Abstract
In order to test if nestin is a useful marker for various types of progenitor cells, we explored nestin expression in the retina during development. Nestin expression was co-evaluated with bromodeoxyuridine (BrdU) labeling and Griffonia simplicifolia isolectin B4 (GSIB4) histochemistry. Nestin immunoreactivity appears in cell soma of dividing neural progenitor cells and their leading processes in retinas from embryonic day (E) 13 to E20, in accordance with a BrdU-labeled pattern. At postnatal day (P) 5, it is restricted to the end feet of Müller cells. BrdU-labeled nuclei were mainly in the inner part of the inner nuclear layer in postnatal neonates. The retinal vessels demarcated with GSIB4-positive endothelial cells were first distributed in the nerve fiber layer from P3. Afterward the vascular branches sprouted and penetrated deeply into the retina. The endothelial cells positive for GSIB4 and the pericytes in the microvessels were additionally immunoreactive for nestin. Interestingly, the presumed migrating microglial cells showing only GSIB4 reactivity preceded the microvessels throughout the neuroblast layer during vascular sprouting and extension. These findings may suggest that nestin expression represents the proliferation and movement potential of the neural progenitor cells as well as the progenitor cells of the endothelial cell and the pericyte during retinal development. Thus, Müller glial cells might be potential neural progenitor cells of the retina, and the retinal microvasculature established by both the endothelial and the pericyte progenitor cells via vasculogenesis along microglia migrating routes sustains its angiogenic potential.
Keywords: Neurogenesis, Vasculogenesis, Retina, Nestin, Proliferation, Migration
Introduction
The presence of neural stem and progenitor cells in the adult brain of vertebrates provides a hopeful prospect for the development of a strategy for neural regeneration in various incurable neurodegenerative diseases because of the latent neurogenic potency of these cells. The regions presenting the neural progenitor cells are restricted, e.g., the neuroepithelial lining of the cerebral ventricles and the hippocampal dentate gyrus are only two such areas [1-3]. In the mammalian brain, the neural progenitor cell characteristics appeared only in subependymal cells in the regions described above throughout adult life [4-6].
It has been proposed that the retina as an out-pocketing of the diencephalon also contains neural progenitor cells. In adult fish and mammalian eyes, the ciliary margin has formerly been accepted as a plausible reservoir of retinal neural progenitor cells [7]. This is because, developmentally, the ciliary margin is a transitional area from the neural retina to the ciliary body and the iris, even though the lining epithelium of these structures commonly originates from the neuroepithelium. Several lines of evidence regarding retinal stem or progenitor cells have persuasively indicated that Müller glial cells are potential retinal progenitor cells in various vertebrate retinas [8-14]. The basis for this hypothesis comes from the findings that Müller glial cells spanning the whole depth of the retina originally differentiated from radial glial cells responsible for neuronal proliferation and migration during retinal development [15]. This has also been confirmed by retina injury model and transgenic experiments. Müller cells dedifferentiate, proliferate, and produce new neurons and glia following retinal injury [13, 16-18]. On the other hand, according to the lineage tracing experiments of Bernardos et al. [14], in which transgenic zebra fish retina expressed low levels of the progenitor cell marker protein Pax6, and even proliferated at a low frequency in uninjured retina, it has been suggested that Müller glial cells reserve a latent neurogenic capacity rather than act as neuronal progenitors.
Recently, the possibility that pluripotent stem cells transdifferentiated into neural cells enlightens restorative therapeutic investigations and their clinical applications in the brain and spinal cord for the treatment of incurable neurodegenerative diseases [19-23]. In this context, neural progenitor cells have gained even more attention because of their utility as transplantable cellular vectors and targets for endogenous induction, for basic research and for clinical applications in diverse neurodegenerative diseases.
When considering endogenous induction by stem cell transplantation, progenitor cell typing might be preceded in the target organ. The present study has examined the progenitor cell typing in the developing retina by using a neural progenitor marker protein nestin [24-26]. The identity of nestin expressing progenitor cells and the functional role of nestin during retinal development were analyzed immunochemically. We aimed to provide the morphological and developmental horizons for the prospect that gene or cell engineering through in situ induction of the progenitor cells, or transplantation of the stem cells into injured retina, enable partial restoration or prevention from aggravated progression of various retinal disorders.
Materials and Methods
Animals
Ten litters of Sprague Dawley rats were used for the different developmental stages. All experimental procedures performed on the animals were conducted following the approval of the Catholic Ethics Committee of the Catholic University of Korea, conforming to the National Institute of Health Guidelines for the Care and Use of Laboratory Animals (NIH publication number 80-23, revised 1996).
BrdU injection
To analyze the pattern of retinal neural cell proliferation during development, 5'-bromo-2'-deoxyuridine (BrdU) was diluted in sterilized saline and injected at a dose of 5 mg/100 g body weight. For embryonic stages, BrdU was administered to pregnant mothers intraperitoneally three times per day on appropriate embryonic days (E) before sacrifice, and for postnatal stages, BrdU was administered to E18 mothers per day.
