Abstract
The present study aims at understanding the timing and nature of mitochondrial DNA (mtDNA) alterations in urothelial cell carcinoma (UCC) and their detection in urine sediments. The entire 16.5 kb mitochondrial genome was sequenced in matched normal lymphocytes, tumor and urine sediments from 31 UCC patients and compared with different clinical stages and histological grades. The mtDNA content index was examined in all the specimens. Sixty five percent (20/31) of the patients harbored at least 1 somatic mtDNA mutation. A total of 25 somatic mtDNA mutations were detected, which were more frequent in the respiratory complex coding regions (Complex-I, III, IV and V) of the mtDNA and significantly affected respiratory complex-III compared to the other complexes (P=0.021–0.039). Compared to stage Ta, mtDNA mutation was higher in stage T1 and significantly higher in stage T2 (P=0.01) patients. MtDNA mutation was also significantly higher (P=0.04) in stage T2 compared to stage T1 patients. Ninety percent (18/20) of the patients harboring mtDNA mutation in the tumor also had mutation in their urine sediments. Eighty percent (20/25) of the tumor-associated mtDNA mutations was detectable in the urine sediments. Compared to the normal lymphocytes, the mtDNA content increased significantly in the tumor (P=0.0013) and corresponding urine sediments (P=0.0025) in 19/25 (76%) patients analyzed. Our results indicate that mtDNA alterations occur frequently in progressive stages of UCC patients and are readily detectable in the urine sediments. MtDNA mutations appear to provide a promising tool for developing early detection and monitoring strategies for UCC patients.
Keywords: Urothelial cell carcinoma, mitochondria, mtDNA alteration, urine detection
Introduction
The mitochondria are unique organelles that have their own DNA (mtDNA), inherited maternally, replicated and transcribed semi-autonomously.1–3 The human mtDNA is a 16.5 kb double stranded closed circular molecule that encodes 12S and 16S rRNAs, 22 tRNAs and 13 proteins (Total 37 genes) required for the oxidative phosphorylation system (OXPHOS).2–3 Most human cells contain hundreds of copies of mtDNA and nearly all of these mtDNA copies are identical i.e. homoplasmic at birth.2–3 Introduction of a new mtDNA mutation in a cell may result in a state of mixed population of mtDNA known as heteroplasmy.3 During replicative segregation, the mtDNA balance can be biased towards homoplasmic mutant or wild type.3 MtDNA is particularly susceptible to damage by environmental carcinogens because it contains no introns, protective histones and is exposed to endogenous reactive oxygen species (ROS) generated as a byproduct of the OXPHOS system.2 Possibly as a consequence of exposure to endogenous ROS, the frequency of mtDNA mutations in cancer cells has been reported to be tenfold higher than nuclear DNA mutations.2
Urothelial cell carcinoma (UCC) ranks ninth in worldwide cancer incidence with 357,000 cases reported worldwide in 2002.4 In the United States, UCC is the fourth most commonly diagnosed cancer in men and eighth most common cancer in women.5 In 2010, an estimated number of new cases and related death will be 70,530 and 14,680 respectively in the US.6 It is almost 12 years, since Polyak et al have reported mtDNA mutation for the first time in colon cancer cell lines and patients.7 Since then, several studies have identified mtDNA sequence variants in different human cancers.7 However, little information is available on a comprehensive analysis of mtDNA alterations in UCC and corresponding body fluids such as urine.
In the present study, we examined the pattern of mtDNA alterations (mtDNA mutation and DNA content index) in UCC patients at various stages along with paired urine sediments. Frequent somatic mtDNA mutations were detected in the progressive stages of the UCC. The majority of the mutations were readily detectable in the corresponding urine sediments. The mtDNA content index also increased significantly in the tumors and corresponding urine sediments compared to the normal lymphocytes of the UCC patients.
Materials and Methods
Patient history and tumor samples
We have obtained matched lymphocytes, tumor tissues and urine sediments from 31 UCC patients after signed informed consent for a Johns Hopkins’ IRB approved protocol. All specimens were from primary UCC patients at their different stages, who underwent curative surgery and did not receive any prior treatment and all were transitional cell carcinomas (TCC). Matched lymphocytes and urine sediments from 10, age matched cancer-free individuals were also collected. The clinical stages and histological grades of all the patients along with the frequency of mtDNA mutations in tumor and urine are shown in Table 1–2.
Table 1.
