Abstract
Histone methylation is implicated in both gene activation and repression, depending on the specific lysine residue that gets methylated. Recent years have witnessed an explosive expansion of the list of remarkably site-specific histone methyltransferases and demethylases, which greatly facilitates the study on the biological functions of histone methylation in gene expression and cell differentiation in mammalian cells. Adipogenesis represents an excellent model system to understand transcriptional and epigenetic regulation of gene expression and cell differentiation. While transcriptional regulation of adipogenesis has been extensively studied, the roles of epigenetic mechanisms in particular histone methylation in regulation of adipogenesis have just begun to be understood. This review will summarize the recent progress on epigenetic regulation of adipogenesis by histone methylation, with a focus on histone H3K4 and H3K27. The available evidence suggests that site-specific histone methylations play critical roles in adipogenesis and control the expression of both positive and negative master regulators of adipogenesis.
Introduction
Type 2 diabetes, which accounts for 90–95% of all diabetes, is one of the leading causes of morbidity and mortality worldwide. Obesity is the single most important risk factor for type 2 diabetes. Understanding the molecular mechanisms underlying adipogenesis (generation of fat tissue) may lead to novel approaches to the treatment of obesity and lipodystrophy, the two diseases that are tightly associated with type 2 diabetes. Transcriptional regulation of adipogenesis has been extensively reviewed [1, 2]. This review will focus on the role of histone lysine methylation in regulation of adipogenesis. I start with an introduction on the dynamic regulation of histone methylations by site-specific histone methyltransferases and demethylases. After a brief overview of the major positive and negative regulators of adipogenesis, I discuss the roles of histone methylations in particular histone H3K4 and H3K27 methylations, and the associated histone methyltransferases and demethylases, in controlling the expression of the master positive and negative regulators of adipogenesis.
1. Dynamic regulation of histone methylation by site-specific methyltransferases and demethylases
Epigenetic mechanisms, including histone modifications (such as acetylation, methylation and phosphorylation) (Figure 1), chromatin remodeling, histone variant incorporation, non-coding RNAs and DNA methylation, play critical roles in regulating both global and tissue- and developmental stage-specific gene expression [3]. Histone acetylation occurs on lysine (K) residues and is dynamically regulated by histone acetyltransferases (HATs) and deacetylases [4]. Recent evidences suggest that although HATs are often capable of acetylating multiple K residues in vitro, they possess remarkable site-specificities in cells. For example, the paralogous HATs CBP and p300 are redundant and are specifically required for H3K18ac and H3K27ac in cells while another pair of paralogous HATs GCN5 and PCAF are also redundant but are specifically required for H3K9ac in cells [5]. Histone acetylation generally correlates with gene activation, with some histone acetylations, such as H3K18ac and H3K27ac, are likely the cause of gene activation, while other histone acetylations, such as H3K9ac, are likely the consequence of gene activation [5].
Figure 1. Histone modifications.

Acetylation (ac) generally correlates with gene activation. Histone lysine (K) methylations (me) that correlate with gene activation are shown in green while those correlated with gene repression are shown in red.
Unlike histone acetylations, histone lysine (K) methylations can be correlated with either gene activation or gene repression, depending on the specific K residue that becomes methylated [3, 6]. Methylations on histone H3K4, H3K36 and H3K79 are generally associated with gene activation, whereas methylations on histone H3K9 and H3K27 are generally associated with gene repression (Figure 1).
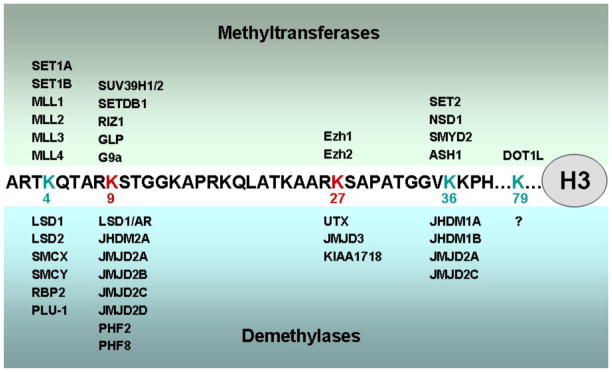
Histone lysine methylation is dynamically regulated by site-specific methyltransferases and demethylases [7, 8]. In mammals, the Drosophila Trithorax-related Set1-like histone methyltransferase (HMT) complexes specifically methylate H3K4 [9, 10]. The Polycomb repressive complex 2 (PRC2) is the predominant H3K27 methyltransferase in mammalian cells [11]. Multiple site-specific histone demethylases have also been identified. These enzymes are capable of removing methylations on H3K4, H3K9, H3K27 and H3K36 in a site-specific manner. Figure 2 lists the HMTs and histone demethylases that have been identified so far [7]. Almost all of these enzymes were identified within the last decade. While their biochemical properties have been extensively studied in vitro, their substrate- and site-specificities in cells are incompletely understood. More importantly, the biological functions of these histone modifying enzymes in regulating gene expression, cell differentiation and animal development have remained largely unexplored. Adipogenesis provides an excellent model system to study the biological functions of these enzymes.
