Abstract
The application of fluid shear stress on leukocytes is critical for physiological functions including initial adhesion to the endothelium, the formation of pseudopods, and migration into tissues. The formyl peptide receptor (FPR) on neutrophils, which binds to formyl-methionyl-leucyl-phenylalanine (fMLP) and plays a role in neutrophil chemotaxis, has been implicated as a fluid shear stress sensor that controls pseudopod formation. The role of shear forces on earlier indicators of neutrophil activation, such as L-selectin shedding and αMβ2 integrin activation, remains unclear. Here, human neutrophils exposed to uniform shear stress (0.1–4.0 dyn/cm2) in a cone-and-plate viscometer for 1–120 min showed a significant reduction in both αMβ2 integrin activation and L-selectin shedding after stimulation with 0.5 nM of fMLP. Neutrophil resistance to activation was directly linked to fluid shear stress, as the response increased in a shear stress force- and time-dependent manner. Significant shear-induced loss of FPR surface expression on neutrophils was observed, and high-resolution confocal microscopy revealed FPR internalized within neutrophils. These results suggest that physiological shear forces alter neutrophil activation via FPR by reducing L-selectin shedding and αMβ2 integrin activation in the presence of soluble ligand.
Introduction
The adhesion of leukocytes to the luminal surface of the microvasculature plays an important role in the inflammatory response and lymphocyte homing to lymphatic tissues (1–3). The initial step of the leukocyte adhesion cascade involves the capture and rolling of leukocytes on the receptor-bearing endothelial cell layer, with L-selectin acting as an important mediator on the leukocyte surface (4). Experiments with L-selectin knockout mice show severely impaired leukocyte migration into the inflamed endothelial wall, along with virtually no lymphocyte migration to lymphatic tissues (5,6). While E-selectin and P-selectin are cell adhesion molecules expressed on activated endothelial cells, L-selectin is constitutively expressed on the microvilli tips of neutrophils. In contrast to E-selectin and P-selectin, L-selectin is rapidly cleaved from the neutrophil surface due to inflammatory stimuli and cellular activation (7). In addition, the firm adhesion of neutrophils to endothelial cells in the vasculature is mediated via intracellular adhesion molecule-1 (ICAM-1) on endothelial cells binding to β2 integrins on neutrophils, such as CD11b/CD18 or Mac-1, and CD11a/CD18 or LFA-1 (8,9). Together, the downregulation of L-selectin and the active conformational change of the αM subunit of αMβ2 integrins, leading to increased binding affinity, are key indicators of neutrophil activation (10,11). Stimulus by fMLP, which binds to the formyl peptide receptor (FPR) on neutrophils, has been shown to lead to β2 integrin conformational changes and downregulation of L-selectin (12–15).
FPR, a chemoattractant G-protein coupled receptor (GPCR), exhibits high constitutive activity in addition to activity due to agonist binding (16,17). Under static conditions in the absence of fluid shear stress, neutrophils spread their cytoplasm and are able to migrate on a glass substrate, in part due to constitutive GPCR activity. In the presence of fluid shear stress, neutrophils have been shown to rapidly retract lamellipodia, assume a round resting state, and decrease GPCR constitutive activity (18–20). In neutrophils treated with pertussis toxin, a Gi inhibitor, the fluid shear stress-induced pseudopod retraction response was significantly attenuated, demonstrating the role of GPCR activity changes due to fluid shear stress (19). Transfection of cDNA for FPR into a cell line with very low levels of FPR and low pseudopod activity led to the projection of pseudopods, which then retracted after exposure to fluid shear stress (19). FPR depletion via siRNA delivery in differentiated HL60 cells also significantly reduced fluid shear stress-induced pseudopod retraction.
Although the ability of activated leukocytes to retract pseudopods in response to fluid shear stress has been documented in several studies, a variety of different neutrophil morphological responses have been obtained. Circulating leukocytes have been shown to undergo significant shape change and morphology disruption after exposure to extended periods of fluid shear stress (21). Leukocytes treated with dexamethasone or centrifugation have been shown to reverse their shear stress response and can activate and project pseudopods when exposed to fluid shear stress (22,23). Leukocytes adhered to a glass substrate that show no active deformation of pseudopods have been shown to become activated and extend pseudopods upon sudden application of fluid shear stress (24). Leukocytes activated specifically by low concentrations of GPCR ligands such as platelet-activating factor (PAF) and fMLP have been shown to retract pseudopods upon application of fluid shear stress (25).
The effect of fluid shear stress on earlier indicators of neutrophil activation, such as L-selectin shedding and αMβ2 integrin activation induced by low concentrations of fMLP, has not yet been addressed. In this study, we examined the quantitative dynamics of the shear stress-dependent response of fMLP-induced L-selectin shedding and αMβ2 integrin activation in neutrophils.
Materials and Methods
A detailed description of the materials and methods used in this study can be found in the Supporting Material. Briefly, neutrophils were isolated from human peripheral blood by centrifugation at 480 × g for 50 min at 23°C in a Marathon 8K centrifuge (Fisher Scientific, Pittsburgh, PA) using 1-Step Polymorphs (Accurate Chemical & Scientific, Westbury, NY). Neutrophils were resuspended at a concentration of 0.5 × 106 cells/mL in HBSS containing 0.5% HSA, 2 mM Ca2+, 1 mM Mg2+, and 10 mM HEPES (Invitrogen, Carlsbad, CA), buffered to pH 7.4. Neutrophils were incubated with 25 μM GM6001, 5 mM 1,10-phenanthroline (1,10-Ph), or 35 μM TAPI-0 for 30 min for protease inhibition studies. Neutrophils were then exposed to fluid shear stress using a cone-and-plate viscometer consisting of a stationary plate beneath a rotating cone maintained at 23°C or 37°C by a circulating water bath (Brookfield, Middleboro, MA). The cone-and-plate viscometer design allows for a uniform shear rate to be applied to the entire sample. The shear rate, G, does not depend on the distance from the cone center, and is given by
where ω is the angular velocity of the cone (rad/s) and θ is the angle of the cone (rad). A laminar flow field is expected for all experimental conditions. Under these conditions for a Newtonian fluid, the shear stress, τ, is proportional to the shear rate being applied:
where μ is the viscosity of the medium. Before the experiments, the stationary plate and rotating cone were incubated with 5% BSA at room temperature for 1 h. Neutrophil suspensions of 500 μL were placed on the plate and allowed to equilibrate before the onset of shear stress. To determine the shear stress threshold required for neutrophil resistance, neutrophils were exposed to shear stresses ranging from 0.1 to 4.0 dyn/cm2 over 2 h. To determine the shear stress duration threshold and time dependence of the shear stress response, neutrophils were exposed to a shear stress of 4.0 dyn/cm2 for increasing time intervals of 1–120 min in duration. To maintain a constant shear rate while increasing the shear stress, the medium viscosity was increased by adding dextran polymer to the suspension. After shearing, aliquots of neutrophils were immediately exposed to fMLP, IL-8, or no chemoattractant for a period of 10 min. The combination of fluid shear stress followed by chemotactic stimulation is a situation encountered by neutrophils in the microvasculature in vivo. Sheared and nonsheared neutrophils were immediately labeled with fluorescent antibodies to quantify L-selectin, activated CD11b subunits of β2 integrins, FPR, CXCR1, and CXCR2 expression using an Accuri C6 flow cytometer (Accuri Cytometers, Ann Arbor, MI). The average number of FPR, CXCR1, and CXCR2 receptors on the surface of neutrophils was determined via flow cytometry using fluorescent beads with defined numbers of antigen-binding capacities (ABCs) to generate a fluorescence calibration curve.
