Abstract
OBJECTIVES
to examine whether improved diabetes control is related to better cognitive outcomes.
DESIGN
randomized control trial
SETTING
a randomized trial of telemedicine vs. usual care in elderly persons with type 2 diabetes.
PARTICIPANTS
Participants were 2169 persons 55 years and older with type 2 diabetes from New York City and Upstate New York.
INTERVENTION
The diabetes case management intervention was implemented by a diabetes nurse, via a telemedicine unit in the participant’s home, and in coordination with the primary care physician.
MEASUREMENTS
Hemoglobin A1c (HbA1c), systolic blood pressure (SBP), and low density lipoprotein cholesterol (LDL), were measured at a baseline visit and at up to 5 annual follow-up visits. Global cognition was measured at those visits with the Comprehensive Assessment and Referral Evaluation (CARE).
RESULTS
In mixed models the intervention was related to slower global cognitive decline in the intervention group (p = 0.01). Improvements in HbA1c (p = 0.03), but not SBP or LDL, mediated the effect of the intervention on cognitive decline.
CONCLUSION
Improved diabetes control in the elderly following existing guidelines through a telemedicine intervention was associated with less global cognitive decline. The main mediator of this effect seemed to be improvements in HbA1c.
Keywords: Diabetes treatment, cognitive impairment, clinical trials
INTRODUCTION
Type 2 diabetes (T2D) is related to higher cognitive impairment and dementia risk 1, 2. This is important given that T2D affects 25% of the elderly in the United States (US)3. Whether T2D control affects cognitive outcomes is unclear. We explored this in the Informatics in Diabetes Education and Telemedicine Study (IDEATel), a randomized trial of telemedicine vs. usual care in 2169 persons. IDEATel showed improved diabetes control in hemoglobin A1C (HbA1c), blood pressure (BP), and low density lipoprotein (LDL) after 5 years of intervention4. We sought to assess whether the intervention was related to better global cognitive outcomes. We also tested which T2D control parameter mediated the effect of the intervention on global cognition.
METHODS
IDEATel was a randomized controlled trial with blinded outcomes assessment. Detailed descriptions were published4. Subjects were enrolled through primary care providers (PCP) in New York City (Columbia University Medical Center), and Upstate New York (State University of New York Upstate Medical University at Syracuse). The Institutional Review Boards at participating institutions approved the study. Participants provided informed consent. An independent Data and Safety Monitoring Board monitored the study.
Setting and Participants
The sample consisted of 2169 subjects recruited and randomized between December, 2000 and October, 2005. Inclusion criteria were: age ≥ 55 years, Medicare beneficiary, diabetes defined by physician’s diagnosis and being on treatment with diet or diabetes medications, residence in a federally designated medically underserved area, and fluency in English or Spanish. Exclusion criteria were: moderate or severe cognitive impairment, severe visual, mobility, or motor coordination impairment, severe co-morbid condition, severe expressive or receptive communication impairment, lack of electrical outlet for telemedicine unit, and spending more than 3 months a year at a location different from their residence. A flow diagram of the study is shown in Appendix Figure 1.
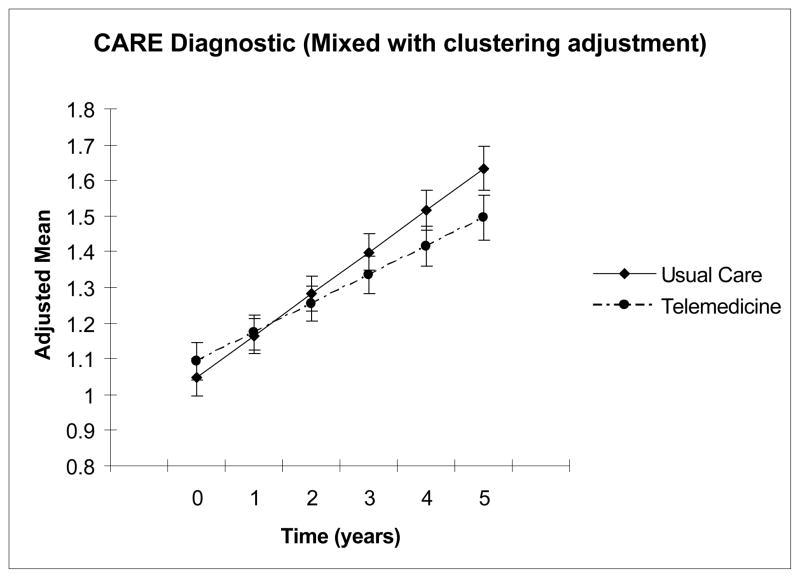
Figure 1.
