Abstract
Tasks that tax working memory (WM) have consistently been found to decrease mind wandering. These findings may indicate mind wandering requires WM resources for maintenance and cannot persist when the resources are being consumed by a task. An alternative explanation for these findings, however, is that mind wandering persists without the support of WM but is nonetheless decreased during any demanding task because good performance requires the restriction of attention from task-unrelated thought (TUT). The present study tests these two competing hypotheses by asking whether individuals with greater WM resources mind wander more during an undemanding task, as would only be predicted by theory that WM supports TUT. We found that higher WM capacity individuals reported more TUT in undemanding tasks, suggesting that WM enables the maintenance of mind wandering.
The average mind wanders half of daily life, often thinking quite spontaneously about personal priorities unrelated to the task at hand (Killingsworth & Gilbert, 2010; Giambra, 1995; Klinger & Cox, 1987). Such task-unrelated thought (TUT) presents a paradox: while TUT’s spontaneous nature suggests it is a resource-free process, TUT’s priority-driven nature suggests it is a resource-intensive process (Smallwood & Schooler, 2006). Indeed, priority-driven attention that maintains and manipulates information not present is classically considered to require working memory (WM) resources (Baddeley & Hitch, 1974), and WM-related brain areas are active during TUT (Christoff, Gordon, Smallwood, Smith, & Schooler, 2009; Stawarczyk, Majerus, Maquet, & D’Argembeau, 2011). The tension between the spontaneous and goal-directed features of TUT has stimulated debate of whether mind wandering consumes WM resources.
One perspective suggests TUT requires WM resources to persist. According to this view, a fragment of TUT can spontaneously occur but requires WM resources to elaborate into a train of TUT (Teasdale, Proctor, Lloyd, & Baddeley, 1993; Smallwood & Schooler, 2006). The theory rests on findings that TUT increases when WM resources are available, such as during WM-undemanding tasks (Teasdale et al., 1993, Mason et al., 2007). Conversely, TUT decreases when WM resources are scarce, such as during WM-demanding tasks (Teasdale et al., 1995). Further, when TUT does occur during tasks relying on WM, performance can decline (Smallwood et al., 2004; Cheyne, Solman, Carriere, & Smilek, 2009), suggesting that maintaining TUT may divert cognitive resources needed for tasks (Smallwood, Beach, Schooler, & Handy, 2008; Teasdale et al., 1995).
An alternative perspective suggests TUT does not require WM resources. According to this view, TUT spontaneously occurs and persists in a resource-free manner, and only enters awareness when WM fails to restrict attention to a task (McVay & Kane, 2009, 2010). This theory can likewise explain why TUT decreases during WM-demanding tasks, as good task performance requires WM to unfailingly restrict attention from TUT. Perhaps the strongest evidence for the theory comes from studies of individual differences in working memory capacity (WMC). A recent laboratory study indicated that individuals who possess greater WM resources report less TUT during a go/no-go task commonly used to study mind wandering (the Sustained Attention to Response Task or SART, McVay & Kane, 2009).
This evidence that WM may inhibit mind wandering seemingly contradicts any role for WM in maintaining TUT. However, to conclude that WM solely inhibits TUT is premature. The SART’s low frequency of trials that refresh task goals (11% no-go trials in McVay & Kane, 2009) encourages using WM resources to proactively maintain no-go-relevant task goals in order to overcome the habitual go response reinforced by ~90% of trials. Thus, the SART places demands on WM resources which otherwise might have facilitated TUT.
In contrast, tasks with a high frequency of trials that refresh task goals (50% or 100%) relieve WM from proactively maintaining task goals (Kane & Engle, 2003). Such WM-undemanding contexts are well suited for exploring whether greater WM resources, when free, support greater TUT. Therefore, to evaluate the two competing models of TUT, we gave participants from a range of WMCs WM-undemanding tasks permissive to mind wandering. We used a visual search task (50% incongruent targets) in experiment 1 and a task of tapping in time with normal breathing (100% targets) in experiment 2. We then asked whether participants with greater WMC mind wandered more in these WM-undemanding contexts, as predicted only by theory that WM can support TUT.
