Abstract
Human rhinovirus (HRV) is a nonenveloped, single stranded RNA virus belonging to the family Picornaviridae. HRV infections can cause both upper and lower respiratory illnesses in children and adults. Lower respiratory illnesses are more likely to occur in specific high risk groups, including infants, and children and adults with asthma. The relationships between rates of infection and the risk of clinical illness and exacerbation are not completely understood. Recent studies employing polymerase chain reaction and other molecular techniques indicate that there are new branches on the HRV family tree, and one characteristic of recently detected viruses is that they cannot be detected by standard tissue culture. Here we review the current literature and discuss new advances in understanding the link between HRV and asthma.
Keywords: Asthma, human rhinovirus, infection
INTRODUCTION
Human rhinovirus (HRV) was first isolated in 1956 from a patient with a common cold. Over the next 50 years, a total of 100 serotypes were defined using viral culture techniques.1 In the past 20 years, the development of more sensitive nucleic-acid based diagnosis methods has led to new insights into the genetic diversity of HRV, and the role of these viruses in respiratory disease. For example, various studies beginning in 2006 have detected multiple members of a whole new species of HRV.1 In addition, HRV infections were found to be closely related to wheezing illnesses in infants and young children,2,3 and exacerbations of chronic respiratory diseases such as asthma, chronic obstructive pulmonary diseases, and cystic fibrosis.4,5 Despite the extensive clinical evidence linking HRV infection to lower respiratory illness, the precise role of HRV as a lower airway pathogen is still controversial, mainly because HRV are so often recovered in individuals who have very mild illness or are asymptomatic. In this review, new advances in understanding the link between HRV and asthma will be discussed.
PROPERTIES OF HRVS
HRV are 7.2 kb positive-sense single stranded RNA viruses that belong to the Picornaviridae family. 'Pico' is the Latin root for 'small', and so the literal translation of Pico-rna-viridae is "small-RNA-virus". Although the previous classification system listed a separate Rhinovirus genus, the revised classification system has HRV as three separate species in the Enterovirusgenus.6,7 There are more than 100 classical HRV serotypes that were detected initially by culture, and these include the closely related viruses HRV-1A and HRV-1B, HRV-2 to HRV-100 and HRV-Hanks.8
The capsid of HRV is an icosahedron that is formed by 4 capsid proteins: VP1, VP2, VP3, and VP4. HRV are now classified into A and B species viruses according to the nucleotide sequence that encodes the VP4/VP2 capsid protein. The first HRV-C species virus was identified in 2006 by Arden et al.1, and close to 50 members of this species have been defined based on partial genetic sequencing. Epidemiologic studies indicate that HRV-C viruses are often detected in infants and young children with symptoms of lower respiratory illness,6,7,9 and a study of Australian children suggests that HRV-C viruses are found more often than other HRV in children with exacerbations of asthma.10 These findings raise the possibility that HRV-C may be more virulent than other HRV. As additional full length sequence information becomes available, it is possible that some viruses may be classified as a separate HRV-D species based on genetic differences.8 HRV can also be grouped according to the 'receptor' that is used by HRV to invade host cells. More than 90% of the HRV use human intercellular adhesion molecule (ICAM-1, CD54) as their receptor and are known as 'major group' viruses (e.g. HRV-A16).11 Approximately 10% of the RVs bind to members of the low-density lipoprotein receptor (LDLR) family, and are known as 'minor group' viruses (e.g. HRV-A1). Notably, HRV-C types bind to a distinct receptor that has not yet been identified.6,8
Recently, complete genome sequences were determined for canonical HRV-A and -B species viruses from the American Type Culture Collection (ATCC), as well as several field isolates.11 These sequences were used to draw a phylogenetic tree for HRV, and also provided new insights into similarities and differences among the three HRV species. For example, there were species-specific sequences in a pyrimidine-rich region located next to the cloverleaf structure in the 5'-untranslated region (5'UTR). In addition, there was clear evidence that some HRV genomes were the product of recombination between two parental HRV types. Continued work on HRV genomics may provide new insights into mechanisms of pathogenicity, and possible identify new targets for medical therapies.6
EPIDEMIOLOGY OF HRV INFECTION
HRV infection is the most common cause of respiratory illness in children and adults of all ages.12 HRV infections have long been known to occur most frequently during the spring and autumn seasons.13 A recent study obtained weekly samples from children during several seasons, and found that HRV infections continue to be quite common in the summer and winter seasons.12 HRV infections also can be asymptomatic; a review of several studies reveals that 12%-22% of samples acquired from asymptomatic children were positive for HRV,4,14 and rates of asymptomatic infection can be even higher in young infants.15 Similar findings have been reported for seronegative adult volunteers after experimental inoculation with HRV-A16.10 These studies also demonstrate that HRV infections have a relatively short latency period (1-3 days) between inoculation and the onset of symptoms. Most HRV illnesses last 3-7 days, and the intensity of viral shedding roughly correlates with the intensity of symptoms. Low level shedding of HRV can continue for 1-2 weeks after symptom resolution, and even longer in some infants.16 HRV does not cause chronic infection in immune competent individuals.
