Abstract
TWIK-1 two-pore domain K+ channels generally produce nonmeasurable or very low levels of K+ currents in heterologous expression systems under physiologically ionic conditions. Two controversial mechanisms have been proposed to account for this behavior: TWIK-1 K+ channels are expressed in the cell surface but silenced by sumoylation at a lysine residue (TWIK-1 K274); constitutive and rapid internalization of TWIK-1 causes TWIK-1 channel silencing. Here we report that TWIK-1 K+ channels heterologously expressed in Chinese hamster ovary cells, which are silent in physiological K+ gradients, are able to conduct large monovalent cation currents when extracellular ionic conditions change. These results support the hypothesis that TWIK-1 K+ channels are expressed in the cell surface but silent, and suggest that the TWIK-1 gating behavior rather than the lack of cell surface expression of TWIK-1 results in nondetectable TWIK-1 K+ currents in heterologous expression systems.
Mammalian two-pore domain K+ channels, which are encoded by 15 KCNK genes, mediate leak K+ conductance. They are regulated by physical and chemical stimuli and play important roles in a variety of physiological and pathophysiological functions (1).
TWIK-1, the first cloned mammalian two-pore domain K+ channel, is highly expressed in the brain, kidney, and heart (2–4). Mice deficient for TWIK-1 exhibit impaired regulation of phosphate and water transports in kidney (5). Our previous studies indicate that TWIK-1 K+ channels contribute to a large passive K+ conductance in rat hippocampal astrocytes and account for an inward leak Na+ current in human cardiac myocytes under hypokalemic conditions (6,7).
When heterologously expressed in mammalian cells or Xenopus oocytes, TWIK-1 K+ channels generate unusually low levels of K+ currents (2,8). Two mechanisms have been used to explain this feature of TWIK-1 K+ channels:
The first hypothesis is that TWIK-1 K+ channels are expressed in the cell surface but silent. Rajan et al. (8) have shown that TWIK-1 K+ channels with a GFP-tag in the N-terminal reach the plasma membrane of Xenopus oocytes. However, the TWIK-1 K+ channels in the cell surface have a very low open probability because they are silenced by sumoylation, a posttranslational modification of lysine residues by conjugation of a small ubiquitin modifier protein. Desumoylation in TWIK-1 K274 residue leads to the opening of TWIK-1 K+ channels and such a regulation can occur reversely in inside-out patches of membrane excised from transfected Chinese hamster ovary (CHO) cells (8,9), suggesting that small ubiquitin modifier protein regulates gating behavior of TWIK-1 K+ channels (10).
Recently, an alternative hypothesis has been proposed: constitutive and rapid retrieval or internalization of TWIK-1 from the cell surface causes the absence or lack of TWIK-1 K+ channels in the plasma membrane so that no detectable TWIK-1 K+ current can be measured. Mutation of a diisoleucine repeat located in the cytoplasmic C-terminus (TWIK-1⋅I293A⋅I294A) stabilizes TWIK-1 in the plasma membrane and results in robust TWIK-1 K+ currents (11), suggesting that the diisoleucine-based motif controls the surface expression or retrieval of TWIK-1 K+ channels.
Here we reexamined the hypothesis that TWIK-1 K+ channels are expressed in the cell surface, even though in the absence of measurable TWIK-1 K+ currents, by employing a different strategy. If this hypothesis is reasonable, it is possible to measure ionic currents through TWIK-1 channels when these silent TWIK-1 K+ channels, which produce nondetectable K+ currents in transfected CHO cells in physiological K+ gradients, become functional in response to physical or chemical stimuli. Otherwise, the lack of cell surface expression of TWIK-1 should not produce any measurable TWIK-1 current under the same stimuli. Therefore, we tested whether silent TWIK-1 K+ channels are able to functionally conduct monovalent cation currents. In this study, we demonstrated that silent TWIK-1 K+ channels conduct large Na+, K+, Rb+, and NH4+ currents in transfected CHO cells when extracellular ionic conditions change.