Figure 2. Histone lysine methyltransferases and demethylases.
Histone lysine methylation is dynamically regulated by site-specific methyltransferases and demethylases.
2. Positive and negative regulators of adipogenesis
Adipocytes are believed to derive from multipotent mesenchymal stem cells (MSCs). Differentiation of MSCs to adipocytes involves two stages: determination and terminal differentiation. Determination refers to the commitment of MSCs to the adipocyte lineage, which results in the conversion of MSCs into preadipocytes. In the terminal differentiation stage, the fibroblast-like preadipocytes differentiate and convert into fat-laden adipocytes [2].
Much of our knowledge on adipogenesis comes from studies on differentiation of preadipocytes or mouse embryonic fibroblasts (MEFs) in cell culture. The immortalized white preadipocyte cell line 3T3-L1 is widely used. Primary white and brown preadipocytes can also be easily isolated from the stromal vascular fractions of the inguinal white adipose tissue (WAT) of young adult mice and the interscapular brown adipose tissue (BAT) of new born mouse pups, respectively. The primary brown preadipocytes can further be immortalized using SV40T antigen [12]. Differentiation of these primary and immortalized preadipocytes towards mature adipocytes is efficient in cell culture and appears to faithfully recapitulate adipogenesis in mice [2].
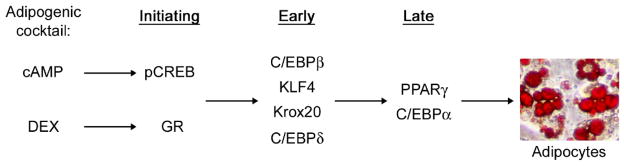
In a standard adipogenesis assay, differentiation is induced by treating confluent preadipocytes with an adipogenic cocktail of isobutylmethylxanthine (IBMX), dexamethasone (DEX) and insulin [1]. IBMX increases intracellular cAMP level to activate protein kinase A, which phosphorylates and activates cAMP response element-binding protein (CREB). DEX binds and activates glucocorticoid receptor (GR). Phosphorylated CREB (pCREB, the active form) and DEX-bound GR serve as initiating adipogenic transcription factors and induce the expression of early adipogenic transcription factors C/EBPβ, KLF4, Krox20 and C/EBPδ within hours of initiation of adipogenesis [1, 13]. The elevated levels of these early adipogenic transcription factors induce expression of two principal adipogenic transcription factors, PPARγ (Peroxisome Proliferator-Activated Receptor-γ) and C/EBPα. PPARγ belongs to the nuclear receptor super family of ligand-activated transcription factors. It is considered the master regulator of adipogenesis and is both necessary and sufficient for adipogenesis [1, 14]. PPARγ cooperates with another principal adipogenic transcription factor C/EBPα to directly and synergistically activate expression of hundreds of adipocyte genes responsible for establishing the mature adipocyte phenotype [15, 16]. Thus, adipogenesis is positively regulated by a cascade of sequentially expressed adipogenic transcription factors (Figure 3).
Figure 3. Adipogenesis is positively regulated by a cascade of sequentially expressed adipogenic transcription factors.
Adipocytes have been stained with Oil Red O and show red color. pCREB, phosphorylated CREB.
Multiple negative regulators of adipogenesis have also been identified [2]. The Wnt/β-catenin signaling is one of the best studied and appears to play a major role in negative regulation of adipogenesis. The Wnt family of secreted proteins regulates cell proliferation, differentiation, and fate determination during embryonic development and adult tissue homeostasis [17]. The Wnt family has nineteen members in humans and mice. Among them, Wnt1, Wnt6, Wnt10a and Wnt10b have been shown to inhibit adipogenesis [18, 19]. Activation of Wnt/β-catenin signaling by over-expression of Wnt1 or Wnt10b prevents the induction of PPARγ and C/EBPα but not C/EBPβ, which works upstream of PPARγ and C/EBPα [20]. In addition, β-catenin interacts with and inhibits the activity of PPARγ, the master regulator of adipogenesis [21]. Conversely, inhibition of Wnt/β-catenin signaling promotes adipogenesis [20].
Thus, adipogenic transcription factors in particular PPARγ and C/EBPα promote adipogenesis and Wnt/β-catenin signaling inhibits adipogenesis. Recent studies suggest that site-specific histone methylations control the expression of these positive and negative master regulators of adipogenesis (see below).