Pattern of mtDNA mutation in the UCC patients and corresponding urine sediments.
| Patient ID1 |
Number of mtDNA mutation in Tumor/position |
Number of mtDNA mutation in Urine2/Potition |
Stage | Grade |
|---|---|---|---|---|
| UCC3 | 1/A950G | 1/A950G | Ta | III |
| UCC4 | 1/G1606A | 1/G1606A | Ta | III |
| UCC9 | 1/A2644G | 1/A2644G | Ta | III |
| UCC12 | 0 | 0 | Ta | II |
| UCC13 | 1/A2707G | 1/A2707G | Ta | II |
| UCC17 | 1/T3590C | 1/T3590C | Ta | II |
| UCC21 | 0 | 0 | Ta | II |
| UCC24 | 0 | 0 | Ta | II |
| UCC25 | 0 | 0 | Ta | II |
| UCC28 | 0 | 0 | Ta | II |
| UCC1 | 1/A3755C | 1/A3755C | T1 | II |
| UCC5 | 1/C4493T | 1/C4493T | T1 | II |
| UCC7 | 1/A5257G | 1/A5257G | T1 | II |
| UCC10 | 2/T6459C; T6562C | 2/T6459C; T6562C | T1 | II |
| UCC15 | 0 | 0 | T1 | III |
| UCC18 | 0 | 0 | T1 | II |
| UCC20 | 2/C2544T; A3011C | 0 | T1 | II |
| UCC22 | 0 | 0 | T1 | III |
| UCC2 | 1/G8833A | 1/G8833A | T2 | II |
| UCC6 | 1/A9604G | 1/A9604G | T2 | III |
| UCC8 | 0 | 0 | T2 | III |
| UCC11 | 3/G9655A; C11300T; A4337G | 2/G9655A; C11300T; | T2 | III |
| UCC14 | 0 | 0 | T2 | III |
| UCC16 | 1/A14469G | 1/A14469G | T2 | III |
| UCC19 | 1/T14787C | 1/T14787C | T2 | II |
| UCC23 | 0 | 0 | T2 | II |
| UCC26 | 1/C15329T | 1/C15329T | T2 | II |
| UCC27 | 2/G5619A; A14706G | 0 | T2 | III |
| UCC29 | 1/T15722C | 1/T15722C | T2 | III |
| UCC30 | 1/C5317T | 1/C5317T | T2 | II |
| UCC31 | 1/T12083C | 1/T12083C | T2 | III |
UCC: Urothelial cell carcinoma;
MtDNA was isolated from matched urine sediment of the UCC patients and sequenced on the Affymetrix Mitochip v2.0 sequencing array platform along with the corresponding tumor and lymphocytes.
Table 2.
Pattern of somatic mtDNA mutation in the tumor and corresponding urine samples of the UCC patients
| Nucleotide position1 |
Haplotype2 | rCRS3 | Normal4 | Tumor | Urine | mtDNA region |
Amino acid change |
|---|---|---|---|---|---|---|---|
| 950 | H | a | a | g | g | 12SrRNA | - |
| 1606 | H | g | g | a | a | tRNAVal | - |
| 2544 | H | c | c | t | c | 16S rRNA | - |
| 2644 | V | a | a | g | g | 16S rRNA | - |
| 2707 | H | a | a | c | c | 16SrRNA | - |
| 3011 | V | a | a | c | a | 16SrRNA | - |
| 3590 | H | t | t | c | c | ND1 | Leu-Pro |
| 3755 | H | a | a | c | c | ND1 | Leu-Pro |
| 4337 | V | a | a | g | a | tRNAGln | - |
| 4493 | H | c | c | t | t | ND2 | Val-Val |
| 5257 | V | a | a | g | g | ND2 | Leu-Ter5 |
| 5317 | H | c | c | t | t | ND2 | Ala-Gly |
| 5619 | V | g | g | a | g | tRNAAla | - |
| 6459 | H | t | t | c | c | COI | Trp-Arg |
| 6562 | H | t | t | c | c | COI | Phe-Ser |
| 8833 | H | g | g | a | a | ATP6 | Ala-Thr |
| 9604 | H | a | a | g | g | COIII | Asp-Ser |
| 9655 | H | g | g | a | a | COIII | Ser-Asp |
| 11300 | V | c | c | t | t | ND4 | Leu-Phe |
| 12083 | H | t | t | c | c | ND4 | Ser-Pro |
| 14469 | H | a | a | g | g | ND6 | Tyr-His |
| 14706 | H | a | a | g | a | tRNAGlu | - |
| 14787 | H | t | t | c | c | CYTB | Ile-Thr |
| 15329 | H | c | c | t | t | CYTB | Leu-Phe |
| 15722 | H | t | t | c | c | CYTB | Tyr-Arg |
All mtDNA sequence variants were interrogated in the Human Mitochondrial Genome Database (http://www.genpat.uu.se/mtDB/); rCRS:
The mtDNA haplogroup was determined using haplogroup diagnostic SNPs from the Human mitochondrial genome database.