Methods
CHO cells with at least 80% confluence were transfected by Lipofectamine 2000 (Invitrogen, Carlsbad, CA) with 3 μg of TWIK-1 plasmids and 1 μg of pEGFP plasmids and studied 24 h later. GFP expression was used to identify effectively transfected CHO cells. Whole-cell patch-clamp recordings were performed and data were analyzed, as described previously (7). Briefly, whole-cell currents of TWIK-1 K+ channels in transfected CHO cells were recorded with a standard 2.2 s voltage ramp from −140 mV to +80 mV each 15 s. In Figs. 1 and 2, whole-cell currents at +80 mV in Na+-based bath solutions with 5 mM [K+]o are <250 pA. The pipette solution contained 140 mM KCl, 1 mM MgCl2, 10 mM EGTA, 1 mM K2-ATP, and 5 mM HEPES (pH7.4). The Na+-based bath solution contained 135 mM NaCl, 5 mM KCl, 2 mM CaCl2, 1 mM MgCl2, and 10 mM HEPES (pH7.4). The total concentration of Na+ and K+ in bath solutions is 140 mM so bath solutions with various [K+]o were obtained by exchanges of equimolar K+ and Na+. The Rb+ or NH4+-based bath solutions were obtained by replacing extracellular Na+ by equimolar monovalent cations.
Figure 1.
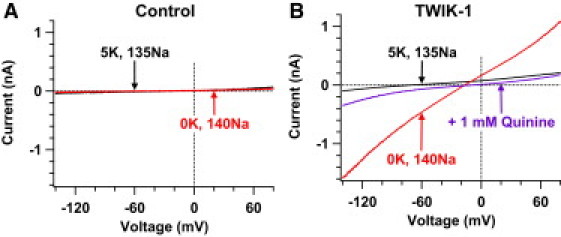
Silent TWIK-1 K+ channels conduct Na+ and K+ currents in lowered [K+]o. Whole-cell currents are shown in CHO cells transfected with GFP plasmids alone (A) or with both TWIK-1 and GFP plasmids (B) before (black lines) and after (red lines) removing 5 mM extracellular K+. At the end of the experiments, quinine blockade confirmed TWIK-1 currents (purple line) in panel B.
Figure 2.
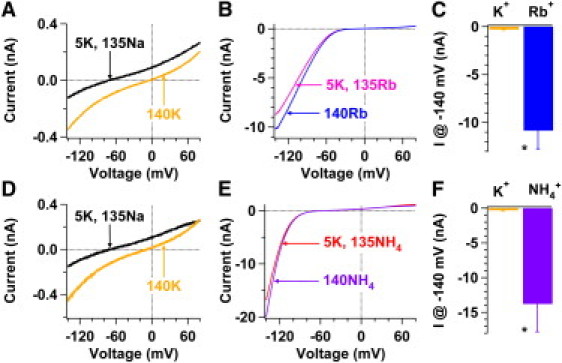
Silent TWIK-1 K+ channels conduct Rb+ or NH4+ currents. (A and B) Whole-cell currents of TWIK-1 channels were sequentially recorded from the same transfected CHO cell in bath solutions containing 5 mM K+ and 135 mM Na+ (black line), 140 mM K+ (orange line), 5 mM K+ and 135 mM Rb+ (pink line), and 140 mM Rb+ (blue line). (C) Summary of currents measured at −140 mV in panels A and B in bath solutions containing 140 mM K+ (orange bar) or 140 mM Rb+ (blue bar) (−311 ± 53 pA vs. −10,843 ± 1850 pA, n = 4). (D and E) Experiments similar to those in panels A and B were performed except that NH4+ replaced Rb+. (F) Summary of currents measured at −140 mV in panels D and E in bath solutions containing 140 mM K+ (orange bar) or 140 mM NH4+ (purple bar) (−323 ± 88 pA vs. −13,761 ± 4046 pA, n = 4). (∗P < 0.001).
Results and Discussion
Silent TWIK-1 K+ channels conduct large inward Na+ and outward K+ currents
We have previously shown that in the transfected CHO cells, by which large TWIK-1 K+ currents are measured, TWIK-1 K+ channels change ion selectivity and conduct inward leak Na+ currents in lowered extracellular K+ concentrations ([K+]o) (7). Thus, we investigated whether silent TWIK-1 K+ channels heterologously expressed in CHO cells exhibit the same characteristic in lowered [K+]o, become permeable to extracellular Na+, and conduct inward Na+ currents. Consistent with previous reports (7,8,11), TWIK-1 K+ channels do not produce measurable currents in most of the transfected CHO cells in Na+-based bath solutions with physiological [K+]o. Whole-cell currents recorded at −140 mV and +80 mV are −95 ± 6 pA and 188 ± 19 pA (n = 4), respectively (black line, Fig. 1 B), within the range of background noises. However, removing 5 mM K+ in the bath solution converted silent TWIK-1 K+ channels into nonselective cation channels that conducted large inward Na+ and outward K+ currents with a reversal potential of −15.6 ± 1.5 mV (n = 4) (red line, Fig. 1 B) and a Na+ to K+ relative permeability of ∼0.53. It took ∼10 min for TWIK-1 channels to complete the selectivity switch process. Moreover, whole-cell currents recorded in −140 mV (−843 ± 191 pA) and +80 mV (637 ± 119 pA) were significantly increased by 8.8-fold and 3.3-fold, respectively. These results are consistent with previous observations in those transfected CHO cells that large TWIK-1 K+ currents are detected (7). In contrast, these results were not seen in CHO cells transfected with GFP alone (n = 20; Fig. 1 A). Such an amount of inward Na+ and outward K+ currents in 0 mM [K+]o indicates that TWIK-1 K+ channels are expressed on the surface of transfected CHO cells. This finding supports the hypothesis that TWIK-1 K+ channels reach the cell surface but are not able to conduct measurable K+ currents in physiological K+ gradients.