MOUSE MODEL OF HRV INFECTION
Until recently, it was generally accepted that HRV infections were limited to the chimpanzee and human, and did not occur in the mouse. The reason for this species limitation is that HRV does not bind to murine ICAM-1. In contrast, minor group HRV can bind to murine LDLR, but replication was found to be very limited unless the mouse was immunosuppressed. An improved mouse model of HRV infection was reported in 2008.17 Bartlett et al.17 used a transgenic mouse that expressed human ICAM-1, and successfully produced HRV infections that mimicked many features of human HRV infection. In addition, infection of mice with the minor-group virus HRV-1B was also achieved by increasing the viral inoculum.17 In the mouse, HRV infection induced respiratory tract neutrophilic inflammation, and production of interferons and neutrophil chemokines. Viral replication was also documented, although the duration is short (24 hours or less) compared to human infection. Additional studies have demonstrated that in animals sensitized to ovalbumin, HRV infection augmented eosinophilic airway inflammation, increased production of Th2 cytokines, and induced airway hyperreactivity.17 Although the mouse model has some limitations, these early findings suggest that it may be helpful in defining HRV pathogenesis and mechanisms for HRV-induced exacerbations of asthma.
HRV-INDUCED ANTIVIRAL AND INFLAMMATORY RESPONSES
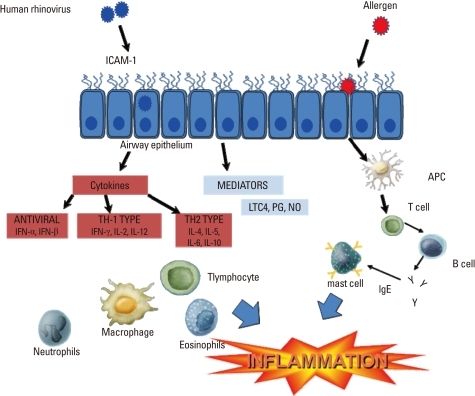
Cells recruited to the airway are activated by the infection and secrete a variety of cytokines and mediators that may have antiviral effects, but also contribute to airway inflammation and respiratory symptoms.18,19 Activated macrophages can secrete IFN-α, IFN-γ, TNF-α, MIP-1α, IL-1, and IL-8. Neutrophil numbers increase rapidly during the acute phase of a cold, and these cells secrete elastase, cathepsin G, and neutrophil proteinase 3. Eosinophils generally do not increase in number during the acute cold, but increases in eosinophils have been reported during recovery. Notably, eosinophilic inflammation has been linked to an increased risk of virus-induced exacerbation, and increased severity of experimentally-induced colds (Figure).10,20
Figure.
