persistent effects of antipsychotic medication on striatal volume cannot be ruled out (Kornhuber et al., 2006).
Our findings suggest that CN structural changes in BPAD-1 may be subtle. While differences between patients and controls are not identified in volumetric analysis they may be revealed by shape analysis. The results of the study showed that there is little to no effect of adult-onset BPAD-I or medications for BPAD-I on the overall volume of CN for patients, but that there may be shape changes in the ventral region of CN for patients. The trend level difference in both volume and shape between patients with a history of psychosis and no history could be due to a long-term effect of exposure to anti-psychotic medications. While it was known that all patients had been exposed to antipsychotics, none had received antipsychotics within the 12 months prior to scanning, although the true extent of the long-lasting effect of typical antipsychotics is unknown (Kornhuber et al., 2006), and may be relevant to the present findings.
While there are many studies investigating anatomical changes in the BG in BPAD, few studies have focused on direct comparisons between BPAD patients and a healthy control group on the basis of CN volume. One of the few studies that is directly comparable with the current study was by Aylward et al. (1994), who compared the CN volumes of 30 BPAD patients and 30 age-matched controls with no family or personal history of psychiatric illness. This study identified a significant diagnosis by sex interaction for CN volume, suggesting that male bipolar patients had significantly larger caudate volumes bilaterally compared to the male comparison subjects. In the current study, there were no significant findings on the basis of diagnosis, despite similar sample sizes (30 BPAD patients and 30 HC for Aylward and 27 BPAD patients and 24 controls for this study), and age between groups (BPAD: 39 ± 11, HC: 38 ± 9 for Aylward, and BPAD: 38 ± 11, HC: 39 ± 11 for this study). There are several differences between the two studies which may explain the differing findings. First, Aylward et al. (1994) used the Structured Clinicial Interview for DSM-III-R, which did not distinguish between BPAD Type I and Type II, nor of presentations of BPAD Not Otherwise Specified and cyclothymia, which are now thought to exist on a ‘bipolar spectrum’ (Bader and Dunner, 2007). Earlier studies may have included a more heterogeneous group of patients who are clinically and functionally distinct (Vieta and Suppes, 2008). Secondly, Aylward et al.’s MRI sequences generated 5 mm thick slices, which might otherwise be expected to reduce sensitivity to volumetric changes compared to the 1 mm thick slices in our study.
As a result of the better resolution of our images we used a manual tracing technique which differed in several ways. Our MRI tracing process used clearly defined anatomical boundaries and the rostral and caudal cut-offs relied on visual inspection of the anatomy as opposed to selecting an arbitrary number of slices after a certain landmark. This is a critical issue given the variability of the placement of landmarks such as the junction between the CN, nucleus accumbens and the lateral ventricles. A major challenge was defining the border between the CN and the nucleus accumbens where artefacts and variability in intensities forced some approximation in 6–7% of the slices per scan. Despite this issue, intra- and interrater reliability remained high.