A potential challenge in interpreting enhanced TWIK-1 currents (red line, Fig. 1 B), which were induced by removing 5 mM extracellular K+, is that externalization or insertion of TWIK-1 K+ channels into the plasma membrane could also result in increases of TWIK-1 currents. However, two lines of evidence argue against this possibility: First, externalization of TWIK-1 K+ channels should only increase the number of TWIK-1 K+ channels in the cell surface and the amplitude of TWIK-1 K+ currents; it should not result in dynamic changes in ion selectivity of TWIK-1 K+ channels. Thus, externalization of TWIK-1 fails to explain altered ion selectivity of TWIK-1 K+ channels in lowered [K+]o. Instead, increases in detectable TWIK-1 currents may reflect the changes in single channel properties of TWIK-1 channels such as open probability and unitary current. Second, our previous report has shown that the K+ selectivity of TWIK-1 channels could be restored ∼77 min later, after [K+]o was changed back from 0 mM to 5 mM (7). Because CHO cells are dialyzed by electrode solutions containing 10 mM EGTA for such a long time, most endogenous enzymes should be inactivated and biochemical processes should be terminated. It is unlikely that trafficking (externalization or internalization) of TWIK-1 K+ channels could occur after >1 h of establishments of whole-cell configuration.
Silent TWIK-1 K+ channels conduct extremely large inward Rb+ and NH4+ currents
We have previously shown that in the transfected CHO cells, in which large TWIK-1 K+ currents are detected, TWIK-1 K+ channels are permeable to Rb+ and NH4+ in both normal and lowered [K+]o (7). Then we studied whether silent TWIK-1 K+ channels heterologously expressed in CHO cells are permeable to Rb+ and NH4+. In Na+-based bath solutions with 5 mM [K+]o, TWIK-1 K+ channels are basically silent, as whole-cell currents recorded at +80 mV are only 199 ± 52 pA (n = 4) (black line, Fig. 2 A) and 177 ± 38 pA (n = 4) (black line, Fig. 2 D), respectively. However, these silent TWIK-1 K+ channels exhibit extremely large Rb+ or NH4+ conductance in Rb+ or NH4+-based solutions, and have a reversal potential of −11.7 ± 2.5 mV or −56.0 ± 1.0 mV in 5 mM [K+]o (pink line, Fig. 2 B; red line, Fig. 2 E), respectively, in agreement with a previous report (7). Although the relative permeability of Rb+ or NH4+ to K+ of TWIK-1 K+ channels is ∼0.60 or ∼0.1, respectively, at −140 mV these silent TWIK-1 K+ channels conducted significantly 35-fold larger inward Rb+ currents (blue bar, Fig. 2 C) or 40-fold larger inward NH4+ currents (purple bar, Fig. 2 F) in the bath solution containing 140 mM Rb+ or NH4+ than inward K+ currents (orange bars, Fig. 2, C and F) in the bath solution containing in 140 mM K+. In contrast, these results are not observed in CHO cells transfected with GFP alone (n = 15; data not shown). Such large amounts of inward Rb+ and NH4+ currents demonstrate that TWIK-1 K+ channels are expressed on the surface of transfected CHO cells. This finding not only supports the hypothesis that silent TWIK-1 K+ channels reside in the plasma membrane but also suggests that these silent TWIK-1 K+ channels can be activated by extracellular Rb+ and NH4+.