3. Regulation of adipogenesis by H3K4 methylation
Mono-, di- and tri-methylations on histone H3K4 (H3K4me1, H3K4me2 and H3K4me3, respectively) are generally correlated with gene activation. Genome-wide analyses show that H3K4me1 and H3K4me2 are associated with open chromatin and are often enriched on cis-regulatory regions [22]. H3K4me1, along with H3K27ac, is often enriched on enhancers [23]. H3K4me3 is enriched around transcription start sites and correlates well with gene expression level [24]. PPARγ has mainly two isoforms, PPARγ1 and PPARγ2, which are transcribed from two different promoters [25]. While the two isoforms are expressed at comparable levels in white adipocytes, PPARγ1 is the predominant one in brown adipocytes [26, 27]. PPARγ1 is expressed at low level in preadipocytes and its expression increases markedly during adipogenesis. PPARγ2 is absent in preadipocytes but is dramatically induced during adipogenesis. H3K4me3 levels on PPARγ1 and PPARγ2 promoters correlate remarkably well with both the dynamic changes and the relative levels of PPARγ1 and PPARγ2 expression [22, 27].
The enzymes responsible for H3K4 methylation have been identified. In yeast, a single Set1/COMPASS complex, through its enzymatic subunit Set1, is responsible for all mono-, di- and tri-methylations on histone H3K4. Drosophila has three Set1-like H3K4 methyltransferase complexes, which use dSet1, Trithorax (Trx), or Trithorax-related (Trr) as the enzymatic subunit. Mice and humans have six Set1-like histone H3K4 methyltransferase complexes. Based on the protein sequence homologies among the enzymatic subunits and the subunit compositions, the six complexes can be categorized into three subgroups: SET1A and SET1B, MLL1 and MLL2, and MLL3 and MLL4 (MLL4 is also known as ALR and KMT4D and is sometimes named as MLL2 in the literature), which correspond to the Drosophila dSet1, Trx and Trr complexes, respectively. The two members of each subgroup share identical subunit composition except for the enzymatic subunits [9, 10, 28].
PTIP and a novel protein PA1 are both unique components of the MLL3/MLL4-containing histone H3K4 methyltransferase complexes [10, 29, 30]. PTIP is required for PPARγ and C/EBPα expression in MEFs. Further, PTIP is required for the robust induction of PPARγ and C/EBPα during adipogenesis of preadipocytes. Deletion of PTIP reduces H3K4me3 levels on PPARγ and C/EBPα promoters, which correlate well with the reduced gene expression levels. Accordingly, PTIP-deficient MEFs and white and brown preadipocytes all show severe defects in adipogenesis. Rescue of the adipogenesis defect in PTIP-null MEFs requires co-expression of PPARγ and C/EBPα. Finally, deletion of PTIP in mouse adipose tissue significantly reduces tissue weight. Thus, by controlling the induction of PPARγ and C/EBPα, the two principal adipogenic transcriptional factors, histone methylation regulator PTIP plays a critical role in adipogenesis [27].
Several other unique components of the MLL3/MLL4 complexes are also required for adipogenesis. Deletion of the Ncoa6 subunit leads to defect in PPARγ-stimulated adipogenesis in MEFs [31]. Ncoa6 interacts directly with PPARγ and is likely mediating the interaction between PPARγ and MLL3/MLL4 complexes [32]. Consistently, deletion of MLL3 leads to a significantly decreased amount of white adipose tissue in mice [33]. Together, these results suggest a critical role of the MLL3/MLL4-containing histone H3K4 methyltransferase complexes in adipogenesis.
Several questions remain to be answered on the precise mechanism by which PTIP and associated MLL3/MLL4 complexes regulate adipogenesis. First, besides their association with MLL3/MLL4 complexes, PTIP and PA1 also form a small and separate complex that exists outside of the MLL3/MLL4 complexes [34]. It remains to be determined whether regulation of PPARγ and C/EBPα expression by PTIP is mediated by the associated MLL3/MLL4 complexes. Second, the role of the novel protein PA1 in adipogenesis remains to be shown. Third, the molecular mechanism by which H3K4 methyltransferases MLL3/MLL4 regulate adipogenesis remains to be defined. Is the increase of H3K4me3 on PPARγ1 and PPARγ2 promoters during adipogenesis a cause or a consequence of gene activation? Fourth, the MLL3/MLL4 complexes not only contain H3K4 methyltransferases MLL3/MLL4 but also histone H3K27 demethylase UTX [10, 29, 35, 36]. Since PPARγ promoter is enriched with H3K4me3 but lacks H3K27me3 during adipogenesis [22], it will be interesting to investigate whether H3K4 methyltransferases MLL3/MLL4 synergize with H3K27 demethylase UTX to facilitate PPARγ expression and adipogenesis. Finally, the roles of SET1A/B- and MLL1/2-containing H3K4 methyltransferase complexes in adipogenesis remain to be understood.