Sheared and nonsheared neutrophil suspensions were fixed with paraformaldehyde and analyzed for morphological characteristics and receptor internalization using brightfield and confocal microscopy, respectively. Outlines of neutrophils were created from thresholded brightfield images using edge-detection functions in Metamorph (Universal Imaging, West Chester, PA). Changes in neutrophil shape were determined using the “shape factor” program in Metamorph, where the shape factor is given by
where P is the perimeter and A is the area of the object (neutrophil). Shape factor values close to 1 represent a perfect circle, whereas values <1 represent elongated or ruffled shapes.
For internalization studies, neutrophil samples were distributed onto slides using a Shandon CytoSpin III centrifuge (Shandon, Pittsburgh, PA). Samples were permeabilized in 0.2% Triton X-100 for 5 min and then incubated in 1% BSA for 1 h. After incubation in primary anti-human FPR for 12 h, slides were incubated with a secondary IgG-fluorescein isothiocyanate (FITC) antibody for 30 min at 4°C and then mounted onto coverslips. Samples were examined with a Zeiss 710 Spectral Confocal Microscope System (Carl Zeiss MicroImaging, Jena, Germany) at 65× magnification with an FITC filter. Metamorph software was used to examine FPR internalization and fluorescence intensities within neutrophils. To measure fluorescence intensity within the cell, the cell membrane was thresholded using edge-detection functions to exclude the fluorescent membrane from calculations.
Results
Fluid shear stress reduces fMLP-induced L-selectin shedding and αMβ2 integrin activation in neutrophils
We initially studied the fluid shear stress response of neutrophils to L-selectin shedding and αMβ2 integrin activation after exposure to a low concentration of fMLP. Neutrophil suspensions were exposed to static conditions or to 4.0 dyn/cm2 of fluid shear stress in a cone-and-plate viscometer for 2 h at 23°C and then stimulated with or without 0.5 nM fMLP for 10 min. Stimulus from fMLP, an inflammatory mediator, is known to lead to downregulation of L-selectin and conformational change in the αM subunit of αMβ2 integrins (12–14). Neutrophils exposed to static conditions (Fig. 1 A) and 4.0 dyn/cm2 (Fig. 1 B) of fluid shear stress in the absence of fMLP did not show appreciable differences in L-selectin shedding and αMβ2 integrin activation, as expected. However, neutrophils exposed to fMLP after fluid shear stress (Fig. 1 D) showed a measurable reduction in L-selectin shedding and αMβ2 integrin activation compared to neutrophils exposed to fMLP after exposure to static conditions (Fig. 1 C). No significant difference in L-selectin shedding and αMβ2 integrin activation was found between sheared and nonsheared neutrophils, whereas a significant reduction in fMLP-induced L-selectin shedding (Fig. 1 G) and αMβ2 integrin activation (Fig. 1 H) was found in sheared neutrophils compared to neutrophils under static conditions. Experiments were also conducted at 37°C to compare to 23°C experiments, and similar differences were seen between neutrophils under shear and static conditions in terms of L-selectin shedding and αMβ2 integrin activation (Fig. S2). Experiments at 37°C were performed for 20 min rather than 2 h to minimize sample evaporation.
Figure 1.
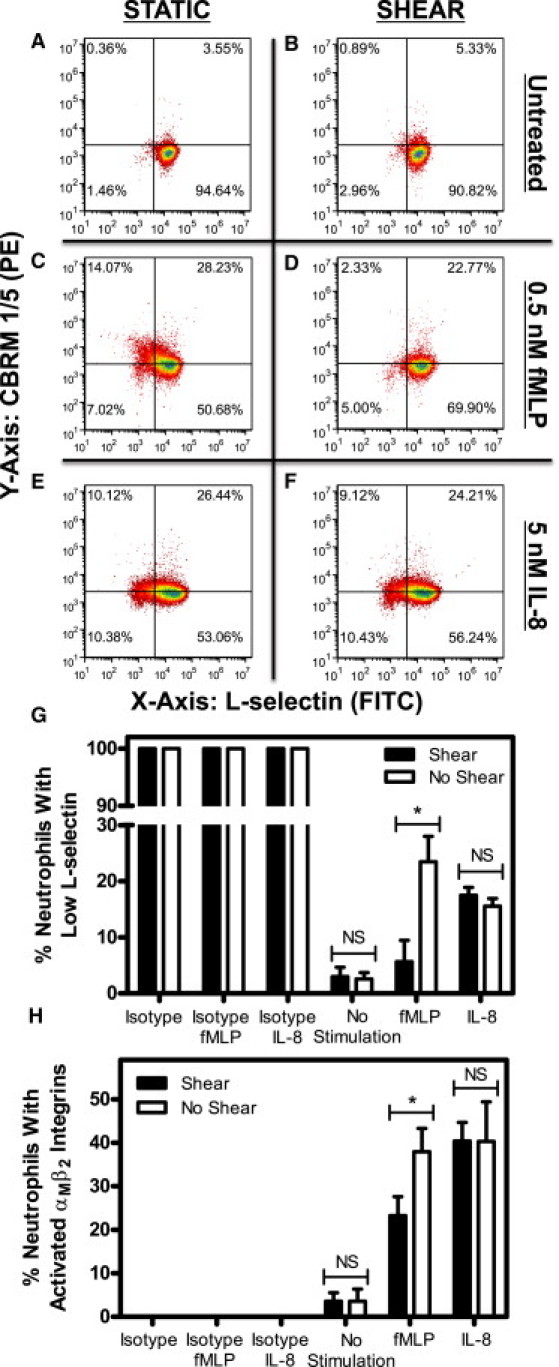
Fluid shear stress alters neutrophil resistance to L-selectin shedding and αMβ2 integrin activation. Neutrophils were exposed to static conditions (A) or 4.0 dyn/cm2 of shear (B) for 2 h, and were then immediately exposed to 0.5 nM of fMLP (C and D) or IL-8 (E and F) for 10 min. All conditions were repeated with n = 5 donors for L-selectin shedding (G) and αMβ2 integrin activation (H). The upper two quadrants of each flow cytometry plot represent positive CBRM1/5 staining, whereas the lower two signify little to no CBRM1/5 staining. The two righthand quadrants of each plot represent positive L-selectin staining and the two lefthand quadrants represent the absence of L-selectin staining. Quadrants were determined by labeling neutrophils with isotype antibodies corresponding to L-selectin and CBRM1/5 antibodies, which label for all nonspecific binding sites on the neutrophil surface. Low L-selectin represents fluorescence values no greater than isotype values. PE, R-Phycoerythrin fluorescence channel; FITC, fluorescein isothiocyanate fluorescence channel. Error bars represent 95% confidence intervals. ∗P < 0.05 for all measurements; NS, nonsignificant difference.