Comparison of changes in adjusted means in the Comprehensive Assessment and Referral Evaluation (CARE)-Diagnostic Scale between the intervention and control from mixed models (adjusted for clustering) during a maximum follow-up of 5 years.
Randomization and Interventions
Participants were randomized by PCP clusters. The telemedicine intervention has been described elsewhere.4 The target HgbA1c was ≤ 7%, except for participants with reduced life expectancy and/or severe hypoglycemic unawareness, for whom that target was ≤ 8%. At study onset the BP goal was < 130/85 mmHg, or < 125/75 mmHg in the presence of proteinuria (> 1g/24h) or renal insufficiency. In 2003 the BP goal changed to < 130/80 mmHg, except for proteinuria or renal insufficiency. The LDL goals were < 130 mg/dl for primary prevention, and < 100 mg/dl for those with cardiovascular disease. In 2001, the LDL goal changed to < 100 mg/dl.5 In 2004 the LDL goal for participants with high cardiovascular risk changed to < 70 mg/dl. 6
Usual Care
Participating PCPs cared for patients in both trial arms. PCPs were mailed diabetes care guidelines. Usual care group participants received clinical care from PCPs, without guidance from study personnel.
Diabetes control parameters
Diabetes control parameters were HbgA1c, BP, and LDL. Follow-up examinations were conducted at one-year-intervals. Data from baseline and five follow-up visits through February 28, 2007 were used in the analyses.
Subjects came to the baseline and follow-up visits fasting, having held their glycemic control medications, but taking their BP medications. Detailed description of parameter measurements can be found elsewhere.4
Outcomes
The Comprehensive Assessment and Referral Evaluation (CARE)-Diagnostic Scale developed by Gurland and colleagues7 was used for cognitive screening. The CARE-Diagnostic has 14 items, including age recall, year of birth, address, telephone number, date, and if memory problems affect remembering “things like names of people in your family or close friends?”, “to shop”, “to get chores done around the house”, and “keep track of your personal business, like paying bills or handling money.” Higher scores represent worse cognitive impairment. A moderate cut score of six was used to exclude subjects with moderate to severe cognitive impairment. This measure has been used with community samples of diverse race/ethnic backgrounds. The scale was used for cognitive screening in two large epidemiological studies of dementia among Latinos, African-Americans and non-Latino Whites in North Manhattan. Cronbach’s alphas were .83, .84, and .83, for the Latino, African-American and White subgroups, respectively8. The Cronbach’s alpha estimates for the current sample were lower (ranging from 0.56 to 0.75) because of lower item base rates resulting from baseline exclusion of individuals with moderate to severe cognitive impairment. The sensitivity of the scale with clinical diagnosis was .87, the specificity was .79. The scale is relatively unbiased across education and race/ethnic groups8.
Statistical analyses
First, variable distribution was examined. We compared baseline characteristics between the trial arms using chi-squared for categorical outcomes and t-test for continuous outcomes. For variables not normally distributed we used the Wilcoxon rank-sum test.
We examined the longitudinal relationship between cognitive decline and treatment group. First examined was a model treating cognition as a continuous variable using SAS PROC MIXED. We conducted sensitivity analyses modeling the outcome as a Poisson distribution using SAS PROC GLIMMIX. Analyses used an intent-to-treat approach. We used several approaches to model missing data and results were similar.
We examined which diabetes control parameter mediated the effect of the intervention on cognitive decline. Only the three primary outcomes in IDEATel were examined; the pre-specified alpha level for each outcome was set at 0.05. Methods for examination of mediation effects have received recent attention in terms of statistical power 9, 10 and performance. Although evidence from Monte Carlo studies support simple joint significance tests of the mediating path coefficients11, 12, also examined were two other formal tests of mediation effects13, 14. The mediating paths examined were between the intervention and the diabetes control parameter (e.g. HbA1C), and between the diabetes control parameter and cognition. If both paths were statistically significant, we considered the diabetes control parameter to be mediating the effect of the intervention on cognition. These formulas are appropriate for multilevel analyses with random effects such as those used to provide estimates of the path coefficients15. For these analyses, Aroian Z, MacKinnon & Lockwood asymmetric distribution of products11, and joint significance of the coefficient relating the intervention with the mediator (a), and the mediator with the outcomes (b) 11 were used. Confidence intervals for the product of a and b were determined using the method of MacKinnon, Lockwood, and Williams16.
