Abstract
Background
Little attention has been paid to selecting and developing health-related quality of life (HRQOL) measurement tools for young adult survivors of childhood cancer (YASCC). The primary purpose of this study was to develop a HRQOL tool for YASCC based on three legacy instruments.
Methods
Data collected from 151 YASCC were analyzed. HRQOL was measured using the Medical Outcomes Study SF-36, Quality of Life in Adult Cancer Survivors, and Quality of Life-Cancer Survivor. We used the following stages to develop our HRQOL tool: mapping items from three instruments into a common HRQOL construct, checking dimensionality using confirmatory factor analyses (CFA), and equating items using Rasch modeling.
Results
We assigned 123 items to a HRQOL construct comprised of six generic and eight survivor-specific domains. CFA retained 107 items that meet the assumptions of unidimensionality and local independence. Rasch analysis retained 68 items that satisfied the indices of information-weighted/outlier-sensitive fit statistic mean square. However, items in most domains possess relatively easy measurement properties, whereas YASCC’s underlying HRQOL was on the middle to higher levels.
Conclusions
Psychometric properties of the established tool for measuring HRQOL of YASCC were not satisfied. Future studies need to refine this tool, especially adding more challenging items.
Keywords: Childhood cancer, measurement, quality of life, Rasch analysis, young adult survivor
INTRODUCTION
As a result of advances in oncology therapies and post-treatment care, the number of young adult survivors of childhood cancer (YASCC) has increased significantly. The 5-year survival rate of American YASCC has improved from 58% in the 1970s to 80% in the 2000s [1]. The increased survival rates of childhood cancer are usually accompanied by late effects (LEs) and associated growth and developmental delays. These adverse effects are caused by cancer therapies and/or cancer itself, and may appear several years after the cancer diagnosis and successful treatment. For YASCC, typical LEs include neurocognitive, cardiopulmonary, endocrine, and musculoskeletal impairments, as well as recurrent or secondary cancers [2]. Oeffinger et al. [3] and Geenen et al., [4] reported that 62% and 75% of YASCC, respectively, developed at least one adverse outcome or chronic disease associated with LEs.
LEs may lead to impaired daily functioning and health-related quality of life (HRQOL) in childhood cancer survivors [5–9]. Many studies are dedicated to investigating HRQOL of YASCC using generic or condition-specific instruments. Typical generic instruments include the Medical Outcomes Study SF-36, Symptom Checklist-90 Revised, Health Utility Index, etc. However, the use of generic instruments tends to indicate inconclusive findings in HRQOL when comparing YASCC to healthy controls (i.e., better HRQOL [10,11], impaired HRQOL [12–14], or no difference [15–17]). Although the findings can be confounded by factors such as cancer type, treatment modality, age at diagnosis, survival time, etc., it is likely that generic instruments are not sensitive enough to capture concerns which are important and unique to YASCC.
In contrast, several studies used treatment-based instruments to measure HRQOL of YASCC, such as the European Organization for Research and Treatment of Care Quality of Life Core-30 (EORTC QOL-C30) [18] and the Functional Assessment of Cancer Therapy (FACT) [19,20]. However, these instruments initially were developed for cancer patients receiving active treatment and primarily focus on the side effects of treatments such as symptoms experienced by patients rather than the longer-term LEs experienced by survivors. The emphasis on symptoms and subsequent failure to recognize survivors’ LEs can negatively impact clinical decision-making and survivorship planning.
A limited number of HRQOL instruments have been developed for long-term cancer survivors. The Quality of Life-Cancer Survivors (QOL-CS) [21], Quality of Life in Adult Cancer Survivors (QLACS) [22], and Impact of Cancer for Childhood Cancer Survivors (IOC-CS) [23] are three notable survivor-based instruments. However, the QOL-CS and QLACS were developed for survivors of adult-onset cancer rather than YASCC, and thus it cannot be assumed these instruments can appropriately capture YASCC’s perception about their HRQOL. The IOC-CS was developed to measure unique psychosocial issues and changes experienced by YASCC such as life challenge, talking with parents, personal growth, financial problems, etc. Evidence suggests that content of the IOC-CS is partially covered by the QLACS [24] and that it should be used as a supplement to generic HRQOL measures [23].
From a measurement perspective, the use of legacy HRQOL instruments for YASCC may be limited because they were developed by classical test theory (CTT) [25–27]. Scores derived from CTT typically cannot differentiate between a subject’s underlying trait (e.g., physical functioning) and measurement properties of the instrument (e.g., item difficulty). In addition, CTT is test/scale-driven rather than item-driven, meaning that the entire set of items in a specific domain must be administered for all subjects to ensure reliability even though some items do not match subjects’ underlying functioning.
Item response theory (IRT) is an item-level analysis for investigating the relationship between subjects’ responses to an item and their underlying HRQOL [25–27]. IRT is useful in identifying whether items designed to measure a specific domain of HRQOL (e.g., physical functioning) from different instruments possess the same measurement property (e.g., item difficulty). Moreover, IRT can help develop item banks, which are a collection of well tested items measuring the same construct from different sources, and suggest whether adding other items can improve measurement precision of specific levels of underlying HRQOL [28,29]. Developing item banks provides a great foundation for dynamic assessments of HRQOL, such as computerized adaptive testing. Dynamic assessment is an important concept of measurement for cancer survivors because their underlying HRQOL is assumed to change over time.
The purpose of this study was to develop a HRQOL tool for YASCC who were enrolled in either the Cancer/Tumor Registries of the University of Florida (UF) or the Moffitt Cancer Center (MCC), or UF’s Survivorship Program. Specifically, items from two survivor-specific instruments (the QOL-CS and QLACS) and one generic instrument (the SF-36 Health Survey) were investigated and Rasch modeling was implemented for developing our HRQOL tool.
METHODS
Study population
Subjects were recruited using the following criteria of eligibility: 1) between 21 and 30 years old, 2) diagnosed with cancer prior to age 18, and 3) completed active cancer treatment at least two years prior to study participation. All cancer diagnoses were included, except skin cancers, carcinoma in situ, and precancerous conditions. Eligible subjects were identified through UF and MCC Cancer/Tumor Registries or UF’s Survivorship Program.
Data collection
Both the UF and MCC IRBs approved this study. After eligible subjects (570 from UF and 109 from MCC) were identified, an introductory letter was sent to request participation and offer the ability to opt out of the study. Subjects who did not opt out were telephoned for a thirty-minute interview, where they were asked to complete the HRQOL survey, and answer socio demographic information. The interviews were conducted between 06/2009 and 09/2009 (UF subjects) and between 03/2010 and 05/2010 (MCC subjects). Valid contact information was unavailable for 337 subjects (49.6% of total sample). Twenty seven potential subjects were reached but did not meet the eligibility criteria, 48 declined to participate, and 151 completed the survey. The remaining 116 subjects had a working telephone number, but could not be reached. The overall response rate was 47.9%. Of the 151 subjects, 141 were recruited from UF (129 from the Cancer/Tumor Registry and 12 from the CSP) and 10 were recruited from MCC. A $25 gift card was mailed to subjects after the telephone interview was completed. There were no difference in age, gender, and race/ethnicity between eligible YASCC who did or did not participate in this study (all P’s>0.05).