Volumetric ROI studies may not provide adequate functional correlates (Strakowski et al., 2005), or afford enough sensitivity to detect subtle volumetric differences (Hwang et al., 2006). It is argued that for ROI studies the time and labour intensiveness of manual tracing leads to a restriction of sample sizes and makes detecting subtle differences more difficult (Worth et al., 1997). The inclusion of shape analysis in this study extends the capacity of our comparison to detect these subtle differences. Our shape analysis – using a robust methodology used in studies of neurodegenerative (Gerardin et al., 2009) and psychiatric (Levitt et al., 2009) disorders – demonstrated subtle findings not seen in the pure volumetric analysis. Given that the CN is topographically mapped to different cortical regions that subserve its motor, cognitive and affective roles (Draganski et al., 2008; Haber et al., 2000; Leh et al., 2007), a shape analysis approach allows us to potentially implicate the corticostriatal loops that relay through the CN. Bipolar patients in this study showed reductions in caudate regions connecting dorsolateral prefrontal regions, which have been implicated in the pathophysiology of bipolar disorder (Arnone et al., 2009; Strakowski et al., 2005). Our findings were seen on the left side only, which is consistent with the literature implicating left greater than right prefrontal regions (McDonald et al., 2004). This left-sided shape change was present at trend level in the psychotic group compared to the non-psychotic group, in regions connecting to medial frontal, orbitofrontal and prefrontal cortical regions, consistent with studies suggesting structural change in these regions in psychotic bipolar patients (de Azevedo-Marques Perico et al., 2011; Fornito et al., 2009; Tost et al., 2010). While these changes are subtle and not all survived FDR correction, these findings suggest that shape analysis is a useful adjunct to traditional ROI analysis of well-defined structures, particularly those whose connections are topographically arranged. When referencing these results to other disorders in which we have used the same shape analysis methodology, the difference maps demonstrate that the regional deflation seen in the left CN in bipolar patients – in the order of 4–5 mm – is of a similar magnitude to regions of significant deflation seen when patients with frontotemporal dementia (Looi et al., 2011b) and progressive supranuclear palsy (Looi et al., 2011a) are compared to matched controls, but less than that seen in the primary striatal degenerative disorder neuroacanthocytosis (Walterfang et al., 2011). Additionally, the findings seen in this study are regionally much smaller compared to analyses with neurodegenerative disorders. This suggests that these findings represent significant, if circumscribed, neuropathology that when seen in other disorders predicts functional impairment.
Our study has a number of limitations. Given the number of participants, it is possible that our subgroup analyses are underpowered, leading to the possibility of type II error – particularly so in a non-parametric analysis over more than 1000 data points per structure. Additionally, the interrater reliability for the left caudate was < 0.85 (whereas intrarater reliability was high), which suggests that while the between-group comparisons in this study are likely to be valid, the comparability of the findings with those seen utilising this methodology in other datasets may be more limited (Looi et al., 2010, 2011a, 2011b; Walterfang et al., 2011). Furthermore, we cannot exclude that antipsychotic treatment – to which all patients were exposed, albeit none in the year preceding scanning – affected the results of our comparisons, potentially by attenuating between-group differences. Finally, while we are inferring that subtle shape changes are due to microstructural alterations, volumetric MRI technology is limited in its capacity to detect microscopic changes and it is possible that these findings only represent the detectable ‘tip’ of a larger affected region.
Despite insights from functional, receptor and genetic studies, the pathophysiology of BPAD is still largely incomplete. There are multiple interacting factors that do not appear to localise to any single structure (Strakowski et al., 2005), so it is difficult to define any particular hypotheses for our largely negative findings. It is possible that there is no baseline change in the CNV due to BPAD-I in itself, and that volume changes in the general population are due largely to the effects of medication, given evidence that lithium protects against the loss of grey matter implicated in BPAD (Brambilla et al., 2001). However, given the substantial number of patients that were taking lithium (n = 12), such an effect for medicated subjects in the form of increased CNV was expected and the lack of result here was surprising. Overall, the interconnected nature of the corticothalamic and limbic circuits that include the BG and complex functional findings suggest that the relationship between functional and neuroanatomical defects in BPAD is not direct.
We have demonstrated in a study using high-resolution MRI that gross structural changes do not occur in the CN in BPAD-1, although there may be subtle changes detectable by shape analysis, in regions that connect with prefrontal cortical areas that have previously been implicated in the disorder.
Acknowledgments
Funding
The study was supported by an NHMRC Grant and Rotary Funding held by Professor Malhi. Professor Christos Pantelis was supported by a NHMRC Senior Principal Research Fellowship (ID: 628386) and NHMRC Program Grant (ID: 566529).