RESULTS
The maximum follow-up was 5 years. The analyses included 2169 persons. The majority (87%) had one or more follow-ups, and about half (43%) had four or five years of follow-up.
Table 1 shows a comparison of characteristics between the trial arms. There were no significant differences in any baseline characteristic.
Table 1.
Comparison of baseline demographic and clinical characteristics between the intervention and usual care group.
| Characteristic | Intervention N = 1093 |
Usual Care N = 1076 |
|---|---|---|
|
| ||
| Age at randomization, years | 70.6 (6.6) | 70.4 (6.8) |
|
| ||
| Women, (%) | 676 (61.8) | 648 (60.2) |
|
| ||
| Race/Ethnicity, (%) | ||
|
| ||
| African-American (non-Hispanic) | 147 (13.4) | 136 (12.6) |
| Hispanic | 379 (34.7) | 365 (33.9) |
| White (non-Hispanic) | 558 (51.1) | 568 (52.8) |
| Other | 9 (0.8) | 7 (0.7) |
|
| ||
| Upstate New York | 610 (55.8) | 610 (56.7) |
|
| ||
| Education | ||
| Elementary | 574 (52.6) | 565 (52.6) |
| High school | 329 (30.2) | 306 (28.5) |
| College | 168 (15.4) | 186 (17.3) |
|
| ||
| Duration of diabetes, years | 10.9 (9.4) | 10.7 (9.0) |
|
| ||
| Body mass index | 32.4 (7.0) | 32.2 (7.0) |
|
| ||
| Systolic blood pressure | 142.5 (23.6) | 141.4 (23.2) |
|
| ||
| HbA1c | 7.4 (1.5) | 7.4 (1.6) |
|
| ||
| LDL | 103.9 (35.1) | 104.2 (36.4) |
|
| ||
| Microalbumin creatinine ratio | 1.5 (0.6) | 1.5 (0.6) |
|
| ||
| Duration of follow-up (years) | 3.5(1.4) | 3.6 (1.4) |
|
| ||
| CARE score | 0.92 (1.25) | 0.91 (1.24) |
As expected, both trial arms experienced cognitive decline during the study, evidenced by increasing CARE scores. However, the treatment group experienced significantly less decline (Figure 1), indicated by a statistically significant interaction term of randomization group and time with a negative coefficient (Table 2). Sensitivity analyses using a Poisson distribution for the CARE-Diagnostic yielded similar results.
Table 2.
Longitudinal analyses of the CARE Diagnostic Cognitive Impairment Screening scale based on intent-to-treat analyses using SAS Proc mixed with an adjustment for clustering within PCP (n = 2169). The group variable compares baseline scores between the intervention and usual care arms. The time variable estimates the change in CARE Diagnostic scores over time. The interaction term of group by time tests differences in changes in the CARE Diagnostic scores between the intervention and usual care arms. The negative coefficient and the significant p value for the interaction term indicate that cognitive decline, indicated by higher scores in the CARE-Diagnostic, was slower in the intervention group.