Fluid shear stress does not affect IL-8-induced L-selectin shedding and αMβ2 integrin activation
To assess whether fluid shear stress alters L-selectin shedding and αMβ2 integrin activation via other major neutrophil chemoattractant GPCRs, neutrophils were exposed to static conditions or to 4.0 dyn/cm2 of fluid shear stress in a cone-and-plate viscometer for 2 h at 23°C, followed by stimulation either with or without 5 nM of IL-8. IL-8, a chemoattractant found on the surface of endothelial cells (26–28), binds to GPCRs CXCR1 and CXCR2, which are believed to have lower constitutive activity in comparison to FPR (29–32). Neutrophils exposed to static conditions (Fig. 1 E) or 4.0 dyn/cm2 (Fig. 1 F) of fluid shear stress followed by IL-8 treatment did not show significant differences in L-selectin shedding (Fig. 1 G) or αMβ2 integrin activation (Fig. 1 H).
fMLP-induced L-selectin shedding and αMβ2 integrin activation in neutrophils is dose-dependent in fluid shear stress magnitude
To study the effect of shear stress magnitude on fMLP-induced activation, neutrophils were exposed to varying shear stresses of 0.1–4.0 dyn/cm2 for 2 h. These shear stress magnitudes are values typically found in the microcirculation (33). At low shear stresses of 0.10 and 0.25 dyn/cm2, no significant difference in L-selectin shedding (Fig. 2 A) or αMβ2 integrin activation (Fig. 2 B) was observed between cells exposed to shear followed by fMLP stimulation and cells exposed to no shear followed by fMLP stimulation. However, a shear stress of 0.75 dyn/cm2 yielded a significant reduction in L-selectin shedding (Fig. 2 A) and αMβ2 integrin activation (Fig. 2 B) after exposure to fMLP. Shear stress exposures of 2.5 and 4.0 dyn/cm2 showed an even greater reduction of L-selectin shedding (Fig. 2 A) and αMβ2 integrin activation (Fig. 2 B).
Figure 2.
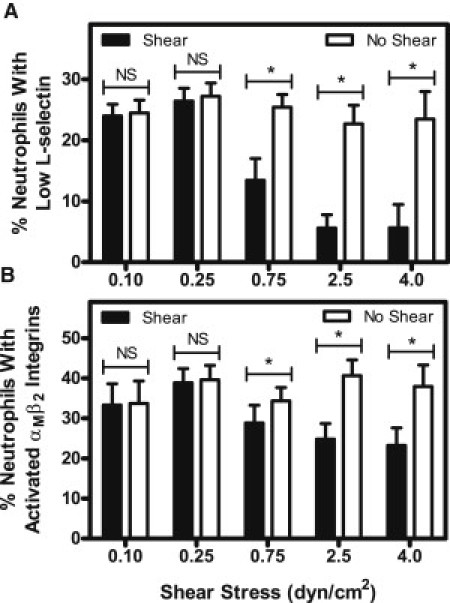
Increasing fluid shear stress reduces fMLP-induced L-selectin shedding (A) and αMβ2 integrin activation (B) of neutrophils. Shear stress magnitude was varied in separate experiments from 0.1 to 4.0 dyn/cm2 for 2 h at 23°C, followed by stimulation with 0.5 nM of fMLP for 10 min. n = 5 donors for each shear stress value. Error bars represent 95% confidence intervals. ∗P < 0.05 for all measurements.
fMLP-induced L-selectin shedding and αMβ2 integrin activation in neutrophils is dependent on shear stress exposure time
To assess the kinetics of the FPR mechanosensing phenomenon, neutrophils were exposed to a constant shear stress of 4.0 dyn/cm2 at 23°C, with the fluid shear stress exposure time increasing from 1 to 120 min, after which they were stimulated with 0.5 nM fMLP. The exposure time was increased to determine a threshold where the shear-induced resistance begins to develop. No significant difference in fMLP-induced L-selectin shedding in neutrophils was found over a shear stress exposure time period of 1–10 min (Fig. 3 A). However, a significant decrease in fMLP-induced L-selectin shedding was found at a threshold shear stress exposure time of 20 min, and the amount of L-selectin shedding was found to be significantly less than that for neutrophils in static conditions for the range 20–120 min. fMLP-induced αMβ2 integrin activation in neutrophils showed no significant difference between neutrophils exposed to shear and static conditions for 1 to 30 min (Fig. 3 B). A threshold exposure time of 60 min was required to produce a significant difference in αMβ2 integrin activation between neutrophils exposed to shear and static conditions, and this response was significant over a range of 60–120 min.
Figure 3.
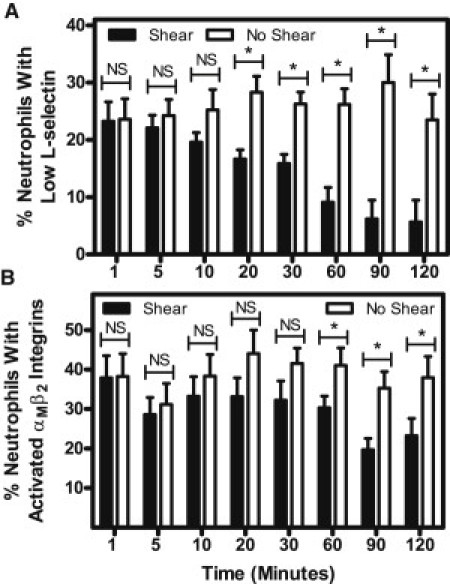
Neutrophil resistance to activation increases with increasing fluid shear stress duration. The time dependence of the mechanical response of neutrophils to fMLP-induced L-selectin shedding (A) and αMβ2 integrin activation (B) was determined by increasing the shear stress exposure time from 1 to 120 min at a uniform shear stress of 4.0 dyn/cm2 at 23°C. n = 3 donors for each time point. Error bars represent 95% confidence intervals. ∗P < 0.05 for all measurements.
Neutrophils develop a resistance to fMLP-induced L-selectin shedding and αMβ2 integrin activation with increasing shear stress magnitude and shear stress exposure time
Due to the variability in response experienced with primary neutrophils, neutrophils exposed to both shear and static conditions from each donor were directly compared over varying shear stress magnitudes and shear stress exposure durations. Neutrophil resistance responses to L-selectin shedding and αMβ2 integrin activation were directly compared using the equation
The resistance equation applies to neutrophils labeled for both L-selectin shedding and αMβ2 integrin activation for varying shear stress magnitudes and shear stress durations.
By varying the shear stress magnitude, it is apparent that shear stress does not confer resistance in neutrophils to fMLP-induced L-selectin shedding (Fig. 4 A) and αMβ2 integrin activation (Fig. 4 C) at lower shear stress values of 0.10 and 0.25 dyn/cm2. Resistance to L-selectin shedding and αMβ2 integrin activation is achieved at 0.75 dyn/cm2, and maximum shear stress response is conferred at 2.5 dyn/cm2. No additional increase in response is observed at a higher shear stress of 4.0 dyn/cm2. No measurable resistance to L-selectin shedding (Fig. 4 A) or αMβ2 integrin activation (Fig. 4 C) was observed in neutrophils without fMLP stimulation. By varying the duration of shear stress exposure, it is apparent that the shear-induced resistance to fMLP-induced L-selectin shedding (Fig. 4 B) and αMβ2 integrin activation (Fig. 4 D) increases with increasing shear stress exposure time. Resistance to activation after 1–120 min of fluid shear exposure was not observed in the absence of fMLP.