Footnotes
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Ahn MS, Breeze JL, Makris N, et al. Anatomic brain magnetic resonance imaging of the basal ganglia in pediatric bipolar disorder. Journal of Affective Disorders. 2007;104:147–154. doi: 10.1016/j.jad.2007.04.015. [DOI] [PubMed] [Google Scholar]
- Alexander GE, Crutcher MD. Functional architecture of basal ganglia circuits: neural substrates of parallel processing. Trends in Neuroscience. 1990;13:266–271. doi: 10.1016/0166-2236(90)90107-l. [DOI] [PubMed] [Google Scholar]
- Altshuler L, Bartzokis G, Greider T, et al. An MRI study of temporal lobe structures in men with bipolar disorder or schizophrenia. Biological Psychiatry. 2000;48:147–162. doi: 10.1016/s0006-3223(00)00836-2. [DOI] [PubMed] [Google Scholar]
- Andreasen N, Swayze V, Flaum M, et al. Ventricular abnormalities in affective disorder: clinical and demographic correlates. American Journal of Psychiatry. 1990;147:893–900. doi: 10.1176/ajp.147.7.893. [DOI] [PubMed] [Google Scholar]
- Arnone D, Cavanagh J, Gerber D, et al. Magnetic resonance imaging studies in bipolar disorder and schizophrenia: a meta-analysis. British Journal of Psychiatry. 2009;195:194–201. doi: 10.1192/bjp.bp.108.059717. [DOI] [PubMed] [Google Scholar]
- Aylward E, Roberts-Twillie J, Barta P. Basal ganglia volumes and white matter hyperintensities in patients with bipolar disorder. American Journal of Psychiatry. 1994;151:687–693. doi: 10.1176/ajp.151.5.687. [DOI] [PubMed] [Google Scholar]
- Bader C, Dunner D. Bipolar disorder not otherwise specified in relation to the bipolar spectrum. Bipolar Disorders. 2007;9:860–867. doi: 10.1111/j.1399-5618.2007.00378.x. [DOI] [PubMed] [Google Scholar]
- Baxter L, Phelps M, Mazziotta J, et al. Cerebral metabolic rates for glucose in mood disorders. Studies with positron emission tomography and fluorodeoxyglucose F 18. Archives of General Psychiatry. 1985;42:441–447. doi: 10.1001/archpsyc.1985.01790280019002. [DOI] [PubMed] [Google Scholar]
- Beyer J, Kuchibhatla M, Payne M, et al. Caudate volume measurement in older adults with bipolar disorder. International Journal of Geriatric Psychiatry. 2004;19:109–114. doi: 10.1002/gps.1030. [DOI] [PubMed] [Google Scholar]
- Blumberg H, Kaufman J, Martin A, et al. Amygdala and hippocampal volumes in adolescents and adults with bipolar disorder. Archives of General Psychiatry. 2003;60:1201–1208. doi: 10.1001/archpsyc.60.12.1201. [DOI] [PubMed] [Google Scholar]
- Blumberg H, Stern E, Martinez D, et al. Increased anterior cingulate and caudate activity in bipolar mania. Biological Psychiatry. 2000;48:1045–1052. doi: 10.1016/s0006-3223(00)00962-8. [DOI] [PubMed] [Google Scholar]
- Bookstein F. Shape and the information in medical images: a decade of the morphometric synthesis. Computer Vision and Image Understanding. 1997;66:97–118. [Google Scholar]
- Bora E, Fornito A, Yucel M, et al. Voxelwise meta-analysis of gray matter abnormalities in bipolar disorder. Biological Psychiatry. 2010;67:1097–1105. doi: 10.1016/j.biopsych.2010.01.020. [DOI] [PubMed] [Google Scholar]
- Brambilla P, Harenski K, Nicoletti M, et al. Differential effects of age on brain gray matter in bipolar patients and healthy individuals. Neuropsychobiology. 2001;43:242–247. doi: 10.1159/000054897. [DOI] [PubMed] [Google Scholar]