Tissue preparation
Four pregnant rats carrying litters of embryos at E13, E15, E18, and E20; pups at postnatal day (P) 0, P3, P5, P7, P14, P21, and P28 were anesthetized by an intraperitoneal injection of 4% chloral hydrate (ml/100 g body weight). The eyes were enucleated, and the animals were killed immediately by an overdose of 4% chloral hydrate. The eyes from the E13 and E15 embryos were prepared as a whole and the others' were then cut along the anterior border of the oraserrata. The posterior segments of the eyes were processed as follows.
Immunocytochemistry
The whole eyes (of embryos) and the posterior segments of the eyes were immersed in 4% paraformaldehyde in 0.1 M phosphate buffer, pH 7.4 (PB) for 30 minutes. The retinas were dissected out and placed in the same fixative for 2 hours at 4℃. The retinas were then washed through several changes of PB and transferred to 30% sucrose in PB for 5 hours at 4℃ for cryoprotection. They were quick-frozen in liquid nitrogen, thawed and rinsed in 0.01 M phosphate-buffered saline (PBS), pH 7.4. The retinas were embedded in 4% agar in PBS or in wax after dehydration. The retinas embedded in agar were cut at 50 µm with a vibratome and stored in PBS at 4℃, and those in wax were cut at 5 µm with a microtome and attached onto gelatin-coated slides for subsequent procedures. Prior to all the immunostaining, the retinal sections were first incubated in 10% normal donkey serum in PBS for 1 hour at room temperature to block nonspecific binding sites.
To trace nestin expression in neural components of the developing retina, sections were incubated with mouse monoclonal anti-nestin antibody (Chemicon, Billerica, MA, USA) diluted to 1 : 200 in PBS overnight at 4℃. After the incubation, the retinas were thoroughly rinsed in several changes of PBS for 45 minutes and incubated with Cy3-conjugated donkey anti-mouse IgG (1 : 1,000, Jackson ImmunoResearch Labs, Baltimore, PA, USA) in PBS for 2 hours at room temperature. The sections were rinsed in PB, attached to gelatin-coated slides and mounted in glycerol.
To check for nestin expression in other components of the developing retina, double-fluorescent staining was performed. The sections of retina processed for nestin immunofluorescent staining were subsequently treated with biotin-labeled Griffonia simplicifolia isolectin B4 (GSIB4; 1 : 50, Sigma, St. Louis, MO, USA) in PBS overnight at 4℃, thoroughly rinsed in PBS, and incubated in streptavidin-conjugated fluorescein isothiocyanate (FITC; 1 : 50, Jackson ImmunoResearch Labs) for 2 hours at room temperature.
To analyze cell proliferation in the developing neural retina, anti-BrdU immunostaining was performed in 5 µm thick wax sections using an avidin-biotin-peroxidase complex (ABC) method. The retinal sections were incubated with rat monoclonal anti-BrdU antibody (1 : 100, Accurate Chemical & Scientific, Westbury, NY, USA) in PBS for 2 hours at room temperature. After rinsing in four changes of PBS, the sections were incubated in biotin-labeled goat anti-rat IgG (1 : 100, Jackson ImmunoResearch Labs) for 1 hour at room temperature. After incubation, the sections were rinsed in PBS, and then incubated in ABC (Vector Laboratories, Burlingame, CA, USA) in PBS for 1 hour at room temperature. The retinal sections were then rinsed in two changes of PBS and three changes of 0.05 M Tris-HCl buffer (TB; pH 7.4) for 5 minutes at room temperature. Sections were then incubated in 0.05% 3,3'-diaminobenzidine tetrahydrochloride (DAB) containing 0.01% H2O2 in TB for 1-2 minutes to produce a chromogenic reaction, washed in TB, and subsequently washed in PBS.
Electron microscopy
For electron microscopic observation of nestin expression, the retinal pieces embedded in 4% agar were cut at 50 µm with a vibratome, and sections were placed in PBS. The sections were incubated in 10% normal donkey serum in PBS for 1 hour at room temperature, and were then incubated in anti-nestin antibody (1 : 200, Millipore, Billerica, MA, USA) in PBS overnight at 4℃. They were rinsed in three changes of PBS for 45 minutes, incubated in biotin-labeled donkey anti-mouse IgG for 1 hour, and washed with three changes of PBS. Subsequently, the sections were incubated in ABC solution for 1 hour, washed in TB, and then incubated in 0.05% DAB containing 0.01% H2O2 for 2 minutes. The color reaction was stopped by replacing the DAB-plus-H2O2 solution with TB.
The immunostained sections were postfixed in 1% glutaraldehyde in PB for 5 minutes, rinsed in PB, and postfixed in 1% OsO4 in PB for 1 hour. They were washed in PB and dehydrated in a graded series of alcohol; at the 70% alcohol step, they were stained en bloc with 1% uranyl acetate in 70% alcohol for 1 hour, infiltrated in propylene oxide, and flat embedded in Epon 812 mixture. After curing at 60℃ for 3 days, well-prepared areas were selected, excised, and attached onto an Epon support. Seminthin sections, 1 µm thick, were made from these, and ultrathin sections, approximately 70-90 nm thick, were made subsequently. The ultrathin sections were located on one-hole grids coated with Formvar and examined using an electron microscope (JEM1010, Jeol, Tokyo, Japan).