Measurement
Items derived from three instruments were used to establish our HRQOL measurement tool (Table 1). The QOL-CS is comprised of four survivor-specific domains: physical, psychological, social, and spiritual well-being [21]. A Likert-type rating scale with 7 response categories was used for each item. The QLACS is comprised of seven generic domains: negative feelings, positive feelings, cognitive problems, physical pain, sexual functioning, social avoidance, and fatigue. In addition to generic domains, this instrument is comprised of four cancer-specific domains: financial problems, distress about family, distress about cancer recurrence, appearance problems, and benefits of cancer [22]. A Likert-type rating scale with 7 response categories was used for each item. The SF-36 is comprised of eight generic domains of HRQOL: physical functioning, role limitations due to physical health problems, bodily pain, general health perceptions, vitality, social functioning, role limitations due to emotional problems, and mental health [30]. A Likerttype rating scale with 3 and 5 response categories was used for different items, respectively. Each subject self reported all items of the three HRQOL instruments by using a time frame of “past four weeks.”
Table 1.
Content of three legacy instruments
| QOL-CS | QLACS | SF-36* | |
|---|---|---|---|
| Domain (# of items) |
|
Generic
Survivor-specific
|
|
An item measuring health transition is not included
We also collected information on self-reported LEs (1 item) and comorbid conditions (1 item) for the purpose of testing known-groups validity. We defined LEs as any medical conditions that are directly related to cancer or previous cancer treatment, which was measured based on the following item: “Do you currently have any serious medical conditions related to your cancer or previous cancer treatments; for example, secondary cancers, recurrence of cancer, or complications such as hearing loss from platinum chemotherapy or radiation to the ear?” We defined comorbid conditions as any medical conditions that are not directly related to cancer or previous cancer treatment, which was measured based on the following item: “Do you currently have any medical conditions that are unrelated to cancer such as asthma?”
Developing a HRQOL measurement tool
We proposed a four-stage approach to develop and validate our HRQOL tool: 1) mapping items from three instruments into a common construct of HRQOL, 2) checking the dimensionality of the common construct, 3) equating items within a specific HRQOL domain using Rasch analysis, and 4) validating each domain of a HRQOL tool.
Stage 1: Mapping items from three instruments into a common construct of HRQOL
Given the fact that the construct and associated items of the three instruments are not the same, it is important to establish a common construct of HRQOL to accommodate all items. This common construct was initially determined by Pearson’s correlation coefficient between the score of each item in the two survivor-specific instruments (i.e., the QOL-CS and QLACS) and scores of the eight domains in the SF-36. We used the SF-36 as a framework of generic HRQOL and then assess its association with items of the QLACS and QOL-CS because, based on face validity, some items from the QOL-CS and QLACS measure generic rather than condition-specific domains. Given a small sample size (151 subjects) and many items under tests (123 items), we did not conduct exploratory factor analyses to investigate relationships between all items and potential latent factors. Alternatively, we assigned items from two survivor-specific instruments to one of the eight domains in the SF-36 if the correlation coefficients were greater than 0.4. Items assigned to the domains of “physical functioning” and “role limitations due to physical health problems” were further grouped into the physical functioning domain. This is because the correlations of an item associated with these two domains differed by less than 0.15, suggesting a negligible difference [31]. Similarly, items assigned to the domains of “mental health” and “role limitations due to emotional problems” were grouped into the psychological functioning domain.
If the items demonstrated low correlation coefficients (< 0.4) between item scores and the SF-36 domain scores, and the contents of items were similar, these items were assigned to one of the survivor-specific domains, including distress about cancer, distress about family, cognitive functioning, sexual functioning, appearance concerns, spiritual well-being, benefit from cancer, and financial concerns. The survivor-specific domains were created based on our literature review [32–34] and the content of the QOL-CS and QLACS.
Stage 2: Checking the dimensionality of the common construct
Each of the designated domains was assumed to measure a single concept of HRQOL (e.g., physical functioning). To confirm this assumption, two measurement properties were tested: unidimensionality and local independence. Unidimensionality refers to only one dominant factor accounting for a subject’s response to items within a specific domain. Local independence refers to a lack of a significant association among item responses after the dominant factor was controlled for. These two concepts are related because the violation of unidimensionality may be indicative of local dependency.
We conducted confirmatory factor analyses (CFA) to assess unidimensionality and local independence of each domain. If the Comparative Fit Indices (CFI) >0.95, Tucker-Lewis-Index (TLI) >0.95, and Root Mean Square Error of Approximation (RMSEA) <0.06, the assumption of unidimensionality is held [35]. If residual correlation <0.2, the assumption of local independency is held. We sequentially removed the items with local dependency and refitted the model of CFA to resolve the violation of unidimensionality.
Stage 3: Equating the items within a specific domain using Rasch analysis
We used Rasch modeling to link items from three legacy instruments, leading to the establishment of a HRQOL tool. This methodology focuses on item level analysis and uses probabilistic models to express a subject’s response to an item as a function of his/her underlying HRQOL (e.g., physical functioning) and item characteristics (i.e., item difficulty) [36]. Mathematically, the value of difficulty is the location of an item on the latent continuum (or metric) of underlying HRQOL, where at a given level of underlying HRQOL, subjects have a 50% probability of endorsing the item [36]. The advantage of Rasch analysis is the calibration of item difficulty and a subject’s underlying HRQOL on the same metric (unit: logit).
We assessed whether the data fit the Rasch model using indices of the information-weighted/outlier-sensitive fit statistic mean square (INFIT/OUTFIT MNSQ). Items with the MNSQ >1.3 indicate misfitting and the possibility that items are measuring other constructs rather than the one intended to be measured. In contrast, items with the MNSQ <0.7 indicate overfitting and the possibility that items are redundant to the construct we intended to measure. In both cases, such items were removed from a specific domain.
We collapsed some response categories based on of the actual sample size and the value of difficulty parameter. Theoretically, a response category that captures a higher level of underlying HRQOL should correspond to a greater value of difficulty parameter than a response category that captures a lower level of underlying HRQOL. Operationally, if the sample size of a specific category is less than 30, the adjacent response categories are collapsed. In addition, if the values of difficulty parameter for some categories are not displayed in the expected order (or individual curves on the figure of the categorical response curve (CRC) are not displayed in the expected order), the adjacent response categories are collapsed.
