Abstract
Mast cells are important effector cells of allergy and are involved in the pathology of many other diseases. Measurement of β-hexosaminidase activity, the most commonly used method for evaluation of murine mast cell activity, requires a large number of cells and thus is of limited utility for studying mast cells in mouse models of disease. In this study we evaluated the sensitivity of histamine release as compared to β-hexosaminidase activity in the measurement of mast cell activation. Whereas a minimum of 6×104 mast cells per ml were required to detect slight increases in β-hexosaminidase activity after anti-IgE and ionomycin stimulation, substantial increases in histamine release could be detected under the same activating conditions with as few as 480 mast cells per ml. These findings demonstrate that measurement of histamine release is substantially more sensitive than assessment of β-hexosaminidase activity for detecting mast cell activation. Additionally, we describe a novel flow cytometric method for detecting murine mast cell activation. When using 7.5×105 peritoneal cells per condition and gating on IgE+ c-kit+ cells, mast cell expression of surface CD200R1 increased after both IgE and non IgE-mediated activation. This flow cytometric procedure was uncomplicated and rapid, with increases in surface CD200R1 expression appearing after as little as 30 minutes of stimulation time. Measuring histamine release and surface CD200R1 expression are sensitive approaches for detection of murine mast cell activation. Further, both approaches can be done on unpurified peritoneal cell populations. By requiring low numbers of cells, these approaches are ideal for investigating mast cell activation in murine models of disease.
Keywords: mast cell, flow cytometry, CD200R1, histamine, β-hexosaminidase, IgE
1. Introduction
Mast cells, originally identified by Paul Ehrlich in 1878, are bone marrow-derived cells that reside in tissues throughout the body with skin, airways, and intestines being the most common sites (Metcalfe et al., 1997; Marshall, 2004). They typically become activated after surface bound IgE is crosslinked by multivalent antigen, which results in rapid release of inflammatory mediators such as histamine and leukotriene C4 and induces classical allergy symptoms (Metcalfe et al., 1997; Galli, 2000; Bischoff, 2007). Mast cells can also become activated by pathways that do not involve IgE and have the ability to release a wide range of cytokines and inflammatory molecules. In addition to being key effector cells of allergy, mast cells have become increasingly recognized as playing important roles in helminth infections, bacterial infections, autoimmunity, neurologic disorders, and vascular diseases (Marshall, 2004; Bischoff, 2007). Identifying easy and rapid techniques to measure activation of murine mast cells could be of substantial benefit for investigations involving disease models in mice.
Currently, the most common approach taken to assess murine mast cell activation is measurement of β-hexosaminidase activity. In this assay, purified mast cells are stimulated in vitro, supernatants are collected, and colored product is measured after addition of a nominally-priced β-hexosaminidase substrate. Although this approach is relatively simple and inexpensive, a major drawback is that it requires high numbers of mast cells. Typical protocols for the measurement of β-hexosaminidase activity require concentrations of 6×105 mast cells/ml per condition tested (Karimi et al., 2000; Mortaz et al., 2005a; Mortaz et al., 2005b). While obtaining such numbers of mast cells is not an issue for in vitro experiments utilizing cells lines or bone-marrow derived mast cells, it is limiting for experiments evaluating the function of mast cells obtained directly from mice. In this study, we tested whether histamine release was a more sensitive measure of murine mast cell activation than β-hexosaminidase assay.
Additionally, we developed a flow cytometric method for measuring murine mast cell activation. While there are numerous flow cytometric methods for detecting activated human mast cells, including measuring surface expression of CD35, CD63, CD69, LAMP-1, and LAMP-2 (Diaz-Agustin et al., 1999; Escribano et al., 2002; Grutzkau et al., 2004), there is no widely used flow cytometric assay in place for detection of murine mast cell activation. Recently, we demonstrated that surface expression of CD200R1, an inhibitory receptor belonging to the imunoglobulin superfamily, can be utilized as an activation marker of basophils (Torrero et al., 2009). As both basophils and mast cells can be activated through cross-linking of surface IgE and as a prior study demonstrated that murine mast cells express CD200R1 (Cherwinski et al., 2005), in this study we evaluated whether CD200R1 can be utilized for detection of murine mast cell activation.
Our results demonstrate that histamine is a far more sensitive measure of murine mast cell activation than β-hexosaminidase and that CD200R1 can be used as a marker of mast cell activation.
2. Materials and Methods
2.1 Animals
Female BALB/c mice were obtained from the NCI Mouse Repository (Frederick, MD) and housed at the Uniformed Services University (USU) Center for Laboratory Medicine. At study endpoints, mice were euthanized with carbon dioxide and cells used for mast cell activation studies obtained by peritoneal lavage. All experiments were performed in accordance with protocols approved by the USU Institutional Animal Care and Use Committee.
2.2 Infection of mice with Litomosoides sigmodontis
Balb/c mice were infected with L. sigmodontis, a filarial roundworm infection of rodents, by intraperitoneal injection of 40 L3 stage larvae. These worms migrate to the pleural cavity over the first few days of infection, where they subsequently reside and develop into adult worms by approximately 4 weeks post-infection. This infection results in the production of large quantities of parasite-specific IgE beginning at 4 weeks post-infection (Torrero et al., 2010), resulting in sensitization of both mast cells and basophils to parasite antigen (LsAg) through binding of specific IgE to FcER1. L3 stage larvae for infection studies were obtained from the pleural cavity of Mongolian jirds (Meriones unguiculatus, TRS Laboratory Inc., Athens, GA) that had been infected by the bite of infectious mites 4 days earlier as previously described (Hubner et al., 2009).