Experiment 1
Method
Participants and Procedure
Ninety-three community participants received $10/hr to complete a 30-minute visual search task followed by a WMC assessment, the Automated Operation Span (OSPAN) task, known to correlate well with established WMC assessments and predict general fluid intelligence (for detailed task descriptions, see Forster & Lavie, 2009, Experiment 4; Unsworth, Heitz, Schrock, & Engle, 2005).
As is standard, nine participants were excluded for scoring below 85% on OSPAN’s secondary math task and 10 were excluded for performing at chance in visual search, leaving 74 participants (28 males), ages 18–61 (M 24.7, SD 8.9), with OSPAN scores 9–73 (M 58.9, SD 13) that were squared to yield a more normal distribution (skew = −.81, kurtosis = −.46).
Visual Search Task
For each trial a central ring of six letters containing the target—either X or N—was presented for 100 ms with a peripheral distractor—either X or N—to the left or right. Participants indicated target identity by key press as quickly and accurately as possible.
Trials varied on 2 dimensions, perceptual load (low, high) and distractor (congruent, incongruent), and occurred in blocks of 48 trials of a single load condition. In low load blocks non-target letters in the central ring were small Os, allowing the target to be easily distinguished. In contrast, on high load blocks non-target letters (H K M V W Z) were angular and target-sized, making the target more difficult to perceive. There were 8 blocks of each load condition, ordered ABBAABBAABBAABBA. Within each block, trials varied in distractor identity (50% incongruent). Stimulus presentation was counterbalanced on identity and position of targets and distractors.
At the end of each block came the thought probe, “What were you thinking just now?” Participants pressed 0 if they had been thinking task-related thoughts, that is, thoughts “about the task you are doing at that exact moment”, such as “where’s the X, oh there it is.” Conversely, participants pressed 1 for task-unrelated thoughts such as “I must stop by the supermarket on the way home” or “I made lots of mistakes at the beginning of the experiment.” TUT scores equaled the percentage of probes on which a participant indicated TUT.
OSPAN Task
For each of 15 trials, a display alternated 3–7 times between single letters for memorization and math equations (e.g. 1 + (3/3) = ?) for verification under response deadline. Deadlines were customized based on the participant’s latencies (M + 2.5 SDs) for 15 math-only practice items. A participant’s OSPAN score equaled total letters recalled in correct sequence at the ends of trials.
Statistical Analyses
TUT was analyzed using a General Linear Model with a within-participant categorical factor of perceptual load, a continuous quantitative factor of mean-centered OSPAN2, and their interaction. Low load error rates as well as reaction time (RT) and distractor response-competition (RC, percent increase in RT in incongruent relative to congruent trials, as in Forster & Lavie, 2009) from only accurate trials served as covariates when necessary.
Results
To test the theory that WM resources are necessary for mind wandering, we asked whether higher WMC predicts more TUT during low perceptual load visual search, a context permissive to mind wandering (Forster & Lavie, 2009). A true absence of positive correlation between WMC and TUT would refute the theory, but in fact higher WMC individuals reported greater TUT during low load, r(72) = .28, p = .01 (see Fig. 1). This did not result from a general bias of higher WMC individuals to report greater TUT. TUT did not depend on WMC during high perceptual load blocks, r(72) = −.03, p = .83, consistent with the Load X WMC interaction, F(1,72) = 9.3, p < .01, ηp2 = .11, and theory that high load induces low TUT regardless of an individual’s WM resources by exhausting limited perceptual capacity in task-relevant processing (Lavie, 2005; Forster & Lavie, 2007, 2009; Lavie, Herst, de Fockert, & Viding, 2004). In sum, participants with more WM resources reported more mind wandering in an undemanding context.