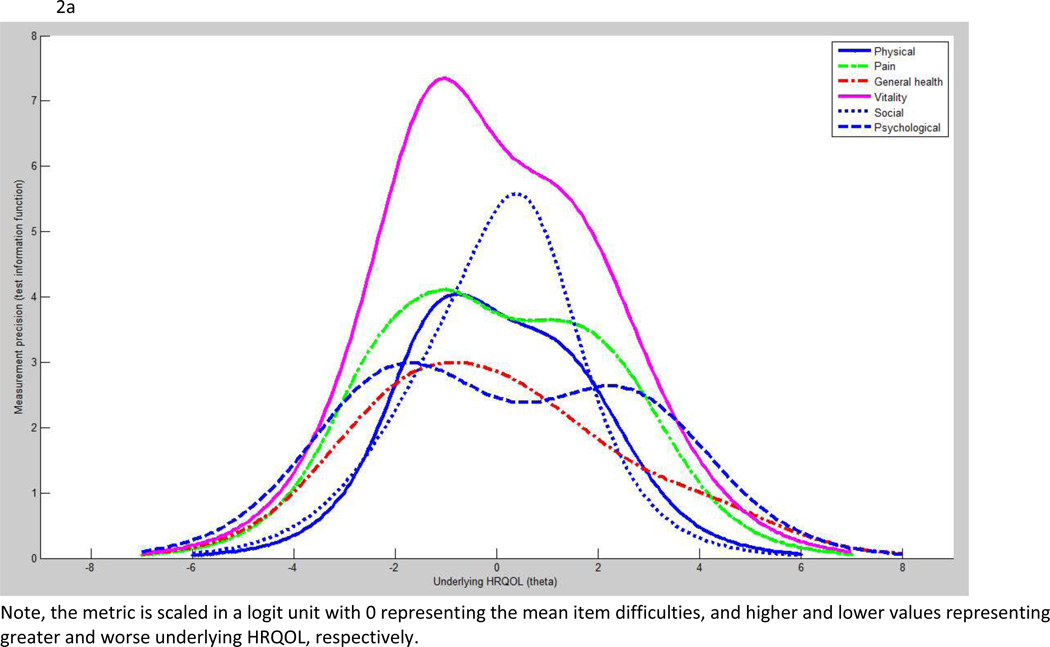
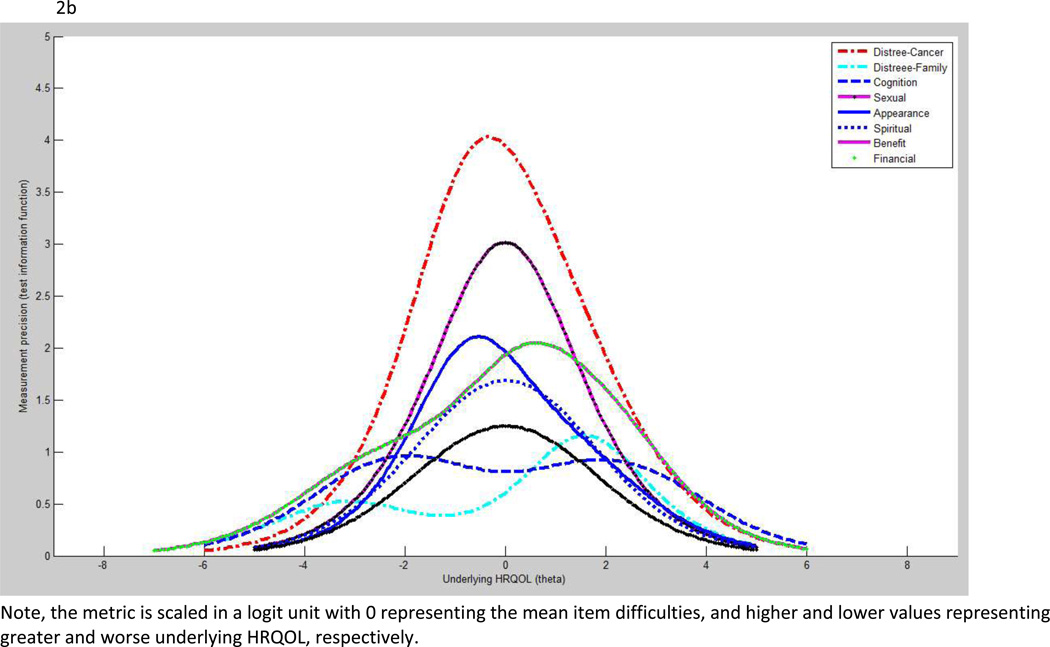
We assessed the quality of each HRQOL domain based on the spread of item difficulty versus underlying HRQOL of subjects (i.e., item-person map), item separation (>2), person separation (>2), item reliability (>0.8), personality reliability (>0.8), and ceiling/floor effects (<30%). We also assessed the measurement precision of each HRQOL domain based on test information function. We used the value 10 and above on the test information function (equivalent to reliability 0.9) to represent the acceptable measurement precision. On the item-person map and test information function, the metric is scaled in a logit unit with 0 representing the mean item difficulties, and higher and lower values representing greater and worse underlying HRQOL, respectively.
Stage 4: Validating each domain of a HRQOL tool
Known-groups validity is defined as the extent to which the items can discriminate between various groups of health conditions, such as LEs and comorbid conditions [37]. We compared the mean differences in HRQOL scores between YASCC with versus without LEs and between YASCC with versus without comorbid conditions. For the purpose of interpretation, we transferred the underlying HRQOL scores of YASCC to T-scores with a mean of 50 and a standard deviation (SD) of 10. Mean differences >5 points (equivalent to >0.5 SD) between the two groups were considered a clinically meaningful difference [38]. We hypothesized that HRQOL of YASCC with LEs (or comorbid conditions) would be more impaired than YASCC without LEs (or without comorbid conditions).
We used the Mplus 6.0 for confirmatory factor analyses, the WINSTEPS 3.60.1 [39] for Rasch analyses, and the STATA 9.0 [40] for the remaining analyses.
RESULTS
Characteristics of subjects
Table 2 shows the characteristics of subjects (N=151). The mean age was 26.1 years old (SD: 2.9) and 54% were male. Race/ethnicity was comprised of 85% White, 10% Black, and 5% other. About 24% and 19% of the subjects self-reported having LEs and/or comorbid conditions, respectively.
Table 2.