4. Regulation of adipogenesis by H3K27 methylation
Tri-methylation on H3K27 (H3K27me3) is a repressive epigenetic mark important for Polycomb-mediated gene silencing [11]. The mammalian Polycomb repressive complex 2 (PRC2) uses its enzymatic subunit Ezh2 to specifically methylate H3K27. Ezh2 is responsible for the majority of H3K27me2 and H3K27me3 in cells [37, 38]. Genome-wide analyses have shown that Ezh2 and H3K27me3 are enriched on a large number of developmental regulators in embryonic stem cells and other cell types [39, 40]. In preadipocytes, Ezh2 and H3K27me3 levels are low on PPARγ gene locus but are high on multiple Wnt gene loci [22, 38]. Deletion of Ezh2 in preadipocytes dramatically decreases H3K27me3 on PRC2 target genes including Wnt1, Wnt6, Wnt10a and Wnt10b, which are negative regulators of adipogenesis. The resulting de-repression and increased expression of Wnt1, Wnt6, Wnt10a and Wnt10b leads to activation of Wnt/β-catenin signaling, which inhibits adipogenesis by preventing the induction of principal adipogenic transcription factors PPARγ and C/EBPα. The adipogenesis defect in Ezh2 null preadipocytes can be rescued by over-expression of PPARγ and C/EBPα [38]. Deletion of Ezh2 also increases expression of other PRC2 target genes including known adipogenesis inhibitors Pref-1 and GATA3. However, the adipogenesis defect in Ezh2 null cells can be partially rescued by over-expression of inhibitors of Wnt/β-catenin signaling, indicating that the de-repression of Wnt genes is responsible at least in part for the differentiation defect in Ezh2 null preadipocytes. Importantly, the HMT activity of Ezh2 is essential for repression of Wnt genes and for adipogenesis [38]. Together, these results indicate that Ezh2 is required for adipogenesis and that the H3K27 methyltransferase PRC2, through the enzymatic activity of Ezh2, directly represses Wnt genes to facilitate adipogenesis. These results also establish a direct, functional link between Polycomb and Wnt proteins, which are two important classes of developmental regulators.
It should be noted that the Polycomb targeted Wnt1 and Wnt10b genes are expressed at significant levels in preadipocytes [18]. Ezh2 protein level in cells, as well as the H3K27me3 level on Wnt genes, remains constant during adipogenesis. However, Wnt1 and Wnt10b expression decreases rapidly during differentiation of both wild-type and Ezh2 null preadipocytes [38]. These results suggest that PRC2 constitutively represses Wnt genes during adipogenesis and that transcription repressors other than PRC2 actively decrease Wnt1 and Wnt10b levels during adipogenesis.
Interestingly, deletion of Ezh2 in cells leads to a marked increase of H3K27ac along with the marked decrease of H3K27me3, not only in whole cell extracts but also on Ezh2-regulated Wnt promoters, in Ezh2 null preadipocytes [38]. Since HATs CBP and p300 are responsible for H3K27ac in cells [5], these results suggest that Ezh2-mediated H3K27 methylation represses Wnt expression by blocking CBP/p300-mediated H3K27ac.
Several questions remain to be answered on the role of H3K27 methylation in adipogenesis. First, Ezh2 is responsible for both H3K27me2 and H3K27me3 but not H3K27me1 in cells. It is currently unclear whether H3K27me2 or H3K27me3 or both are involved in repressing Wnt genes to facilitate adipogenesis. Second, how PRC2 complex is recruited to the Wnt genes is unclear. Ezh2 has been reported to bind long non-coding RNAs (ncRNAs), which may recruit PRC2 to target gene promoters [41]. The potential involvement and the identities of ncRNAs or transcription factors that recruit PRC2 to the Wnt genes remain to be investigated.
5. Summary
Methylations on histone H3K4 are generally associated with gene activation whereas methylations on H3K27 are generally associated with gene repression. PTIP, a protein that associates with histone H3K4 methyltransferases MLL3/MLL4 and histone H3K27 demethylase UTX, is required for PPARγ and C/EBPα expression and adipogenesis [27]. The histone H3K27 methyltransferase PRC2 uses its enzymatic subunit Ezh2 to repress Wnt genes and facilitate adipogenesis [38]. Together, these results provide an initial view of epigenetic regulation of adipogenesis by histone H3K4 and H3K27 methylations, and suggest that site-specific histone methylations control expression of both positive and negative master regulators of adipogenesis (Figure 4).
Figure 4. Epigenetic regulation of adipogenesis by histone H3K4 and H3K27 methylations.

PTIP, a protein that associates with histone H3K4 methyltransferases MLL3/MLL4, is required for PPARγ and C/EBPα expression and adipogenesis. Ezh2 uses its histone H3K27 methyltransferase activity to constitutively repress Wnt genes and facilitate adipogenesis.
6. Future directions
PPARγ and Wnts are master positive and negative regulators of adipogenesis, respectively. The H3K4 methylation regulator PTIP promotes PPARγ expression while the H3K27 methyltransferase Ezh2 represses Wnt expression during adipogenesis (Figure 4). However, the epigenetic factors that repress PPARγ but promote Wnt expression in preadipocytes have not been identified. Analyzing the enrichment and/or the dynamic changes of histone methylation patterns on PPARγ and Wnt promoters in preadipocytes and in the early phase of adipogenesis may provide clues to solving this issue.