| Estimate | Std. Err. | p-value | |
|---|---|---|---|
| Intercept | 1.28 | 0.04 | <0.0001 |
| Group (Intervention vs. usual care) | −0.02 | 0.05 | 0.59 |
| Time (years) | 0.11 | 0.01 | <0.0001 |
| Group by Time | −0.03 | 0.01 | 0.01 |
Mediation analyses
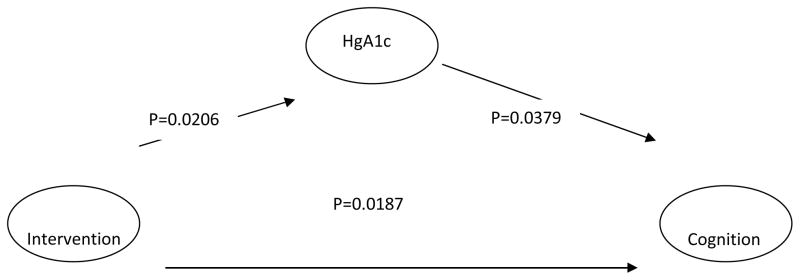
The primary endpoints of IDEATel were HbA1c, BP, and LDL4. The intervention arm resulted in improvements in these parameters compared to usual treatment. HbA1c (p = 0.02), systolic BP (SBP) (p =0.008), and LDL (p=0.0004) showed more beneficial changes in the intervention compared with the usual care group (8). We explored whether a particular parameter mediated the association between the treatment arm and cognitive outcome. When cognition was modeled linearly, only HbA1c was demonstrated to be a significant mediator (p=0.03) (Figure 2). (The 95% confidence limits for the product of the coefficient relating the intervention with HbA1c, and the coefficient relating HbA1c with cognition, were 0.0005 and 0.0278.)
Figure 2.
Path diagram depicting the direct and indirect effects of the telemedicine intervention through Hemoglobin A1c on cognition (n = 2169)a.
a SAS PROC Mixed with a compound symmetry covariance structure was used in the cognition analyses with an adjustment for clustering within PCP. HgA1c was treated as a time-varying covariate in the cognition analyses. SAS PROC Mixed was used to predict HgA1c and included adjustments for clustering and heterogeneity of variances in group and residual variances and exponential terms to model the nonlinear distribution of HgA1c over time. A first order auto-regressive covariance structure was used for the HgA1c analyses. Up to six waves of data (baseline plus five follow-up) were included in the analyses.
Systolic BP and LDL were also examined, but were found not to have a mediating effect on cognition. Sensitivity analyses modeling the CARE-diagnostic in a Poisson distribution yielded similar results.
DISCUSSION
We found that improved HbA1c, SBP, and LDL in the elderly was related to slower global cognitive decline in a 5-year clinical trial. This association was mediated by HbA1c, but not SBP, or LDL.
T2D is associated with higher risk of cognitive decline 17, 18, cognitive impairment without dementia2, 19, mild cognitive impairment (MCI)1, and dementia2 in epidemiologic studies. Furthermore, T2D is associated with both amnestic cognitive impairment, including amnestic MCI1 and dementia due to Alzheimer’s disease (AD) 20, and non-amnestic cognitive impairment, including non-amnestic MCI1 and vascular dementia (VD)2. The association between T2D and VD is stronger than the association with AD21.
While there is ample epidemiologic data relating T2D to an increased risk of cognitive impairment22, 23, it is not clear how treatment affects cognitive impairment risk among persons with T2D. The diabetes control and complications trial (DCCT) in type 1 diabetes demonstrated that improved HbA1c was related to improved cognition in non-amnestic domains24. To the best of our knowledge, there are no published data from clinical trials in the elderly exploring the effect of improved diabetes control on cognition. Our study, like the DCCT, demonstrated that improved HbA1C is related with better global cognition.
Better HbA1c mediated the association between the intervention and better global cognition. HbA1c is an advanced product of glycosilation (AGE). AGEs are produced as elevated glucose promotes the Maillard reaction. In a hyperglycemic environment, diabetic animal and human tissues contain increased AGE and upregulation of its receptor (RAGE).25 AGE in vascular basement membranes promote vascular leakage 26 and contribute to diabetic nephropathy27, 28 retinal neovascularization 29, 30 and diabetic neuropathy.31 Increased RAGE is also observed in AD 32–34. Vasculature with deposited Aβ from patients with AD display increased RAGE antigen compared to age-matched controls 32, 35. Expression of RAGE is enhanced in blood vessels near Aβ deposits in the AD brain 32, 35. Along with increased RAGE in the AD brain, there is a shift of RAGE distribution from neuron to microvasculature36. We cannot make direct inferences about the role of AD pathology in our results. However, the strongest correlate of the CARE-Diagnostic in a subsample of 594 individuals with domain specific neuropsychological data was total recall (Pearson correlation coefficient (r)= −0.42; p <0.0001) and delayed recognition (r=0.39; p <0.0001) of the Buschke Selective Reminding Test37, neuropsychological markers and predictors of AD 38. Non-amnestic tests, better markers of vascular cognitive impairment39, were less strongly correlated with the CARE. For example the color trails 1 and 2, measures of frontal and executive functions, more commonly affected by cerebrovascular disease40, had weaker correlations with the CARE-Diagnostic ( r and p = 0.27 and <0.0001 for color trails 1, and 0.23 and <0.0001 for color trails 2). Thus, our findings indirectly support a role for AGE in cognitive impairment in T2D. This has potentially important therapeutic implications, because AGE-specific therapeutic agents can be used to decrease brain deposition of amyloid beta, the culprit of AD41, and decrease the risk of AD32 in persons with T2D.