Revised Cambridge Reference Sequence;
MtDNA was prepared from matched normal lymphocytes, microdissected tumor tissues and urine sediments for sequencing on the Affymetrix Mitochip v2.0 platform.
Ter; Termination codon.
Mitochondrial whole genome amplification
Fresh frozen tumor tissues were microdissected followed by genomic DNA extraction according to our standard protocol.9 High quality gnomic DNA was also extracted from normal matched lymphocytes following the same protocol. Twenty five-fifty ml of urine samples were centrifuged at 5000 rpm and voided urine sediments were kept in −80°C until DNA extraction following the above protocol. Whole mitochondrial DNA was amplified in three overlapping long PCR fragments, with each reaction containing 50 ng of genomic DNA as per the Affymetrix Mitochip resequencing array 2.0 protocol (Affymetrix.com).9 Subsequent mtDNA digestion, prehybridization, hybridization, washing, and scanning of the MitoChip were performed as described in the Affymetrix CustomSeq Resequencing protocol.9
Data analysis
Data analysis was performed using Affymetrix GSEQ software. Revised Cambridge Reference Sequence (rCRS) was used as the reference sequence. All the resulting mtDNA sequences were interrogated at different Human Mitochondrial Genome Databases as described earlier to identify specific mtDNA sequence variants.1
Determination of mtDNA mutations in tumors and urine sediments
Somatic mtDNA sequence variants were identified as base pair changes in mtDNA of the tumor when compared with mtDNA sequence of the matched normal lymphocytes (6–8; Table 2). Clonal mtDNA sequence variants were identified as identical base pair changes in matched tumor and urine sediments compared with the normal lymphocytes (Table 2). Germ line sequence variants were identified as base pair changes present in the normal lymphocytes as well as in tumor tissues and/or urine sediments compared with rCRS. 1 In each case, mtDNA sequences were interrogated at the available Human Mitochondrial Genome Databases for defining each sequence variant as described earlier.1
Calculation of the mtDNA mutation load in different respiratory complexes of the mitochondrial genome
The total number of somatic mtDNA mutations detected in individual complexes was considered for calculating the mutation load as described earlier. 1 The mtDNA mutation load on each complex was calculated as follows: total number of nucleotides altered per complex/total number of nucleotides per complex ×100. 1
Quantitative real-time PCR
To examine mtDNA content, we used the 7900HT sequence detection system (Applied Biosystems, Foster City, CA) to amplify nuclear DNA (nDNA) encoded β-Actin and mtDNA encoded Cytochrome C oxidase I (COI) as described earlier.10 This assay quantitatively determines the relative rate of amplification of mtDNA sequence compared to a sequence of nDNA.
Statistical analysis
Student’s t-test, Fisher’s exact test or Chi-square test was performed as appropriate to determine the statistical significance of any difference observed. P value less than 0.05 was considered significant. All P values generated were two-sided.
Results
Pattern of somatic mtDNA mutations in the UCC patients
To understand the frequency and nature of mtDNA mutations in the UCC patients, we analyzed microdissected tumor tissues and matched normal lymphocytes from 31 UCC patients on a reliable mtDNA sequencing platform. 9 Average call rate on the Mitochip version 2.0 was 95.6%. The patients belonged to the major European haplogroup H and V (Table 2). Sixty five percent (20/31) of the UCC tumors harbored at least 1 somatic mtDNA mutation (Table 1–2). The mtDNA mutations were mostly heteroplasmic in nature and nucleotide transitions (A↔G; T↔C) (Table 2). A total of 25 somatic mtDNA mutations were detected in different regions of the mitochondrial genome (Table 2). Nine were from the rRNA and tRNA coding regions of the mtDNA; 1 from the 12SrRNA; and 4 each from 16SrRNA and tRNAs. The remainder of the 16 mutations was found in different respiratory complex coding regions; 2 were from ND1, 3 from ND2; 2 were from ND4, 1 from ND6 (Complex I); 3 were from CYTB (Complex III); 2 each from COXI and COXIII (Complex IV) and 1 from ATPase6 (complex V). Of the 16 respiratory complex coding mtDNA mutations, 15 (94%) were non-synonymous in nature (Table 2). The number of mtDNA mutation was higher in the different respiratory complex coding regions (complex I, III, IV and V) compared to the rRNA and tRNA coding regions of the mtDNA (16 vs. 9; Table 2). Other than the “somatic mtDNA mutations”, we also identified a number of known single nucleotide polymorphic sequence variants (SNPs) in these patients (Table S1).