It has been reported that knockdown of histone demethylase LSD1 increases histone H3K9 dimethylation (H3K9me2) on C/EBPα promoter and leads to decreased adipogenesis. Conversely, knockdown of histone H3K9 methyltransferase SetDB1 (also known as ESET) decreases H3K9me2 on C/EBPα promoter and leads to increased adipogenesis. These results suggest opposing roles of LSD1 and SetDB1 in regulating C/EBPα expression and adipogenesis [42]. However, LSD1 mainly demethylates H3K4me1/2 and SetDB1 mainly performs trimethylation on histone H3K9 [7, 43]. The H3K9me2 level in cells is predominantly controlled by the euchromatin-associated H3K9 methyltransferase G9a [44]. Thus, it will be important to determine whether G9a-mediated H3K9me2 plays any role in regulation of PPARγ expression and adipogenesis.
Within hours of initiation of adipogenesis, cAMP-induced pCREB and Dex-bound GR rapidly induce expression of early adipogenic transcription factors C/EBPβ, KLF4, Krox20 and C/EBPδ, which are essential for induction of PPARγ and C/EBPα expression and for adipogenesis (Figure 3). How histone methylations regulate the rapid induction of these early adipogenic transcription factors is completely unknown.
Another important question is how H3K4 or H3K27 methylation targets PPARγ or Wnt genes, respectively. In other words, how H3K4 methyltransferase complexes are recruited to PPARγ genes and how H3K27 methyltransferase complex PRC2 is recruited to Wnt genes are unclear. These histone modifying complexes may be recruited by sequence-specific transcription factors that directly bind to PPARγ or Wnt gene loci. Alternatively, sequence-specific non-coding RNAs may directly recruit these HMT complexes to PPARγ or Wnt genes. Since subunits of these histone modifying complexes contain multiple histone modification-binding domains, it is possible that these HMT complexes are recruited to PPARγ or Wnt genes by recognizing pre-existing histone modifications on target genes, i.e. through cross-talk between histone modifications.
PPARγ is a nuclear receptor and thus a ligand-activated transcription factor. Correlating with ligand-induced nuclear receptor target gene activation, ligand induces sequential enrichment of H3K18/27ac, RNA Pol II, and several histone methylations including H3K4me3, H3K36me3 and H3K79me2 on nuclear receptor target genes [5]. While the exact roles of these gene activation-associated histone methylations remain to be determined, gene repression-associated histone methylations have been implicated in regulating nuclear receptor target gene expression [45]. One good example is the role of histone methyltransferase SetDB1, which is activated by non-canonical Wnt signaling to methylate histone H3K9 to repress target gene activation by PPARγ [46]. The contributions of other site-specific histone methylations to the transcriptional activation or repression of PPARγ target genes important for adipogenesis remain to be defined.
The majority of histone methyltransferases and demethylases were identified in the 21st century and their biological functions are poorly understood. Adipogenesis provides an excellent model system to study the roles of histone methyltransferases and demethylases, and the dynamics of site-specific histone methylations, in regulation of gene expression and cell differentiation. In addition to histone H3K4 and H3K27 methylations described above, it will be important to understand the roles of methylations on histone H3K9, H3K36 and H3K79, and the related histone methyltransferases and demethylases, in regulation of adipogenesis. Straightforward knockout (KO) of these enzymes usually leads to embryonic lethality. Fortunately, conditional KO mouse strains (floxed mice) for many of these histone modifying enzymes and associated factors are becoming available. Preadipocytes carrying conditional KO of histone modifying enzymes can be easily isolated from these mice, thus providing an excellent model system to study epigenetic regulation of cell differentiation in vitro [27, 38]. The results should be verified in vivo by crossing floxed mice with tissue-specific Cre mice to specifically delete the gene-of-interest in adipose tissue [47].
Highlights.