Figure 4.
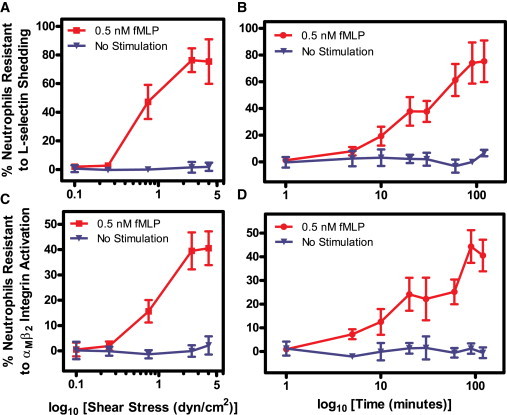
Neutrophils develop resistance to fMLP-induced L-selectin shedding and αMβ2 integrin activation with increasing shear stress magnitude (A and C) and shear stress duration (B and D). Resistance is plotted as a function of the log10 of shear stress (dyn/cm2) or time (min). n = 5 donors for each shear stress point, and n = 3 donors for each time point. Error bars represent 95% confidence intervals.
Neutrophils acquire a shear-induced resistance to fMLP-induced morphological changes
To assess how fluid shear stress alters neutrophil morphology after fMLP stimulation, neutrophils exposed to 4.0 dyn/cm2 shear stress or to static conditions for 2 h at 23°C were fixed with 4% paraformaldehyde and examined for morphological characteristics. Activated neutrophils project pseudopods after exposure to chemoattractants such as fMLP, PAF, C5a anaphylotoxin, leukotriene B4, and IL-8 (34). To quantify this, neutrophil morphology was evaluated using Metamorph by calculating the shape factor of each cell. The shape factor approaches 1 for round, unactivated neutrophils and decreases for more extended, dendritic shapes.
No significant difference in the shape factor was found between neutrophils exposed to shear and static conditions without exposure to fMLP (Fig. 5 A). Neutrophils stimulated with 0.5 nM fMLP showed an average shape factor significantly less than 1. Interestingly, a significant difference in shape factor was found between sheared and unsheared neutrophils after exposure to 0.5 nM fMLP, with the sheared neutrophils exhibiting a shape factor close to unity. Neutrophils assumed a round morphology in the absence of fMLP (Fig. 5, B and C), whereas unsheared neutrophils developed a more extended morphology than did sheared neutrophils in the presence of fMLP (Fig. 5, D and E).
Figure 5.
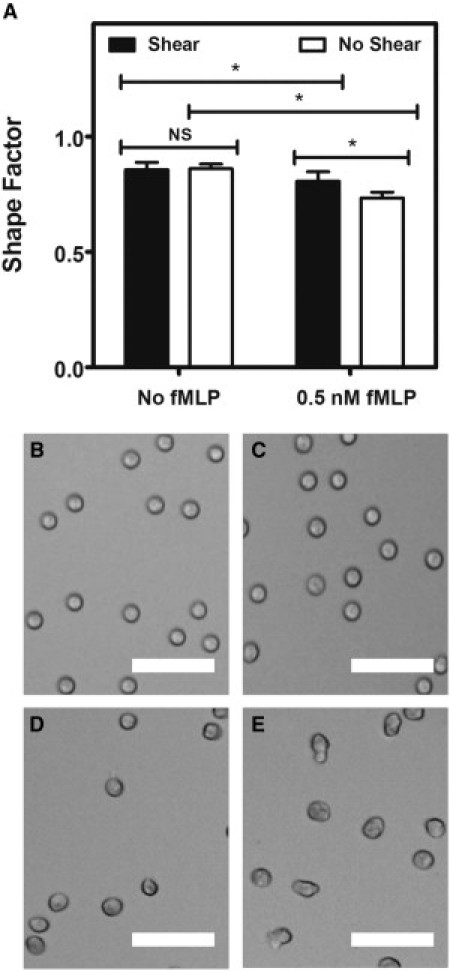
Fluid shear stress alters fMLP-induced neutrophil morphology changes. (A) Shape factor measurements of neutrophils exposed to shear stress or static conditions without and with 0.5 nM fMLP stimulation. (B–E) Brightfield images of neutrophils exposed to shear stress or static conditions without fMLP (B and C, respectively), and neutrophils exposed to shear stress or static conditions with 0.5 nM fMLP (D and E, respectively). All scale bars = 50 μm. n = 3 donors, with 300 neutrophils analyzed for shape factor for each donor condition. Error bars represent 95% confidence intervals. ∗P < 0.05 for all measurements.
Fluid shear stress reduces FPR surface expression
To investigate the mechanotransduction effects on FPR surface expression, neutrophils were exposed to shear stress (4.0 dyn/cm2) and static conditions at 23°C for 2 h and immediately labeled with anti-FPR antibodies to be analyzed via flow cytometry. Sheared neutrophils displayed a reduction in FPR expression (Fig. 6 A) compared to nonsheared samples. QSC bead analysis indicated a significant difference in FPR receptor count, as sheared neutrophils averaged 14,600 receptors/cell, whereas nonsheared samples averaged 20,100 receptors/cell (Fig. 6 D). Sheared neutrophils did not show a reduction in the expression of the two IL-8 receptors, CXCR1 (Fig. 6 B) and CXCR2 (Fig. 6 C), in comparison to nonsheared neutrophils. CXCR1 and CXCR2 receptor counts using QSC beads showed no significant differences between sheared and nonsheared neutrophils.
Figure 6.
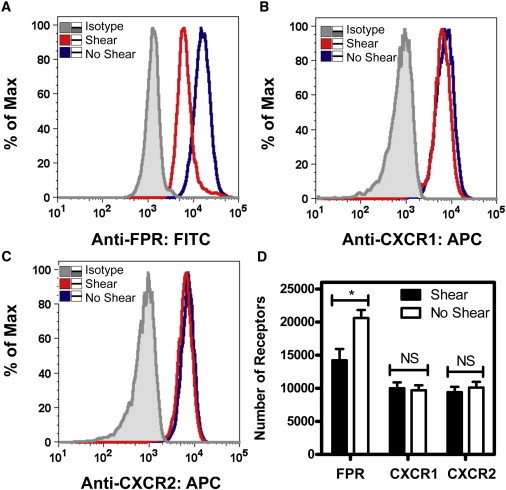
Fluid shear stress induces a loss of FPR surface expression. (A–C) Flow cytometry plots show FPR expression at 4.0 dyn/cm2 for 2 h (A) in sheared compared to nonsheared samples, along with expression of IL-8 receptors CXCR1 (B) and CXCR2 (C) expression in sheared and nonsheared neutrophils. (D) Receptor quantification using QSC beads was used to determine the surface receptor count of FPR, CXCR1, and CXCR2 on neutrophils. n = 3 donors. Error bars represent 95% confidence intervals. ∗P < 0.05 for all measurements.
FPR expression levels are unaltered in the presence of protease inhibitors
To assess whether FPR is enzymatically cleaved by proteases derived from the neutrophil upon exposure to fluid shear stress, neutrophils were treated with 25 μM GM6001, 5 mM 1,10-Ph, or 35 μM TAPI-0 for 30 min before fluid shear stress exposure. Neutrophils exposed to fluid shear stress (4.0 dyn/cm2) and static conditions at 23°C for 2 h were then labeled with anti-FPR antibodies to be analyzed via flow cytometry, followed by surface-receptor quantification. No significant differences were observed in FPR surface expression for neutrophils treated with protease inhibitors compared to untreated neutrophils exposed to shear (Fig. 7), indicating that loss of FPR expression is not due to cleavage by neutrophil proteases under fluid shear.