Pattern of somatic mtDNA mutation in the urine sediments of the UCC patients
We further assessed corresponding urine sediments obtained from the 20 UCC patients having mtDNA mutation in the tumors (Table 1–2). All corresponding urine samples were sequenced on the same Mitochip version 2.0 sequencing platform. Ninety percent (18/20) of the patients with mtDNA mutation in the tumor also detected with the tumor-associated mtDNA mutation in the corresponding urine sediments except one mutation (Patient UCC11, Table 1). Eighty percent (20/25) of the mtDNA mutations present in the tumor were detectable in the urine sediments of the UCC patients (Table 1–2).
Association between clinical characteristics and mtDNA mutation in the UCC patients
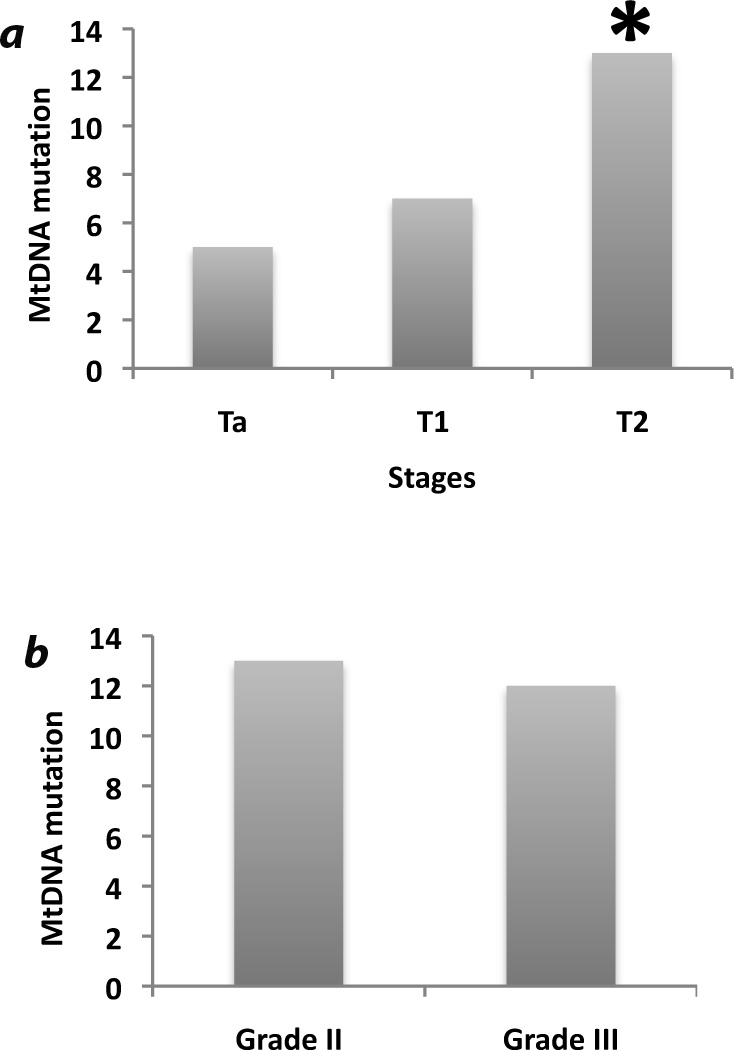
We also compared the pattern of mtDNA mutation with clinical characteristics to understand their correlation with UCC progression. Of the 31 UCC patients, 10 were stage Ta, 8 were T1 and 13 were T2 stage patients (Table 1). We have observed a progressive increase of mtDNA mutation across the different clinical stage. Compared to the Ta stage, the number of patients with mtDNA mutation was higher in the T1 and significantly higher in T2 (P=0.01) stage (Figure 1A). MtDNA mutation was also significantly higher in the T2 stage patients (P=0.04) compared to the T1 stage patients (Figure 1A). No significant association was observed between mtDNA mutation and histological grades of the UCC patients ((P=0.24, Figure 1B). Other than the UCC patients, we also examined matched lymphocytes and urine sediments from 10 cancer-free individuals for mtDNA mutation. No somatic mtDNA mutation was detected in the lymphocytes or urine sediments obtained from 10 cancer-free individuals (Data not shown).
Figure 1.
Prevalence of mtDNA mutations across the different stages and histological grades of the UCC patients. a. Compared to the Ta stage patients, the number of mtDNA mutation was higher in the T1 and significantly higher in T2 (P=0.01) stage. MtDNA mutation was also significantly higher in the T2 stage patients (P=0.04) compared to the T1 stage patients. b. No significant association was observed between mtDNA mutation and histological grades of the UCC patients (P=0.24). *P value <0.05)
MtDNA mutations affect mitochondrial respiratory Complex-III and may be pathogenic
We also determined the theoretical effect of somatic mtDNA mutation on different respiratory complexes encoded by the mitochondrial genome. 1 Only respiratory complex (complex I, III, IV and V) coding non-synonymous mtDNA mutations observed in the patients were considered, and the mutational load for each complex was calculated as described earlier. 1 As shown in Figure 2, the mutational load was significantly higher in Complex III (Cytochrome b, CYTB) compared to the complex-I (P=0.021); complex-IV (P=0.025) and complex-V (P=0.039).