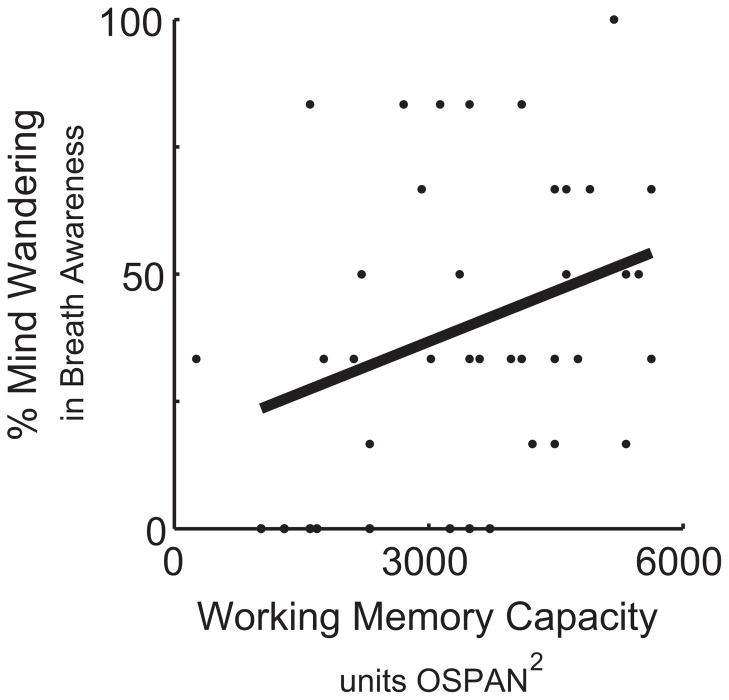
Figure 1.
The relation between working memory (WM) capacity and mind wandering during a WM-undemanding, low perceptual load visual search task in experiment 1. Pearson r(72) = .28, p = .01
While we propose that higher WMC participants mind wandered more because they had more WM resources to mind wander with, an alternative explanation is that higher WMC participants found the low load task easier. Indeed, task ease is known to increase TUT (McKiernan, D’Angelo, Kaufman, & Binder, 2006) and under certain conditions WMC may facilitate visual search performance (Kane, Poole, Tuholski, & Engle, 2006; Sobel, Gerrie, Poole, & Kane, 2007; Poole & Kane, 2009). To explore this alternative explanation, planned comparisons at low load revealed that higher WMC individuals did not significantly differ in errors or RT, ps > .1, but showed less RC to distractors, r(72) = .29, p = .01. None of these performance measures correlated with TUT, however, ps > .2. Nonetheless, we re-analyzed the correlation between WMC and TUT while controlling for the performance measures. Higher WMC still significantly predicted greater TUT, sr(69) = .25, p = .04, suggesting the finding is not simply due to differences in task difficulty.
Experiment 2
To replicate the positive association between WMC and TUT and further rule out task difficulty as an explanation of findings, we designed a WM-undemanding breath awareness task without any detectable WMC-related performance differences. We then asked whether participants with greater WMC mind wandered more during it.
Method
Participants and Procedure
Forty-five community participants received $10/hr to complete a 6-min resting baseline, a 20-minute breath counting task, a 9-minute breath awareness task, questionnaires, and the OSPAN. Only breath awareness and OSPAN data are discussed here.
As is standard, three participants were excluded for scoring below 85% on OSPAN’s secondary math task, leaving 42 participants (17 males), ages 18–65 (M 26.5, SD 10), with OSPAN scores 16–75 (M 57.7, SD 13.4) that were squared to yield a more normal distribution (skew = −.28, kurtosis = −.81).
Breath Awareness
Participants were instructed “be aware… of the movement of breath… breathe normally… [with] each exhale, press the letter L”. Participants were also instructed to self-catch mind wandering: “If you suddenly realize that your attention was completely off task, that’s ok. Press the CONTROL button, and gently bring the attention back to your breath.”
Every ~90 sec (range 60–120 sec) a set of two probes appeared in succession asking, “just now where was your attention?” and “how aware were you of where your attention was?” The first probe in each set was rated on a 6-point Likert scale ranging from “completely on-task” to “completely off-task”, and is the only probe data presented here. TUT scores equaled the percentage of these six probes which a participant rated > 3. Instruction examples of TUT were the same as those given in experiment 1.
A subset of participants wore a respiration belt (BIOPAC) depending on its availability, as it was shared by multiple research studies.
Results
Compliance with motor instructions was confirmed in a subset of participants, with mean keypress rate tracking mean breath rate, r(9) = .99, p < .01. Mean keypress rate did not depend on WMC, r(40)=.02, p = .91.