Subject characteristics (N=151)
| Characteristics | Mean (SD) or % |
|---|---|
| Age in year (SD) | 26.1 (2.9) |
| Gender (%) | |
| Male | 53.6% |
| Female | 46.4% |
| Race (%) | |
| White | 84.8% |
| Black | 9.9% |
| Other (mixed) | 5.3% |
| Education | |
| High school or below | 29.8% |
| Some college | 20.5% |
| Associate degree | 19.2% |
| Bachelor degree | 23.2% |
| Graduate/ professional degree | 7.3% |
| Employment | |
| Employed | 71.1% |
| Unemployed | 28.9% |
| Marital status | |
| Single | 60.3% |
| Married | 34.4% |
| Common law, divorced, or separated | 5.3% |
| Self-reported late effect | |
| Yes | 23.8% |
| No | 76.2% |
| Self-reported comorbid condition | |
| Yes | 18.5% |
| No | 81.5% |
Establishment of a HRQOL tool
In the first stage, we assigned 123 items derived from the three instruments to a framework of HRQOL comprised of six generic and eight survivor-specific domains based on the correlation coefficients and content of the items (Table 3).
Table 3.
Three stages in establishing a HRQOL tool
| Stage 1 | Stage 2 | Stage 3 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Item retained (N=123) |
RMSEA | CFI | TLI | Item retained (N=107) |
Person separation |
Person reliability |
Item separation |
Item reliability |
Ceiling/ floor effect, % |
Item retained (N=68) |
|
|
Generic Physical functioning Pain General health Vitality Social functioning Psychological functioning |
15 7 9 11 8 23 |
0.12 0.22 0.14 0.16 0.16 0.14 |
0.98 0.97 0.95 0.93 0.95 0.90 |
0.99 0.98 0.96 0.97 0.96 0.96 |
12 7 8 11 7 18 |
1.40 2.44 1.69 2.50 1.21 2.13 |
0.66 0.86 0.74 0.86 0.60 0.82 |
4.59 5.12 6.20 3.93 2.05 3.04 |
0.95 0.96 0.97 0.94 0.81 0.90 |
32.5/0 18.5/0.7 0/0 4.6/0 29.8/0 12.6/0 |
7 6 5 9 6 7 |
|
Survivor – specific Distress – Cancer Distress – Family Cognitive functioning Sexual functioning Appearance concerns Spiritual well-being Benefit from cancer Financial problems |
12 4 5 5 5 4 9 6 |
0.18 0 0 0.17 0.09 0 0.08 0.08 |
0.96 1.0 1.0 0.97 0.92 1.0 0.99 0.99 |
0.98 1.0 1.0 0.96 0.90 1.0 0.99 0.99 |
10 4 4 5 5 4 6 6 |
1.54 0.55 1.08 0.78 0.65 0.50 1.32 0.63 |
0.70 0.23 0.54 0.38 0.30 0.20 0.64 0.28 |
3.25 0 0 1.45 2.00 5.05 5.76 2.14 |
0.91 0 0 0.68 0.80 0.96 0.97 0.82 |
32.5/0.7 35.8/15.9 25.2/3.3 50.3/0 39.1/1.3 5.3/4.6 25.8/4.6 55.6/4.0 |
6 2 2 4 3 4 4 3 |
In the second stage, we conducted CFA to assess unidimensionality and local independency on each domain (Table 3). Initially, the domains of physical functioning, general health, social functioning, psychological functioning, distress about cancer, cognitive functioning, and benefit from cancer did not satisfy the assumptions of unidimensionality based on fit indices CFI, TLI, and RMSEA and local independency based on residual correlation. After one of the paired items with local dependency was removed, the dimensionality assessment was further improved. Domains of distress about family, cognitive functioning, and spiritual well-being satisfied all fit indices, whereas the rest of the domains (except appearance concerns) satisfied or marginally satisfied the fit indices. As a result, 107 items were retained in this stage.
In the third stage, we conducted Rasch analysis to further remove items from each domain and assess the quality for each domain (Table 3). We removed the items with MNSQ >1.3 or <0.7 because these items represent either misfitting or overfitting onto the specific construct of HRQOL we intend to measure. For a given domain, the overfitting items came from the same or different legacy instruments (and the same results for the misfitting items). For example, of the domain of distress about cancer, the items “fear of second cancer” and “fear recurrence” were classified as overfitting and subsequently were removed, whereas the item “when felt pain, worried cancer again” was retained. This in part reflects the fact that the items “fear of second cancer” and “fear recurrence” were redundant and provided less information compared to the item “when felt pain, worried cancer again.” In addition, for the same domain, the item “worry about fertility issues” was classified as misfitting. This in part reflects the fact that this item may capture another important issue concerned by YASCC, such as fertility concern. We retained 68 items in this stage.
With respect to quality assessment, most HRQOL domains (except pain, vitality, and psychological functioning) did not satisfy the indices of person separation (<2) and person reliability (<0.8). Person separation index represents the capability of items in the domains to separate the subjects into statistically distinct levels of underlying HRQOL. Higher person separation suggests a measurement scale covering a sufficient range of the measured construct [36]. In contrast, most domains (except distress about family, cognitive functioning and sexual functioning) satisfied the indices of item separation (>2) and item reliability (>0.8). Higher item separation represents the capability of the subjects to separate the items into different levels of difficulty. About half of the HRQOL domains indicated ceiling effects (>30%), implying that more than 30% of the subjects reported the highest possible HRQOL scores. Therefore, these domains may not have been able to discriminate underling the highest scores.
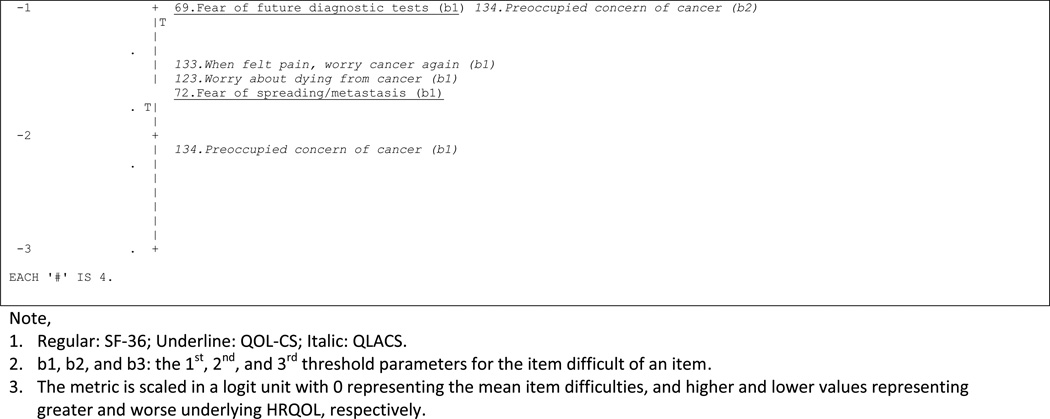
Figures 1a, 1b, 1c, and 1d visually assess whether the spread of item difficulty in the selected domains was wide enough to cover the subjects’ underlying HRQOL. Findings suggest that items in most domains were relatively easier for the subjects, implying that these items measure middle to lower levels of underlying HRQOL, whereas the subjects’ levels of underlying HRQOL were higher. In the example of physical functioning, the distributions of scores and difficulty levels of items were in the opposite directions; of subjects’ mean scores of underlying physical functioning was higher than the domain’s mean item difficulty by 1.5 logit unit.