Figure 2.
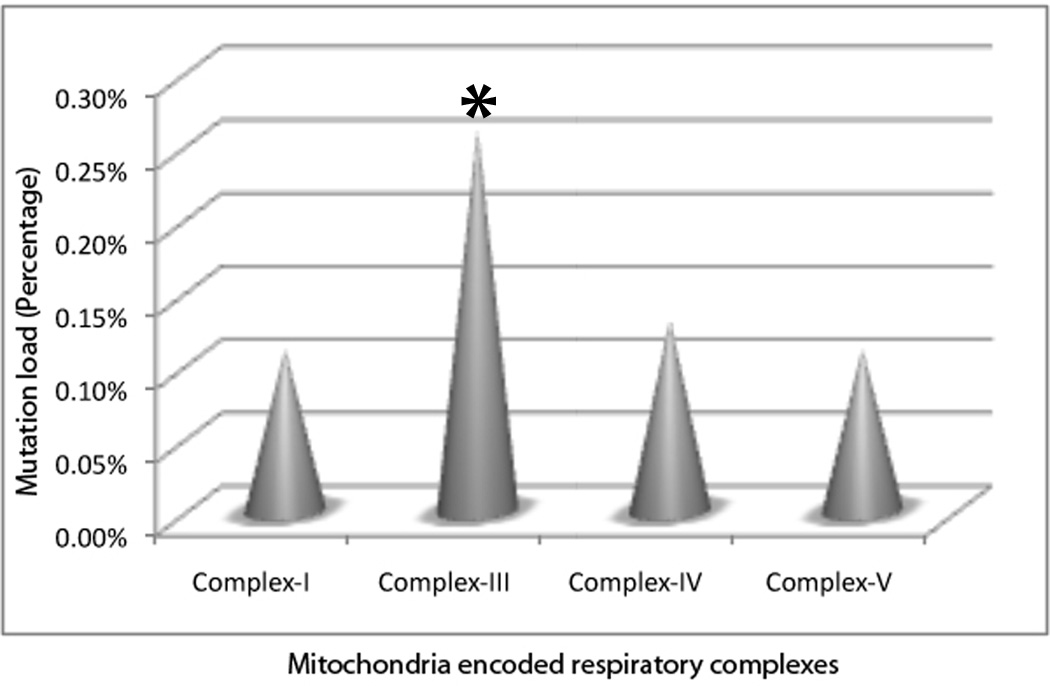
The effect of mtDNA mutation on different mitochondria encoded respiratory complexes. Mutational load was significantly higher in Complex III (Cytochrome b, CYTB, Indicated by asterisk) compared to complex-I (P=0.021); complex-IV (P=0.025) and complex-V (P=0.039).
Pattern of mtDNA content in the patients
Alteration in the mtDNA content index is associated with a commonly seen increase in the number and copy of mtDNA in tumors.10 We performed a quantitative real-time PCR to determine mtDNA content in patients’ tumors, corresponding urine sediments and normal lymphocytes available from 25 UCC patients only. This assay determines the relative rate of amplification of mtDNA sequence compared to a sequence of nDNA. We observed a significant increase (P=0.0013) in the mtDNA content in the tumor compared to the matched lymphocyte of 76% (19/25) of the UCC patients (Figure 3A). We also observed a significant increase (P=0.0025) of the mtDNA content in the corresponding urine sediments of the above 19 UCC patients compared to the normal lymphocytes. The 6 patients without significant change in the mtDNA content index also did not exhibit any somatic mtDNA mutation. We could not detect any significant mtDNA content alteration in the 10 cancer-free individuals analyzed (Figure 3B).
Figure 3.