Another potential mechanism relating T2D to cognitive impairment is cerebrovascular disease (CVD)42. CVD could cause cognitive impairment through white matter hyperintensities, a correlate of microvascular disease, or infarcts 21, 43. CVD could also interact with amyloid pathology to precipitate cognitive impairment44. LDL and SBP, the major predictors of macrovascular disease and infarcts45, did not mediate the effect of the intervention arm on global cognitive decline, suggesting that the difference in these parameters between the treatment groups was not large enough to affect cognition. Although we attempted to identify the individual role of diabetes control parameters as mediators of the intervention on cognitive decline, it is possible that the sum of the improvements of HbA1c, SBP, and LDL caused by the intervention was stronger than the effect of each parameter alone.
Our study has limitations. Cognition was not a primary outcome of the IDEATel trial. The CARE-Diagnostic was primarily used as a cognitive impairment screen but was measured at each visit. Post-hoc analyses of outcomes in clinical trials may result in chance findings. This is a major concern when results for the primary outcomes are negative46. IDEATel showed a significant effect of telemedicine on its primary outcomes4, and we demonstrated that improvement in one of those primary outcomes, HbA1c, explained cognitive outcomes. Another limitation is the use of a global measure of cognition. The Action to Control Cardiovascular Risk in Diabetes Memory Study (ACCORD-MIND)47 will report whether intensive glycemic, lipid, and blood pressure control improved domain-specific cognitive outcomes. Another limitation is the lack of data on hypoglycemia. The DCCT showed that hypoglycemia was not related to cognitive impairment24, but a observational study showed that lower HbA1c and hypoglycemia in persons with T2D was related to a higher risk of dementia48. However, IDEATel glycemic control goals were less strict compared with ACCORD49, which demonstrated increased mortality in its intensive glycemic control arm.
Our findings show that improving T2D control in the elderly following current guidelines may slow global cognitive decline.
Supplementary Material
Acknowledgments
All authors received funding from Cooperative Agreement 95-C-90998 from the Centers for Medicare and Medicaid Services which funded IDEATel. All authors except R. Weinstock currently received funding from grant number P60 MD000206. R. Weinstock is a paid consultant on grant number P60 MD000206.
Sponsor’s Role: the sponsors had no role in the design, methods, subject recruitment, data collections, analysis and preparation of paper.
Funding sources: These analyses were supported by Cooperative Agreement 95-C-90998 from the Centers for Medicare and Medicaid Services and by Northern Manhattan Center of Excellence on Minority Health and Health Disparities (CEMHD), funded by the National Institute for Minority Health and Health Disparities (NIMHD), grant number P60 MD000206.
Footnotes
Author Contributions:
Concept and design: Luchsinger, Teresi.