2.3 L. sigmodontis worm antigen (LsAg) Preparation
After harvesting male and female adult worms from infected animals, worms were mechanically homogenized. PBS-soluble fractions were then collected and sterile filtered as previously described (Torrero et al., 2010).
2.4 Collection of peritoneal cells
Peritoneal cells were collected as previously described (Jensen et al., 2006; de Almeida Buranello et al., 2010). Briefly, after soaking the ventral surface with 70% ethanol, fur-bearing skin and subcutaneous tissue was dissected away. Approximately 5 ml 1×HBSS (Invitrogen, CA) was injected into the peritoneal cavity and the abdomen was manually agitated for 1 minute. After carefully cutting a 5 mm hole in the peritoneum, 1×HBSS and cells were removed with a transfer pipette. The peritoneal cavity was then washed 2–3 more times with 1×HBSS. If significant red blood cell contamination existed, red blood cells were lysed with 0.8% ammonium chloride (NH4Cl).
2.5 Purification of peritoneal mast cells
After centrifugation of peritoneal cells at 400×g for 10 min, supernatant was thoroughly removed and the cell pellet resuspended in 70% percoll (GE Healthcare) solution and overlayed with RPMI (Mediatech Inc., VA), as previously described (Jensen et al., 2006; Gri et al., 2008). Cells were centrifuged for 15 minutes at 580×g. Non mast cells were carefully removed from the Percoll/RPMI interface along with most of the remaining supernatant without disturbing the mast cell pellet, which was then resuspended in RPMI.
2.6 Determining mast cell purity
Flow cytometric enumeration of mast cells
Total numbers of peritoneal cells were enumerated by counting non red cells using a hemocytometer. Cells were then fixed with fixative solution from a whole blood lysing reagent kit (Beckman Coulter, CA) containing 9.25% formaldehyde and 3.75% methanol. Cells were washed and resuspended in 250 µl of 1% bovine serum albumin (BSA, Cohn V fraction, Sigma-Aldrich, MO) in 1x PBS and incubated for 1 hour or overnight at 4°C. Cells were surface stained with anti-CD117 (c-kit) APC (2B8, BD Bioscience, CA) and anti-IgE FITC (R35–72, BD Biosciences) and incubated at 4°C for 30 minutes. After washing cells twice with 1x PBS, cells were resuspended in a final volume of 250 µl 1x PBS. Cell were then analyzed using a BD LSRII flow cytometer and FACSDiva software (BD Biosciences) and the percentage of peritoneal cells that were mast cells determined by measuring the percentage of total cells that were c-kit+IgE+. For all flow cytometry experiments, optimal concentrations of antibody used for staining were determined by individual antibody titration experiments.
Cytospins
Thirty thousand to seventy-five thousand cells were centrifuged at 500 RPM for 5 minutes onto slides using a cytofunnel and then allowed to air dry.
For May Grünwald/Giemsa staining, slides were placed in May Grünwald stain (Sigma-Aldrich) for 5 minutes, then 1×PBS, pH 7.2 for 2 minutes, followed by Giemsa stain (Sigma-Aldrich) diluted 1:20 in distilled, deionized water for 18 minutes. Slides were removed from 1:20 diluted Giemsa stain and briefly dipped in distilled, deionized water and allowed to air dry.
Toluidine blue staining was conducted as previously described (Kulka and Metcalfe, 2010). Briefly, slides were placed in Mota’s fixative for 10 minutes, fixative was removed by rinsing with 66% ethanol, and then slides were washed by dipping in distilled, deionized water. Slides were stained with acid toluidine solution for 10 minutes followed by dipping in distilled, deionized water. Slides were rinsed with 66% ethanol and dipped in distilled, deionized water and then rinsed with 100% ethanol and dipped in distilled, deionized water.
2.7 Measuring histamine concentration and β-hexosaminidase activity
Peritoneal cells and purified mast cells were adjusted to correct starting concentrations using Histamine Release Buffer (Beckman Coulter). Cells were incubated at 37°C for 15 minutes and then stimulated for 30 minutes with media, 1 µg/ml ionomycin (CalBiochem, CA), and 0.5 µg/ml anti-IgE at 37°C. At the end of the incubation period, cells were centrifuged at 400×g for 10 minutes.
For histamine measurement, 150 µl supernatant was first acylated in order to prevent the breakdown of histamine. Histamine concentrations were then measured using a competitive ELISA (EIA Histamine assay, Beckman Coulter) per the manufacterer’s directions. Concentrations of histamine in acylated supernatants were determined from a standard curve after reading absorbances at 405 nm using a Victor3 V plate reader (PerkinElmer, MA).
For β-hexosaminidase activity determination, 50 µl of supernatant was incubated with 200 µl of 1mM 4-nitrophenyl N-acetyl-β-D-glucosaminide enzyme substrate in 0.05M citrate acid buffer (pH 4.6) for 1 hour at 37 °C as previously described (Cohen and Brown, 2001). Reactions were terminated with 0.1M sodium carbonate buffer. Activity of β-hexosaminidase was determined from a standard curve generated with the enzyme product 4-nitrophenol in 0.05M citrate acid buffer after measuring absorbances at 405 nm using Victor3 V plate reader.
Fold increases in histamine release and 4-nitrophenol production were calculated by dividing media stimulation levels.
Percentage of total histamine was calculated by dividing the amount of histamine in supernatant after stimulation with media, anti-IgE, ionomycin, or LsAg by the amount of histamine in a lysed aliquot of cells. Percentage of total β-hexosaminidase activity was calculated by dividing the amount of 4-nitrophenol produced in supernatant after stimulation with media, anti-IgE, or ionomycin by the amount of 4-nitrophenol produced in a lysed aliquot of cells.