Figure 1.
a: Item-person map: physical functioning
b: Item-person map: social functioning
c: Item-person map: distress about cancer
d: Item-person map: benefit from cancer
Measurement precision
Figures 2a and 2b show measurement precision measured by test information function on generic and survivor-specific domains, respectively. Among the generic measures, vitality and social functioning demonstrated superior measurement precision to other domains. Among the survivor-specific domains, distress about cancer and sexual functioning were superior to the remaining domains. However, no domains in this HRQOL tool exceed the acceptable measurement precision which is the value of 10.
Figure 2.
a: Test information function: generic domains
b: Test information function: survivor-specific domains
Known-groups validity
Table 4 shows the known-groups validity of the generic and survivor-specific domains assessed by regression analyses. There was a greater discrimination by generic domains than survivor-specific domains associated with self-reported LEs and comorbid conditions.
Table 4.
Known-groups validity
| Late effect† | Comorbid condition† | |||
|---|---|---|---|---|
| No covariate adjustment |
Covariate adjustment‡ |
No covariate adjustment |
Covariate adjustment‡ |
|
| Generic | ||||
| Physical functioning | 6.35** | 6.50** | 6.20** | 6.33** |
| Pain | 6.05** | 6.40** | 7.01** | 7.34*** |
| General health | 5.92** | 5.36** | 8.49*** | 8.54*** |
| Vitality | 5.54** | 4.97* | 7.00** | 7.39*** |
| Social functioning | 5. 53** | 5.73** | 5.04* | 4.75* |
| Psychological functioning | 6.51** | 7.09*** | 6.17** | 6.40** |
| Survivor – specific | ||||
| Distress – Cancer | 1.00 | 0.39 | 5.60** | 5.67** |
| Distress – Family | 1.11 | 0.89 | 0.33 | 0.43 |
| Cognitive functioning | 1.51 | 1.49 | 3.03 | 2.66 |
| Sexual functioning | 3.36 | 3.49 | 0.66 | 0.95 |
| Appearance concerns | 5.67** | 6.06** | 3.56 | 3.19 |
| Spiritual wellbeing | −1.00 | 0 | −0.92 | −0.70 |
| Benefit from cancer | 1.63 | 2.21 | −1.27 | −1.23 |
| Financial problems | 6.47** | 6.18** | 3.80 | 3.32 |
P<0.05;
P<0.01;
P<0.001
Numbers in cells represent discrepancy in scores of underlying HRQOL between two groups (without late effects vs. with late effects, or without comorbid conditions vs. with comorbid conditions); positive values mean YASCC without late effects (or without comorbid conditions) possess greater underlying HRQOL vs. YASCC with late effects (or with comorbid conditions)
Adjusting for age, gender, race, and educational background
In generic domains, YASCC who had LEs reported significantly impaired HRQOL in all domains compared to YASCC without LEs (P<0.05). Similarly, YASCC who had comorbid conditions reported significantly impaired HRQOL in all domains compared to YASCC without comorbid conditions (P<0.05). With the exception of vitality and social functioning (with covariate adjustment), the difference in HRQOL scores between both groups was all greater than 5.0, indicating a clinically meaningful difference.
In survivor-specific domains, however, YASCC who had LEs reported significantly impaired HRQOL only in the domains of appearance concerns and financial problems (P<0.05) compared to YASCC without LEs. YASCC who had comorbid conditions reported significantly impaired HRQOL only in the domain of distress about cancer (P<0.05) compared to YASCC without comorbid conditions.
DISCUSSION
Many studies are interested in investigating HRQOL of YASCC using “off-the-shelf” instruments [32,33]. However, few studies have rigorously investigated the psychometric properties and the appropriateness of these instruments for YASCC. One exceptional study, conducted by Zebrack et al., found the pre-specified four-factor construct (physical, psychological, social and spiritual wellbeing) of the QOL-CS cannot be replicated by YASCC [41]. Importantly, all of the previous studies were conducted based on CTT rather than IRT methodology. This study used IRT to establish a HRQOL measurement tool for YASCC based on the items derived from the QOL-CS, QLACS, and SF-36.
Our HRQOL tool is comprised of six generic domains and eight survivor-specific domains. Items retained in specific domains met the criteria of unidimensionality and local independence. However, Rasch analyses suggest the majority of the domains, particularly physical functioning, social functioning, distress about cancer, cognitive functioning, sexual functioning, and financial problems, did not satisfy because the domains essentially capture the middle and lower levels of underlying HRQOL. This is problematic since the underlying HRQOL of a great portion of subjects was higher and beyond the capability of our HRQOL tool which is comprised of items from three legacy instruments.
The poor quality of HRQOL domains is evident by the unsatisfied person separation index, person reliability (Table 2), poor person-item match (e.g., Figures 1b and 1c), and measurement precision (Figures 2a and 2b). This finding was not surprising since the items derived from the QOL-CS and QLACS were designed to measure HRQOL of adult-onset cancer survivors rather than YASCC. It is likely that YASCC are more resilient and possess greater health status/health outcomes after they survived from cancer compared to adult-onset cancer survivors [42,43]. Therefore, the domains in our HRQOL tool are comprised of relatively simple items in terms of measurement properties which may not be appropriate for YASCC. The significant ceiling effects echo this observation, suggesting that our HRQOL tool did not cover the full range of the underlying HRQOL that we would like to measure and cannot discriminate subjects whose HRQOL is in the upper levels. The finding of ceiling effect casts a concern about the responsiveness of the measurement if we want to monitor the change in HRQOL among YASCC over their life course.
Compared to survivor-specific domains, generic domains discriminated better between known groups of LEs and comorbid conditions, respectively. Ideally, survivor-specific measures should demonstrate a stronger association with LEs and comorbid conditions than generic measures. The contradictory findings may be due to the fact that, in this study the LEs and comorbid conditions were measured by self-report rather than clinical assessment (e.g., Common Terminology Criteria for Adverse Events [44]). The percentage of LEs reported by our subjects was 24%, which is lower than studies of Oeffinger et al. and Geenen et al. [3,4]. However, the study of Geenen el al. was based on medical records, physician assessment, and a few by self report [4]. The study of Oeffinger et al. did not discriminate between late effects and comorbid conditions, and simply reported “chronic” health conditions [3]. Therefore, the comparisons cannot be completely generalized to our study.