Results
Overview
In the present study, retinal development in the neuroepithelium of the inner layer of the optic cup has been traced from E13. The retina at E18 is classified morphologically as a six-layered structure, constructed from (outermost to innermost) the neuroblast layer (NBL, itself comprising the outer nuclear layer; the outer plexiform layer [OPL], and the inner nuclear layer [INL], from P5), the presumed inner plexiform layer, the presumed ganglion cell layer, and the nerve fiber layer. The retinas at P14 subsequently show a well-established form, composed of the six layers as described above.
Nestin expression and cell proliferation
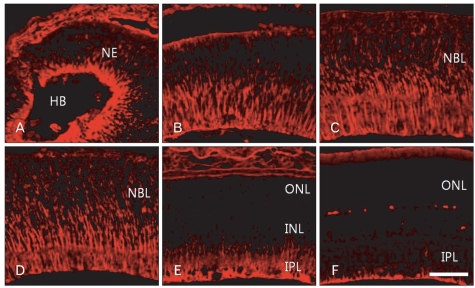
Nestin immunoreactivity was detected in radial fibers throughout retinal development. In the developing retina at E13, nestin immunoreactivity appears in the proximal part of the neuroepithelium (Fig. 1A). With aging, nestin immunoreactivity gradually extended into the middle half of the retina at E18, and even into the distal part of the retina by E20 (Fig. 1B, C). Nestin immunoreactivity throughout the whole depth of the retina was maintained to P3 (Fig. 1D). Subsequently, the immunoreactivity gradually decreased and was restricted to the proximal part and finally to the end feet of Müller radial glial cells (Fig. 1E, F).
Fig. 1.
Confocal microscopic views of the developing retinas processed for nestin immunofluorescent staining. E13 (A), E18 (B), E20 (C), P3 (D), P7 (E), and P14 (F). Nestin immunoreactivity appeared first in the proximal radial fibers of the neuroepithelium (NE) at E13 (A). Subsequently it expanded distally to P3 (D). The nestin immunoreactivity was gradually restricted to proximal fibers (E) and finally to the end feet of Müller glial cells (F). E, embryonic day; HB, hyaloid body; INL, inner nuclear layer; IPL, inner plexiform layer; NBL, neuroblast layer; ONL, outer nuclear layer; P, postnatal day. Scale bar=50 µm.
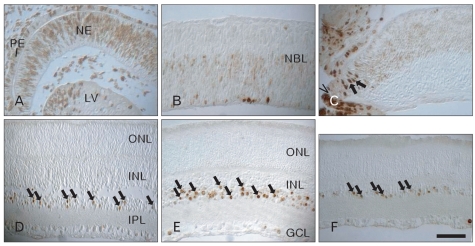
BrdU labeling appeared in neural cell nuclei throughout all the experimental periods. At E13, proliferating cell nuclei identified with BrdU labeling were distributed throughout the entire depth of the neuroepithelium, except its proximal margin near the hyaloid body (Fig. 2A). At E15, BrdU labeling was localized in the inner part of the neuroblast layer. This pattern was maintained until E15. BrdU labeling appeared sporadically in the outer halves of the developing retina at E18 (Fig. 2B). Interestingly, there was almost no BrdU labeling at E20 through the main body of the retina, except for in the ciliary margin (Fig. 2C). Postnatally, BrdU labeling appeared mainly in the innermost part of the INL, and peaked at P14 (Fig. 2D, E). At P28, BrdU labeling was restricted to the innermost cell layer of the INL (Fig. 2F).
Fig. 2.
Light micrographs of BrdU labeling in the developing retinas. Proliferating cell nuclei labeled with BrdU were distributed throughout the whole depth of the neuroepithelium (NE) at E13 (A). BrdU labeling was localized in the inner part of the neuroblast layer (NBL) at E15 (B). There was almost no BrdU labeling at E20 (C) through the main body of the retina, except in the ciliary margin (caret). BrdU labeling (arrows) was detected in the inner nuclear layer (INL) postnatally (D-F) and reached a peak at P14 (E). E, embryonic day; GCL, ganglion cell layer; IPL, inner plexiform layer; LV, lens vesicle; ONL, outer nuclear layer; P, postnatal day; PE, pigment epithelium. Scale bar=50 µm.