Histone methylations regulate gene expression and cell differentiation
Histone methylations are regulated by methyltransferases and demethylase
This review focuses on regulation of adipogenesis by histone methylation
H3K4 and H3K27 methylations control expression of master regulators of adipogenesis
Acknowledgments
Research in Kai Ge laboratory was supported by the Intramural Research Program of the NIDDK, NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Farmer SR. Transcriptional control of adipocyte formation. Cell Metab. 2006;4:263–273. doi: 10.1016/j.cmet.2006.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rosen ED, MacDougald OA. Adipocyte differentiation from the inside out. Nat Rev Mol Cell Biol. 2006;7:885–896. doi: 10.1038/nrm2066. [DOI] [PubMed] [Google Scholar]
- 3.Li B, Carey M, Workman JL. The Role of Chromatin during Transcription. Cell. 2007;128:707–719. doi: 10.1016/j.cell.2007.01.015. [DOI] [PubMed] [Google Scholar]
- 4.Roth SY, Denu JM, Allis CD. Histone Acetyltransferases. Annual Review of Biochemistry. 2001;70:81–120. doi: 10.1146/annurev.biochem.70.1.81. [DOI] [PubMed] [Google Scholar]
- 5.Jin Q, Yu L-R, Wang L, Zhang Z, Kasper LH, Lee J-E, Wang C, Brindle PK, Dent SYR, Ge K. Distinct roles of GCN5/PCAF-mediated H3K9ac and CBP/p300-mediated H3K18/27ac in nuclear receptor transactivation. EMBO J. 2011;30:249. doi: 10.1038/emboj.2010.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kouzarides T. Chromatin Modifications and Their Function. Cell. 2007;128:693–705. doi: 10.1016/j.cell.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 7.Shi Y. Histone lysine demethylases: emerging roles in development, physiology and disease. Nat Rev Genet. 2007;8:829. doi: 10.1038/nrg2218. [DOI] [PubMed] [Google Scholar]
- 8.Klose RJ, Kallin EM, Zhang Y. JmjC-domain-containing proteins and histone demethylation. Nat Rev Genet. 2006;7:715. doi: 10.1038/nrg1945. [DOI] [PubMed] [Google Scholar]
- 9.Mohan M, Herz H-M, Smith ER, Zhang Y, Jackson J, Washburn MP, Florens L, Eissenberg JC, Shilatifard A. The COMPASS family of H3K4 methylases in Drosophila. Mol Cell Biol. 2011:MCB.06092–06011. doi: 10.1128/MCB.06092-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cho Y-W, Hong T, Hong S, Guo H, Yu H, Kim D, Guszczynski T, Dressler GR, Copeland TD, Kalkum M, Ge K. PTIP Associates with MLL3- and MLL4-containing Histone H3 Lysine 4 Methyltransferase Complex. J Biol Chem. 2007;282:20395–20406. doi: 10.1074/jbc.M701574200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schuettengruber B, Chourrout D, Vervoort M, Leblanc B, Cavalli G. Genome regulation by polycomb and trithorax proteins. Cell. 2007;128:735–745. doi: 10.1016/j.cell.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 12.Klein J, Fasshauer M, Ito M, Lowell BB, Benito M, Kahn CR. beta 3-Adrenergic Stimulation Differentially Inhibits Insulin Signaling and Decreases Insulin-induced Glucose Uptake in Brown Adipocytes. J Biol Chem. 1999;274:34795–34802. doi: 10.1074/jbc.274.49.34795. [DOI] [PubMed] [Google Scholar]
- 13.Birsoy Kv, Chen Z, Friedman J. Transcriptional Regulation of Adipogenesis by KLF4. Cell Metabolism. 2008;7:339. doi: 10.1016/j.cmet.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rosen ED, Hsu C-H, Wang X, Sakai S, Freeman MW, Gonzalez FJ, Spiegelman BM. C/EBPalpha induces adipogenesis through PPARgamma : a unified pathway. Genes Dev. 2002;16:22–26. doi: 10.1101/gad.948702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nielsen R, Pedersen TA, Hagenbeek D, Moulos P, Siersbaek R, Megens E, Denissov S, Borgesen M, Francoijs K-J, Mandrup S, Stunnenberg HG. Genome-wide profiling of PPAR{gamma}:RXR and RNA polymerase II occupancy reveals temporal activation of distinct metabolic pathways and changes in RXR dimer composition during adipogenesis. Genes Dev. 2008;22:2953–2967. doi: 10.1101/gad.501108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lefterova MI, Zhang Y, Steger DJ, Schupp M, Schug J, Cristancho A, Feng D, Zhuo D, Stoeckert CJ, Jr, Liu XS, Lazar MA. PPAR{gamma} and C/EBP factors orchestrate adipocyte biology via adjacent binding on a genome-wide scale. Genes Dev. 2008;22:2941–2952. doi: 10.1101/gad.1709008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Logan CY, Nusse R. The Wnt Signaling Pathway in Development and Disease. Annual Review of Cell and Developmental Biology. 2004;20:781–810. doi: 10.1146/annurev.cellbio.20.010403.113126. [DOI] [PubMed] [Google Scholar]