Figure 7.
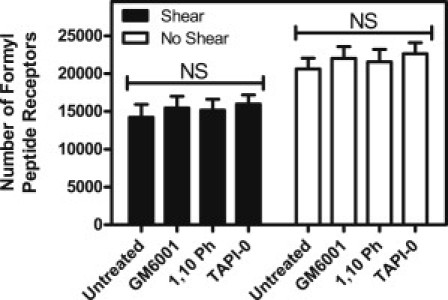
Protease inhibition does not affect FPR surface expression upon neutrophil exposure to fluid shear stress. Untreated neutrophils and neutrophils treated with GM6001, 1,10-Ph, or TAPI-0 were exposed to static conditions and 4.0 dyn/cm2 of shear stress for 2 h. Samples were analyzed for FPR surface expression via flow cytometry. All conditions were repeated for significance with n = 3 donors. Error bars represent 95% confidence intervals. NS, not significant.
Neutrophils experience FPR internalization under fluid shear
To examine potential FPR internalization as a possible cause for FPR surface-expression decrease after fluid shear stress exposure, neutrophils were exposed to either fluid shear stress (4.0 dyn/cm2) or static conditions for 2 h, permeabilized, and then labeled with anti-FPR antibodies for examination via confocal microscopy. Images were thresholded (Fig. 8 C) to exclude the cell membrane from fluorescence measurements. Immunostaining revealed FPR to be clearly localized within the cell in sheared samples (Fig. 8 A), whereas minimal FPR was shown within neutrophils exposed to static conditions (Fig. 8 B). The average pixel intensities calculated from within neutrophils showed a significant increase in fluorescence intensity in sheared neutrophils, compared to neutrophils exposed to static conditions (Fig. 8 D).
Figure 8.
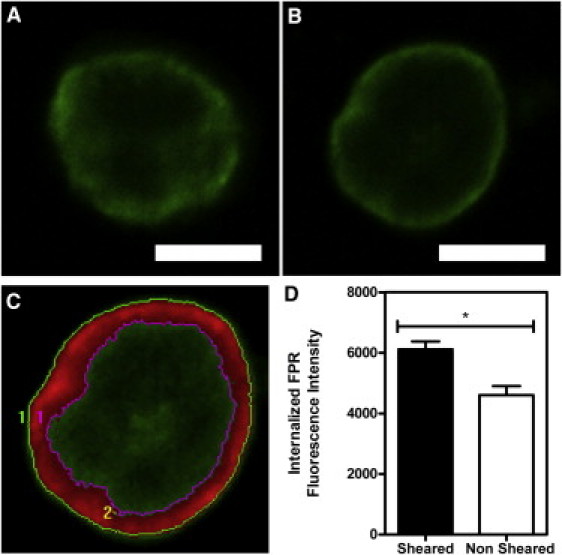
Fluid shear stress induces FPR internalization in neutrophils. (A and B) Neutrophils were exposed to 4.0 dyn/cm2 of fluid shear stress (A) or to static conditions (B) for 2 h. All samples were permeabilized, labeled with FPR antibodies, and examined via confocal microscopy. (C) Images were then thresholded to exclude the cell membrane from measurements. (D) Quantification of the average fluorescence intensity of the inner portion of sheared and nonsheared cells. All scale bars = 5 μm. All conditions were repeated for significance with n = 3 donors. Error bars represent 95% confidence intervals. ∗P < 0.05 for all measurements.
Discussion
The aim of this study was to quantify the shear stress dependent responses of fMLP-induced L-selectin shedding and αMβ2 integrin activation in neutrophils via the FPR. Resistance was acquired by neutrophils during exposure to physiological levels of shear stress in a force- and time-dependent manner, directly implicating fluid shear stress in this response. Interestingly, we found this resistance to be first manifest at a shear stress magnitude of 0.75 dyn/cm2; this value is close to the 0.5 dyn/cm2 minimum level of shear stress found by Schmid-Schonbein and colleagues to induce pseudopod retraction, which is also believed to act through the FPR (18). In addition, a shear stress of >0.4 dyn/cm2 is required for L-selectin adhesion to CD34 on vascular tissue (35). The kinetic studies of shear-induced fMLP resistance showed that measurable effects began in the 5 min range for both L-selectin shedding and αM integrin conformational change, consistent to what was observed in pseudopod retraction experiments (20).
Although neutrophils acquired a resistance to both fMLP-induced L-selectin shedding and αMβ2 integrin activation, the number of cells that were resistant to both effects varied. The fact that fewer cells developed a resistance to αMβ2 integrin activation than to L-selectin shedding could be due to the fact that the extracellular domain of CD18 can be cleaved at physiological shear stresses (36). Cleavage of CD18 on cells due to fluid shear stress may distort the true number of cells that are positive or negative for αMβ2 integrin activation, whereas L-selectin has only been found to be cleaved due to fluid shear stress when rolling on carbohydrate ligand-coated surfaces (37).
In addition to the variations in fMLP-induced L-selectin shedding and αMβ2 integrin activation in neutrophils, it is also apparent that a subset does not acquire any resistance due to fluid shear stress. There is evidence that fluid shear stress on the neutrophil membrane can be nonuniform; variations in the shear stress distribution on the surface of a neutrophil have been found, due to the geometric shape of the membrane (38). Although spherical in shape, neutrophils have a villous membrane made up of numerous fine foldings (39) that may alter the shear stress exerted on cell-surface receptors. Despite these two factors, it is notable that a measurable population of neutrophils developed a resistance to fMLP-induced activation. The ranges of the neutrophils being examined might also explain the differences in their response to fluid shear stress. Recent studies suggest that neutrophil lifespans might be much longer than previously believed, with neutrophils having a circulatory lifespan of up to 5.4 days, compared to the previously generally accepted short lifespan of less than one day (40).
Neutrophils have the ability to enzymatically cleave their surface receptors with proteases derived from within the cell upon exposure to fluid shear, including adhesion receptors such as L-selectin (37) and CD18 (36). Cleavage of FPRs by matrix metalloproteinases (MMPs) has been shown by others to alter the fluid-shear response of neutrophils (41). However, when neutrophils were treated with MMP inhibitors in this study, no significant changes in FPR surface expression were observed compared to untreated samples exposed to fluid shear. This indicates that the loss of FPR surface expression is not due to enzymatic cleavage via proteases derived from the neutrophil due to the onset of shear. Cleavage of FPR observed by other groups was due to the elevated levels of MMPs within the blood of the spontaneously hypertensive rat (41), whereas the neutrophils used in this study were taken from normal human donors.