Nestin expression and lectin histochemistry
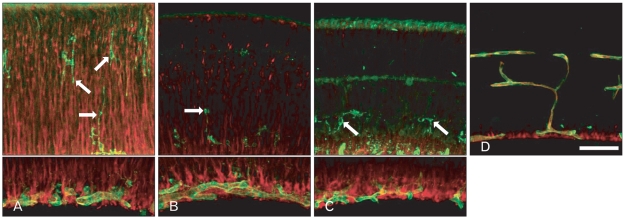
Lectin was mainly labeled in the endothelial cells of the blood vessels throughout retinal development. The retinal blood vessels demarcated with endothelial cells silhouetted by GSIB4-positivity were firstly distributed in the nerve fiber layer of the P3 retina (Fig. 3A). In addition to these endothelial cells, the presumed microglial cells were positive for GSIB4. At P3, the GSIB4-positive cells in the inner plexiform layer showed the appearance of typical microglial cells with numerous short podia, whereas those in the neuroblast layer had the appearance of migrating cells with one or more long processes extended into the inner border of the proliferating zone of the retina. At P5, the outer NBL including the proliferating zone was differentiated from the NBL by the OPL, posing an upper limit of GSIB4-positive microglial cell distribution (Fig. 3B). These patterns were sustained to P7 (Fig. 3C). GSIB4 labeling was detected largely in the endothelial cell lining of the microvessels and in microglial cells near the blood vessels in the ganglion cell layer by P14 afterward (Fig. 3D).
Fig. 3.
Confocal microscopic views of the developing retinas processed for nestin immunofluorescence (red) and lectin (GSIB4, green) histochemistry. Lectin- and nestin-positive endothelial cell-lined retinal vessels developed in the nerve fiber layer from P3 (A), and lectin-positive presumed microglial cells (arrows) were distributed sporadically along the radial fibers with red fluorescence throughout the neuroblast layer. Microglial cells (arrows) with green fluorescence were restricted to the inner half of the inner nuclear layer at P5 (B) and P7 (C). At P14 (D), the microvessels lined with lectin- and nestin-positive endothelial cells were distributed in the ganglion cell layer, the inner plexiform layer, the inner nuclear layer, and the outer plexiform layer. P, postnatal day; GSIB4, Griffonia simplicifolia isolectin B4. Scale bar=50 µm.
Nestin immunoreactivity subsequently appeared in the endothelial cells of the microvessels in the differentiated retina by P3 (Fig. 3A). The labeling characteristics of nestin and GSIB4 in the endothelial cells differed, appearing partially in cytoplasm and in the contour of the cell, respectively. The retinal blood vessels first developed in the nerve fiber layer by P3. The primary branches of the retinal blood vessels sprouted and penetrated into the outer part of the inner retina from P5 (Fig. 3B), and these patterns were sustained to P7 (Fig. 3C). At P14, the retina, the deep capillary network in the OPL, and the superficial network in the inner margin of the INL were well demarcated with nestin- and GSIB4-positive endothelial lining (Fig. 3D).
Nestin immunoelectron microscopy
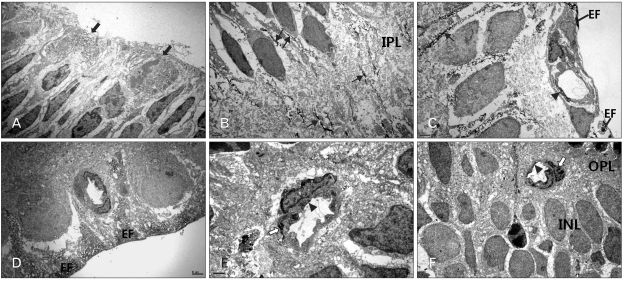
Nestin immunoreactivity was detected in the dividing neural progenitor cells near the ventricular surface of the perinatal retinas and their leading cytoplasmic processes toward their programmed sites during proliferation and migration periods (Fig. 4A, B). Nestin immunoreactivity appeared as fine fibrillar profiles in the cell bodies of dividing neural progenitor cells, whereas it appeared in fiber bundles in their radial fibers. During in situ proliferation and differentiation periods, nestin immunoreactivity appeared in the proximal radial fibers of the Müller cells (Fig. 4C, D). In establishing processes of the vasculature in the retina, the endothelial cells in the microvessels, such as the primary branches of the blood vessels and the superficial and deep capillary networks, showed additional nestin immunoreactivity (Fig. 4C-F). Interestingly, in earlier vasculature, the presumed developing smooth muscle cells of the retinal vessels in the ganglion cell layer were partially immunoreactive (Fig. 4C); in later vasculature, the pericytes of the microvessels were partially immunoreactive (Fig. 4E, F).
Fig. 4.
Electron microphotographs taken from parts of the developing retina processed for nestin immunohistochemistry. Nestin immunoreactivity appeared in the dividing cells (solid arrows) in the ventricular zone of E18 (A) and in the radial fibers (arrows) throughout the neuroblast layer and inner plexiform layer (IPL) (P3) (B) of the developing retina. Nestin immunoreactivity was strongly demarcated the end feet (EF) of the Müller glial cells at P7 (C). Additional nestin imminoreactivity was detected in the endothelial cells (arrowhead) of the retinal vessel in the nerve fiber layer at P7 (C). After the establishment of the capillary networks, the endothelial cells in the retinal vessels showed no nestin immunoreactivity (P14) (D). While in the developing capillary networks of the outer plexiform layer (OPL) at P14 (E) and P21 (F), nestin was also labeled in the pericytes (open arrow) underneath the endothelial cells (arrowhead). E, embryonic day; INL, inner nuclear layer; P, postnatal day. Scale bars=2 µm (A-D, F), 1 µm (E).