Because these results were obtained with rapid changes of bath solutions and the observed effects can be reversibly repeated in Na+-based and Rb4+ (or NH4+)-based solutions with 5 mM K+, externalization or internalization of TWIK-1 K+ channels should not contribute to such a large amount of inward Rb+ or NH4+ currents. Another potential challenge in interpreting large inward Rb+ or NH4+ currents is due to the change in the TWIK-1 channel conductance. However, the change in the TWIK-1 channel conductance alone is unlikely to enhance inward currents by ∼40-fold in response to extracellular ionic changes from 140 mM K+ to 140 mM Rb+ or NH4+. Also, the change in the TWIK-1 channel conductance alone should result in similar increases in both inward and outward currents, but the switches of K+-based bath solutions to Rb+ (or NH4+)-based bath solutions enhanced outward K+ currents only by approximately three- or nine-fold (Fig. 2), inconsistent with increases in inward currents. It is possible that the changes in both open probability and conductance of TWIK-1 channels contribute to large inward Rb+ or NH4+ currents.
We provided two pieces of evidence indicating that silent TWIK-1 K+ channels are able to conduct large monovalent cation currents in transfected CHO cells when extracellular ionic conditions change. These results support the hypothesis that TWIK-1 K+ channels are expressed in the cell surface but silent under physiological K+ gradients. Our findings also suggest that the TWIK-1 gating behavior rather than the lack of cell surface expression of TWIK-1 results in nonmeasurable TWIK-1 K+ currents in the heterologous expression systems.
Acknowledgments
We thank Steve A. Goldstein for comments on the manuscript.
This work was supported by the American Heart Association (0635125N and 11GRNT7270014) start-up funds and a Faculty Research Award Program A award from the University at Albany, State University of New York (to H.C.).
References and Footnotes
- 1.Goldstein S.A., Bayliss D.A., Rajan S. International Union of Pharmacology. LV. Nomenclature and molecular relationships of two-P potassium channels. Pharmacol. Rev. 2005;57:527–540. doi: 10.1124/pr.57.4.12. [DOI] [PubMed] [Google Scholar]
- 2.Lesage F., Guillemare E., Barhanin J. TWIK-1, a ubiquitous human weakly inward rectifying K+ channel with a novel structure. EMBO J. 1996;15:1004–1011. [PMC free article] [PubMed] [Google Scholar]
- 3.Lesage F., Lauritzen I., Lazdunski M. The structure, function and distribution of the mouse TWIK-1 K+ channel. FEBS Lett. 1997;402:28–32. doi: 10.1016/s0014-5793(96)01491-3. [DOI] [PubMed] [Google Scholar]
- 4.Gaborit N., Le Bouter S., Demolombe S. Regional and tissue specific transcript signatures of ion channel genes in the non-diseased human heart. J. Physiol. 2007;582:675–693. doi: 10.1113/jphysiol.2006.126714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nie X., Arrighi I., Vallon V. Expression and insights on function of potassium channel TWIK-1 in mouse kidney. Pflugers Arch. 2005;451:479–488. doi: 10.1007/s00424-005-1480-9. [DOI] [PubMed] [Google Scholar]
- 6.Zhou M., Xu G., Chen H. TWIK-1 and TREK-1 are potassium channels contributing significantly to astrocyte passive conductance in rat hippocampal slices. J. Neurosci. 2009;29:8551–8564. doi: 10.1523/JNEUROSCI.5784-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ma L., Zhang X., Chen H. TWIK-1 two-pore domain potassium channels change ion selectivity and conduct inward leak sodium currents in hypokalemia. Sci. Signal. 2011;4:ra37. doi: 10.1126/scisignal.2001726. [DOI] [PubMed] [Google Scholar]
- 8.Rajan S., Plant L.D., Goldstein S.A. Sumoylation silences the plasma membrane leak K+ channel K2P1. Cell. 2005;121:37–47. doi: 10.1016/j.cell.2005.01.019. [DOI] [PubMed] [Google Scholar]
- 9.Plant L.D., Dementieva I.S., Goldstein S.A. One SUMO is sufficient to silence the dimeric potassium channel K2P1. Proc. Natl. Acad. Sci. USA. 2010;107:10743–10748. doi: 10.1073/pnas.1004712107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wilson V.G., Rosas-Acosta G. Wrestling with SUMO in a new arena. Sci. STKE. 2005;2005:pe32. doi: 10.1126/stke.2902005pe32. [DOI] [PubMed] [Google Scholar]
- 11.Feliciangeli S., Tardy M.P., Lesage F. Potassium channel silencing by constitutive endocytosis and intracellular sequestration. J. Biol. Chem. 2010;285:4798–4805. doi: 10.1074/jbc.M109.078535. [DOI] [PMC free article] [PubMed] [Google Scholar]