In line with theory that WM resources are necessary for mind wandering, we found that higher WMC predicted more probe-caught TUT during breath awareness, r(40) = .33, p = .03 (see Fig. 2). Self-caught TUT did not correlate with WMC, r(40) = −.05, p = .76.
Figure 2.
The relation between working memory (WM) capacity and mind wandering during a WM-undemanding, breath awareness task in experiment 2. Pearson r(40) = .33, p = .03
Discussion
The present study establishes that task-unrelated thought (TUT) increases with increasing working memory capacity (WMC) when tasks make few demands on working memory (WM) resources. These findings support the view that WM enables TUT to persist in situations permissive to mind wandering (Teasdale et al., 1993; Smallwood & Schooler, 2006). Conversely, these findings challenge claims that WM’s sole relation to TUT is inhibitory, restricting attention to task (McVay & Kane, 2009, 2010). Critically, such a theory cannot easily explain why participants with more WM resources, though better at restricting attention, nonetheless mind wandered more under low perceptual load and breath awareness.
In light of existing data, we propose that WM enables a context-dependent moderation of TUT. In WM-undemanding contexts when restricting attention to task is not prioritized, WM resources are free to maintain personal priorities and facilitate TUT. However, in WM-demanding contexts when restricting attention to task is prioritized, WM resources can help maintain the goal to stay on task and inhibit TUT. Such a dual role of WM resources could explain both the positive correlation between WMC and TUT observed in the current study and the negative correlation between WMC and TUT during the Sustained Attention to Response Task (SART) (McVay & Kane, 2009). Even in the SART, though, when TUT does occur we propose it requires WM resources to help it persist. This view is consistent with findings of increased activity in brain areas associated with WM during mind wandering in the SART (Christoff, et al., 2009; Stawarczyk, et al., 2011).
The context-dependent effect of WM on mind wandering is likely to generalize beyond the laboratory. Experience sampling in daily life indicates that higher WMC participants mind wander more on tasks in which they report not trying to concentrate, but mind wander less than low WMC participants when concentrating (Kane et al., 2007). As our data provide a concurrent measurement of TUT and behavior we are able to verify that the positive WMC/TUT association is not simply a side effect of WMC-related differences in task performance, a finding with potential ecological validity given that TUT in the laboratory can predict TUT in life (McVay, Kane, & Kwapil, 2009).
The present data suggest that in circumstances conducive to mind wandering WM resources can help maintain TUT. An outstanding question is how. We suggest that if WM resources are maintaining personal priorities, then they will prime the elaboration of TUT fragments into coherent trains of TUT based on those priorities. Supporting this view, when a context makes WM resources available, TUT forms more connected sequences (Teasdale et al., 1993). TUT also becomes more future-oriented (Smallwood, Nind, & O’Connor, 2009), especially for those with greater WMC (Baird, Smallwood, & Schooler, in press), in line with findings that future-oriented TUT increases when personal priorities are primed (Stawarczyk, Majerus, Maj, Van der Linden, & D’Argembeau, 2011).
However, free WM resources are not obligated to support TUT. As in the high load condition of experiment 1, task-relevant perceptual processing may cut off TUT—presumably at early perceptual processing stages (Lavie, 2005)—rendering it unavailable for elaboration by WM. Or task-unrelated personal priorities may be recognized as unhelpful and released from WM maintenance. Training in these and other strategies holds promise for reducing mind wandering associated with unhappiness (Killingsworth & Gilbert, 2010).
The relationship between WM and TUT merits more research. Given that half of daily life is typically spent mind wandering, opportunity abounds for exploring the way WM shapes and perpetuates our internal worlds.
Acknowledgments
The authors thank the Lavie and Engle labs for sharing E-prime scripts; A. Shackman for manuscript feedback; M. Hartwig, E. Stoll, and E. Lied for data collection; and the participants.
Funding
This work was supported by the Fetzer Institute [grant number PO1-AT004952], NIH [grant number RO1-MH043454], and the Roke Foundation.
Contributor Information
Daniel B. Levinson, University of Wisconsin Madison
Jonathan Smallwood, Max Planck Institute.