- Chakos M, Lieberman J, Builder R, et al. Increase in caudate nuclei volumes of first-episode schizophrenic patients taking antipsychotic drugs. American Journal of Psychiatry. 1994;151:1430–1436. doi: 10.1176/ajp.151.10.1430. [DOI] [PubMed] [Google Scholar]
- Chang K, Barnea-Goraly N, Karchemskiy A, et al. Cortical magnetic resonance imaging findings in familial pediatric bipolar disorder. Biological Psychiatry. 2005a;58:197–203. doi: 10.1016/j.biopsych.2005.03.039. [DOI] [PubMed] [Google Scholar]
- Chang K, Karchemskiy A, Barnea-Goraly N, et al. Reduced amygdalar gray matter volume in familial pediatric bipolar disorder. Journal of the American Academy of Child and Adolescent Psychiatry. 2005b;44:565–573. doi: 10.1097/01.chi.0000159948.75136.0d. [DOI] [PubMed] [Google Scholar]
- Coffman J, Bornstein R, Olson S, et al. Cognitive impairment and cerebral structure by MRI in bipolar disorder. Biological Psychiatry. 1990;27:1188–1196. doi: 10.1016/0006-3223(90)90416-y. [DOI] [PubMed] [Google Scholar]
- De Azevedo-Marques Perico C, Duran FL, Zanetti MV, et al. A population-based morphometric MRI study in patients with first-episode psychotic bipolar disorder: comparison with geographically matched healthy controls and major depressive disorder subjects. Bipolar Disorders. 2011;13:28–40. doi: 10.1111/j.1399-5618.2011.00896.x. [DOI] [PubMed] [Google Scholar]
- DelBello M, Zimmerman M, Mills N, et al. Magnetic resonance imaging analysis of amygdala and other subcortical brain regions in adolescents with bipolar disorder. Bipolar Disorders. 2004;6:43–52. doi: 10.1046/j.1399-5618.2003.00087.x. [DOI] [PubMed] [Google Scholar]
- Dewan M, Haldipur C, Lane E, et al. Bipolar affective disorder: 1. Comprehensive quantitative computed tomography. Acta Psychiatrica Scandinavica. 1988;77:677–682. doi: 10.1111/j.1600-0447.1988.tb05186.x. [DOI] [PubMed] [Google Scholar]
- Draganski B, Kherif F, Kloppel S, et al. Evidence for segregated and integrative connectivity patterns in the human basal ganglia. Journal of Neuroscience. 2008;28:7143–7152. doi: 10.1523/JNEUROSCI.1486-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eritaia J, Wood S, Stuart G, et al. An optimized method for estimating intracranial volume from magnetic resonance images. Magnetic Resonance in Medicine. 2000;44:973–977. doi: 10.1002/1522-2594(200012)44:6<973::aid-mrm21>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- First M, Spitzer R, Gibbon M, et al. Structured Clinical Interview for DSM-IV. Washington DC: American Psychiatric Press; 1998. [Google Scholar]
- Foland L, Altshuler L, Sugar C, et al. Increased volume of the amygdala and hippocampus in bipolar patients treated with lithium. Neuroreport. 2007;19:221–224. doi: 10.1097/WNR.0b013e3282f48108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fornito A, Yucel M, Wood SJ, et al. Anterior cingulate cortex abnormalities associated with a first psychotic episode in bipolar disorder. British Journal of Psychiatry. 2009;194:426–433. doi: 10.1192/bjp.bp.107.049205. [DOI] [PubMed] [Google Scholar]
- Frangou S. The Maudsley Bipolar Disorder Project. Epilepsia. 2005;46(Suppl 4):19–25. doi: 10.1111/j.1528-1167.2005.463005.x. [DOI] [PubMed] [Google Scholar]
- Gardner H, Kleinmann N, Brook R, et al. The economic impact of bipolar disorder in an employed population from an employer perspective. Journal of Clinical Psychiatry. 2006;67:1209–1218. doi: 10.4088/jcp.v67n0806. [DOI] [PubMed] [Google Scholar]