There is great debate regarding which survivor-specific instrument is superior to others in measuring HRQOL for cancer survivors [45,46]. Our Rasch analysis suggests that both survivor-specific instruments make a unique contribution to the establishment of our HRQOL tool. As demonstrated in Figures 1b and 1c, many items from both instruments indeed capture different levels of underlying HRQOL. This observation further suggests that the use of items from multiple instruments will allow measuring a broader scope of HRQOL for cancer survivors than the use of a single instrument alone.
Instrument development is an on-going process. It is necessary to refine our HRQOL tool for YASCC given the significant ceiling effects combined with poor item-person matching at the higher levels of underlying HRQOL. Future studies need to add more difficult or challenging items to fill in the gap located at higher levels of the metric. Adding new items can help expand the tool’s ability to measure a full range of underlying HRQOL, improve measurement precision, and increase responsiveness of our HRQOL tool [28,47].
In addition to inserting new items to the HRQOL tool, future studies should include the domains which are important or unique to YASCC, but not emphasized in our or other HRQOL tools. Qualitative studies, one the one hand, suggest that YASCC demonstrate resilience in their personal growth and positive orientation towards their future [43]. That is, many YASCC posses characteristics of optimism [48], hope for the future [49], self-confidence [50], maturity [48,51], and ability to manage challenging issues in daily living [52]. On the other hand, YASCC have reported difficulties in some areas throughout their survivorship trajectory. For example, YASCC have reported concerns about their fertility status [43,50,53,54] and passing cancer onto their future children [43,50,54]. Consequently, survivor’s uncertainty regarding their fertility has caused strain in their intimate relationships [54]. YASCC have also expressed concern regarding their body appearance [48,55,56]. Finally, YASCC have reported concerns about communication with providers [54,56] and health care transitions [48,51]. Although these concepts are important and unique to YASCC, they have traditionally been excluded from the HRQOL framework used for YASCC [32,33]. This study serves a foundation for refining the measurement tools to assess generic and survivor-specific HRQOL for YASCC.
Several study limitations merit attention. First, we recruited YASCC primarily using the Cancer/Tumor Registries of two academic medical centers. Thus, the findings may not be generalizable to populations from other settings, such as the community setting. Second, the invalid contact information was high and the true response rate may be lower than 49.6% as indicate in Methods. The low response rate is largely due to inaccurate contact information in the two registries, reflecting a unique characteristic of YASCC populations. Typically these YASCC have their parent’s home address when diagnosed and then become independent from their parents and migrate to different places. Although there were no difference in age, gender, and race/ethnicity between YASCC responders and non-responders as indicated in methods, there may be inherent differences between these two groups which will limit the generalizability. Third, we used Rasch modeling (including item difficulty parameter alone) rather than two-parameter IRT models (including item difficulty and discrimination parameters) given a small sample size. However, we cannot rule out that the composition of items in a specific HRQOL domain may have changed if items with poor discrimination parameter were deleted. Finally, the LEs and comorbid conditions used to test known-groups validity were based on subjects’ self report. Their responses were not confirmed by a clinician or the medical record.
In conclusion, there is a great need to use appropriate HRQOL instruments for YASCC. This study developed a HRQOL tool to measure HRQOL based on three legacy instruments, yet the psychometric properties of our HRQOL tool was not satisfied. Future studies should focus on refining this HRQOL tool for measuring HRQOL more meaningfully.
Acknowledgement
The authors thank Ms. Katie Eddleton and Ms. Devin Murphy for assisting data collection and comments on the manuscript.
Funding sources:
This work was supported in part by the University of Florida and Moffitt Cancer Centers Collaborative Initiative (IH, GQ, ES, and PS) and the National Institute of Health K23 HD057146 (IH).
REFERENCES
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Weiner SL, Simone JV. National Cancer Policy Board (U.S.) Childhood cancer survivorship: improving care and quality of life. Washington, D.C.: National Academies Press; 2003. p. 206. [PubMed] [Google Scholar]
- 3.Oeffinger KC, Mertens AC, Sklar CA, Kawashima T, Hudson MM, Meadows AT, Friedman DL, Marina N, Hobbie W, Kadan-Lottick NS, Schwartz CL, Leisenring W, Robison LL, Childhood Cancer Survivor Study Chronic health conditions in adult survivors of childhood cancer. N Engl J Med. 2006;355:1572–1582. doi: 10.1056/NEJMsa060185. [DOI] [PubMed] [Google Scholar]
- 4.Geenen MM, Cardous-Ubbink MC, Kremer LC, van den Bos C, van der Pal HJ, Heinen RC, Jaspers MW, Koning CC, Oldenburger F, Langeveld NE, Hart AA, Bakker PJ, Caron HN, van Leeuwen FE. Medical assessment of adverse health outcomes in long-term survivors of childhood cancer. JAMA. 2007;297:2705–2715. doi: 10.1001/jama.297.24.2705. [DOI] [PubMed] [Google Scholar]
- 5.Blaauwbroek R, Stant AD, Groenier KH, Kamps WA, Meyboom B, Postma A. Health-related quality of life and adverse late effects in adult (very) long-term childhood cancer survivors. Eur J Cancer. 2007;43:122–130. doi: 10.1016/j.ejca.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 6.Boman KK. Assessing psychological and health-related quality of life (HRQL) late effects after childhood cancer. Acta Paediatr. 2007;96:1265–1268. doi: 10.1111/j.1651-2227.2007.00447.x. [DOI] [PubMed] [Google Scholar]