Mechanisms of rhinovirus-induced airway inflammation. Human rhinovirus binds to ICAM-1 and other receptors to begin the replication cycle. Viral infections induce a variety of mediators, cytokines, and chemokines from epithelial cells and airway leukocytes that initiate an inflammatory response, including chemotaxis of neutrophils, and eosinophils. An antiviral response is also mounted by both epithelial and dendritic cells, producing type I interferons (IFNs). Airway eosinophils and allergic sensitization are risk factors for more severe rhinovirus illnesses, possibly by suppressing antiviral responses, which may be deficient in asthma.
ICAM, intercellular adhesion molecule; IL, interleukin; LTC4, leukotriene C4; PG, prostaglandins; NO, nitric oxide.
HRV is recognized by several pattern recognition receptors, including toll-like receptor (TLR)-3, TLR-7/8, retinoic acid inducible gene 1 protein (RIG-1) and melanoma differentiation-associated gene 5 (MDA-5).18 Following binding to receptors on the cell membrane, HRV is internalized into endosomes, and then releases RNA into the cell cytoplasm in a process known as "uncoating". RNA is recognized through several different pathways. TLR-7 and TLR-8 are expressed on the endosomal membrane and bind single-stranded RNA. These receptors activate several intracellular signaling proteins including myeloid differentiation primary response gene-88, and a cascade of proteins including TNF receptor associated factor (TRAF)-6, interleukin-1 receptor-associated kinase (IRAK)-1, IRAK-4 and interferon regulatory factor (IRF)-7.21,22 In addition, double-stranded RNA produced during viral replication can activate TLR-3 on the endosomal membrane, leading to activation of TRIF, IKKγ, IKKβ, IKKα, and ultimately NF-κB. In the cytoplasm, HRV RNA binds to and activates RIG-1 or by MDA-5. These "RNA helicases" set off a similar signaling cascade including MAVS, IKKγ, IKKβ, IKKα, TBK-1/IKKε, and IRF-3. The net result of activation of RNA-sensing proteins is the production of interferons and other effector molecules with antiviral properties. HRV-infected epithelial cells can also secrete a variety of acute phase cytokines (e.g. TNF-α, IL-6), chemokines (e.g. IL-8, RANTES, MIP-1α, and IP-10) and growth factors (G-CSF, GM-CSF) that initiate the respiratory tract inflammatory response, and recruit and activate cellular inflammatory responses.21,22
ROLES OF HRV IN THE DEVELOPMENT OF ASTHMA
More severe viral illnesses in infancy have been linked to increased risk of developing asthma. For example, children who were diagnosed with lower respiratory tract infection by a physician before 3 years old were more likely to have asthma between 6-11 years of age.23,24 More recent studies indicate that the etiology of wheezing illnesses can provide additional information about asthma risk. Respiratory syncytial virus (RSV) is the most common cause of bronchiolitis, and it has recently been appreciated that HRV infections are the second most common etiology. Other viruses that also are associated with wheezing illnesses include coronaviruses, parainfluenzaviruses, metapneumoviruses, adenoviruses, and infections with multiple viruses. Long term follow-up of infants with bronchiolitis provided evidence that bronchiolitis caused by HRV infections was associated with a greater risk of wheeze compared to RSV bronchiolitis,25 and this association was confirmed in two ongoing birth cohort studies.25,26 In the Childhood Origins of ASThma (COAST) cohort study, HRV wheezing illnesses in the first three years was associated with a significantly greater risk for asthma at age 6 (odd ratio [OR]=9.8) compared to infants who wheezed with RSV (OR=2.6).26 In the Childhood Asthma Study (CAS) conducted in Perth Australia, the risk of asthma at the age of 5 was increased for infants who developed wheezing illnesses with either HRV or RSV. The risk of developing asthma was greatest for infants who wheezed and also developed allergen sensitization before 2 years of age.27 These findings provide convincing evidence that wheezing with HRV infections in infancy is a strong risk factor for asthma, and in addition, allergic sensitization and viral wheezing both contribute to asthma risk.