2.8 Measuring mast cell activation by flow cytometry
After counting total numbers of peritoneal cells using a hemocytometer, peritoneal cells were diluted with RPMI to a concentration of 3.75x106/ml and 200 µl of cells were aliquoted per tube (750,000 cells/tube). Cells were incubated for 15 minutes at 37°C and then stimulated with either several concentrations of anti-IgE (0.0078, 0.031, 0.125, 0.5, 2 µg/ml, R35–72, BD Biosciences), 0.5 µg/ml ionomycin, or 0.5 µM fMLP. Cells were incubated at 37°C for 0.5, 1, 2, 3 or 4 hours for timecourse experiments and for 3 hours for all other experiments. At the end of the incubation period, cells were fixed with 100 µl of fixative solution from a whole blood lysing reagent kit (Beckman Coulter, CA), containing 9.25% formaldehyde and 3.75% methanol. Cells were washed and resuspended in 250 µl 1% bovine serum albumin (BSA, Cohn V fraction, Sigma-Aldrich) in 1×PBS and blocked for 1 hour or overnight at 4°C. After incubation with 1% BSA, cells were surface stained with anti-CD117 APC, anti-IgE FITC, and anti-CD200R1 (OX-110, AbdSerotec) for 30 minutes at 4°C. After washing cells twice with 1×PBS, cells were resuspended to a final volume of 250 µl with 1×PBS and analyzed using a BD LSRII flow cytometer and FACSDiva software (BD Biosciences). Gates for CD200R1 positivity were established using the fluorescence minus 1 (FMO) technique (Baumgarth and Roederer, 2000).
2.9 Statistical Analyses
Two-tailed, paired Student’s t test was used to determine statistical significance between two groups. All statistics were performed using GraphPad Prism 4.03. P-values less than 0.05 were considered significant.
3. Results
3.1 Identification and enumeration of peritoneal cells and peritoneal mast cells
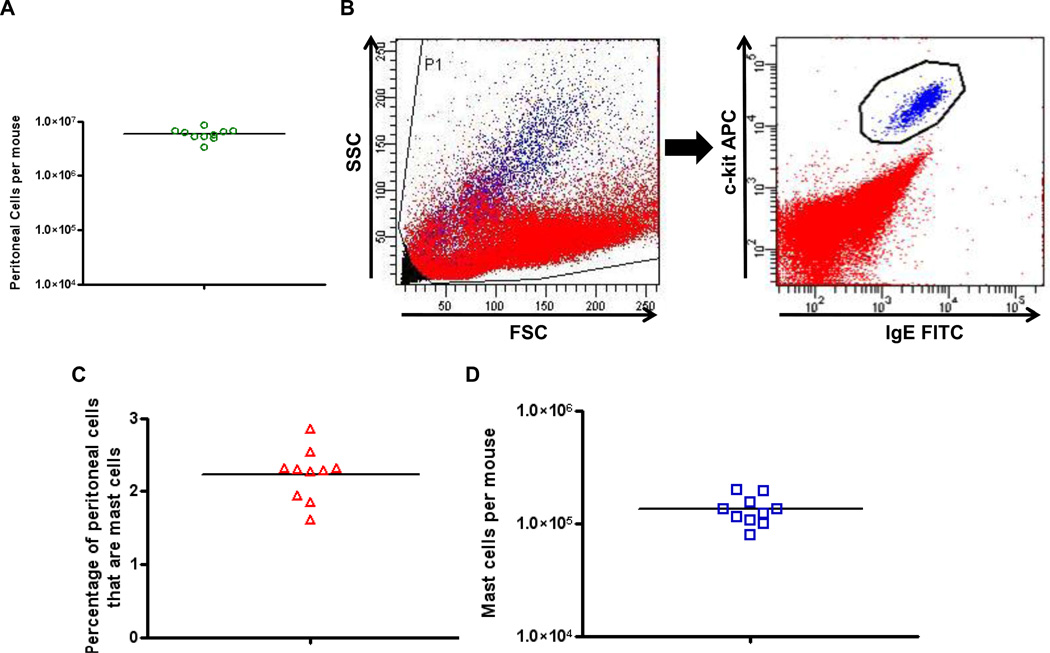
Peritoneal cells were obtained from individual BALB/c mice by lavage of the peritoneal cavity with cold 1×HBSS. Total peritoneal cells from each mouse were enumerated using a hemocytometer and averaged 5.95×106 per mouse (Fig. 1A). Mast cells were identified as IgE+ c-kit+ cells by flow cytometry (Fig. 1B). The mean percentage of peritoneal cells that were mast cells was 2.23% (Fig. 1C), resulting in a total of 132,685 peritoneal mast cells on average per mouse (Fig. 1D).
Figure 1.
Identification and enumeration of peritoneal cells and peritoneal mast cells. A. Numbers of total peritoneal cells from individual mice. Peritoneal cells were obtained by lavage and counted with a hemocytometer. B. Gating strategy to identify mast cells. After initial forward and side scatter gating (left panel), mast cells were identified as IgE+ and c-kit+ cells (right panel). Mast cells shown in blue, other peritoneal cells shown in red. C. Percentages of peritoneal cells that were mast cells determined by flow cytometry. D. Total calculated number of peritoneal mast cells per mouse. For all panels, n = 10.