Richard J. Davidson, University of Wisconsin Madison
References
- Cheyne AJ, Solman GJF, Carriere JSA, Smilek D. Anatomy of an error: a bidirectional state model of task engagement/disengagement and attention-related errors. Cognition. 2009;111(1):98–113. doi: 10.1016/j.cognition.2008.12.009. [DOI] [PubMed] [Google Scholar]
- Baddeley AD, Hitch GJ. Recent advances in learning and motivation. New York: Academic Press; 1974. Working memory; pp. 47–89. [Google Scholar]
- Baird B, Smallwood J, Schooler JW. Back to the future: Autobiographical planning and the functionality of mind-wandering. Consciousness and Cognition. doi: 10.1016/j.concog.2011.08.007. (in press) [DOI] [PubMed] [Google Scholar]
- Christoff K, Gordon AM, Smallwood J, Smith R, Schooler JW. Experience sampling during fMRI reveals default network and executive system contributions to mind wandering. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(21):8719–8724. doi: 10.1073/pnas.0900234106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forster S, Lavie N. High perceptual load makes everybody equal: eliminating individual differences in distractibility with load. Psychological Science: A Journal of the American Psychological Society/APS. 2007;18(5):377–381. doi: 10.1111/j.1467-9280.2007.01908.x. [DOI] [PubMed] [Google Scholar]
- Forster S, Lavie N. Harnessing the wandering mind: the role of perceptual load. Cognition. 2009;111(3):345–355. doi: 10.1016/j.cognition.2009.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giambra LM. A laboratory method for investigating influences on switching attention to task-unrelated imagery and thought. Consciousness and Cognition. 1995;4(1):1–21. doi: 10.1006/ccog.1995.1001. [DOI] [PubMed] [Google Scholar]
- Kane MJ, Engle RW. Working-memory capacity and the control of attention: the contributions of goal neglect, response competition, and task set to Stroop interference. Journal of Experimental Psychology General. 2003;132(1):47–70. doi: 10.1037/0096-3445.132.1.47. [DOI] [PubMed] [Google Scholar]
- Kane MJ, Brown LH, McVay JC, Silvia PJ, Myin-Germeys I, Kwapil TR. For whom the mind wanders, and when: an experience-sampling study of working memory and executive control in daily life. Psychological Science: A Journal of the American Psychological Society/APS. 2007;18(7):614–621. doi: 10.1111/j.1467-9280.2007.01948.x. [DOI] [PubMed] [Google Scholar]
- Kane MJ, Poole BJ, Tuholski SW, Engle RW. Working memory capacity and the top-down control of visual search: Exploring the boundaries of “executive attention”. Journal of Experimental Psychology Learning, Memory, and Cognition. 2006;32(4):749–777. doi: 10.1037/0278-7393.32.4.749. [DOI] [PubMed] [Google Scholar]
- Killingsworth MA, Gilbert DT. A wandering mind is an unhappy mind. Science (New York, NY) 2010;330(6006):932. doi: 10.1126/science.1192439. [DOI] [PubMed] [Google Scholar]
- Klinger E, Cox WM. Dimensions of thought flow in everyday life. Imagination, Cognition and Personality. 1987;7(2):105–128. doi: 10.2190/7K24-G343-MTQW-115V. [DOI] [Google Scholar]
- Lavie N. Distracted and confused?: selective attention under load. Trends in Cognitive Sciences. 2005;9(2):75–82. doi: 10.1016/j.tics.2004.12.004. [DOI] [PubMed] [Google Scholar]
- Lavie N, Hirst A, de Fockert JW, Viding E. Load theory of selective attention and cognitive control. Journal of Experimental Psychology General. 2004;133(3):339–354. doi: 10.1037/0096-3445.133.3.339. [DOI] [PubMed] [Google Scholar]