Whether viral infections actually cause asthma is still hotly debated. It is clear that some risk factors for viral wheezing are also risk factors for asthma. For example, low lung function in infancy has been linked to both of these outcomes. On the other hand, recurrent viral lower respiratory illness in infancy could damage the lower airways, and the ensuing inflammatory and repair processes could lead to remodeling of airway structures to promote chronic airway obstruction and asthma.28-30 Studies are underway to better delineate risk factors for viral wheezing and the relationship of acute viral wheeze to chronic changes in airway structure and function.31
HRV AND EXACERBATIONS OF ASTHMA
HRV-induced interferon responses are important contributors to the early innate response to infection. In asthma, HRV-induced secretion of type I and III IFN secretion may be impaired, leading to more severe infection and a more prolonged inflammatory response.32,33 During exacerbations, there is also evidence that neutrophilic responses to viral infection may be enhanced,34 especially in the lower airways. Increased severity of infection, or a greater inflammatory response to infection, could ultimately lead to airway edema and respiratory smooth muscle contraction which consequently aggravate asthma.17 In addition, there is epidemiologic evidence that synergistic interactions between allergy and viral infection promote wheeze,35,36 and several mechanisms have been proposed to explain this effect. For example, the Th2 cytokine IL-4 greatly enhances HRV-induced secretion of thymic stromal lymphopoietin in cultured epithelial cells.37 This cytokine has been linked to the amplification of allergic inflammatory responses. Moreover, engagement of high affinity IgE receptors on plasmacytoid dendritic cells inhibits the ability of these cells to produce interferons in response to respiratory viruses.38 These experimental findings provide plausible mechanisms for interactions between viral infections and allergic mechanisms in the pathogenesis of HRV-induced wheezing illnesses.
TREATMENT OF VIRUS INDUCED ASTHMA
Medications including first generation antihistamines and nonsteroidal anti-inflammatory agents are commonly used to alleviate the symptoms of viral infection. Unfortunately, these agents do not decrease duration of symptoms or affect the natural history of cold symptoms.39,40 As discussed earlier in this review, HRV infections induce numerous cytokines and chemokines that cause airway inflammation and it is likely that the virus-induced inflammation contributes to cold symptoms.5,19 Based on these findings, studies have been conducted to determine whether anti-inflammatory medications can be used to lessen cold symptoms or virus-induced exacerbations of asthma.9,40,41 Corticosteroids administered either topically or systemically do not affect the course of illness produced by experimental infection with HRV. In contrast, there are in vitro data to suggest that the combination of fluticasone and salmeterol reduces virus-induced inflammatory responses, including neutrophil and lymphocyte chemokine secretion by airway epithelial cells.41,42
Treatment of infants with recurrent viral wheezing has yielded mixed results. Regular use of inhaled corticosteroid reduces daily symptoms and has modest effects on the number of wheezing episodes, although temporary slowing of growth has been observed.43,44 One study of infants hospitalized for wheezing found that infants with HRV infections had a distinct response to treatment with systemic corticosteroid.43 Compared to placebo, corticosteroid treatment had no beneficial effects noted during the acute illness, but children who were hospitalized with HRV infections and treated with corticosteroid were less likely to develop recurrent wheezing episodes. The latter effect was not observed in children who had RSV infections. In other reports montelukast prevented acute wheezing episodes in children during seasons of high viral prevalence.41,45,46 In children with moderate to severe asthma, the addition of omalizumab to standard asthma therapy reduced asthma exacerbations, including those associated with viral infections.47 Notably, omalizumab treatment eliminated the rise in spring and fall asthma exacerbations, which are often caused by infections with HRV. More clinical data are needed to clarify the utility of anti-inflammatory medications in the prevention or treatment of virus-induced wheezing episodes.
CONCLUSIONS
New findings from clinical research studies indicate that HRV infections are the most common cause of wheezing illnesses in children with asthma. There are also indications that recurrent HRV lower respiratory infections could promote the development of asthma in some infants. Advances in HRV genetics together with more sophisticated murine and in vitro models of infection raise have provided new insights into HRV molecular virology and host-virus interactions. Collectively, these new models are expected to contribute not only to understanding the pathogenesis of HRV-induced exacerbations of asthma, but also clarify the potential role for HRV infection as a risk factor for asthma development.