- 18.Prestwich TC, Macdougald OA. Wnt/beta-catenin signaling in adipogenesis and metabolism. Curr Opin Cell Biol. 2007;19:612–617. doi: 10.1016/j.ceb.2007.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cawthorn WP, Bree AJ, Yao Y, Du B, Hemati N, Martinez-Santibañez G, MacDougald OA. Wnt6, Wnt10a and Wnt10b inhibit adipogenesis and stimulate osteoblastogenesis through a β-catenin-dependent mechanism. Bone. 2012 doi: 10.1016/j.bone.2011.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ross SE, Hemati N, Longo KA, Bennett CN, Lucas PC, Erickson RL, MacDougald OA. Inhibition of Adipogenesis by Wnt Signaling. Science. 2000;289:950–953. doi: 10.1126/science.289.5481.950. [DOI] [PubMed] [Google Scholar]
- 21.Liu J, Wang H, Zuo Y, Farmer SR. Functional interaction between peroxisome proliferator-activated receptor gamma and beta-catenin. Mol Cell Biol. 2006;26:5827–5837. doi: 10.1128/MCB.00441-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mikkelsen TS, Xu Z, Zhang X, Wang L, Gimble JM, Lander ES, Rosen ED. Comparative Epigenomic Analysis of Murine and Human Adipogenesis. Cell. 2010;143:156. doi: 10.1016/j.cell.2010.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Heintzman ND, Hon GC, Hawkins RD, Kheradpour P, Stark A, Harp LF, Ye Z, Lee LK, Stuart RK, Ching CW, Ching KA, Antosiewicz-Bourget JE, Liu H, Zhang X, Green RD, Lobanenkov VV, Stewart R, Thomson JA, Crawford GE, Kellis M, Ren B. Histone modifications at human enhancers reflect global cell-type-specific gene expression. Nature. 2009;459:108. doi: 10.1038/nature07829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barski A, Cuddapah S, Cui K, Roh T-Y, Schones DE, Wang Z, Wei G, Chepelev I, Zhao K. High-Resolution Profiling of Histone Methylations in the Human Genome. Cell. 2007;129:823–837. doi: 10.1016/j.cell.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 25.Zhu Y, Qi C, Korenberg JR, Chen X, Noya D, Rao MS, Reddy JK. Structural Organization of Mouse Peroxisome Proliferator-Activated Receptor {gamma} (mPPAR{gamma}) Gene: Alternative Promoter Use and Different Splicing Yield Two mPPAR{gamma} Isoforms. Proceedings of the National Academy of Sciences. 1995;92:7921–7925. doi: 10.1073/pnas.92.17.7921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jitrapakdee S, Slawik M, Medina-Gomez G, Campbell M, Wallace JC, Sethi JK, O'Rahilly S, Vidal-Puig AJ. The peroxisome proliferator-activated receptor-gamma regulates murine pyruvate carboxylase gene expression in vivo and in vitro. J Biol Chem. 2005;280:27466–27476. doi: 10.1074/jbc.M503836200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cho YW, Hong S, Jin Q, Wang L, Lee JE, Gavrilova O, Ge K. Histone Methylation Regulator PTIP Is Required for PPARgamma and C/EBPalpha Expression and Adipogenesis. Cell Metab. 2009;10:27–39. doi: 10.1016/j.cmet.2009.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cho YW, Hong S, Ge K. Affinity Purification of MLL3/MLL4 Histone H3K4 Methyltransferase Complex. Methods Mol Biol. 2012;809:465–472. doi: 10.1007/978-1-61779-376-9_30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Issaeva I, Zonis Y, Rozovskaia T, Orlovsky K, Croce CM, Nakamura T, Mazo A, Eisenbach L, Canaani E. Knockdown of ALR (MLL2) reveals ALR target genes and leads to alterations in cell adhesion and growth. Mol Cell Biol. 2007;27:1889–1903. doi: 10.1128/MCB.01506-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Patel SR, Kim D, Levitan I, Dressler GR. The BRCT-Domain Containing Protein PTIP Links PAX2 to a Histone H3, Lysine 4 Methyltransferase Complex. Developmental Cell. 2007;13:580. doi: 10.1016/j.devcel.2007.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Qi C, Surapureddi S, Zhu Y-J, Yu S, Kashireddy P, Rao MS, Reddy JK. Transcriptional Coactivator PRIP, the Peroxisome Proliferator-activated Receptor {gamma} (PPAR{gamma})-interacting Protein, Is Required for PPAR{gamma}-mediated Adipogenesis. J Biol Chem. 2003;278:25281–25284. doi: 10.1074/jbc.C300175200. [DOI] [PubMed] [Google Scholar]
- 32.Zhu Y, Kan L, Qi C, Kanwar YS, Yeldandi AV, Rao MS, Reddy JK. Isolation and characterization of peroxisome proliferator-activated receptor (PPAR) interacting protein (PRIP) as a coactivator for PPAR. J Biol Chem. 2000;275:13510–13516. doi: 10.1074/jbc.275.18.13510. [DOI] [PubMed] [Google Scholar]