Acquisition of subjects and/or data: Palmas, Teresi, Silver, Kong, Eimicke, Weinstock, Shea Analysis and interpretation of data: Luchsinger, Palmas, Teresi, Kong, Eimicke, Weinstock, Shea
Preparation of manuscript: Luchsinger, Palmas, Teresi, Eimicke, Weinstock, Shea
Trial registration: NCT00271739 (clinicaltrials.gov)
References
- 1.Luchsinger JA, Reitz C, Patel B, Tang M-X, Manly JJ, Mayeux R. Relation of Diabetes to Mild Cognitive Impairment. Arch Neurol. 2007;64:570–575. doi: 10.1001/archneur.64.4.570. [DOI] [PubMed] [Google Scholar]
- 2.Luchsinger JA, Tang MX, Stern Y, Shea S, Mayeux R. Diabetes mellitus and risk of Alzheimer’s disease and dementia with stroke in a multiethnic cohort. Am J Epidemiol. 2001;154:635–641. doi: 10.1093/aje/154.7.635. [DOI] [PubMed] [Google Scholar]
- 3.Prevention CfDCa. Fact Sheet Press Release: Number of People with Diabetes Increases to 24 million. 2008;2008 [Google Scholar]
- 4.Shea S, Weinstock RS, Teresi JA, et al. A randomized trial comparing telemedicine case management with usual care in older, ethnically diverse, medically underserved patients with diabetes mellitus: 5 year results of the IDEATel study. J Am Med Inform Assoc. 2009;16:446–456. doi: 10.1197/jamia.M3157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III) Jama. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 6.Grundy SM, Cleeman JI, Merz CN, et al. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III guidelines. Circulation. 2004;110:227–239. doi: 10.1161/01.CIR.0000133317.49796.0E. [DOI] [PubMed] [Google Scholar]
- 7.Gurland B, Kuriansky J, Sharpe L, Simon R, Stiller P, Birkett P. The Comprehensive assessment and Referral Evaluation (CARE)--rationale, development and reliability. Int J Aging Hum Dev. 1977;8:9–42. doi: 10.2190/cl3j-0e20-97xx-mv5l. [DOI] [PubMed] [Google Scholar]
- 8.Gurland BJ, Wilder DE, Cross PE, Teresi JA, Barrett VW. Screening scales for dementia: towards reconciliation of conflicting cross-cultural findings. Int J Geriatr Psychiatry. 1992;7:105–113. [Google Scholar]
- 9.Vittinghoff E, Sen S, McCulloch CE. Sample size calculations for evaluating mediation. Stat Med. 2009;28:541–557. doi: 10.1002/sim.3491. [DOI] [PubMed] [Google Scholar]
- 10.Sjolander A. Bounds on natural direct effects in the presence of confounded intermediate variables. Stat Med. 2009;28:558–571. doi: 10.1002/sim.3493. [DOI] [PubMed] [Google Scholar]
- 11.MacKinnon DP, Lockwood CM, Hoffman JM, West SG, Sheets V. A comparison of methods to test mediation and other intervening variable effects. Psychol Methods. 2002;7:83–104. doi: 10.1037/1082-989x.7.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pituch KA, Whittaker TA, Stapleton LM. A comparison of methods to test for mediation in multisite experiments. Multivariable Behavioral Research. 2005;40:1–23. doi: 10.1207/s15327906mbr4001_1. [DOI] [PubMed] [Google Scholar]
- 13.Sobel ME. Asymptotic confidence intervals for indirect effects in structural equation models. In: Leinhardt S, editor. Sociological methodology. Washington, DC: American Sociological Association; 1982. pp. 290–312. [Google Scholar]
- 14.Aroian LA. The probability function of the product of two normally distributed variables. Ann Mathematical Statistics. 1944;18:265–271. [Google Scholar]
- 15.Krull JL, MacKinnon DP. Multilevel mediation modeling in group-based intervention studies. Eval Rev. 1999;23:418–444. doi: 10.1177/0193841X9902300404. [DOI] [PubMed] [Google Scholar]
- 16.MacKinnon DP, Lockwood CM, Williams J. Confidence limits for the indirect effect: Distribution of the product and resampling methods. Multivariate Behavioral Research. 2008;39:99–128. doi: 10.1207/s15327906mbr3901_4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yaffe K, Blackwell T, Kanaya AM, Davidowitz N, Barrett-Connor E, Krueger K. Diabetes, impaired fasting glucose, and development of cognitive impairment in older women. Neurology. 2004;63:658–663. doi: 10.1212/01.wnl.0000134666.64593.ba. [DOI] [PubMed] [Google Scholar]