ACKNOWLEDGMENTS
This study was supported by NIH grants U19 AI070503-01, P01 HL070831, and HHSN272200900052C.
Footnotes
There are no financial or other issues that might lead to conflict of interest.
References
- 1.Arden KE, McErlean P, Nissen MD, Sloots TP, Mackay IM. Frequent detection of human rhinoviruses, paramyxoviruses, coronaviruses, and bocavirus during acute respiratory tract infections. J Med Virol. 2006;78:1232–1240. doi: 10.1002/jmv.20689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Garbino J, Gerbase MW, Wunderli W, Deffernez C, Thomas Y, Rochat T, Ninet B, Schrenzel J, Yerly S, Perrin L, Soccal PM, Nicod L, Kaiser L. Lower respiratory viral illnesses: improved diagnosis by molecular methods and clinical impact. Am J Respir Crit Care Med. 2004;170:1197–1203. doi: 10.1164/rccm.200406-781OC. [DOI] [PubMed] [Google Scholar]
- 3.Lee BE, Robinson JL, Khurana V, Pang XL, Preiksaitis JK, Fox JD. Enhanced identification of viral and atypical bacterial pathogens in lower respiratory tract samples with nucleic acid amplification tests. J Med Virol. 2006;78:702–710. doi: 10.1002/jmv.20595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Johnston SL, Sanderson G, Pattemore PK, Smith S, Bardin PG, Bruce CB, Lambden PR, Tyrrell DA, Holgate ST. Use of polymerase chain reaction for diagnosis of picornavirus infection in subjects with and without respiratory symptoms. J Clin Microbiol. 1993;31:111–117. doi: 10.1128/jcm.31.1.111-117.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tan WC. Viruses in asthma exacerbations. Curr Opin Pulm Med. 2005;11:21–26. doi: 10.1097/01.mcp.0000146781.11092.0d. [DOI] [PubMed] [Google Scholar]
- 6.Palmenberg AC, Rathe JA, Liggett SB. Analysis of the complete genome sequences of human rhinovirus. J Allergy Clin Immunol. 2010;125:1190–1199. doi: 10.1016/j.jaci.2010.04.010. quiz 200-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arden KE, Mackay IM. Newly identified human rhinoviruses: molecular methods heat up the cold viruses. Rev Med Virol. 2010;20:156–176. doi: 10.1002/rmv.644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bartlett NW, Johnston SL. Rhinoviruses. In: Mahy BWJ, editor. Encyclopedia of virology. Oxford: Elsevier; 2008. pp. 467–475. [Google Scholar]
- 9.Brownlee JW, Turner RB. New developments in the epidemiology and clinical spectrum of rhinovirus infections. Curr Opin Pediatr. 2008;20:67–71. doi: 10.1097/MOP.0b013e3282f41cb6. [DOI] [PubMed] [Google Scholar]
- 10.DeMore JP, Weisshaar EH, Vrtis RF, Swenson CA, Evans MD, Morin A, Hazel E, Bork JA, Kakumanu S, Sorkness R, Busse WW, Gern JE. Similar colds in subjects with allergic asthma and nonatopic subjects after inoculation with rhinovirus-16. J Allergy Clin Immunol. 2009;124:245–252. 52.e1–52.e3. doi: 10.1016/j.jaci.2009.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee WM, Wang W. Human rhinovirus type 16: mutant V1210A requires capsid-binding drug for assembly of pentamers to form virions during morphogenesis. J Virol. 2003;77:6235–6244. doi: 10.1128/JVI.77.11.6235-6244.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Winther B, Hayden FG, Hendley JO. Picornavirus infections in children diagnosed by RT-PCR during longitudinal surveillance with weekly sampling: Association with symptomatic illness and effect of season. J Med Virol. 2006;78:644–650. doi: 10.1002/jmv.20588. [DOI] [PubMed] [Google Scholar]