- 7.Ishida Y, Honda M, Ozono S, Okamura J, Asami K, Maeda N, Sakamoto N, Inada H, Iwai T, Kamibeppu K, Kakee N, Horibe K. Late effects and quality of life of childhood cancer survivors: Part 1. Impact of stem cell transplantation. Int J Hematol. 2010;91:865–876. doi: 10.1007/s12185-010-0584-y. [DOI] [PubMed] [Google Scholar]
- 8.Ishida Y, Sakamoto N, Kamibeppu K, Kakee N, Iwai T, Ozono S, Maeda N, Okamura J, Asami K, Inada H, Honda M, Horibe K. Late effects and quality of life of childhood cancer survivors: Part 2. Impact of radiotherapy. Int J Hematol. 2010;92:95–104. doi: 10.1007/s12185-010-0611-z. [DOI] [PubMed] [Google Scholar]
- 9.Pemberger S, Jagsch R, Frey E, Felder-Puig R, Gadner H, Kryspin-Exner I, Topf R. Quality of life in long-term childhood cancer survivors and the relation of late effects and subjective well-being. Support Care Cancer. 2005;13:49–56. doi: 10.1007/s00520-004-0724-0. [DOI] [PubMed] [Google Scholar]
- 10.Apajasalo M, Sintonen H, Siimes MA, Hovi L, Holmberg C, Boyd H, Makela A, Rautonen J. Health-related quality of life of adults surviving malignancies in childhood. Eur J Cancer. 1996;32A:1354–1358. doi: 10.1016/0959-8049(96)00024-x. [DOI] [PubMed] [Google Scholar]
- 11.Elkin TD, Phipps S, Mulhern RK, Fairclough D. Psychological functioning of adolescent and young adult survivors of pediatric malignancy. Med Pediatr Oncol. 1997;29:582–588. doi: 10.1002/(sici)1096-911x(199712)29:6<582::aid-mpo13>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 12.Novakovic B, Fears TR, Horowitz ME, Tucker MA, Wexler LH. Late effects of therapy in survivors of ewing's sarcoma family tumors. J Pediatr Hematol Oncol. 1997;19:220–225. doi: 10.1097/00043426-199705000-00008. [DOI] [PubMed] [Google Scholar]
- 13.Stam H, Grootenhuis MA, Caron HN, Last BF. Quality of life and current coping in young adult survivors of childhood cancer: Positive expectations about the further course of the disease were correlated with better quality of life. Psychooncology. 2006;15:31–43. doi: 10.1002/pon.920. [DOI] [PubMed] [Google Scholar]
- 14.Zeltzer LK, Chen E, Weiss R, Guo MD, Robison LL, Meadows AT, Mills JL, Nicholson HS, Byrne J. Comparison of psychologic outcome in adult survivors of childhood acute lymphoblastic leukemia versus sibling controls: A cooperative children's cancer group and national institutes of health study. J Clin Oncol. 1997;15:547–556. doi: 10.1200/JCO.1997.15.2.547. [DOI] [PubMed] [Google Scholar]
- 15.Felder-Puig R, Formann AK, Mildner A, Bretschneider W, Bucher B, Windhager R, Zoubek A, Puig S, Topf R. Quality of life and psychosocial adjustment of young patients after treatment of bone cancer. Cancer. 1998;83:69–75. doi: 10.1002/(sici)1097-0142(19980701)83:1<69::aid-cncr10>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 16.Maunsell E, Pogany L, Barrera M, Shaw AK, Speechley KN. Quality of life among long-term adolescent and adult survivors of childhood cancer. J Clin Oncol. 2006;24:2527–2535. doi: 10.1200/JCO.2005.03.9297. [DOI] [PubMed] [Google Scholar]
- 17.Moe PJ, Holen A, Glomstein A, Madsen B, Hellebostad M, Stokland T, Wefring KW, Steen-Johnsen J, Nielsen B, Howlid H, Borsting S, Hapnes C. Long-term survival and quality of life in patients treated with a national all protocol 15–20 years earlier: IDM/HDM and late effects? Pediatr Hematol Oncol. 1997;14:513–524. doi: 10.3109/08880019709030908. [DOI] [PubMed] [Google Scholar]
- 18.Veenstra KM, Sprangers MA, van der Eyken JW, Taminiau AH. Quality of life in survivors with a van ness-borggreve rotationplasty after bone tumour resection. J Surg Oncol. 2000;73:192–197. doi: 10.1002/(sici)1096-9098(200004)73:4<192::aid-jso2>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 19.Mulrooney DA, Ness KK, Neglia JP, Whitton JA, Green DM, Zeltzer LK, Robison LL, Mertens AC. Fatigue and sleep disturbance in adult survivors of childhood cancer: A report from the childhood cancer survivor study (CCSS) Sleep. 2008;31:271–281. doi: 10.1093/sleep/31.2.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.O'Leary TE, Diller L, Recklitis CJ. The effects of response bias on self-reported quality of life among childhood cancer survivors. Qual Life Res. 2007;16:1211–1220. doi: 10.1007/s11136-007-9231-3. [DOI] [PubMed] [Google Scholar]
- 21.Ferrell BR, Dow KH, Grant M. Measurement of the quality of life in cancer survivors. Qual Life Res. 1995;4:523–531. doi: 10.1007/BF00634747. [DOI] [PubMed] [Google Scholar]
- 22.Avis NE, Smith KW, McGraw S, Smith RG, Petronis VM, Carver CS. Assessing quality of life in adult cancer survivors (QLACS) Qual Life Res. 2005;14:1007–1023. doi: 10.1007/s11136-004-2147-2. [DOI] [PubMed] [Google Scholar]
- 23.Zebrack BJ, Donohue JE, Gurney JG, Chesler MA, Bhatia S, Landier W. Psychometric evaluation of the impact of cancer (IOC-CS) scale for young adult survivors of childhood cancer. Qual Life Res. 2010;19:207–218. doi: 10.1007/s11136-009-9576-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Crespi CM, Ganz PA, Petersen L, Castillo A, Caan B. Refinement and psychometric evaluation of the impact of cancer scale. J Natl Cancer Inst. 2008;100:1530–1541. doi: 10.1093/jnci/djn340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bhatia S, Robison LL, Francisco L, Carter A, Liu Y, Grant M, Baker KS, Fung H, Gurney JG, McGlave PB, Nademanee A, Ramsay NK, Stein A, Weisdorf DJ, Forman SJ. Late mortality in survivors of autologous hematopoietic-cell transplantation: Report from the bone marrow transplant survivor study. Blood. 2005;105:4215–4222. doi: 10.1182/blood-2005-01-0035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hays RD, Morales LS, Reise SP. Item response theory and health outcomes measurement in the 21st century. Med Care. 2000;38:II28–II42. doi: 10.1097/00005650-200009002-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Embretson SE, Reise SP. Item response theory for psychologists. Mahwah, N.J.: Lawrence Erlbaum Associates; 2000. p. 371. [Google Scholar]
- 28.Bjorner JB, Chang CH, Thissen D, Reeve BB. Developing tailored instruments: Item banking and computerized adaptive assessment. Qual Life Res. 2007;16(Suppl 1):95–108. doi: 10.1007/s11136-007-9168-6. [DOI] [PubMed] [Google Scholar]