Discussion
The present study demonstrated that nestin expression showed two different phases in cell types and in the patterning of these cell types during retinal development. Nestin expression appeared in fiber bundles in the radial processes and fibril aggregations in the dividing cell body. Nestin fiber bundles appeared first in the proximal radial fibers of the neuroepithelium, expanded into the distal radial fibers until P3, when they reached a peak for postmitotic neural cell proliferation, and thereafter finally regressed into the end feet of the Müller cells in the developing retinas. Additionally, the endothelial lining of the retinal vessels also showed nestin expression. It appeared first in the optic nerve fiber layer and its branched microvessels, showing that nestin expression penetrated deep into the retina, forming the superficial and deep capillary networks. Just beneath the endothelial lining of establishing capillaries, the pericytes also showed nestin expression.
Neural progenitor cells in the developing central nervous system have been generally accepted as radial glia, from which neuronal and glial cells are generated [27-30]. In the neuroepithelium of the early developing brain, multipotent neural progenitor cells are widespread and differentiated into radial glia. Radial glial cells in the developing neocortex are in turn mitotically active for neurogenesis, as traced by BrdU-green fluorescent protein- and carbocyanine-labeling techniques [28, 29]. Durings cortical neurogenesis, neurons generated from radial glial cells either translocated their somata to the pial surface [29, 31] or migrated along their parent radial fiber into the cortical plate [28]. After cortical neurogenesis, radial glial cells as astroglial progenitor cells differentiated into glial cells. In accordance with these findings in cerebral cortical development, we have confirmed that radial glia in the developing rat retina play the role of retina neural progenitor cells, as shown by nestin-positive dividing cell somata in the ventricular zone facing to subretinal space and nestin-positive radial fibers in the vitreous surface corresponding to the pial surface of the brain cortex.
Interestingly, nestin-immunoreactive materials in the mitotic cell somata in the ventricular zone of the developing retinas assume a fine fibrillar state, whereas in the radial fibers, they are fiber bundles. Considering cytoarchitectural states of nestin intermediate filaments, it has been previously reported that nestin cannot form filaments because of its short N-terminus, an essential domain for intermediate filament assembly; however, nestin readily forms copolymer intermediate filaments combined with the other types of intermediate filaments such as vimentin [32-36]. On the other hand, nestin has been traced and found to be expressed earlier than vimentin in the developing neural retinas, suggesting that nestin is a marker for neural progenitor cells and vimentin for the differentiation of the precursor radial glial cells to neurons, Müller cells, and astrocytes [26]. Taken together, these findings suggest that the retinogenesis in our nestin immunoelectron microscopic observations seems to be established by two modes of radial migration, as described by Nadarajah et al. [31]: the early mitotic radial glial nuclear translocation and the later neuronal radial movement along the radial glial scaffold. After the establishment of a layered structure in the neural retina, the radial glial cells differentiated into the Müller glial cells with fading nestin positivity limited to their end feet.
Nestin immunoreactive radial fibers in the developing rat retina may form a radial scaffold for newly generated neurons from nestin-immunoreactive retinal neural precursors. This hypothesis can be explained when the spatial sequence of the nestin expression pattern is evaluated, combined with the temporal sequence of the retinal neuronal birth date by BrdU labeling. In our rodent retinogenesis findings, nestin expression in the radial fibers at E13 was first restricted to vitreous proximal fibers, gradually expanded over the distal two thirds of the whole depth until P3, and was finally limited to the end feet of Müller glial cells. In contrast, BrdU-labeled cells were largely restricted to INL postnatally and peaked at P14, suggesting that cells with the most recent birth date are located in the INL terminates around P14. Taken together, the spatial sequence of nestin expression expansion found in our study is consistent with the temporal sequence of the retinogenesis, as described by Rapaport et al. [37], who stated that rat retinal cell genesis commenced around E10, that half of the cells were born by P1, and that retinogenesis was complete near P12. They also reported that the temporal order of different cell phenotypes was as follows: retinal ganglion cells, horizontal cells, cones, amacrine cells, rods, bipolar cells, and Müller glia. With these results, it is suggested that nestin in retinogenesis plays an important role in the cytoskeletal components responsible for migration of newly generated neurons from radial glial cells, and that retinogenesis is terminated as remaining faint nestin immunoreactivity is found in the end feet of Müller glial cells.
In addition to neural precursor radial glial cells, endothelial cells in the growing retinal blood vessels also showed nestin immunoreactivity in the present study. Nestin expression in the endothelial cells of the blood vessels has been previously reported in developing and adult brain, brain tumors, and some other organs [38-43]. From these reports and our study, it can be deduced that nestin acts in the formation of the cytoskeleton of newly formed endothelial cells and further plays a role as a novel and reliable marker of neovascularization showing proliferating angiogenic endothelium.
Moreover, nestin immunoreactive endothelial linings sprout outward following the microglial migration path during the perinatal development of vasculature in our study, in accordance with the other reports [44, 45]. Another interesting feature in our study is that nestin expression appears also in the pericytes around the growing capillary networks. Earlier nestin expression outside the endothelium was found only in the developing smooth muscle cells of the retinal blood vessels in the presumed nerve fiber layer and ganglion cell layer. With these additional features, we suggest that nestin may confer not only proliferation, but also movement potential to these precursor cells in both the neurogenesis and the angiogenesis during retinal development.