3.2 70% Percoll gradient results in a highly purified population of peritoneal mast cells
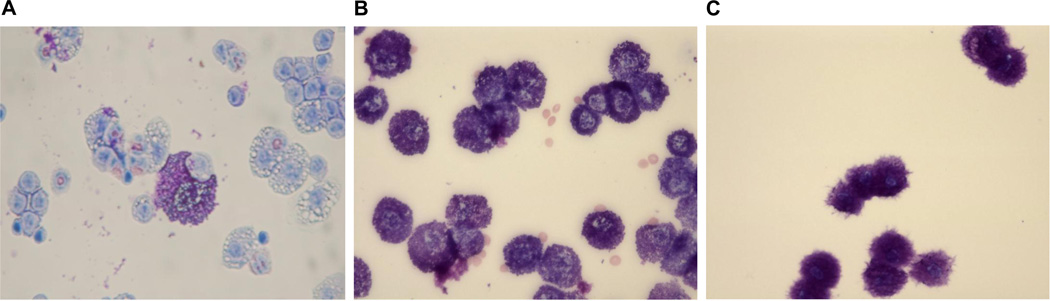
Use of a percoll gradient to purify peritoneal mast cells has been previously described (Jensen et al., 2006; Gri et al., 2008). As seen in figure 2, this approach results in a highly purified population of mast cells. Peritoneal cells were obtained by lavage of the peritoneal cavity of BALB/c mice. Mast cells were determined to be 2.5% of all peritoneal cells by May Grünwald/Giemsa staining of cytospins (Fig. 2A). A 70% percoll gradient was used to isolate mast cells from other peritoneal cells. Mast cell purity was measured as greater than 98% by both May Grünwald/Giemsa staining (Fig. 2B) and toluidine blue staining (Fig. 2C).
Figure 2.
A 70% Percoll gradient results in a highly purified population of peritoneal mast cells. A. Cytospin of total peritoneal cells stained with May Grünwald/Giemsa stain. Mast cells determined to be 2.5% of all peritoneal cells. B. Cytospin of May Grünwald/Giemsa stained mast cells purified from the peritoneal cavity with percoll. Mast cells determined to be >98% of all cells. C. Additional staining of purified mast cells with Toluidine Blue Stain. Percentage of cells that were mast cells was identical to May Grünwald/Giemsa stainied cells. Magnification = 400x.
3.3 Histamine release is a more sensitive test of mast cell activation than β-hexosaminidase activity
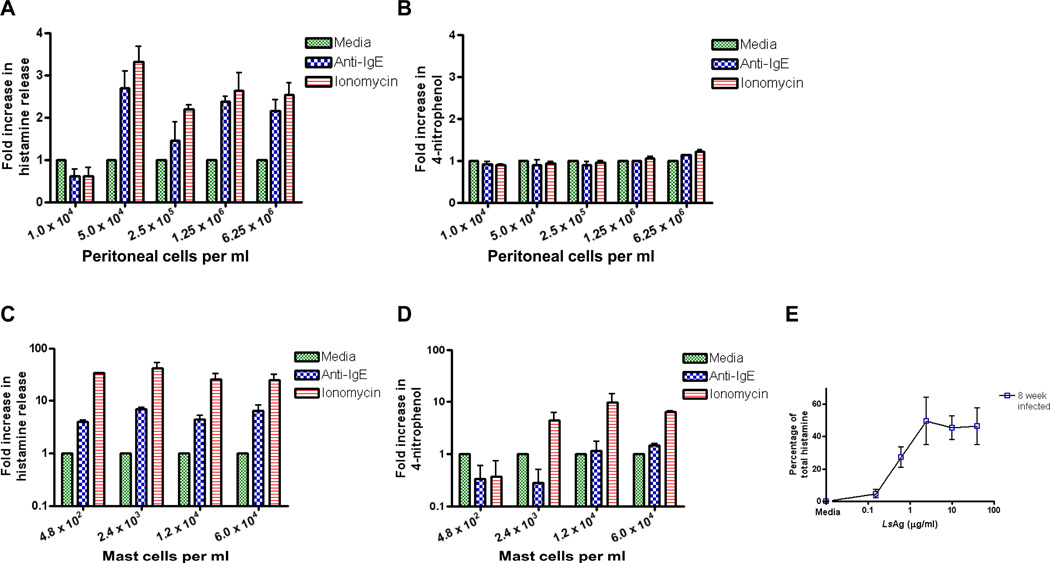
Sensitivities of two mast cell degranulation assays were compared since ex vivo assays are often limited by cell number. Histamine release and β-hexosaminidase activity were measured in peritoneal cells and purified peritoneal mast cells after 30 min of incubation with media, 0.5 µg/ml anti-IgE, or 1 µg/ml ionomycin. Histamine release in response to both anti-IgE and ionomycin stimulation could be detected from as few as 5×104 peritoneal cells/ml (mean concentration of histamine release when stimulated with media = 47.2 nM vs. 118.8 nM when stimulated with anti-IgE and 150.5 nM when stimulated with ionomycin, for average fold increases of 2.5 and 3.2, Fig. 3A). However, only slight increases in β-hexosaminidase activity could be detected even when concentrations of 6.25×106 peritoneal cells/ml were used (mean concentration of 4-nitrophenol with media = 57.2 µM vs. 64.5 µM when stimulated with anti-IgE and 69.2 µM when stimulated with ionomycin, for average fold increases of less than 1.3 for both conditions, Fig. 3B).
Figure 3.