- Genovese C, Lazar N, Nichols T. Thresholding of statistical maps in functional neuroimaging using the false discovery rate. Neuroimage. 2002;15:870–878. doi: 10.1006/nimg.2001.1037. [DOI] [PubMed] [Google Scholar]
- Gerardin E, Chetelat G, Chupin M, et al. Multidimensional classification of hippocampal shape features discriminates Alzheimer’s disease and mild cognitive impairment from normal aging. Neuroimage. 2009;47:1476–1486. doi: 10.1016/j.neuroimage.2009.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gur R, Maany V, Mozley D, et al. Subcortical magnetic resonance imaging volumes in neuroleptic-naïve and treated patients with schizophrenia. American Journal of Psychiatry. 1998;155:1711–1717. doi: 10.1176/ajp.155.12.1711. [DOI] [PubMed] [Google Scholar]
- Haber SN, Fudge JL, McFarland NR. Striatonigrostriatal pathways in primates form an ascending spiral from the shell to the dorsolateral striatum. Journal of Neuroscience. 2000;20:2369–2382. doi: 10.1523/JNEUROSCI.20-06-02369.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannan KL, Wood SJ, Yung AR, et al. Caudate nucleus volume in individuals at ultra-high risk of psychosis: a cross-sectional magnetic resonance imaging study. Psychiatry Research. 2010;182:223–230. doi: 10.1016/j.pscychresns.2010.02.006. [DOI] [PubMed] [Google Scholar]
- Harvey I, Persaud R, Ron M, et al. Volumetric MRI measurements in bipolars compared with schizophrenics and healthy controls. Psychological Medicine. 1994;24:689–699. doi: 10.1017/s0033291700027847. [DOI] [PubMed] [Google Scholar]
- Holmes C, Hoge R, Collins L, et al. Enhancement of MR images using registration for signal averaging. Journal of Computer Assisted Tomography. 1998;22:324–333. doi: 10.1097/00004728-199803000-00032. [DOI] [PubMed] [Google Scholar]
- Hwang K, Lyoo I, Dager S, et al. Basal ganglia shape alterations in bipolar disorder. American Journal of Psychiatry. 2006;163:276–285. doi: 10.1176/appi.ajp.163.2.276. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Smith S. A global optimisation method for robust affine registration of brain images. Medical Image Analysis. 2001;5:143–156. doi: 10.1016/s1361-8415(01)00036-6. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady M, et al. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17:825–841. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- Koo MS, Levitt JJ, Salisbury DF, et al. A cross-sectional and longitudinal magnetic resonance imaging study of cingulate gyrus gray matter volume abnormalities in first-episode schizophrenia and first-episode affective psychosis. Archives of General Psychiatry. 2008;65:746–760. doi: 10.1001/archpsyc.65.7.746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornhuber J, Wiltfang J, Riederer P, et al. Neuroleptic drugs in the human brain. European Archives of Psychiatry and Clinical Neuroscience. 2006;256:274–280. doi: 10.1007/s00406-006-0661-7. [DOI] [PubMed] [Google Scholar]
- Langan C, McDonald C. Neurobiological trait abnormalities in bipolar disorder. Molecular Psychiatry. 2009;14:833–846. doi: 10.1038/mp.2009.39. [DOI] [PubMed] [Google Scholar]
- Lavretsky H, Kurbanyan K, Ballmaier M, et al. Sex differences in brain structure in geriatric depression. American Journal of Geriatric Psychiatry. 2004;12:653–657. doi: 10.1176/appi.ajgp.12.6.653. [DOI] [PubMed] [Google Scholar]