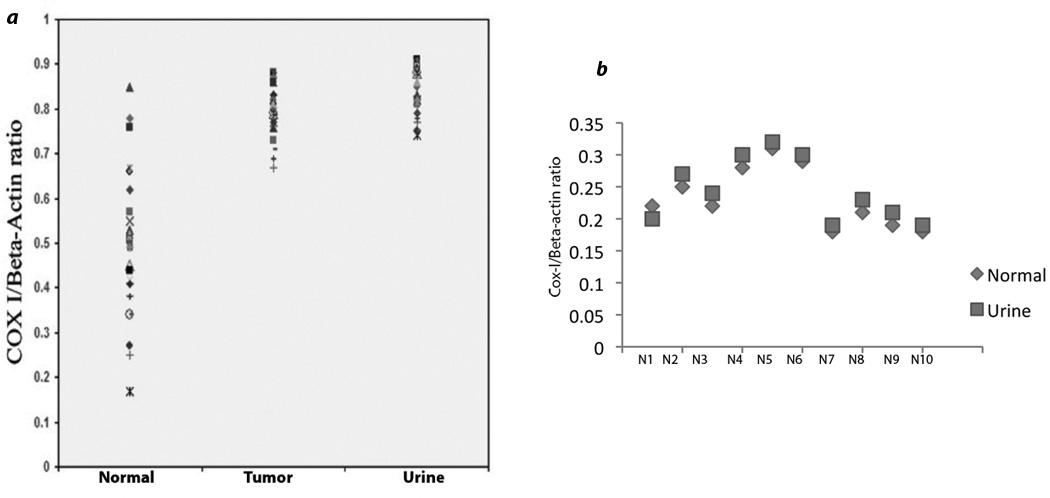
Pattern of mtDNA content index in the UCC patients. The employed assay quantitatively determines the relative rate of amplification of mtDNA sequence (COX-I) compared to a sequence of nDNA (Beta-actin). a. The mtDNA content index was significantly higher (P=0.0013) in the tumors of the 76% (19/25) of the UCC patients compared to the matched normal lymphocytes. MtDNA content was also significantly higher (P=0.0025) in the corresponding urine sediments compared to the normal lymphocytes in all the 19 patients. b. Compared to the normal lymphocytes, mtDNA content did not differ significantly (P=0.52) in the corresponding urine sediments of the 10 cancer-free normal individuals (N1#x02013;N10).
Germline mtDNA sequence variants in the patients
Other than the somatic mtDNA mutation, we also examined the pattern of germ line mtDNA sequence variation in matched normal lymphocytes and tumors as well as corresponding urine samples by comparing each patient with the reference rCRS. A total of three coding germ line mtDNA sequence variants were detected in these patients (Table 3). Two out of the three germ line sequence variants were non-synonymous in nature and were from the Complex-I and complex-III, respectively.
Table 3.
Germ line mtDNA sequence variants in the UCC patients
| Locus | Nucleotide position |
Nucleotide change | Amino acid change |
|---|---|---|---|
| ND4 | 11465 | T-C | Synonymous |
| ND6 | 14564 | A-G | V-A |
| CYTB | 15299 | T-C | L-S |
Discussion
In the present study, we examined the pattern of mtDNA alterations in tumors and corresponding urine sediments obtained from 31 primary UCC patients on the Affymetrix Mitochip 2.0 sequencing platform.9 and by real time PCR analysis. This high-throughput mtDNA resequencing platform overcomes problems with the conventional DNA sequencing method as reliable mutation detection is possible.1,9. Stringent criteria were followed and appropriate databases were used to classify every single mtDNA sequence variant, considering the recent findings of erroneous reporting of mtDNA mutation in different cancers.1 Based on the standard data interpretation criterion, we detected both somatic and germ line mtDNA sequence variants in these patients and also confirmed that the resultant sequence variations are not known single nucleotide polymorphisms in different populations as per the available mtDNA databases.1
Early detection of UCC remains a challenge since most patients present only with non-visible microhematuria and this finding alone is also nonspecific. Moreover, progression of superficial UCC tumors to muscle invasive disease remains a major problem in UCC management. In this context, evaluation of urine sediments using mtDNA mutation could be useful as mtDNA content and mutation analysis seems to have favorable characteristics as a biomarker, with excellent cancer specificity and reliable technology for high throughput detection.11 Initial clonal detection of tumor cells using nuclear genome was demonstrated earlier using p53 mutation.12 But due to the large copy number of mutated mtDNA compared with the nDNA in cancer cells, mtDNA detection is more suitable for cancer diagnostic applications.11 Moreover, profiling of a smaller panel of mtDNA mutations affecting different respiratory complexes along with nDNA changes could also be useful for screening populations at risk such as heavy smokers. Recently, we identified tumor associated clonal mtDNA mutation in histologically normal respiratory epithelium and surgical margins of smokers with lung and head and neck cancer respectively.1,10
In the present study, the mtDNA analysis was performed in a blinded fashion.1, and numerous somatic mtDNA mutations were detected in the UCC patients and found to be associated with the disease stage progression. A number of mutations were found in the 16SrRNAs and tRNAs encoding regions of the mtDNA, a frequent target in different tumors.13 Strikingly, the majority of the tumor associated mtDNA mutations were detectable in the corresponding urine sediments. It is very unlikely that the high-resolution Mitochip v2.0 system has a low sensitivity for mutation detection; otherwise, we would not have detected matched mtDNA mutations in a few cells procured from the urine sediments of the same patient. However, due to the lack of availability of precancerous specimens, we could not examine the earliest time point of mtDNA mutation. Yet, it seems that mtDNA mutation screening may be a cornerstone of non-invasive detection strategies for UCC.