- 33.Lee J, Saha PK, Yang Q-H, Lee S, Park JY, Suh Y, Lee S-K, Chan L, Roeder RG, Lee JW. Targeted inactivation of MLL3 histone H3-Lys-4 methyltransferase activity in the mouse reveals vital roles for MLL3 in adipogenesis. Proceedings of the National Academy of Sciences. 2008;105:19229–19234. doi: 10.1073/pnas.0810100105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gong Z, Cho Y-W, Kim J-E, Ge K, Chen J. Accumulation of Pax2 Transactivation Domain Interaction Protein (PTIP) at Sites of DNA Breaks via RNF8-dependent Pathway Is Required for Cell Survival after DNA Damage. J Biol Chem. 2009;284:7284–7293. doi: 10.1074/jbc.M809158200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee MG, Villa R, Trojer P, Norman J, Yan KP, Reinberg D, Di Croce L, Shiekhattar R. Demethylation of H3K27 Regulates Polycomb Recruitment and H2A Ubiquitination. Science. 2007 doi: 10.1126/science.1149042. [DOI] [PubMed] [Google Scholar]
- 36.Hong S, Cho Y-W, Yu L-R, Yu H, Veenstra TD, Ge K. Identification of JmjC domain-containing UTX and JMJD3 as histone H3 lysine 27 demethylases. Proc Natl Acad Sci U S A. 2007;104:18439–18444. doi: 10.1073/pnas.0707292104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shen X, Liu Y, Hsu YJ, Fujiwara Y, Kim J, Mao X, Yuan GC, Orkin SH. EZH1 mediates methylation on histone H3 lysine 27 and complements EZH2 in maintaining stem cell identity and executing pluripotency. Mol Cell. 2008;32:491–502. doi: 10.1016/j.molcel.2008.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang L, Jin Q, Lee J-E, Su Ih, Ge K. Histone H3K27 methyltransferase Ezh2 represses Wnt genes to facilitate adipogenesis. Proceedings of the National Academy of Sciences. 2010;107:7317–7322. doi: 10.1073/pnas.1000031107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Boyer LA, Plath K, Zeitlinger J, Brambrink T, Medeiros LA, Lee TI, Levine SS, Wernig M, Tajonar A, Ray MK, Bell GW, Otte AP, Vidal M, Gifford DK, Young RA, Jaenisch R. Polycomb complexes repress developmental regulators in murine embryonic stem cells. Nature. 2006;441:349. doi: 10.1038/nature04733. [DOI] [PubMed] [Google Scholar]
- 40.Bracken AP, Dietrich N, Pasini D, Hansen KH, Helin K. Genome-wide mapping of Polycomb target genes unravels their roles in cell fate transitions. Genes & Development. 2006;20:1123–1136. doi: 10.1101/gad.381706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gupta RA, Shah N, Wang KC, Kim J, Horlings HM, Wong DJ, Tsai M-C, Hung T, Argani P, Rinn JL, Wang Y, Brzoska P, Kong B, Li R, West RB, van de Vijver MJ, Sukumar S, Chang HY. Long non-coding RNA HOTAIR reprograms chromatin state to promote cancer metastasis. Nature. 2010;464:1071. doi: 10.1038/nature08975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Musri MM, Carmona MC, Hanzu FA, Kaliman P, Gomis R, Parrizas M. Histone demethylase LSD1 regulates adipogenesis. J Biol Chem. 2010;285:30034–30041. doi: 10.1074/jbc.M110.151209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Matsui T, Leung D, Miyashita H, Maksakova IA, Miyachi H, Kimura H, Tachibana M, Lorincz MC, Shinkai Y. Proviral silencing in embryonic stem cells requires the histone methyltransferase ESET. Nature. 2010;464:927–931. doi: 10.1038/nature08858. [DOI] [PubMed] [Google Scholar]
- 44.Rice JC, Briggs SD, Ueberheide B, Barber CM, Shabanowitz J, Hunt DF, Shinkai Y, Allis CD. Histone methyltransferases direct different degrees of methylation to define distinct chromatin domains. Mol Cell. 2003;12:1591–1598. doi: 10.1016/s1097-2765(03)00479-9. [DOI] [PubMed] [Google Scholar]
- 45.Garcia-Bassets I, Kwon Y-S, Telese F, Prefontaine GG, Hutt KR, Cheng CS, Ju B-G, Ohgi KA, Wang J, Escoubet-Lozach L, Rose DW, Glass CK, Fu X-D, Rosenfeld MG. Histone Methylation-Dependent Mechanisms Impose Ligand Dependency for Gene Activation by Nuclear Receptors. Cell. 2007;128:505. doi: 10.1016/j.cell.2006.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Takada I, Mihara M, Suzawa M, Ohtake F, Kobayashi S, Igarashi M, Youn M-Y, Takeyama K-i, Nakamura T, Mezaki Y, Takezawa S, Yogiashi Y, Kitagawa H, Yamada G, Takada S, Minami Y, Shibuya H, Matsumoto K, Kato S. A histone lysine methyltransferase activated by non-canonical Wnt signalling suppresses PPAR-[gamma] transactivation. Nat Cell Biol. 2007;9:1273–1285. doi: 10.1038/ncb1647. [DOI] [PubMed] [Google Scholar]
- 47.He W, Barak Y, Hevener A, Olson P, Liao D, Le J, Nelson M, Ong E, Olefsky JM, Evans RM. Adipose-specific peroxisome proliferator-activated receptor gamma knockout causes insulin resistance in fat and liver but not in muscle. Proc Natl Acad Sci U S A. 2003;100:15712–15717. doi: 10.1073/pnas.2536828100. [DOI] [PMC free article] [PubMed] [Google Scholar]