From the evidence described above, it is possible that the proliferative and movement potency of the Müller glial cells, endothelial cells, and pericytes in the retina are evoked in response to exogenous stimuli, even if these cells are to be latent after fully differentiating. In the case of endothelial cells and pericytes, reentering into their cell cycles remains open because of subsequent consistency of their own cell lineages, while that of Müller glial cells is much more complicated because of their responsibility for generating a variety of neuronal phenotypes. In any case, some reports described reentry of the Müller glial cells into the cell cycle following retinal damage, although this may be limited [11, 46, 47]. Therefore, there will be remarkable progress in restoration from neurodegenerative damage and even diseases in applications where stem cell or gene therapy is engineered to mimic the retinal microenvironment for the reentry of latent Müller glial cells into their cell cycles.
Acknowledgements
This study was supported by a grant from the Ministry of Knowledge Economy, Republic of Korea (10030064).
References
- 1.Lois C, Alvarez-Buylla A. Proliferating subventricular zone cells in the adult mammalian forebrain can differentiate into neurons and glia. Proc Natl Acad Sci U S A. 1993;90:2074–2077. doi: 10.1073/pnas.90.5.2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Luskin MB. Restricted proliferation and migration of postnatally generated neurons derived from the forebrain subventricular zone. Neuron. 1993;11:173–189. doi: 10.1016/0896-6273(93)90281-u. [DOI] [PubMed] [Google Scholar]
- 3.Goldman SA, Zukhar A, Barami K, Mikawa T, Niedzwiecki D. Ependymal/subependymal zone cells of postnatal and adult songbird brain generate both neurons and nonneuronal siblings in vitro and in vivo. J Neurobiol. 1996;30:505–520. doi: 10.1002/(SICI)1097-4695(199608)30:4<505::AID-NEU6>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 4.Morshead CM, Reynolds BA, Craig CG, McBurney MW, Staines WA, Morassutti D, Weiss S, van der Kooy D. Neural stem cells in the adult mammalian forebrain: a relatively quiescent subpopulation of subependymal cells. Neuron. 1994;13:1071–1082. doi: 10.1016/0896-6273(94)90046-9. [DOI] [PubMed] [Google Scholar]
- 5.Doetsch F, Caillé I, Lim DA, García-Verdugo JM, Alvarez-Buylla A. Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell. 1999;97:703–716. doi: 10.1016/s0092-8674(00)80783-7. [DOI] [PubMed] [Google Scholar]
- 6.Chiasson BJ, Tropepe V, Morshead CM, van der Kooy D. Adult mammalian forebrain ependymal and subependymal cells demonstrate proliferative potential, but only subependymal cells have neural stem cell characteristics. J Neurosci. 1999;19:4462–4471. doi: 10.1523/JNEUROSCI.19-11-04462.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xu H, Sta Iglesia DD, Kielczewski JL, Valenta DF, Pease ME, Zack DJ, Quigley HA. Characteristics of progenitor cells derived from adult ciliary body in mouse, rat, and human eyes. Invest Ophthalmol Vis Sci. 2007;48:1674–1682. doi: 10.1167/iovs.06-1034. [DOI] [PubMed] [Google Scholar]
- 8.Das AV, Mallya KB, Zhao X, Ahmad F, Bhattacharya S, Thoreson WB, Hegde GV, Ahmad I. Neural stem cell properties of Müller glia in the mammalian retina: regulation by Notch and Wnt signaling. Dev Biol. 2006;299:283–302. doi: 10.1016/j.ydbio.2006.07.029. [DOI] [PubMed] [Google Scholar]
- 9.Faillace MP, Julian D, Korenbrot JI. Mitotic activation of proliferative cells in the inner nuclear layer of the mature fish retina: regulatory signals and molecular markers. J Comp Neurol. 2002;451:127–141. doi: 10.1002/cne.10333. [DOI] [PubMed] [Google Scholar]
- 10.Fischer AJ. Neural regeneration in the chick retina. Prog Retin Eye Res. 2005;24:161–182. doi: 10.1016/j.preteyeres.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 11.Fischer AJ, Reh TA. Potential of Müller glia to become neurogenic retinal progenitor cells. Glia. 2003;43:70–76. doi: 10.1002/glia.10218. [DOI] [PubMed] [Google Scholar]
- 12.Marquardt T, Gruss P. Generating neuronal diversity in the retina: one for nearly all. Trends Neurosci. 2002;25:32–38. doi: 10.1016/s0166-2236(00)02028-2. [DOI] [PubMed] [Google Scholar]