Our studies show that FPR surface expression downregulation could be due to the observed internalization of FPR by neutrophils upon exposure to fluid shear stress. FPR internalization is typically associated with desensitization of the receptor upon exposure to fMLP (42). When FPR is activated due to fMLP, the receptor becomes phosphorylated by GPCR kinases, which increases FPR affinity for the cytosolic protein arrestin (43). Arrestin binding to GPCRs prevents the receptors from additional association with G-proteins, making arrestins a major component in GPCR desensitization. FPR desensitization has also been shown to occur as a form of cross-desensitization among chemoattractant receptors. HEK293 cells coexpressing fMLP and C5a receptors have shown that activation of C5a receptors resulted in cross-desensitization of Ca2+ mobilization stimulated by FPR, and vice versa (44). Neutrophils expressing combinations of chemoattractant receptors have shown that peptide chemoattractants such as fMLP, C5a, and IL-8 desensitized Ca2+ mobilization to one another and to PAF (45,46). Recently, exposure to fluid shear stress has also been shown to cause FPR on leukocytes to be internalized into perinuclear compartments (47), and similar internalization responses due to shear stress have been observed in other cell types (48). Based on the loss of FPR expression on neutrophils after the application of fluid shear stress, FPR internalization due to shear forces may also cause another form of desensitization, and thus attenuate the effects of fMLP on L-selectin shedding and αM integrin conformational change. Since fMLP is known to induce L-selectin shedding and αM integrin conformational change (12–15) and FPR was internalized upon exposure to shear stress in this study, a downregulation of FPR on the surface available for receptor-ligand binding will contribute to the neutrophil response to activation. Although FPR internalization could play a major role in the downregulation of FPR on the cell surface, other causes for FPR downregulation could also be contributors. For example, it has been suggested that fluid shear may change the conformation in the seven-transmembrane-domain region of FPR, which may also cause FPR inactivation (20).
FPR has been implicated as a sensor for fluid shear stress, but other chemoattractant GPCRs, such as CXCR1 and CXCR2, did not display unique properties while exposed to shear stress in this study. Differences in their response to fluid shear stress could involve differences in their signaling pathways, as neutrophil chemotaxis in response to either fMLP or IL-8 is known to display different properties. Chemotaxis assays have shown neutrophil migration to IL-8 to be dependent on PI3K, but FPR signaling drastically differs, as treatment with Pan-PI3K inhibitors only delays initial neutrophil migration to fMLP (49–53). Physiologically, FPR surface expression on neutrophils might alter under shear, but not that of CXCR1 and CXCR2, because of the differences in fMLP and IL-8 presentation in tissue compared to in the bloodstream. There is extremely low fMLP concentration present in the bloodstream, as fMLP is derived from bacterial protein degradation or from mitochondrial proteins upon tissue damage. While under higher shear stresses within the bloodstream, FPR might remain internalized due to low fMLP concentrations, but once it is under much lower shear stress conditions from interstitial flow within the tissue, FPR could present along the surface of the neutrophil membrane to enhance migration along the chemotactic gradient. Conversely, the differences in IL-8 concentration are not as pronounced. Significant amounts of IL-8 are known to line venular endothelial cells in the bloodstream while also occurring within the tissue during inflammatory conditions (26), necessitating that CXCR1 and CXCR2 levels remain similar under both shear and nonshear conditions.
To investigate the importance of FPR internalization in the shear-induced resistance to activation, one may attempt to match the number of receptor-ligand complexes at equilibrium for sheared and nonsheared neutrophils by increasing the fMLP concentration used to stimulate sheared neutrophils. If simple monovalent receptor-ligand binding is assumed, a monovalent ligand L binds reversibly to a monovalent receptor R to form a receptor-ligand complex C (54). Neglecting ligand depletion in the neutrophil suspension, and assuming the ligand concentration remains constant at its initial value, L0, yields an estimate for the number of receptor-ligand complexes at equilibrium, Ceq, from the equation Ceq = RTL0/KD + L0, where KD is the equilibrium dissociation constant (nM) of the receptor-ligand interactions and RT is the number of receptors present on the cell surface (number/cell).
FPR on neutrophils displays a KD in the range 0.5–1.0 nM (55,56). With the FPR receptor count for sheared neutrophils determined in this study, one can estimate the L0 value necessary to reach the same Ceq found in nonsheared neutrophils. Using KD = 1.0 nM, we predicted the fMLP concentration needed to elicit the same Ceq in sheared neutrophils as in nonsheared neutrophils to be L0 = 0.8 nM. Equal responses of L-selectin shedding and αMβ2 integrin activation in sheared neutrophils exposed to 0.8 nM fMLP compared to nonsheared ones exposed to 0.5 nM fMLP would suggest that FPR internalization is responsible for the shear-induced resistance. Conversely, a lower degree of L-selectin shedding and αMβ2 integrin activation in the sheared neutrophils would suggest that FPR internalization combines with other factors to contribute to the resistance response. Here, sheared neutrophils stimulated with 0.8 nM fMLP showed a reduction in selectin shedding and integrin activation compared to nonsheared neutrophils stimulated with 0.5 nM fMLP, but they exhibited greater selectin shedding and integrin activation than did sheared neutrophils stimulated with 0.5 nM fMLP (Fig. S3). This analysis suggests that FPR internalization, along with other factors, contribute to shear-induced resistance to activation in the neutrophil. However, it should be noted that we have neglected receptor dynamics that occur during the 10-min stimulation with fMLP. Taking into account the synthesis, intracellular sorting, and differential endocytosis of FPRs (57) could help to further evaluate the impact of the effects of FPR downregulation in the neutrophil shear-induced resistance to activation.
Conclusion
The results from this study suggest that fluid shear stress has a significant effect on the activation of circulating neutrophils. Neutrophils acquired a fluid shear stress-induced resistance to activation via FPRs. The resistance was shown to be dependent on shear stress magnitude, as the resistance response increased with increasing shear stress. The mechanical response was also shown to be dependent on shear stress duration, as neutrophils increased their resistance with increased shear stress exposure time. A decrease in FPR surface expression was observed under fluid shear stress, and high-resolution confocal microscopy revealed that FPR was internalized within cells. Although other studies on mechanotransduction in neutrophils have mostly focused on morphological changes, this study focused on earlier indicators of activation, specifically fMLP-induced L-selectin shedding and αMβ2 integrin activation. The complete signaling pathways of these receptors deserve further study, as do the molecules that mediate GPCR internalization. Other receptors that have shown high constitutive activity should be investigated to understand their contributions to the mechanosensing responses of cells within the vascular microenvironment.
Acknowledgments
The authors acknowledge Jeff Mattison for work with blood sample collection and donor recruitment.
This study was supported by the National Institutes of Health, grant No. HL018128.