- 29.Edelen MO, Reeve BB. Applying item response theory (IRT) modeling to questionnaire development, evaluation, and refinement. Qual Life Res. 2007;16(Suppl 1):5–18. doi: 10.1007/s11136-007-9198-0. [DOI] [PubMed] [Google Scholar]
- 30.Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36) I. Conceptual framework and item selection. Med Care. 1992;30:473–483. [PubMed] [Google Scholar]
- 31.Badia X, Prieto L, Roset M, Diez-Perez A, Herdman M. Development of a short osteoporosis quality of life questionnaire by equating items from two existing instruments. J Clin Epidemiol. 2002;55:32–40. doi: 10.1016/s0895-4356(01)00432-2. [DOI] [PubMed] [Google Scholar]
- 32.Clinton-McHarg T, Carey M, Sanson-Fisher R, Shakeshaft A, Rainbird K. Measuring the psychosocial health of adolescent and young adult (AYA) cancer survivors: A critical review. Health Qual Life Outcomes. 2010;8:25. doi: 10.1186/1477-7525-8-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Langeveld NE, Stam H, Grootenhuis MA, Last BF. Quality of life in young adult survivors of childhood cancer. Support Care Cancer. 2002;10:579–600. doi: 10.1007/s00520-002-0388-6. [DOI] [PubMed] [Google Scholar]
- 34.Zebrack B, Hamilton R, Smith AW. Psychosocial outcomes and service use among young adults with cancer. Semin Oncol. 2009;36:468–477. doi: 10.1053/j.seminoncol.2009.07.003. [DOI] [PubMed] [Google Scholar]
- 35.Hu L, Bentler BM. Cutoff criteria for fit indexes in covariance structure analysis: Conventional criteria versus new alternatives. Struct Equ Modeling. 1999;6:1–55. [Google Scholar]
- 36.Smith EV, Smith RM. Introduction to Rasch measurement: theory, models and applications. Maple Grove, Minn: JAM Press; 2004. p. 689. [Google Scholar]
- 37.Fayers PM, Machin D. Quality of life: the assessment, analysis, and interpretation of patient-reported outcomes. Chichester; Hoboken, NJ: J. Wiley; 2007. [Google Scholar]
- 38.Norman GR, Sloan JA, Wyrwich KW. Interpretation of changes in health-related quality of life: The remarkable universality of half a standard deviation. Med Care. 2003;41:582–592. doi: 10.1097/01.MLR.0000062554.74615.4C. [DOI] [PubMed] [Google Scholar]
- 39.Linacre JM. A user's guide to WINSTEPS. Chicago IL: WINSTEPS; 2003. [Google Scholar]
- 40.STATCorp. Stata Statistical Software: Release 9.0. College Station, Texas: Stata Corporation; 2005. [Google Scholar]
- 41.Zebrack BJ, Chesler MA. A psychometric analysis of the quality of life-cancer survivors (QOL-CS) in survivors of childhood cancer. Qual Life Res. 2001;10:319–329. doi: 10.1023/a:1012228823115. [DOI] [PubMed] [Google Scholar]
- 42.Berkowitz CD. Pediatrics: a primary care approach. Elk Grove Village, Ill.: American Academy of Pediatrics; 2008. p. 837. [Google Scholar]
- 43.Parry C. Embracing uncertainty: An exploration of the experiences of childhood cancer survivors. Qual Health Res. 2003;13:227–246. doi: 10.1177/1049732302239600. [DOI] [PubMed] [Google Scholar]
- 44.Trotti A, Colevas AD, Setser A, Rusch V, Jaques D, Budach V, Langer C, Murphy B, Cumberlin R, Coleman CN, Rubin P. CTCAE v3.0: Development of a comprehensive grading system for the adverse effects of cancer treatment. Semin Radiat Oncol. 2003;13:176–181. doi: 10.1016/S1053-4296(03)00031-6. [DOI] [PubMed] [Google Scholar]
- 45.Fredheim OM, Borchgrevink PC, Saltnes T, Kaasa S. Validation and comparison of the health-related quality-of-life instruments EORTC QLQ-C30 and SF-36 in assessment of patients with chronic nonmalignant pain. J Pain Symptom Manage. 2007;34:657–665. doi: 10.1016/j.jpainsymman.2007.01.011. [DOI] [PubMed] [Google Scholar]
- 46.Kuenstner S, Langelotz C, Budach V, Possinger K, Krause B, Sezer O. The comparability of quality of life scores. A multitrait multimethod analysis of the EORTC QLQ-C30, SF-36 and FLIC questionnaires. Eur J Cancer. 2002;38:339–348. doi: 10.1016/s0959-8049(01)00369-0. [DOI] [PubMed] [Google Scholar]
- 47.Martin M, Kosinski M, Bjorner JB, Ware JE, Jr, Maclean R, Li T. Item response theory methods can improve the measurement of physical function by combining the modified health assessment questionnaire and the SF-36 physical function scale. Qual Life Res. 2007;16:647–660. doi: 10.1007/s11136-007-9193-5. [DOI] [PubMed] [Google Scholar]
- 48.Enskar K, Bertero C. Young adult survivors of childhood cancer; experiences affecting self-image, relationships, and present life. Cancer Nurs. 2010;33:E18–E24. doi: 10.1097/NCC.0b013e3181b6365a. [DOI] [PubMed] [Google Scholar]
- 49.Chou LN, Hunter A. Factors affecting quality of life in Taiwanese survivors of childhood cancer. J Adv Nurs. 2009;65:2131–2141. doi: 10.1111/j.1365-2648.2009.05078.x. [DOI] [PubMed] [Google Scholar]
- 50.Casillas JN, Zebrack BJ, Zeltzer LK. Health-related quality of life for Latino survivors of childhood cancer. J Psychosoc Oncol. 2006;24:125–145. doi: 10.1300/J077v24n03_06. [DOI] [PubMed] [Google Scholar]
- 51.Cantrell MA, Conte TM. Between being cured and being healed: The paradox of childhood cancer survivorship. Qual Health Res. 2009;19:312–322. doi: 10.1177/1049732308330467. [DOI] [PubMed] [Google Scholar]
- 52.Parry C, Chesler MA. Thematic evidence of psychosocial thriving in childhood cancer survivors. Qual Health Res. 2005;15:1055–1073. doi: 10.1177/1049732305277860. [DOI] [PubMed] [Google Scholar]
- 53.Green D, Galvin H, Horne B. The psycho-social impact of infertility on young male cancer survivors: A qualitative investigation. Psychooncology. 2003;12:141–152. doi: 10.1002/pon.622. [DOI] [PubMed] [Google Scholar]
- 54.Zebrack BJ, Casillas J, Nohr L, Adams H, Zeltzer LK. Fertility issues for young adult survivors of childhood cancer. Psychooncology. 2004;13:689–699. doi: 10.1002/pon.784. [DOI] [PubMed] [Google Scholar]
- 55.Boydell KM, Stasiulis E, Greenberg M, Greenberg C, Spiegler B. I'll show them: The social construction of (in)competence in survivors of childhood brain tumors. J Pediatr Oncol Nurs. 2008;25:164–174. doi: 10.1177/1043454208315547. [DOI] [PubMed] [Google Scholar]
- 56.Drew S. 'Having cancer changed my life, and changed my life forever': Survival, illness legacy and service provision following cancer in childhood. Chronic Illn. 2007;3:278–295. doi: 10.1177/1742395307085236. [DOI] [PubMed] [Google Scholar]