Clonal mtDNA changes suggest a functional role in UCC progression. Most of the mtDNA mutations observed in this study were at the rRNA, tRNA and different respiratory complex coding regions of the mtDNA, and no mutation was detected in the regulatory D-Loop region where transcription of the all the 13 mtDNA-encoded genes starts. We hypothesize that for progression, cells prefer a functional D-Loop region with growth promoting mtDNA mutations in other target regions. The respiratory complex coding mtDNA mutation load was significantly higher in Cytochrome b (CYTB, Complex-III). Interestingly, we have detected mtDNA mutations in a small subset of UCC patients in the CYTB region.14 Forced overexpression of one of these CYTB mutations triggered increased cell growth and proliferation in both non-tumorigenic human and tumorigenic mouse UCC cells by producing high amounts of reactive oxygen species (ROS).15 Thus, it appears that non-synonymous somatic mtDNA mutation in specific respiratory complex can severely affect the OXPHOS functioning for favoring tumor progression. Other than UCC, CYTB mutation was also reported in different tumor types.8,10,13 However, the somatic mtDNA mutations observed in the present study were not reported to date in other tumor types or mitochondrial disease.
The presence of germ line mtDNA sequence variants could be an indicator of a high susceptibility genetic background.1 The presence of clonal germ line mtDNA sequence variants in tumors and urine sediments may increase the susceptibility of acquiring additional independent somatic mtDNA and/or nDNA alterations necessary for tumorigenic transformation. Notably, all 3 germ line sequence variants were from complex-I (ND4 and ND6) and complex-III (CYTB), the major sites for ROS production.3 Moreover, a deletion mutation from CYTB region also shown to induced increased ROS production in bladder cancer15. This increased ROS generated by various mtDNA mutations including CYTB may interplay with a number of growth regulatory and modifier genes for favoring tumor growth and expansion.3,15
Other than mtDNA mutation, increased mtDNA content is also an indicator of increased mitochondrial function necessary for sustained tumor growth.16–17 Increased mtDNA content was reported in different tumors, but so far not simultaneously assessed in matched tumor and urine sediments of UCC patients.10, 16–17 A recent study reported increased mtDNA content in urine sediment obtained from some UCC patients compared to normal lymphocytes.18 However, matched tumor samples were not assessed in parallel.18 Notably, a number of studies reported increased or decreased mtDNA copy number and somatic mtDNA mutations involving D Loop and other coding regions of the mtDNA in various cancers pointing towards the contribution of mtDNA alteration in tumorigenesis (Table S2).19 Thus, simultaneous detection of both mtDNA mutation and copy number alteration could be useful for developing mitochondria based disease management strategies. In the present study, concurrence of increased mtDNA content and frequent mtDNA mutations in both the tumors and corresponding urine sediments of UCC patients indicates that these events most commonly occur simultaneously. If so, just measuring the simple mtDNA index alone may help validate our findings and pave the way for further development of this approach in the early detection and monitoring of UCC in the clinic. Given the fact that mutated mtDNA copy number is abundant in cancer cells, a highly sensitive detection of mtDNA alteration in urine is possible and may potentially be a useful tool for cancer detection and prognostication.
Supplementary Material
Acknowledgement
This work was supported by NIH PO1 grant 5PO1CA077664 and EDRN UO1grant 5UO1CA084986 (DS); US-Egypt Joint Science and Technology fund-58-3148-169 and A D Williams fund-646299 (SD).
References
- 1.Dasgupta, Koch R, Westra WH, Califano JA, Ha PK, Sidransky D, Koch WM. Mitochondrial DNA mutation in margins and tumors of recurrent HNSCC patients. Can Prev Res. 2010;3:1205–1211. doi: 10.1158/1940-6207.CAPR-10-0018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Verma M, Kagan J, Sidransky D, Srivastava S. Proteomic analysis of cancer-cell mitochondria. Nat Rev Cancer. 2003;3:789–795. doi: 10.1038/nrc1192. [DOI] [PubMed] [Google Scholar]
- 3.Wallace DC. A mitochondrial paradigm of metabolic and degenerative diseases, aging, and cancer: a dawn for evolutionary medicine. Annu Rev Genet. 2005;39:359–407. doi: 10.1146/annurev.genet.39.110304.095751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ploeg M, Aben KK, Kiemeney LA. The present and future burden of urinary bladder cancer in the world. World J Urol. 2009;27:289–293. doi: 10.1007/s00345-009-0383-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Madeb R, Golijanin D, Knopf J, Messing EM. Current state of screening bladder cancer. Expert Rev Anticancer Ther. 2007;7:981–987. doi: 10.1586/14737140.7.7.981. [DOI] [PubMed] [Google Scholar]
- 6. www.cancer.gov.