Histamine release is a more sensitive test of mast cell activation than β-hexosaminidase activity. (A) Histamine release and (B) β-hexosaminidase activity measured in the supernatants of peritoneal cells after 30 minute stimulation with media, 0.5 µg/ml anti-IgE, and 1 µg/ml ionomycin. (C) Histamine release and (D) β-hexosaminidase activity measured in the supernatants of purified peritoneal mast cells after 30 minute stimulation with media, 0.5 µg/ml anti-IgE, and 1 µg/ml ionomycin. E. Histamine release from 4×103 mast cells/ml purified from the peritoneal cavity of mice infected for 8 weeks with Litomosoides sigmodontis after stimulation with LsAg. For panels A-D, fold increases in histamine release and 4-nitrophenol production were calculated by dividing anti-IgE and ionomycin stimulation levels by media stimulation levels. n = at least 3 independent experiments per condition for all experiments. Means +/− SEMs are shown.
The same assays were then performed on purified mast cells. Increases in histamine concentration could be detected after anti-IgE and ionomycin stimulation at concentrations of mast cells as low as 480 mast cells/ml (mean concentration of histamine release when incubated with media = 3.4 nM vs. 13.2 nM when stimulated with anti-IgE and 114.6 nM when stimulated with ionomycin, for average fold increases of 3.9 and 33.7, Fig. 3C). While increases in β-hexosaminidase activity over media were detected after ionomycin stimulation with 6×104, 1.2×104, and 2.4×103 mast cells/ml, changes in β-hexosaminidase activity after anti-IgE stimulation could only be detected with the highest concentration of 6×104 mast cells per ml (mean concentration of 4-nitrophenol when incubated with media = 3.9 µM vs. 5.5 µM when stimulated with 0.5 µg/ml anti-IgE, average fold increase 1.4, Fig. 3D). As seen in supplemental figure 1, histamine release was also more sensitive at detect mast cell activation than β-hexosaminidase assay when the output value measured was percentage release of total histamine, or for the β-hexosaminidase assay, percentage of total 4-nitrophenol produced when cell lysate was utilized.
Histamine release was also a sensitive method for detecting activation of mast cells that had become sensitized to worm antigen during infection of mice with the filarial nematode Litomosoides sigmodontis. This infection sensitizes mast cells and basophils to LsAg by inducing high levels of LsAg-specific IgE (Hubner et al., 2010). As seen in figure 3E, mast cell activation curves to LsAg were generated with as few as 4×103 mast cells/ml (Fig. 3E).
3.4 Mast cell surface CD200R1 time-course expression following activation with different anti-IgE doses
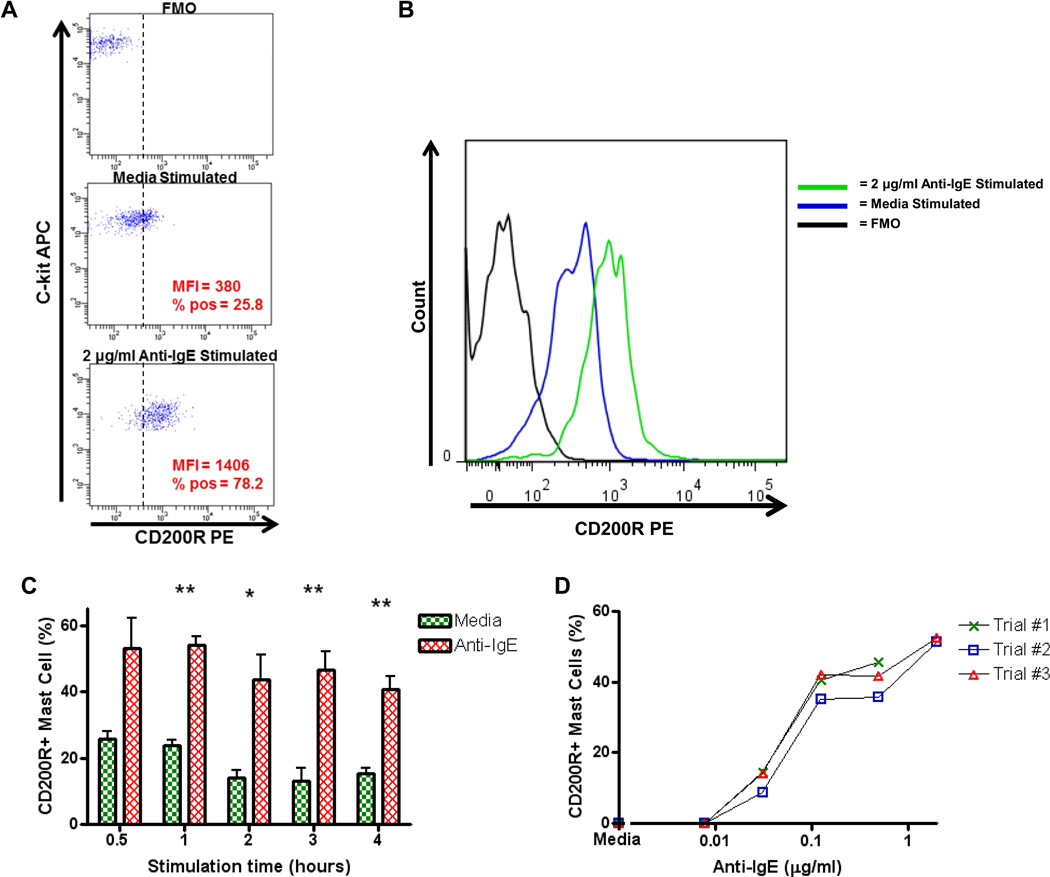
Recently, we demonstrated that surface expression of CD200R1, an inhibitory receptor belonging to the immunoglobulin superfamily, can be utilized as a marker of basophil activation (Torrero et al., 2009). To ascertain whether CD200R1 could also be used as a murine mast cell activation marker, peritoneal cells were stimulated with anti-IgE for various amounts of time and CD200R1 expression on mast cells evaluated by flow cytometry. Peritoneal mast cells were identified as IgE+ c-kit+ cells. As seen in the lower panel of figure 4A and in figure 4B, mast cell expression of CD200R1 markedly increases after activation with anti-IgE. The percentage of mast cells expressing CD200R1 and the mean fluorescence intensity of surface CD200R1 staining of all mast cells both increase substantially. To evaluate the kinetics of CD200R1 upregulation on mast cells after IgE-stimulation, surface CD200R1 expression was measured on mast cells after various incubation periods. Surface CD200R1 expression was upregulated in as little as 30 minutes following stimulation with 0.125 µg/ml anti-IgE (mean percentage of CD200R1+ mast cells = 25.8 after media incubation vs. 53.0 when stimulated with anti-IgE, Fig. 4C). CD200R1 remained upregulated for as long as 4 hours after stimulation, and significant differences between media stimulation and anti-IgE stimulation were found when stimulating for 1, 2, 3 and 4 hours (mean percentages of CD200R1+ mast cells after media incubation = 23.7, 13.9, 12.9, 15.3 vs. 54.0, 43.5, 46.5, 40.8 after stimulation with anti-IgE, p = 0.001, p = 0.03, p = 0.006, p = 0.01, Fig. 4C). Expression of CD200R1 also increased in a dose-dependent manner in response to increasing concentrations of anti-IgE stimulation (Fig. 4D).