Footnotes
This is an Open Access article distributed under the terms of the Creative Commons-Attribution Noncommercial License (http://creativecommons.org/licenses/by-nc/2.0/), which permits unrestricted noncommercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Supporting Material
References
- 1.Springer T.A. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell. 1994;76:301–314. doi: 10.1016/0092-8674(94)90337-9. [DOI] [PubMed] [Google Scholar]
- 2.McEver R.P. Selectins: lectins that initiate cell adhesion under flow. Curr. Opin. Cell Biol. 2002;14:581–586. doi: 10.1016/s0955-0674(02)00367-8. [DOI] [PubMed] [Google Scholar]
- 3.Vestweber D.A., Blanks J.E. Mechanisms that regulate the function of the selectins and their ligands. Physiol. Rev. 1999;79:181–213. doi: 10.1152/physrev.1999.79.1.181. [DOI] [PubMed] [Google Scholar]
- 4.von Andrian U.H., Chambers J.D., Butcher E.C. L-selectin mediates neutrophil rolling in inflamed venules through sialyl LewisX-dependent and -independent recognition pathways. Blood. 1993;82:182–191. [PubMed] [Google Scholar]
- 5.Tedder T.F., Steeber D.A., Pizcueta P. L-selectin-deficient mice have impaired leukocyte recruitment into inflammatory sites. J. Exp. Med. 1995;181:2259–2264. doi: 10.1084/jem.181.6.2259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Steeber D.A., Green N.E., Tedder T.F. Humoral immune responses in L-selectin-deficient mice. J. Immunol. 1996;157:4899–4907. [PubMed] [Google Scholar]
- 7.Palecanda A., Walcheck B., Jutila M.A. Rapid activation-independent shedding of leukocyte L-selectin induced by cross-linking of the surface antigen. Eur. J. Immunol. 1992;22:1279–1286. doi: 10.1002/eji.1830220524. [DOI] [PubMed] [Google Scholar]
- 8.Hughes B.J., Hollers J.C., Smith C.W. Recruitment of CD11b/CD18 to the neutrophil surface and adherence-dependent cell locomotion. J. Clin. Invest. 1992;90:1687–1696. doi: 10.1172/JCI116041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Springer T.A. Traffic signals on endothelium for lymphocyte recirculation and leukocyte emigration. Annu. Rev. Physiol. 1995;57:827–872. doi: 10.1146/annurev.ph.57.030195.004143. [DOI] [PubMed] [Google Scholar]
- 10.Elemer G.S., Edgington T.S. Monoclonal antibody to an activation neoepitope of αMβ2 inhibits multiple αMβ2 functions. J. Immunol. 1994;152:5836–5844. [PubMed] [Google Scholar]
- 11.Diamond M.S., Garcia-Aguilar J., Springer T.A. The I domain is a major recognition site on the leukocyte integrin Mac-1 (CD11b/CD18) for four distinct adhesion ligands. J. Cell Biol. 1993;120:1031–1043. doi: 10.1083/jcb.120.4.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fan H., Derynck R. Ectodomain shedding of TGF-α and other transmembrane proteins is induced by receptor tyrosine kinase activation and MAP kinase signaling cascades. EMBO J. 1999;18:6962–6972. doi: 10.1093/emboj/18.24.6962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jutila M.A., Rott L., Butcher E.C. Function and regulation of the neutrophil MEL-14 antigen in vivo: comparison with LFA-1 and MAC-1. J. Immunol. 1989;143:3318–3324. [PubMed] [Google Scholar]
- 14.Kishimoto T.K., Jutila M.A., Butcher E.C. Neutrophil Mac-1 and MEL-14 adhesion proteins inversely regulated by chemotactic factors. Science. 1989;245:1238–1241. doi: 10.1126/science.2551036. [DOI] [PubMed] [Google Scholar]
- 15.Kishimoto T.K., Jutila M.A., Butcher E.C. Identification of a human peripheral lymph node homing receptor: a rapidly down-regulated adhesion molecule. Proc. Natl. Acad. Sci. USA. 1990;87:2244–2248. doi: 10.1073/pnas.87.6.2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wenzel-Seifert K., Hurt C.M., Seifert R. High constitutive activity of the human formyl peptide receptor. J. Biol. Chem. 1998;273:24181–24189. doi: 10.1074/jbc.273.37.24181. [DOI] [PubMed] [Google Scholar]
- 17.Wenzel-Seifert K., Arthur J.M., Seifert R. Quantitative analysis of formyl peptide receptor coupling to giα1, giα2, and giα3. J. Biol. Chem. 1999;274:33259–33266. doi: 10.1074/jbc.274.47.33259. [DOI] [PubMed] [Google Scholar]
- 18.Moazzam F., DeLano F.A., Schmid-Schönbein G.W. The leukocyte response to fluid stress. Proc. Natl. Acad. Sci. USA. 1997;94:5338–5343. doi: 10.1073/pnas.94.10.5338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Makino A., Prossnitz E.R., Schmid-Schönbein G.W. G protein-coupled receptors serve as mechanosensors for fluid shear stress in neutrophils. Am. J. Physiol. Cell Physiol. 2006;290:C1633–C1639. doi: 10.1152/ajpcell.00576.2005. [DOI] [PubMed] [Google Scholar]
- 20.Makino A., Shin H.Y., Schmid-Schönbein G.W. Mechanotransduction in leukocyte activation: a review. Biorheology. 2007;44:221–249. [PubMed] [Google Scholar]
- 21.Dewitz T.S., Hung T.C., McIntire L.V. Mechanical trauma in leukocytes. J. Lab. Clin. Med. 1977;90:728–736. [PubMed] [Google Scholar]
- 22.Fukuda S., Schmid-Schönbein G.W. Centrifugation attenuates the fluid shear response of circulating leukocytes. J. Leukoc. Biol. 2002;72:133–139. [PubMed] [Google Scholar]
- 23.Fukuda S., Mitsuoka H., Schmid-Schönbein G.W. Leukocyte fluid shear response in the presence of glucocorticoid. J. Leukoc. Biol. 2004;75:664–670. doi: 10.1189/jlb.1003464. [DOI] [PubMed] [Google Scholar]
- 24.Coughlin M.F., Schmid-Schönbein G.W. Pseudopod projection and cell spreading of passive leukocytes in response to fluid shear stress. Biophys. J. 2004;87:2035–2042. doi: 10.1529/biophysj.104.042192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fukuda S., Yasu T., Schmid-Schönbein G.W. Mechanisms for regulation of fluid shear stress response in circulating leukocytes. Circ. Res. 2000;86:E13–E18. doi: 10.1161/01.res.86.1.e13. [DOI] [PubMed] [Google Scholar]
- 26.Middleton J., Neil S., Rot A. Transcytosis and surface presentation of IL-8 by venular endothelial cells. Cell. 1997;91:385–395. doi: 10.1016/s0092-8674(00)80422-5. [DOI] [PubMed] [Google Scholar]
- 27.Rot A. Neutrophil attractant/activation protein-1 (interleukin-8) induces in vitro neutrophil migration by haptotactic mechanism. Eur. J. Immunol. 1993;23:303–306. doi: 10.1002/eji.1830230150. [DOI] [PubMed] [Google Scholar]
- 28.Tanaka Y., Adams D.H., Shaw S. Proteoglycans on endothelial cells present adhesion-inducing cytokines to leukocytes. Immunol. Today. 1993;14:111–115. doi: 10.1016/0167-5699(93)90209-4. [DOI] [PubMed] [Google Scholar]