- 13.Ooto S, Akagi T, Kageyama R, Akita J, Mandai M, Honda Y, Takahashi M. Potential for neural regeneration after neurotoxic injury in the adult mammalian retina. Proc Natl Acad Sci U S A. 2004;101:13654–13659. doi: 10.1073/pnas.0402129101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bernardos RL, Barthel LK, Meyers JR, Raymond PA. Late-stage neuronal progenitors in the retina are radial Müller glia that function as retinal stem cells. J Neurosci. 2007;27:7028–7040. doi: 10.1523/JNEUROSCI.1624-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bringmann A, Pannicke T, Grosche J, Francke M, Wiedemann P, Skatchkov SN, Osborne NN, Reichenbach A. Müller cells in the healthy and diseased retina. Prog Retin Eye Res. 2006;25:397–424. doi: 10.1016/j.preteyeres.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 16.Wu DM, Schneiderman T, Burgett J, Gokhale P, Barthel L, Raymond PA. Cones regenerate from retinal stem cells sequestered in the inner nuclear layer of adult goldfish retina. Invest Ophthalmol Vis Sci. 2001;42:2115–2124. [PubMed] [Google Scholar]
- 17.Otteson DC, Hitchcock PF. Stem cells in the teleost retina: persistent neurogenesis and injury-induced regeneration. Vision Res. 2003;43:927–936. doi: 10.1016/s0042-6989(02)00400-5. [DOI] [PubMed] [Google Scholar]
- 18.Yurco P, Cameron DA. Responses of Müller glia to retinal injury in adult zebrafish. Vision Res. 2005;45:991–1002. doi: 10.1016/j.visres.2004.10.022. [DOI] [PubMed] [Google Scholar]
- 19.Mezey E, Chandross KJ, Harta G, Maki RA, McKercher SR. Turning blood into brain: cells bearing neuronal antigens generated in vivo from bone marrow. Science. 2000;290:1779–1782. doi: 10.1126/science.290.5497.1779. [DOI] [PubMed] [Google Scholar]
- 20.Brazelton TR, Rossi FM, Keshet GI, Blau HM. From marrow to brain: expression of neuronal phenotypes in adult mice. Science. 2000;290:1775–1779. doi: 10.1126/science.290.5497.1775. [DOI] [PubMed] [Google Scholar]
- 21.Sanchez-Ramos J, Song S, Cardozo-Pelaez F, Hazzi C, Stedeford T, Willing A, Freeman TB, Saporta S, Janssen W, Patel N, Cooper DR, Sanberg PR. Adult bone marrow stromal cells differentiate into neural cells in vitro. Exp Neurol. 2000;164:247–256. doi: 10.1006/exnr.2000.7389. [DOI] [PubMed] [Google Scholar]
- 22.Woodbury D, Schwarz EJ, Prockop DJ, Black IB. Adult rat and human bone marrow stromal cells differentiate into neurons. J Neurosci Res. 2000;61:364–370. doi: 10.1002/1097-4547(20000815)61:4<364::AID-JNR2>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 23.Hung SC, Cheng H, Pan CY, Tsai MJ, Kao LS, Ma HL. In vitro differentiation of size-sieved stem cells into electrically active neural cells. Stem Cells. 2002;20:522–529. doi: 10.1634/stemcells.20-6-522. [DOI] [PubMed] [Google Scholar]
- 24.Hockfield S, McKay RD. Identification of major cell classes in the developing mammalian nervous system. J Neurosci. 1985;5:3310–3328. doi: 10.1523/JNEUROSCI.05-12-03310.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lendahl U, Zimmerman LB, McKay RD. CNS stem cells express a new class of intermediate filament protein. Cell. 1990;60:585–595. doi: 10.1016/0092-8674(90)90662-x. [DOI] [PubMed] [Google Scholar]
- 26.Bozanić D, Bocina I, Saraga-Babić M. Involvement of cytoskeletal proteins and growth factor receptors during development of the human eye. Anat Embryol (Berl) 2006;211:367–377. doi: 10.1007/s00429-006-0087-z. [DOI] [PubMed] [Google Scholar]
- 27.Qian X, Shen Q, Goderie SK, He W, Capela A, Davis AA, Temple S. Timing of CNS cell generation: a programmed sequence of neuron and glial cell production from isolated murine cortical stem cells. Neuron. 2000;28:69–80. doi: 10.1016/s0896-6273(00)00086-6. [DOI] [PubMed] [Google Scholar]
- 28.Miyata T, Kawaguchi A, Okano H, Ogawa M. Asymmetric inheritance of radial glial fibers by cortical neurons. Neuron. 2001;31:727–741. doi: 10.1016/s0896-6273(01)00420-2. [DOI] [PubMed] [Google Scholar]
- 29.Noctor SC, Flint AC, Weissman TA, Dammerman RS, Kriegstein AR. Neurons derived from radial glial cells establish radial units in neocortex. Nature. 2001;409:714–720. doi: 10.1038/35055553. [DOI] [PubMed] [Google Scholar]
- 30.Campbell K, Götz M. Radial glia: multi-purpose cells for vertebrate brain development. Trends Neurosci. 2002;25:235–238. doi: 10.1016/s0166-2236(02)02156-2. [DOI] [PubMed] [Google Scholar]