- 7.Polyak K, Li Y, Zhu H, Lengauer C, Willson JK, Markowitz SD, Trush MA, Kinzler KW, Vogelstein B. Somatic mutations of the mitochondrial genome in human colorectal tumors. Nature Genet. 1998;20:291–293. doi: 10.1038/3108. [DOI] [PubMed] [Google Scholar]
- 8.Chatterjee A, Mambo E, Sidransky D. Mitochondrial DNA mutations in human cancer. Oncogene. 2006;25:4663–4674. doi: 10.1038/sj.onc.1209604. [DOI] [PubMed] [Google Scholar]
- 9.Zhou S, Kassauei K, Cutler DJ, Kennedy GC, Sidransky D, Maitra A, Califano J. An oligonucleotide microarray for high-throughput sequencing of the mitochondrial genome. J Mol Diagn. 2006;8:476–482. doi: 10.2353/jmoldx.2006.060008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dasgupta S, Yung RC, Westra WH, Rini DA, Brandes J, Sidransky D. Following mitochondrial footprints through a long mucosal path to lung cancer. PLoS One. 2009;4:e6533. doi: 10.1371/journal.pone.0006533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.He Y, Wu J, Dressman DC, Iacobuzio-Donahue C, Markowitz SD, Velculescu VE, Diaz LA, Jr, Kinzler KW, Vogelstein B, Papadopoulos N. Heteroplasmic mitochondrial DNA mutations in normal and tumour cells. Nature. 2010;464:610–614. doi: 10.1038/nature08802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brennan JA, Mao L, Hruban RH, Boyle JO, Eby YJ, Koch WM, Goodman SN, Sidransky D. Molecular assessment of histopathological staging in squamous-cell carcinoma of the head and neck. N Engl J Med. 1995;332:429–435. doi: 10.1056/NEJM199502163320704. [DOI] [PubMed] [Google Scholar]
- 13.Zhou S, Kachhap S, Sun W, Wu G, Chuang A, Poeta L, Grumbine L, Mithani SK, Chatterjee A, Koch W, Westra WH, Maitra A, et al. Frequency and phenotypic implications of mitochondrial DNA mutations in human squamous cell cancers of the head and neck. Proc Natl Acad Sci U S A. 2007;104:7540–7545. doi: 10.1073/pnas.0610818104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fliss MS, Usadel H, Caballero OL, Wu L, Buta MR, Eleff SM, Jen J, Sidransky D. Facile detection of mitochondrial DNA mutations in tumors and bodily fluids. Science. 2000;287:2017–2019. doi: 10.1126/science.287.5460.2017. [DOI] [PubMed] [Google Scholar]
- 15.Dasgupta S, Hoque MO, Upadhyay S, Sidransky D. Mitochondrial cytochrome B gene mutation promotes tumor growth in bladder cancer. Cancer Res. 2008;68:700–706. doi: 10.1158/0008-5472.CAN-07-5532. [DOI] [PubMed] [Google Scholar]
- 16.Mambo E, Chatterjee A, Xing M, Tallini G, Haugen BR, Yeung SC, Sukumar S, Sidransky D. Tumor-specific changes in mtDNA content in human cancer. Int J Cancer. 2005;6:920–924. doi: 10.1002/ijc.21110. [DOI] [PubMed] [Google Scholar]
- 17.Jiang WW, Masayesva B, Zahurak M, Carvalho AL, Rosenbaum E, Mambo E, Zhou S, Minhas K, Benoit N, Westra WH, Alberg A, Sidransky D, et al. Increased mitochondrial DNA content in saliva associated with head and neck cancer. Clin Cancer Res. 2005;117:2486–2491. doi: 10.1158/1078-0432.CCR-04-2147. [DOI] [PubMed] [Google Scholar]
- 18.Yoo JH, Suh B, Park TS, Shin MG, Choi YD, Lee CH, Choi JR. Analysis of fluorescence in situ hybridization, mtDNA quantification, and mtDNA sequence for the detection of early bladder cancer. Cancer Genet Cytogenet. 2010;198:107–117. doi: 10.1016/j.cancergencyto.2009.12.017. [DOI] [PubMed] [Google Scholar]
- 19.Chatterjee a, Dasgupta S, Sidransky D. mitochondrial subversion in cancer. Cancer Prev Res. 2011;4:638–654. doi: 10.1158/1940-6207.CAPR-10-0326. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.