- 29.Hall D.A., Beresford I.J.M., Giles H. Signalling by CXC-chemokine receptors 1 and 2 expressed in CHO cells: a comparison of calcium mobilization, inhibition of adenylyl cyclase and stimulation of GTPγS binding induced by IL-8 and GROα. Br. J. Pharmacol. 1999;126:810–818. doi: 10.1038/sj.bjp.0702329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Seifert R., Wenzel-Seifert K. Constitutive activity of G-protein-coupled receptors: cause of disease and common property of wild-type receptors. Naunyn Schmiedebergs Arch. Pharmacol. 2002;366:381–416. doi: 10.1007/s00210-002-0588-0. [DOI] [PubMed] [Google Scholar]
- 31.Burger M., Burger J.A., Schraufstatter I.U. Point mutation causing constitutive signaling of CXCR2 leads to transforming activity similar to Kaposi's sarcoma herpesvirus-G protein-coupled receptor. J. Immunol. 1999;163:2017–2022. [PubMed] [Google Scholar]
- 32.Chen G., Way J., Kenakin T. Use of constitutive G protein-coupled receptor activity for drug discovery. Mol. Pharmacol. 2000;57:125–134. [PubMed] [Google Scholar]
- 33.Kim M.B., Sarelius I.H. Distributions of wall shear stress in venular convergences of mouse cremaster muscle. Microcirculation. 2003;10:167–178. doi: 10.1038/sj.mn.7800182. [DOI] [PubMed] [Google Scholar]
- 34.Zhelev D.V., Alteraifi A.M., Chodniewicz D. Controlled pseudopod extension of human neutrophils stimulated with different chemoattractants. Biophys. J. 2004;87:688–695. doi: 10.1529/biophysj.103.036699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Finger E.B., Puri K.D., Springer T.A. Adhesion through L-selectin requires a threshold hydrodynamic shear. Nature. 1996;379:266–269. doi: 10.1038/379266a0. [DOI] [PubMed] [Google Scholar]
- 36.Fukuda S., Schmid-Schönbein G.W. Regulation of CD18 expression on neutrophils in response to fluid shear stress. Proc. Natl. Acad. Sci. USA. 2003;100:13152–13157. doi: 10.1073/pnas.2336130100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee D., Schultz J.B., King M.R. Mechanical shedding of L-selectin from the neutrophil surface during rolling on sialyl Lewis x under flow. J. Biol. Chem. 2007;282:4812–4820. doi: 10.1074/jbc.M609994200. [DOI] [PubMed] [Google Scholar]
- 38.Su S.S., Schmid-Schönbein G.W. Fluid stresses on the membrane of migrating leukocytes. Ann. Biomed. Eng. 2008;36:298–307. doi: 10.1007/s10439-007-9406-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schmid-Schönbein G.W., Shih Y.Y., Chien S. Morphometry of human leukocytes. Blood. 1980;56:866–875. [PubMed] [Google Scholar]
- 40.Pillay J., den Braber I., Koenderman L. In vivo labeling with 2H2O reveals a human neutrophil lifespan of 5.4 days. Blood. 2010;116:625–627. doi: 10.1182/blood-2010-01-259028. [DOI] [PubMed] [Google Scholar]
- 41.Chen A.Y., DeLano F.A., Schmid-Schönbein G.W. Receptor cleavage reduces the fluid shear response in neutrophils of the spontaneously hypertensive rat. Am. J. Physiol. Cell Physiol. 2010;299:C1441–C1449. doi: 10.1152/ajpcell.00157.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ali H., Richardson R.M., Snyderman R. Chemoattractant receptor cross-desensitization. J. Biol. Chem. 1999;274:6027–6030. doi: 10.1074/jbc.274.10.6027. [DOI] [PubMed] [Google Scholar]
- 43.Lefkowitz R.J. G protein-coupled receptors. III. New roles for receptor kinases and β-arrestins in receptor signaling and desensitization. J. Biol. Chem. 1998;273:18677–18680. doi: 10.1074/jbc.273.30.18677. [DOI] [PubMed] [Google Scholar]
- 44.Didsbury J.R., Uhing R.J., Snyderman R. Receptor class desensitization of leukocyte chemoattractant receptors. Proc. Natl. Acad. Sci. USA. 1991;88:11564–11568. doi: 10.1073/pnas.88.24.11564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Richardson R.M., Ali H., Snyderman R. Cross-desensitization of chemoattractant receptors occurs at multiple levels. Evidence for a role for inhibition of phospholipase C activity. J. Biol. Chem. 1995;270:27829–27833. doi: 10.1074/jbc.270.46.27829. [DOI] [PubMed] [Google Scholar]
- 46.Richardson R.M., Haribabu B., Snyderman R. Cross-desensitization among receptors for platelet activating factor and peptide chemoattractants. Evidence for independent regulatory pathways. J. Biol. Chem. 1996;271:28717–28724. doi: 10.1074/jbc.271.45.28717. [DOI] [PubMed] [Google Scholar]
- 47.Su S.S., Schmid-Schönbein G.W. Internalization of formyl peptide receptor in leukocytes subject to fluid stresses. Cell Mol. Bioeng. 2010;3:20–29. doi: 10.1007/s12195-010-0111-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sprague E.A., Steinbach B.L., Schwartz C.J. Influence of a laminar steady-state fluid-imposed wall shear stress on the binding, internalization, and degradation of low-density lipoproteins by cultured arterial endothelium. Circulation. 1987;76:648–656. doi: 10.1161/01.cir.76.3.648. [DOI] [PubMed] [Google Scholar]
- 49.Funamoto S., Meili R., Firtel R.A. Spatial and temporal regulation of 3-phosphoinositides by PI 3-kinase and PTEN mediates chemotaxis. Cell. 2002;109:611–623. doi: 10.1016/s0092-8674(02)00755-9. [DOI] [PubMed] [Google Scholar]
- 50.Huang Y.E., Iijima M., Devreotes P. Receptor-mediated regulation of PI3Ks confines PI(3,4,5)P3 to the leading edge of chemotaxing cells. Mol. Biol. Cell. 2003;14:1913–1922. doi: 10.1091/mbc.E02-10-0703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Merlot S., Firtel R.A. Leading the way: directional sensing through phosphatidylinositol 3-kinase and other signaling pathways. J. Cell Sci. 2003;116:3471–3478. doi: 10.1242/jcs.00703. [DOI] [PubMed] [Google Scholar]
- 52.Sasaki A.T., Chun C., Firtel R.A. Localized Ras signaling at the leading edge regulates PI3K, cell polarity, and directional cell movement. J. Cell Biol. 2004;167:505–518. doi: 10.1083/jcb.200406177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Heit B., Liu L., Kubes P. PI3K accelerates, but is not required for, neutrophil chemotaxis to fMLP. J. Cell Sci. 2008;121:205–214. doi: 10.1242/jcs.020412. [DOI] [PubMed] [Google Scholar]
- 54.Lauffenburger D., Linderman J. Oxford University Press; New York: 1993. Receptors: Models for Binding, Trafficking, and Signaling. [Google Scholar]
- 55.Sklar L.A., Finney D.A., Cochrane C.G. The dynamics of ligand-receptor interactions. Real-time analyses of association, dissociation, and internalization of an N-formyl peptide and its receptors on the human neutrophil. J. Biol. Chem. 1984;259:5661–5669. [PubMed] [Google Scholar]
- 56.Koo C., Lefkowitz R.J., Snyderman R. The oligopeptide chemotactic factor receptor on human polymorphonuclear leukocyte membranes exists in two affinity states. Biochem. Biophys. Res. Commun. 1982;106:442–449. doi: 10.1016/0006-291x(82)91130-5. [DOI] [PubMed] [Google Scholar]
- 57.Zigmond S.H., Sullivan S.J., Lauffenburger D.A. Kinetic analysis of chemotactic peptide receptor modulation. J. Cell Biol. 1982;92:34–43. doi: 10.1083/jcb.92.1.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