- 13.Gwaltney JM, Jr, Hendley JO, Simon G, Jordan WS., Jr Rhinovirus infections in an industrial population. I. The occurrence of illness. N Engl J Med. 1966;275:1261–1268. doi: 10.1056/NEJM196612082752301. [DOI] [PubMed] [Google Scholar]
- 14.van Benten I, Koopman L, Niesters B, Hop W, van Middelkoop B, de Waal L, van Drunen K, Osterhaus A, Neijens H, Fokkens W. Predominance of rhinovirus in the nose of symptomatic and asymptomatic infants. Pediatr Allergy Immunol. 2003;14:363–370. doi: 10.1034/j.1399-3038.2003.00064.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lemanske RF, Jr, Jackson DJ, Gangnon RE, Evans MD, Li Z, Shult PA, Kirk CJ, Reisdorf E, Roberg KA, Anderson EL, Carlson-Dakes KT, Adler KJ, Gilbertson-White S, Pappas TE, Dasilva DF, Tisler CJ, Gern JE. Rhinovirus illnesses during infancy predict subsequent childhood wheezing. J Allergy Clin Immunol. 2005;116:571–577. doi: 10.1016/j.jaci.2005.06.024. [DOI] [PubMed] [Google Scholar]
- 16.Jartti T, Lehtinen P, Vuorinen T, Koskenvuo M, Ruuskanen O. Persistence of rhinovirus and enterovirus RNA after acute respiratory illness in children. J Med Virol. 2004;72:695–699. doi: 10.1002/jmv.20027. [DOI] [PubMed] [Google Scholar]
- 17.Bartlett NW, Walton RP, Edwards MR, Aniscenko J, Caramori G, Zhu J, Glanville N, Choy KJ, Jourdan P, Burnet J, Tuthill TJ, Pedrick MS, Hurle MJ, Plumpton C, Sharp NA, Bussell JN, Swallow DM, Schwarze J, Guy B, Almond JW, Jeffery PK, Lloyd CM, Papi A, Killington RA, Rowlands DJ, Blair ED, Clarke NJ, Johnston SL. Mouse models of rhinovirus-induced disease and exacerbation of allergic airway inflammation. Nat Med. 2008;14:199–204. doi: 10.1038/nm1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baum A, García-Sastre A. Induction of type I interferon by RNA viruses: cellular receptors and their substrates. Amino Acids. 2010;38:1283–1299. doi: 10.1007/s00726-009-0374-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kelly JT, Busse WW. Host immune responses to rhinovirus: mechanisms in asthma. J Allergy Clin Immunol. 2008;122:671–682. doi: 10.1016/j.jaci.2008.08.013. quiz 83-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rakes GP, Arruda E, Ingram JM, Hoover GE, Zambrano JC, Hayden FG, Platts-Mills TA, Heymann PW. Rhinovirus and respiratory syncytial virus in wheezing children requiring emergency care. IgE and eosinophil analyses. Am J Respir Crit Care Med. 1999;159:785–790. doi: 10.1164/ajrccm.159.3.9801052. [DOI] [PubMed] [Google Scholar]
- 21.Lau C, Wang X, Song L, North M, Wiehler S, Proud D, Chow CW. Syk associates with clathrin and mediates phosphatidylinositol 3-kinase activation during human rhinovirus internalization. J Immunol. 2008;180:870–880. doi: 10.4049/jimmunol.180.2.870. [DOI] [PubMed] [Google Scholar]
- 22.Nurani G, Lindqvist B, Casasnovas JM. Receptor priming of major group human rhinoviruses for uncoating and entry at mild low-pH environments. J Virol. 2003;77:11985–11991. doi: 10.1128/JVI.77.22.11985-11991.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gern JE. Rhinovirus respiratory infections and asthma. Am J Med. 2002;112(Suppl 6A):19S–27S. doi: 10.1016/s0002-9343(01)01060-9. [DOI] [PubMed] [Google Scholar]