Figure 4.
Mast cell surface CD200R1 expression following activation with anti-IgE. A. Dot plot graphs of CD200R1 expression on peritoneal mast cells. Peritoneal cells were incubated for 3 h with media alone or 2 µg/ml of anti-IgE and then analyzed by flow cytometry. Mast cells were gated as IgE+ c-kit+ (as per figure 1B) and then evaluated for surface CD200R1 expression. Top panel shows PE staining in the absence of anti-CD200R1-PE staining (FMO, fluorescence minus one). The FMO panel was used to set a cut-off for CD200R1 positivity (dashed vertical line). MFI values are for the entire population of mast cells. % pos = % of all mast cells positive for CD200R1. B. Activation of identical mast cells shown in panel A displayed as a histogram. C. Percentages of mast cells expressing CD200R1 after stimulation of peritoneal cells with media and 0.125 µg/ml anti-IgE for 0.5, 1, 2, 3, and 4 hours (n = 3 per condition). Means +/− SEMs are shown. D. Percentages of mast cells expressing CD200R1 in response to stimulation with 0.0078, 0.031, 0.125, 0.5, and 2.0 µg/ml anti-IgE for 3 hours and after subtracting media stimulation levels. * = p < 0.05, ** = p < 0.01.
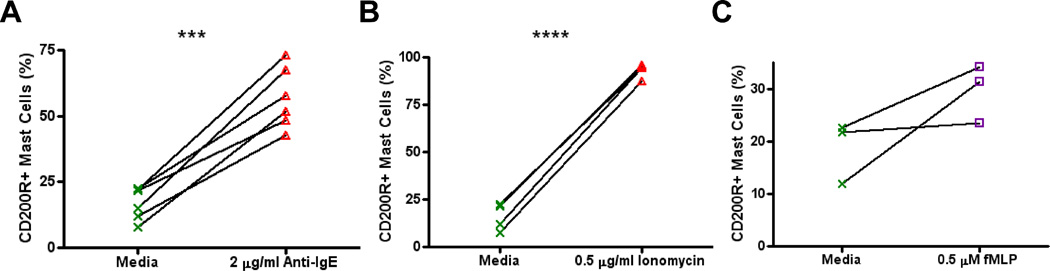
3.5 Increased surface CD200R1 expression following IgE-mediated and non IgE-mediated stimulation
While significant increases in CD200R1 expression were observed after stimulation with anti-IgE (mean percentage of CD200R1+ mast cells incubated with media = 16.9 vs. 57.0 when stimulated with 2 µg/ml anti-IgE, p = 0.0003, Fig. 5A), increases in the percentage of mast cells expressing CD200R1 also occurred after IgE-independent stimulation of cells with ionomycin or fMLP. Whereas a mean of 17.2 percent of mast cells expressed CD200R1 after incubation with media, a mean of 93.7 percent were CD200R1+ after stimulation with ionomycin (p<0.0001, Fig. 5B). Similarly, the mean percentage of mast cells expressing CD200R1 after fMLP stimulation was 29.7 vs only 18.8 after media incubation (Fig. 5C). These trends were also observed when assessing changes in CD200R1 expression on a per cell basis by MFI (Supplemental Fig. 2).
Figure 5.
Increased surface CD200R1 expression following IgE-mediated and non IgE-mediated stimulation. Percentages of mast cells expressing surface CD200R1 after stimulation with (A) 2 µg/ml anti-IgE, (B) 0.5 µg/ml ionomycin, and (C) 0.5 µM fMLP. *** = p < 0.001, **** = p < 0.0001.
4. Discussion
Mast cells are effector cells of allergic disease (Metcalfe et al., 1997; Galli, 2000; Bischoff, 2007), are involved in the pathology of many other diseases (Bischoff, 2007), and can play critical roles in host defense (Woodbury et al., 1984; Echtenacher et al., 1996; Malaviya et al., 1996; Maurer et al., 1998; Knight et al., 2000; McDermott et al., 2003; Edelson et al., 2004; Specht et al., 2011). Their activation status is a diagnostic criterion of some diseases like mastocytosis (Diaz-Agustin et al., 1999; Escribano et al., 2002; Grutzkau et al., 2004) and may be important for the progression or control of other diseases. In this study, we found that histamine release and surface CD200R1 staining are sensitive methods for detection of murine mast cell activation.