- 31.Nadarajah B, Brunstrom JE, Grutzendler J, Wong RO, Pearlman AL. Two modes of radial migration in early development of the cerebral cortex. Nat Neurosci. 2001;4:143–150. doi: 10.1038/83967. [DOI] [PubMed] [Google Scholar]
- 32.Marvin MJ, Dahlstrand J, Lendahl U, McKay RD. A rod end deletion in the intermediate filament protein nestin alters its subcellular localization in neuroepithelial cells of transgenic mice. J Cell Sci. 1998;111(Pt 14):1951–1961. doi: 10.1242/jcs.111.14.1951. [DOI] [PubMed] [Google Scholar]
- 33.Eliasson C, Sahlgren C, Berthold CH, Stakeberg J, Celis JE, Betsholtz C, Eriksson JE, Pekny M. Intermediate filament protein partnership in astrocytes. J Biol Chem. 1999;274:23996–24006. doi: 10.1074/jbc.274.34.23996. [DOI] [PubMed] [Google Scholar]
- 34.Steinert PM, Chou YH, Prahlad V, Parry DA, Marekov LN, Wu KC, Jang SI, Goldman RD. A high molecular weight intermediate filament-associated protein in BHK-21 cells is nestin, a type VI intermediate filament protein. Limited co-assembly in vitro to form heteropolymers with type III vimentin and type IV alpha-internexin. J Biol Chem. 1999;274:9881–9890. doi: 10.1074/jbc.274.14.9881. [DOI] [PubMed] [Google Scholar]
- 35.Herrmann H, Aebi U. Intermediate filaments and their associates: multi-talented structural elements specifying cytoarchitec ture and cytodynamics. Curr Opin Cell Biol. 2000;12:79–90. doi: 10.1016/s0955-0674(99)00060-5. [DOI] [PubMed] [Google Scholar]
- 36.Chou YH, Khuon S, Herrmann H, Goldman RD. Nestin promotes the phosphorylation-dependent disassembly of vimentin intermediate filaments during mitosis. Mol Biol Cell. 2003;14:1468–1478. doi: 10.1091/mbc.E02-08-0545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rapaport DH, Wong LL, Wood ED, Yasumura D, La Vail MM. Timing and topography of cell genesis in the rat retina. J Comp Neurol. 2004;474:304–324. doi: 10.1002/cne.20134. [DOI] [PubMed] [Google Scholar]
- 38.Tohyama T, Lee VM, Rorke LB, Marvin M, McKay RD, Trojanowski JQ. Nestin expression in embryonic human neuroepithelium and in human neuroepithelial tumor cells. Lab Invest. 1992;66:303–313. [PubMed] [Google Scholar]
- 39.Mokrý J, Nemecek S. Angiogenesis of extra- and intraembryonic blood vessels is associated with expression of nestin in endothelial cells. Folia Biol (Praha) 1998;44:155–161. [PubMed] [Google Scholar]
- 40.Mokrý J, Nemecek S. Cerebral angiogenesis shows nestin expression in endothelial cells. Gen Physiol Biophys. 1999;18(Suppl 1):25–29. [PubMed] [Google Scholar]
- 41.Gu H, Wang S, Messam CA, Yao Z. Distribution of nestin immunoreactivity in the normal adult human forebrain. Brain Res. 2002;943:174–180. doi: 10.1016/s0006-8993(02)02615-x. [DOI] [PubMed] [Google Scholar]
- 42.Sugawara K, Kurihara H, Negishi M, Saito N, Nakazato Y, Sasaki T, Takeuchi T. Nestin as a marker for proliferative endothelium in gliomas. Lab Invest. 2002;82:345–351. doi: 10.1038/labinvest.3780428. [DOI] [PubMed] [Google Scholar]
- 43.Mokrý J, Cízková D, Filip S, Ehrmann J, Osterreicher J, Kolár Z, English D. Nestin expression by newly formed human blood vessels. Stem Cells Dev. 2004;13:658–664. doi: 10.1089/scd.2004.13.658. [DOI] [PubMed] [Google Scholar]
- 44.Chan-Ling T, Page MP, Gardiner T, Baxter L, Rosinova E, Hughes S. Desmin ensheathment ratio as an indicator of vessel stability: evidence in normal development and in retinopathy of prematurity. Am J Pathol. 2004;165:1301–1313. doi: 10.1016/S0002-9440(10)63389-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Checchin D, Sennlaub F, Levavasseur E, Leduc M, Chemtob S. Potential role of microglia in retinal blood vessel formation. Invest Ophthalmol Vis Sci. 2006;47:3595–3602. doi: 10.1167/iovs.05-1522. [DOI] [PubMed] [Google Scholar]
- 46.Dyer MA, Cepko CL. Control of Müller glial cell proliferation and activation following retinal injury. Nat Neurosci. 2000;3:873–880. doi: 10.1038/78774. [DOI] [PubMed] [Google Scholar]
- 47.Chang ML, Wu CH, Jiang-Shieh YF, Shieh JY, Wen CY. Reactive changes of retinal astrocytes and Muller glial cells in kainate-induced neuroexcitotoxicity. J Anat. 2007;210:54–65. doi: 10.1111/j.1469-7580.2006.00671.x. [DOI] [PMC free article] [PubMed] [Google Scholar]