- 24.Lemanske RF. Viral infections and asthma inception. J Allergy Clin Immunol. 2004;114:1023–1026. doi: 10.1016/j.jaci.2004.08.031. [DOI] [PubMed] [Google Scholar]
- 25.Kotaniemi-Syrjänen A, Vainionpää R, Reijonen TM, Waris M, Korhonen K, Korppi M. Rhinovirus-induced wheezing in infancy--the first sign of childhood asthma. J Allergy Clin Immunol. 2003;111:66–71. doi: 10.1067/mai.2003.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jackson DJ, Gangnon RE, Evans MD, Roberg KA, Anderson EL, Pappas TE, Printz MC, Lee WM, Shult PA, Reisdorf E, Carlson-Dakes KT, Salazar LP, DaSilva DF, Tisler CJ, Gern JE, Lemanske RF., Jr Wheezing rhinovirus illnesses in early life predict asthma development in high-risk children. Am J Respir Crit Care Med. 2008;178:667–672. doi: 10.1164/rccm.200802-309OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kusel MM, de Klerk NH, Kebadze T, Vohma V, Holt PG, Johnston SL, Sly PD. Early-life respiratory viral infections, atopic sensitization, and risk of subsequent development of persistent asthma. J Allergy Clin Immunol. 2007;119:1105–1110. doi: 10.1016/j.jaci.2006.12.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gern JE. Rhinovirus and the initiation of asthma. Curr Opin Allergy Clin Immunol. 2009;9:73–78. doi: 10.1097/ACI.0b013e32831f8f1b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jackson DJ. The role of rhinovirus infections in the development of early childhood asthma. Curr Opin Allergy Clin Immunol. 2010;10:133–138. doi: 10.1097/ACI.0b013e3283352f7c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sly PD, Kusel M, Holt PG. Do early-life viral infections cause asthma? J Allergy Clin Immunol. 2010;125:1202–1205. doi: 10.1016/j.jaci.2010.01.024. [DOI] [PubMed] [Google Scholar]
- 31.Bartlett NW, McLean GR, Chang YS, Johnston SL. Genetics and epidemiology: asthma and infection. Curr Opin Allergy Clin Immunol. 2009;9:395–400. doi: 10.1097/ACI.0b013e32833066fa. [DOI] [PubMed] [Google Scholar]
- 32.Contoli M, Message SD, Laza-Stanca V, Edwards MR, Wark PA, Bartlett NW, Kebadze T, Mallia P, Stanciu LA, Parker HL, Slater L, Lewis-Antes A, Kon OM, Holgate ST, Davies DE, Kotenko SV, Papi A, Johnston SL. Role of deficient type III interferon-lambda production in asthma exacerbations. Nat Med. 2006;12:1023–1026. doi: 10.1038/nm1462. [DOI] [PubMed] [Google Scholar]
- 33.Wark PA, Johnston SL, Bucchieri F, Powell R, Puddicombe S, Laza-Stanca V, Holgate ST, Davies DE. Asthmatic bronchial epithelial cells have a deficient innate immune response to infection with rhinovirus. J Exp Med. 2005;201:937–947. doi: 10.1084/jem.20041901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Denlinger LC, Sorkness RL, Lee WM, Evans M, Wolff M, Mathur S, Crisafi G, Gaworski K, Pappas TE, Vrtis R, Kelly EA, Gern JE, Jarjour NN. Lower Airway Rhinovirus Burden and the Seasonal Risk of Asthma Exacerbation. Am J Respir Crit Care Med. 2011 doi: 10.1164/rccm.201103-0585OC. Forthcoming. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Olenec JP, Kim WK, Lee WM, Vang F, Pappas TE, Salazar LE, Evans MD, Bork J, Roberg K, Lemanske RF, Jr, Gern JE. Weekly monitoring of children with asthma for infections and illness during common cold seasons. J Allergy Clin Immunol. 2010;125:1001–1006.e1. doi: 10.1016/j.jaci.2010.01.