- 18.Arvanitakis Z, Wilson RS, Bienias JL, Evans DA, Bennett DA. Diabetes mellitus and risk of Alzheimer disease and decline in cognitive function. Arch Neurol. 2004;61:661–666. doi: 10.1001/archneur.61.5.661. [DOI] [PubMed] [Google Scholar]
- 19.MacKnight C, Rockwood K, Awalt E, McDowell I. Diabetes mellitus and the risk of dementia, Alzheimer’s disease and vascular cognitive impairment in the Canadian Study of Health and Aging. Dement Geriatr Cogn Disord. 2002;14:77–83. doi: 10.1159/000064928. [DOI] [PubMed] [Google Scholar]
- 20.Luchsinger JA, Reitz C, Honig LS, Tang MX, Shea S, Mayeux R. Aggregation of vascular risk factors and risk of incident Alzheimer disease. Neurology. 2005;65:545–551. doi: 10.1212/01.wnl.0000172914.08967.dc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Luchsinger JA. Adiposity, hyperinsulinemia, diabetes and Alzheimer’s disease: an epidemiological perspective. Eur J Pharmacol. 2008;585:119–129. doi: 10.1016/j.ejphar.2008.02.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cukierman T, Gerstein HC, Williamson JD. Cognitive decline and dementia in diabetes-systematic overview of prospective observational studies. Diabetologia. 2005;48:2460–2469. doi: 10.1007/s00125-005-0023-4. [DOI] [PubMed] [Google Scholar]
- 23.Biessels GJ, Staekenborg S, Brunner E, Brayne C, Scheltens P. Risk of dementia in diabetes mellitus: a systematic review. Lancet Neurol. 2006;5:64–74. doi: 10.1016/S1474-4422(05)70284-2. [DOI] [PubMed] [Google Scholar]
- 24.The Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Study Research G. Long-Term Effect of Diabetes and Its Treatment on Cognitive Function. N Engl J Med. 2007;356:1842–1852. doi: 10.1056/NEJMoa066397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Negrean M, Stirban A, Stratmann B, et al. Effects of low- and high-advanced glycation endproduct meals on macro- and microvascular endothelial function and oxidative stress in patients with type 2 diabetes mellitus. Am J Clin Nutr. 2007;85:1236–1243. doi: 10.1093/ajcn/85.5.1236. [DOI] [PubMed] [Google Scholar]
- 26.Vlassara H, Striker LJ, Teichberg S, Fuh H, Li YM, Steffes M. Advanced glycation end products induce glomerular sclerosis and albuminuria in normal rats. Proc Natl Acad Sci U S A. 1994;91:11704–11708. doi: 10.1073/pnas.91.24.11704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Peppa M, Brem H, Cai W, et al. Prevention and reversal of diabetic nephropathy in db/db mice treated with alagebrium (ALT-711) Am J Nephrol. 2006;26:430–436. doi: 10.1159/000095786. [DOI] [PubMed] [Google Scholar]
- 28.Thallas-Bonke V, Lindschau C, Rizkalla B, et al. Attenuation of extracellular matrix accumulation in diabetic nephropathy by the advanced glycation end product cross-link breaker ALT-711 via a protein kinase C-alpha-dependent pathway. Diabetes. 2004;53:2921–2930. doi: 10.2337/diabetes.53.11.2921. [DOI] [PubMed] [Google Scholar]
- 29.Stitt AW. Advanced glycation: an important pathological event in diabetic and age related ocular disease. Br J Ophthalmol. 2001;85:746–753. doi: 10.1136/bjo.85.6.746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schalkwijk CG, Lieuw-a-Fa M, van Hinsbergh VW, Stehouwer CD. Pathophysiological role of Amadori-glycated proteins in diabetic microangiopathy. Semin Vasc Med. 2002;2:191–197. doi: 10.1055/s-2002-32042. [DOI] [PubMed] [Google Scholar]
- 31.Sullivan KA, Feldman EL. New developments in diabetic neuropathy. Curr Opin Neurol. 2005;18:586–590. doi: 10.1097/01.wco.0000178825.56414.52. [DOI] [PubMed] [Google Scholar]
- 32.Yan SD, Chen X, Fu J, et al. RAGE and amyloid-beta peptide neurotoxicity in Alzheimer’s disease. Nature. 1996;382:685–691. doi: 10.1038/382685a0. [DOI] [PubMed] [Google Scholar]