Comparison studies clearly demonstrated that histamine release was far more sensitive than β-hexosaminidase assay for detecting activation of in vitro stimulated mast cells. Histamine release after anti-IgE and ionomycin stimulation could be measured from as few as 480 purified mast cells per ml, while the minimum concentration of mast cells required to detect an increase in β-hexosaminidase activity after identical stimulation was 6×104 per ml. Typical protocols assessing β-hexosaminidase activity require a concentration of 6×105 mast cells per ml (Karimi et al., 2000; Mortaz et al., 2005a; Mortaz et al., 2005b) in order to observe significant activation of cells – a concentration that requires 1,250 times more mast cells than histamine release assays would require. Given that the average mouse we analyzed had 132,000 mast cells in its peritoneum (Fig. 1D), ex-vivo studies would require pooling cells from several mice to study mast cell activation if β-hexosaminidase assays were used, whereas numerous histamine release studies could be conducted using the mast cells obtained from just one mouse. Additionally, our results also demonstrate that histamine release can be used to study mast cell activation using non-purified peritoneal cells. Use of freshly obtained peritoneal cells has the advantage of decreasing ex vivo manipulation of cells, though it would require measurement of percentages of mast cells in each peritoneal cell population to make comparisons between different mice.
In this study we also developed a flow cytometric method for measuring murine mast cell activation. When using 7.5×105 peritoneal cells per condition and gating on IgE+ c-kit+ cells, mast cell expression of surface CD200R1 increased after IgE and non IgE-mediated activation. This procedure was uncomplicated and quick, with increased CD200R1 appearing after as little as 30 minutes of stimulation time. This flow cytometric technique does not require mast cell purification, and has the advantage of enumerating mast cells while measuring their activation.
Highlights for Journal of Immunological Methods.
Histamine release is more sensitive for the measurement of murine mast cell activation than β-hexosaminidase.
Surface CD200R1 expression can be utilized as a murine mast cell activation marker by flow cytometry.
Histamine release and flow cytometry for CD200R1 can be performed on unpurified peritoneal cells.
These approaches require few cells, so they are ideal for investigating mast cell activation in animal models of disease.
Supplementary Material
Acknowledgments
The authors thank Karen Wolcott, Kateryna Lund (Biomedical Instrumentation Center), and Kristin Killoran (Department of Microbiology) at the Uniformed Services University for assistance with flow cytometry.
This work was supported by National Institutes of Allergy and Infectious Diseases, National Institutes of Health Grant R01AI076522.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Baumgarth N, Roederer M. A practical approach to multicolor flow cytometry for immunophenotyping. J Immunol Methods. 2000;243:77–97. doi: 10.1016/s0022-1759(00)00229-5. [DOI] [PubMed] [Google Scholar]
- Bischoff SC. Role of mast cells in allergic and non-allergic immune responses: comparison of human and murine data. Nat Rev Immunol. 2007;7:93–104. doi: 10.1038/nri2018. [DOI] [PubMed] [Google Scholar]
- Cherwinski HM, Murphy CA, Joyce BL, Bigler ME, Song YS, Zurawski SM, Moshrefi MM, Gorman DM, Miller KL, Zhang S, Sedgwick JD, Phillips JH. The CD200 receptor is a novel and potent regulator of murine and human mast cell function. J Immunol. 2005;174:1348–1356. doi: 10.4049/jimmunol.174.3.1348. [DOI] [PubMed] [Google Scholar]
- Cohen JS, Brown HA. Phospholipases stimulate secretion in RBL mast cells. Biochemistry. 2001;40:6589–6597. doi: 10.1021/bi0103011. [DOI] [PubMed] [Google Scholar]
- de Almeida Buranello PA, Moulin MR, Souza DA, Jamur MC, Roque-Barreira MC, Oliver C. The lectin ArtinM induces recruitment of rat mast cells from the bone marrow to the peritoneal cavity. PLoS One. 2010;5:e9776. doi: 10.1371/journal.pone.0009776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz-Agustin B, Escribano L, Bravo P, Herrero S, Nunez R, Navalon R, Navarro L, Torrelo A, Cantalapiedra A, Del Castillo L, Villarrubia J, Navarro JL, San Miguel JF, Orfao A. The CD69 early activation molecule is overexpressed in human bone marrow mast cells from adults with indolent systemic mast cell disease. Br J Haematol. 1999;106:400–405. doi: 10.1046/j.1365-2141.1999.01572.x. [DOI] [PubMed] [Google Scholar]
- Echtenacher B, Mannel DN, Hultner L. Critical protective role of mast cells in a model of acute septic peritonitis. Nature. 1996;381:75–77. doi: 10.1038/381075a0. [DOI] [PubMed] [Google Scholar]
- Edelson BT, Li Z, Pappan LK, Zutter MM. Mast cell-mediated inflammatory responses require the alpha 2 beta 1 integrin. Blood. 2004;103:2214–2220. doi: 10.1182/blood-2003-08-2978. [DOI] [PubMed] [Google Scholar]
- Escribano L, Diaz-Agustin B, Nunez R, Prados A, Rodriguez R, Orfao A. Abnormal expression of CD antigens in mastocytosis. Int Arch Allergy Immunol. 2002;127:127–132. doi: 10.1159/000048183. [DOI] [PubMed] [Google Scholar]