- Mason MF, Norton MI, Van Horn JD, Wegner DM, Grafton ST, Macrae CN. Wandering minds: the default network and stimulus-independent thought. Science (New York, NY) 2007;315(5810):393–5. doi: 10.1126/science.1131295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKiernan KA, D’Angelo BR, Kaufman JN, Binder JR. Interrupting the “stream of consciousness”: an fMRI investigation. Neuro Image. 2006;29(4):1185–1191. doi: 10.1016/j.neuroimage.2005.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McVay JC, Kane MJ. Conducting the train of thought: Working memory capacity, goal neglect, and mind wandering in an executive-control task. Journal of Experimental Psychology Learning, Memory, and Cognition. 2009;35(1):196–204. doi: 10.1037/a0014104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McVay JC, Kane MJ. Does mind wandering reflect executive function or executive failure? Comment on Smallwood and Schooler (2006) and Watkins (2008) Psychological Bulletin. 2010;136(2):188–197. doi: 10.1037/a0018298. discussion 198–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McVay JC, Kane MJ, Kwapil TR. Tracking the train of thought from the laboratory into everyday life: an experience-sampling study of mind wandering across controlled and ecological contexts. Psychonomic Bulletin & Review. 2009;16(5):857–863. doi: 10.3758/PBR.16.5.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole BJ, Kane MJ. Working-memory capacity predicts the executive control of visual search among distractors: the influences of sustained and selective attention. Quarterly Journal of Experimental Psychology (2006) 2009;62(7):1430–1454. doi: 10.1080/17470210802479329. [DOI] [PubMed] [Google Scholar]
- Smallwood J, Schooler JW. The restless mind. Psychological Bulletin. 2006;132(6):946–58. doi: 10.1037/0033-2909.132.6.946. [DOI] [PubMed] [Google Scholar]
- Smallwood J, Beach E, Schooler JW, Handy TC. Going AWOL in the brain: mind wandering reduces cortical analysis of external events. Journal of Cognitive Neuroscience. 2008;20(3):458–469. doi: 10.1162/jocn.2008.20037. [DOI] [PubMed] [Google Scholar]
- Smallwood J, Davies JB, Heim D, Finnigan F, Sudberry M, O’Connor R, Obonsawin M. Subjective experience and the attentional lapse: task engagement and disengagement during sustained attention. Consciousness and Cognition. 2004;13(4):657–690. doi: 10.1016/j.concog.2004.06.003. [DOI] [PubMed] [Google Scholar]
- Smallwood J, Nind L, O’Connor RC. When is your head at? An exploration of the factors associated with the temporal focus of the wandering mind. Consciousness and Cognition. 2009;18(1):118–125. doi: 10.1016/j.concog.2008.11.004. [DOI] [PubMed] [Google Scholar]
- Sobel KV, Gerrie MP, Poole BJ, Kane MJ. Individual differences in working memory capacity and visual search: the roles of top-down and bottom-up processing. Psychonomic Bulletin & Review. 2007;14(5):840–845. doi: 10.3758/bf03194109. [DOI] [PubMed] [Google Scholar]
- Stawarczyk D, Majerus S, Maj M, Van der Linden M, D’Argembeau A. Mind-wandering: phenomenology and function as assessed with a novel experience sampling method. Acta Psychologica. 2011;136(3):370–381. doi: 10.1016/j.actpsy.2011.01.002. [DOI] [PubMed] [Google Scholar]
- Stawarczyk D, Majerus S, Maquet P, D’Argembeau A. Neural correlates of ongoing conscious experience: both task-unrelatedness and stimulus-independence are related to default network activity. PloS One. 2011;6(2):e16997. doi: 10.1371/journal.pone.0016997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teasdale JD, Dritschel BH, Taylor MJ, Proctor L, Lloyd CA, Nimmo-Smith I, Baddeley AD. Stimulus-independent thought depends on central executive resources. Memory & Cognition. 1995;23(5):551–559. doi: 10.3758/bf03197257. [DOI] [PubMed] [Google Scholar]
- Teasdale JD, Proctor L, Lloyd CA, Baddeley AD. Working memory and stimulus-independent thought: Effects of memory load and presentation rate. European Journal of Cognitive Psychology. 1993;5(4):417. doi: 10.1080/09541449308520128. [DOI] [Google Scholar]
- Unsworth N, Heitz RP, Schrock JC, Engle RW. An automated version of the operation span task. Behavior Research Methods. 2005;37(3):498–505. doi: 10.3758/bf03192720. [DOI] [PubMed] [Google Scholar]