- 33.Schmidt AM, Yan SD, Yan SF, Stern DM. The biology of the receptor for advanced glycation end products and its ligands. Biochim Biophys Acta. 2000;1498:99–111. doi: 10.1016/s0167-4889(00)00087-2. [DOI] [PubMed] [Google Scholar]
- 34.Lue LF, Walker DG, Brachova L, et al. Involvement of microglial receptor for advanced glycation endproducts (RAGE) in Alzheimer’s disease: identification of a cellular activation mechanism. Exp Neurol. 2001;171:29–45. doi: 10.1006/exnr.2001.7732. [DOI] [PubMed] [Google Scholar]
- 35.Deane R, Du Yan S, Submamaryan RK, et al. RAGE mediates amyloid-beta peptide transport across the blood-brain barrier and accumulation in brain. Nat Med. 2003;9:907–913. doi: 10.1038/nm890. [DOI] [PubMed] [Google Scholar]
- 36.Donahue JE, Flaherty SL, Johanson CE, et al. RAGE, LRP-1, and amyloid-beta protein in Alzheimer’s disease. Acta Neuropathol (Berl) 2006;112:405–415. doi: 10.1007/s00401-006-0115-3. [DOI] [PubMed] [Google Scholar]
- 37.Buschke H, Fuld PA. Evaluating storage, retention, and retrieval in disordered memory and learning. Neurology. 1974;24:1019–1025. doi: 10.1212/wnl.24.11.1019. [DOI] [PubMed] [Google Scholar]
- 38.Small SA, Mayeux R. A clinical approach to memory decline. J Pract Psychiatry Behav Health. 1999;5:87–94. [Google Scholar]
- 39.Sachdev PS, Brodaty H, Valenzuela MJ, et al. The neuropsychological profile of vascular cognitive impairment in stroke and TIA patients. Neurology. 2004;62:912–919. doi: 10.1212/01.wnl.0000115108.65264.4b. [DOI] [PubMed] [Google Scholar]
- 40.Hachinski V, Iadecola C, Petersen RC, et al. National Institute of Neurological Disorders and Stroke-Canadian Stroke Network vascular cognitive impairment harmonization standards. Stroke. 2006;37:2220–2241. doi: 10.1161/01.STR.0000237236.88823.47. [DOI] [PubMed] [Google Scholar]
- 41.Selkoe DJ. The origins of Alzheimer disease: a is for amyloid. Jama. 2000;283:1615–1617. doi: 10.1001/jama.283.12.1615. [DOI] [PubMed] [Google Scholar]
- 42.Craft S. The Role of Metabolic Disorders in Alzheimer Disease and Vascular Dementia: Two Roads Converged. Arch Neurol. 2009;66:300–305. doi: 10.1001/archneurol.2009.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Luchsinger JA, Brickman AM, Reitz C, et al. Subclinical cerebrovascular disease in mild cognitive impairment. Neurology. 2009;73:450–456. doi: 10.1212/WNL.0b013e3181b1636a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Arvanitakis Z, Schneider JA, Wilson RS, et al. Diabetes is related to cerebral infarction but not to AD pathology in older persons. Neurology. 2006;67:1960–1965. doi: 10.1212/01.wnl.0000247053.45483.4e. [DOI] [PubMed] [Google Scholar]
- 45.Sacco RL, Benjamin EJ, Broderick JP, et al. American Heart Association Prevention Conference. IV. Prevention and Rehabilitation of Stroke. Risk factors. Stroke. 1997;28:1507–1517. doi: 10.1161/01.str.28.7.1507. [DOI] [PubMed] [Google Scholar]
- 46.Moye LA. Alpha calculus in clinical trials: considerations and commentary for the new millennium. Stat Med. 2000;19:767–779. doi: 10.1002/(sici)1097-0258(20000330)19:6<767::aid-sim518>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 47.Williamson JD, Miller ME, Bryan RN, et al. The Action to Control Cardiovascular Risk in Diabetes Memory in Diabetes Study (ACCORD-MIND): rationale, design, and methods. Am J Cardiol. 2007;99:112i–122i. doi: 10.1016/j.amjcard.2007.03.029. [DOI] [PubMed] [Google Scholar]
- 48.Whitmer RA, Karter AJ, Yaffe K, Quesenberry CP, Jr, Selby JV. Hypoglycemic episodes and risk of dementia in older patients with type 2 diabetes mellitus. JAMA. 2009;301:1565–1572. doi: 10.1001/jama.2009.460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.The Action to Control Cardiovascular Risk in Diabetes Study G. Effects of Intensive Glucose Lowering in Type 2 Diabetes. N Engl J Med. 2008;358:2545–2559. doi: 10.1056/NEJMoa0802743. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.