- Galli SJ. Mast cells and basophils. Curr Opin Hematol. 2000;7:32–39. doi: 10.1097/00062752-200001000-00007. [DOI] [PubMed] [Google Scholar]
- Gri G, Piconese S, Frossi B, Manfroi V, Merluzzi S, Tripodo C, Viola A, Odom S, Rivera J, Colombo MP, Pucillo CE. CD4+CD25+ regulatory T cells suppress mast cell degranulation and allergic responses through OX40-OX40L interaction. Immunity. 2008;29:771–781. doi: 10.1016/j.immuni.2008.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grutzkau A, Smorodchenko A, Lippert U, Kirchhof L, Artuc M, Henz BM. LAMP-1 and LAMP-2, but not LAMP-3, are reliable markers for activation-induced secretion of human mast cells. Cytometry A. 2004;61:62–68. doi: 10.1002/cyto.a.20068. [DOI] [PubMed] [Google Scholar]
- Hubner MP, Torrero MN, McCall JW, Mitre E. Litomosoides sigmodontis: a simple method to infect mice with L3 larvae obtained from the pleural space of recently infected jirds (Meriones unguiculatus) Exp Parasitol. 2009;123:95–98. doi: 10.1016/j.exppara.2009.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubner MP, Torrero MN, Mitre E. Type 2 immune-inducing helminth vaccination maintains protective efficacy in the setting of repeated parasite exposures. Vaccine. 2010;28:1746–1757. doi: 10.1016/j.vaccine.2009.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen BM, Swindle EJ, Iwaki S, Gilfillan AM. Generation, isolation, and maintenance of rodent mast cells and mast cell lines. Curr Protoc Immunol. 2006 doi: 10.1002/0471142735.im0323s74. Chapter 3, Unit 3 23. [DOI] [PubMed] [Google Scholar]
- Karimi K, Redegeld FA, Blom R, Nijkamp FP. Stem cell factor and interleukin-4 increase responsiveness of mast cells to substance P. Exp Hematol. 2000;28:626–634. doi: 10.1016/s0301-472x(00)00161-2. [DOI] [PubMed] [Google Scholar]
- Knight PA, Wright SH, Lawrence CE, Paterson YY, Miller HR. Delayed expulsion of the nematode Trichinella spiralis in mice lacking the mucosal mast cell-specific granule chymase, mouse mast cell protease-1. J Exp Med. 2000;192:1849–1856. doi: 10.1084/jem.192.12.1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulka M, Metcalfe DD. Isolation of tissue mast cells. Curr Protoc Immunol. 2010 doi: 10.1002/0471142735.im0725s90. Chapter 7, Unit 7 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malaviya R, Ikeda T, Ross E, Abraham SN. Mast cell modulation of neutrophil influx and bacterial clearance at sites of infection through TNF-alpha. Nature. 1996;381:77–80. doi: 10.1038/381077a0. [DOI] [PubMed] [Google Scholar]
- Marshall JS. Mast-cell responses to pathogens. Nat Rev Immunol. 2004;4:787–799. doi: 10.1038/nri1460. [DOI] [PubMed] [Google Scholar]
- Maurer M, Echtenacher B, Hultner L, Kollias G, Mannel DN, Langley KE, Galli SJ. The c-kit ligand, stem cell factor, can enhance innate immunity through effects on mast cells. J Exp Med. 1998;188:2343–2348. doi: 10.1084/jem.188.12.2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDermott JR, Bartram RE, Knight PA, Miller HR, Garrod DR, Grencis RK. Mast cells disrupt epithelial barrier function during enteric nematode infection. Proc Natl Acad Sci U S A. 2003;100:7761–7766. doi: 10.1073/pnas.1231488100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metcalfe DD, Baram D, Mekori YA. Mast cells. Physiol Rev. 1997;77:1033–1079. doi: 10.1152/physrev.1997.77.4.1033. [DOI] [PubMed] [Google Scholar]
- Mortaz E, Redegeld FA, Nijkamp FP, Engels F. Dual effects of acetylsalicylic acid on mast cell degranulation, expression of cyclooxygenase-2 and release of pro-inflammatory cytokines. Biochem Pharmacol. 2005a;69:1049–1057. doi: 10.1016/j.bcp.2004.12.018. [DOI] [PubMed] [Google Scholar]
- Mortaz E, Redegeld FA, van der Heijden MW, Wong HR, Nijkamp FP, Engels F. Mast cell activation is differentially affected by heat shock. Exp Hematol. 2005b;33:944–952. doi: 10.1016/j.exphem.2005.05.004. [DOI] [PubMed] [Google Scholar]
- Specht S, Frank JK, Alferink J, Dubben B, Layland LE, Denece G, Bain O, Forster I, Kirschning CJ, Martin C, Hoerauf A. CCL17 Controls Mast Cells for the Defense against Filarial Larval Entry. J Immunol. 2011;186:4845–4852. doi: 10.4049/jimmunol.1000612. [DOI] [PubMed] [Google Scholar]
- Torrero MN, Hubner MP, Larson D, Karasuyama H, Mitre E. Basophils amplify type 2 immune responses, but do not serve a protective role, during chronic infection of mice with the filarial nematode Litomosoides sigmodontis. J Immunol. 2010;185:7426–7434. doi: 10.4049/jimmunol.0903864. [DOI] [PubMed] [Google Scholar]
- Torrero MN, Larson D, Hubner MP, Mitre E. CD200R surface expression as a marker of murine basophil activation. Clin Exp Allergy. 2009;39:361–369. doi: 10.1111/j.1365-2222.2008.03154.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodbury RG, Miller HR, Huntley JF, Newlands GF, Palliser AC, Wakelin D. Mucosal mast cells are functionally active during spontaneous expulsion of intestinal nematode infections in rat. Nature. 1984;312:450–452. doi: 10.1038/312450a0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.