- Leh SE, Ptito A, Chakravarty MM, et al. Fronto-striatal connections in the human brain: a probabilistic diffusion tractography study. Neuroscience Letters. 2007;419:113–118. doi: 10.1016/j.neulet.2007.04.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehericy S, Ducros M, Van De Moortele PF, et al. Diffusion tensor fiber tracking shows distinct corticostriatal circuits in humans. Annals of Neurology. 2004;55:522–529. doi: 10.1002/ana.20030. [DOI] [PubMed] [Google Scholar]
- Levitt JJ, Styner M, Niethammer M, et al. Shape abnormalities of caudate nucleus in schizotypal personality disorder. Schizophrenia Research. 2009;110:127–139. doi: 10.1016/j.schres.2008.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Looi JC, Macfarlane MD, Walterfang M, et al. Morphometric analysis of subcortical structures in progressive supranuclear palsy: in vivo evidence of neostriatal and mesencephalic atrophy. Psychiatry Research. 2011a;194:163–175. doi: 10.1016/j.pscychresns.2011.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Looi JC, Walterfang M, Styner M, et al. Shape analysis of the neostriatum in subtypes of frontotemporal lobar degeneration: neuroanatomically significant regional morphologic change. Psychiatry Research. 2011b;191:98–111. doi: 10.1016/j.pscychresns.2010.09.014. [DOI] [PubMed] [Google Scholar]
- Looi JC, Walterfang M, Styner M, et al. Shape analysis of the neostriatum in frontotemporal lobar degeneration, Alzheimer’s disease, and controls. Neuroimage. 2010;51:970–986. doi: 10.1016/j.neuroimage.2010.02.017. [DOI] [PubMed] [Google Scholar]
- Malhi GS, Valenzuela M, Wen W, et al. Magnetic resonance spectroscopy and its applications in psychiatry. Australian and New Zealand Journal of Psychiatry. 2002;36:31–43. doi: 10.1046/j.1440-1614.2002.00992.x. [DOI] [PubMed] [Google Scholar]
- Marchand WR, Yurgelun-Todd D. Striatal structure and function in mood disorders: a comprehensive review. Bipolar Disorders. 2010;12:764–785. doi: 10.1111/j.1399-5618.2010.00874.x. [DOI] [PubMed] [Google Scholar]
- McDonald C, Zanelli J, Rabe-Hesketh S, et al. Meta-analysis of magnetic resonance imaging brain morphometry studies in bipolar disorder. Biological Psychiatry. 2004;56:411–417. doi: 10.1016/j.biopsych.2004.06.021. [DOI] [PubMed] [Google Scholar]
- McNeill TH, Brown SA, Rafols JA, et al. Atrophy of medium spiny I striatal dendrites in advanced Parkinson’s disease. Brain Research. 1988;455:148–152. doi: 10.1016/0006-8993(88)90124-2. [DOI] [PubMed] [Google Scholar]
- Miklowitz DJ, Johnson SL. The psychopathology and treatment of bipolar disorder. Annual Review of Clinical Psychology. 2006;2:199–235. doi: 10.1146/annurev.clinpsy.2.022305.095332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasrallah HA, McCalley-Whitters M, Jacoby CG. Cortical atrophy in schizophrenia and mania: a comparative CT study. Journal of Clinical Psychiatry. 1982;43:439–441. [PubMed] [Google Scholar]
- Noga JT, Vladar K, Torrey EF. A volumetric magnetic resonance imaging study of monozygotic twins discordant for bipolar disorder. Psychiatry Research. 2001;106:25–34. doi: 10.1016/s0925-4927(00)00084-6. [DOI] [PubMed] [Google Scholar]
- O’Connell RA, Van Heertum RL, Luck D, et al. Single-photon emission computed tomography of the brain in acute mania and schizophrenia. Journal of Neuroimaging. 1995;5:101–104. doi: 10.1111/jon199552101. [DOI] [PubMed] [Google Scholar]