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sly PD, Boner AL, Björksten B, Bush A, Custovic A, Eigenmann PA, Gern JE, Gerritsen J, Hamelmann E, Helms PJ, Lemanske RF, Martinez F, Pedersen S, Renz H, Sampson H, von Mutius E, Wahn U, Holt PG. Early identification of atopy in the prediction of persistent asthma in children. Lancet. 2008;372:1100–1106. doi: 10.1016/S0140-6736(08)61451-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kato A, Favoreto S, Jr, Avila PC, Schleimer RP. TLR3- and Th2 cytokine-dependent production of thymic stromal lymphopoietin in human airway epithelial cells. J Immunol. 2007;179:1080–1087. doi: 10.4049/jimmunol.179.2.1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gill MA, Bajwa G, George TA, Dong CC, Dougherty II, Jiang N, Gan VN, Gruchalla RS. Counterregulation between the FcepsilonRI pathway and antiviral responses in human plasmacytoid dendritic cells. J Immunol. 2010;184:5999–6006. doi: 10.4049/jimmunol.0901194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bisgaard H, Hermansen MN, Loland L, Halkjaer LB, Buchvald F. Intermittent inhaled corticosteroids in infants with episodic wheezing. N Engl J Med. 2006;354:1998–2005. doi: 10.1056/NEJMoa054692. [DOI] [PubMed] [Google Scholar]
- 40.Hansbro NG, Horvat JC, Wark PA, Hansbro PM. Understanding the mechanisms of viral induced asthma: new therapeutic directions. Pharmacol Ther. 2008;117:313–353. doi: 10.1016/j.pharmthera.2007.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cheuk DK, Tang IW, Chan KH, Woo PC, Peiris MJ, Chiu SS. Rhinovirus infection in hospitalized children in Hong Kong: a prospective study. Pediatr Infect Dis J. 2007;26:995–1000. [Google Scholar]
- 42.Edwards MR, Johnson MW, Johnston SL. Combination therapy: Synergistic suppression of virus-induced chemokines in airway epithelial cells. Am J Respir Cell Mol Biol. 2006;34:616–624. doi: 10.1165/rcmb.2005-0385OC. [DOI] [PubMed] [Google Scholar]
- 43.Jartti T, Lehtinen P, Vanto T, Vuorinen T, Hiekkanen H, Hartiala J, Mäkelä MJ, Ruuskanen O. Atopic characteristics of wheezing children and responses to prednisolone. Pediatr Pulmonol. 2007;42:1125–1133. doi: 10.1002/ppul.20706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Miller JL. Inhaled corticosteroids may cause only temporary slowing of growth in children, studies suggest. Am J Health Syst Pharm. 2000;57:2142, 2149. doi: 10.1093/ajhp/57.23.2142. [DOI] [PubMed] [Google Scholar]
- 45.Bisgaard H Study Group on Montelukast and Respiratory Syncytial Virus. A randomized trial of montelukast in respiratory syncytial virus postbronchiolitis. Am J Respir Crit Care Med. 2003;167:379–383. doi: 10.1164/rccm.200207-747OC. [DOI] [PubMed] [Google Scholar]
- 46.Bisgaard H, Zielen S, Garcia-Garcia ML, Johnston SL, Gilles L, Menten J, Tozzi CA, Polos P. Montelukast reduces asthma exacerbations in 2- to 5-year-old children with intermittent asthma. Am J Respir Crit Care Med. 2005;171:315–322. doi: 10.1164/rccm.200407-894OC. [DOI] [PubMed] [Google Scholar]
- 47.Busse WW, Morgan WJ, Gergen PJ, Mitchell HE, Gern JE, Liu AH, Gruchalla RS, Kattan M, Teach SJ, Pongracic JA, Chmiel JF, Steinbach SF, Calatroni A, Togias A, Thompson KM, Szefler SJ, Sorkness CA. Randomized trial of omalizumab (anti-IgE) for asthma in inner-city children. N Engl J Med. 2011;364:1005–1015. doi: 10.1056/NEJMoa1009705. [DOI] [PMC free article] [PubMed] [Google Scholar]