- Packard MG, Knowlton BJ. Learning and memory functions of the basal ganglia. Annual Review of Neuroscience. 2002;25:563–593. doi: 10.1146/annurev.neuro.25.112701.142937. [DOI] [PubMed] [Google Scholar]
- Pantazopoulos H, Lange N, Baldessarini RJ, et al. Parvalbumin neurons in the entorhinal cortex of subjects diagnosed with bipolar disorder or schizophrenia. Biological Psychiatry. 2007;61:640–652. doi: 10.1016/j.biopsych.2006.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parent A, Hazrati LN. Functional anatomy of the basal ganglia. I. The cortico-basal ganglia-thalamo-cortical loop. Brain Research Brain Research Reviews. 1995;20:91–127. doi: 10.1016/0165-0173(94)00007-c. [DOI] [PubMed] [Google Scholar]
- Passe TJ, Rajagopalan P, Tupler LA, et al. Age and sex effects on brain morphology. Progress in Neuropsychopharmacology and Biological Psychiatry. 1997;21:1231–1237. doi: 10.1016/s0278-5846(97)00160-7. [DOI] [PubMed] [Google Scholar]
- Pearlson GD, Barta PE, Powers RE, et al. Ziskind-Somerfeld Research Award 1996. Medial and superior temporal gyral volumes and cerebral asymmetry in schizophrenia versus bipolar disorder. Biological Psychiatry. 1997;41:1–14. doi: 10.1016/s0006-3223(96)00373-3. [DOI] [PubMed] [Google Scholar]
- Pearlson GD, Garbacz DJ, Breakey WR, et al. Lateral ventricular enlargement associated with persistent unemployment and negative symptoms in both schizophrenia and bipolar disorder. Psychiatry Research. 1984a;12:1–9. doi: 10.1016/0165-1781(84)90133-1. [DOI] [PubMed] [Google Scholar]
- Pearlson GD, Garbacz DJ, Tompkins RH, et al. Clinical correlates of lateral ventricular enlargement in bipolar affective disorder. American Journal of Psychiatry. 1984b;141:253–256. doi: 10.1176/ajp.141.2.253. [DOI] [PubMed] [Google Scholar]
- Pearlson GD, Wong DF, Tune LE, et al. In vivo D2 dopamine receptor density in psychotic and nonpsychotic patients with bipolar disorder. Archives of General Psychiatry. 1995;52:471–477. doi: 10.1001/archpsyc.1995.03950180057008. [DOI] [PubMed] [Google Scholar]
- Rajkowska G, Halaris A, Selemon LD. Reductions in neuronal and glial density characterize the dorsolateral prefrontal cortex in bipolar disorder. Biological Psychiatry. 2001;49:741–752. doi: 10.1016/s0006-3223(01)01080-0. [DOI] [PubMed] [Google Scholar]
- Rowe MK, Chuang DM. Lithium neuroprotection: molecular mechanisms and clinical implications. Expert Reviews in Molecular Medicine. 2004;6:1–18. doi: 10.1017/S1462399404008385. [DOI] [PubMed] [Google Scholar]
- Sanches M, Roberts RL, Sassi RB, et al. Developmental abnormalities in striatum in young bipolar patients: a preliminary study. Bipolar Disorders. 2005;7:153–158. doi: 10.1111/j.1399-5618.2004.00178.x. [DOI] [PubMed] [Google Scholar]
- Sax KW, Strakowski SM, Zimmerman ME, et al. Frontosubcortical neuroanatomy and the continuous performance test in mania. American Journal of Psychiatry. 1999;156:139–141. doi: 10.1176/ajp.156.1.139. [DOI] [PubMed] [Google Scholar]
- Slaght SJ, Paz T, Mahon S, et al. Functional organization of the circuits connecting the cerebral cortex and the basal ganglia: implications for the role of the basal ganglia in epilepsy. Epileptic Disorders. 2002;4(Suppl 3):s9–22. [PubMed] [Google Scholar]
- Smith SM. Fast robust automated brain extraction. Human Brain Mapping. 2002;17:143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strakowski SM, Delbello MP, Adler CM. The functional neuroanatomy of bipolar disorder: a review of neuroim-aging findings. Molecular Psychiatry. 2005;10:105–116. doi: 10.1038/sj.mp.4001585. [DOI] [PubMed] [Google Scholar]
- Strakowski SM, Delbello MP, Sax KW, et al. Brain magnetic resonance imaging of structural abnormalities in bipolar disorder. Archives of General Psychiatry. 1999;56:254–260. doi: 10.1001/archpsyc.56.3.254. [DOI] [PubMed] [Google Scholar]
- Strakowski SM, Delbello MP, Zimmerman ME, et al. Ventricular and periventricular structural volumes in first- versus multiple-episode bipolar disorder. American Journal of Psychiatry. 2002;159:1841–1847. doi: 10.1176/appi.ajp.159.11.1841. [DOI] [PubMed] [Google Scholar]
- Strakowski SM, Wilson DR, Tohen M, et al. Structural brain abnormalities in first-episode mania. Biological Psychiatry. 1993;33:602–609. doi: 10.1016/0006-3223(93)90098-x. [DOI] [PubMed] [Google Scholar]
- Styner M, Oguz I, Xu S, et al. Framework for the statistical shape analysis of brain structures using SPHARM-PDM. Insight Journal. 2006:1–21. [PMC free article] [PubMed] [Google Scholar]
- Swayze VW, 2nd, Andreasen NC, Alliger RJ, et al. Subcortical and temporal structures in affective disorder and schizophrenia: a magnetic resonance imaging study. Biological Psychiatry. 1992;31:221–240. doi: 10.1016/0006-3223(92)90046-3. [DOI] [PubMed] [Google Scholar]
- Szily E, Keri S. Emotion-related brain regions. Ideggyogy Sz. 2008;61:77–86. [PubMed] [Google Scholar]
- Tost H, Ruf M, Schmal C, et al. Prefrontal-temporal gray matter deficits in bipolar disorder patients with persecutory delusions. Journal of Affective Disorders. 2010;120:54–61. doi: 10.1016/j.jad.2009.04.009. [DOI] [PubMed] [Google Scholar]
- Vieta E, Suppes T. Bipolar II disorder: arguments for and against a distinct diagnostic entity. Bipolar Disorders. 2008;10:163–178. doi: 10.1111/j.1399-5618.2007.00561.x. [DOI] [PubMed] [Google Scholar]
- Walterfang M, Looi JC, Styner M, et al. Shape alterations in the striatum in chorea-acanthocytosis. Psychiatry Research. 2011;192:29–36. doi: 10.1016/j.pscychresns.2010.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilke M, Kowatch RA, Delbello MP, et al. Voxel-based morphometry in adolescents with bipolar disorder: first results. Psychiatry Research. 2004;131:57–69. doi: 10.1016/j.pscychresns.2004.01.004. [DOI] [PubMed] [Google Scholar]
- Woo TU, Walsh JP, Benes FM. Density of glutamic acid decarboxylase 67 messenger RNA-containing neurons that express the N-methyl-D-aspartate receptor subunit NR2A in the anterior cingulate cortex in schizophrenia and bipolar disorder. Archives of General Psychiatry. 2004;61:649–657. doi: 10.1001/archpsyc.61.7.649. [DOI] [PubMed] [Google Scholar]
- Worth A, Makris N, Caviness V, et al. Neuroanatomical segmentation in MRI: technological objectives. International Journal of Pattern Recognition and Artificial Intelligence. 1997;11:1161–1187. [Google Scholar]
- Young LT, Bakish D, Beaulieu S. The neurobiology of treatment response to antidepressants and mood stabilizing medications. Journal of Psychiatry and Neuroscience. 2002;27:260–265. [PMC free article] [PubMed] [Google Scholar]
- Yucel K, Taylor VH, McKinnon MC, et al. Bilateral hippocampal volume increase in patients with bipolar disorder and short-term lithium treatment. Neuropsychopharmacology. 2008;33:361–367. doi: 10.1038/sj.npp.1301405. [DOI] [PubMed] [Google Scholar]