Abstract
Mesenchymal stem cells (MSCs) derived from human pluripotent stem cells (hPSC-derived MSCs) will be one promising alternative cell source for MSC-based therapies. Here, an efficient protocol is demonstrated for generating hPSC-derived MSCs under a feeder-free culture system by regulating signaling pathways. Simultaneous treatments with Activin A, BIO (6-bromoindirubin-3′-oxime), and bone morphogenetic protein 4 (ABB) activated the transcription of mesoderm-lineage genes such as T, MIXL1, and WNT3 in hPSCs. The ABB-treated hPSCs could develop into CD105+ cells with a high efficiency of 20% in the MSC-induction medium. The properties of the hPSC-derived CD105+ cells were similar to those of adult MSCs in terms of surface antigens. Also, hPSC-derived MSCs had the potential to differentiate into adipocytes, osteoblasts, and chondrocytes in vitro. The results demonstrated that functional MSCs could be generated efficiently from hPSCs by the combined modulation of signaling pathways.
Introduction
Mesenchymal stem cells (MSCs) were first identified in bone marrow [1] and have great potential for use in MSC-based therapies. Moreover, MSCs can be isolated from various adult tissues such as adipose tissues, cord blood, peripheral blood, neonatal tissues, and human placenta, among others [2–5]. MSCs are multipotent in that they can differentiate into several mesenchymal lineage cell types such as osteocytes, chondrocytes, tendonocytes, adipocytes, myocytes, and fibroblasts. In addition, they can transdifferentiate into neural cells [6,7], cardiomyocytes [8], endothelial cells [9], and hepatocytes [10] under the appropriate medium.
Adult MSCs are considered to be immunologically inert, because they express class I but not class II major histocompatibility complex antigens or costimulatory molecules [11–13]. In fact, it is postulated that MSCs have a potent immunosuppressive effect in vivo [14]. However, there are several limitations to the clinical application of adult MSCs. The presence of MSCs in adult tissues is very rare [15] and the in vitro expansion of adult MSCs is restricted because of replicative senescence [16–20]. To try to get around these limitations, MSCs derived from human pluripotent stem cells (hPSCs) could be employed as an alternative source in cell therapy.
hPSCs, such as embryonic stem cells (ESCs) and induced PSCs (iPSCs), are pluripotent in that they self-renew indefinitely and differentiate into various cell types of the 3 germ layers. Human ESCs (hESCs) retain their pluripotency via coordinated networks of intracellular signaling pathways such as fibroblast growth factor receptor, transforming growth factor β (TGF-β), wingless-int-very popular signaling pathways in cells (WNT), and others [21–25]. Therefore, it is conceivable that a signaling pathway or a combination of multiple signaling pathways might be associated with the differentiation of hESCs into a specialized lineage, either directly or indirectly. In fact, the differentiation of hESCs into trophoblasts, primitive endodermal cells, and mesodermal cells can be induced by treatment with bone morphogenetic protein 2/4 (BMP2/4), a member of the TGF-β superfamily [26,27]. Both the activation of activin/nodal signaling and the suppression of PI3K signaling resulted in the induction of hESCs to definitive endodermal cells [28]. Treatments with Noggin and SB431542 inhibit SMAD signaling in hESCs and human iPSCs (hiPSCs), thereby resulting in differentiation into neural cells [29]. The combined regulation of MEK/ERK and BMP4 signaling pathways can induce hPSCs into functional CD34+ progenitors with high efficiency [30]. A multipotent mesoderm-committed progenitor population, CD326−CD56+, was generated from hESCs in the presence of Activin A, BMP4, vascular endothelial growth factor (VEGF), and fibroblast growth factor 2 [31], and Activin A/Nodal and BMP4 induce cardiogenic mesoderm in both human and mouse PSCs [32]. Additionally, there is a common approach in which specialized cell types can be isolated from embryoid body-derived differentiated cells using antibodies against cell type-specific surface antigens [33,34].
Several approaches have been tried to generate MSCs from hPSCs using a coculturing system with OP9 feeder cells and/or spontaneous differentiation protocols [35–38]. Further, MSCs can be derived from hPSCs by culturing in medium supplemented with growth factors such as basic fibroblast growth factor (bFGF), epidermal growth factor (EGF), and platelet-derived growth factor AB (PDGF-AB) [39,40]. However, these protocols seem to be inadequate for clinical application because of low efficiency and contamination by animal sources. In this study, MSCs could be derived from hPSCs by an efficient protocol carried out under a feeder- and serum-free system. The modulation of TGF-β, WNT, and BMP4 signaling pathways was effective in inducing hPSCs to differentiate into the mesodermal lineage under a feeder-free system. When cultured in MSC-induction medium containing bFGF and EGF, hPSC-derived mesodermal cells developed into CD105+ MSCs with a high efficiency (>20%). Like adult MSCs, hPSC-derived CD105+ MSCs were positive for CD29, CD44, CD73, CD90, and human leukocyte antigen (HLA)-ABC, but negative for CD34, CD45, CD31, and HLA-DR. Further, they could differentiate into adipocytes, osteoblasts, and chondrocytes in vitro. Thus, hPSC-derived MSCs are multipotent and functional. This signaling-controlled, serum/feeder-free system will be adequate for understanding the developmental process of hPSCs into a specialized lineage because of diminished effects of unknown factors, which may arise from serum and coculture with feeder.
Materials and Methods
Maintenance of hPSCs
In this study, CHA4-hESC (passages 30 to 60) [41] and OSKM-hiPSC (passages 20 to 50) [30] lines were used. The hPSCs were cultured in unconditioned medium (UM) containing 4–10 ng/mL bFGF (Invitrogen, Carlsbad, CA) on Mitomycin C (Sigma, St. Louis, MO)-treated STO (ATCC CRL-1503) feeders at 37°C, in air containing 5% CO2. The UM consisted of Dulbecco's modified Eagle medium: nutrient mixture F-12 (DMEM/F12) medium containing 20% knockout serum replacement, 1% nonessential amino acids, and 0.1 mM β-mercaptoethanol (all from Invitrogen, Carlsbad, NM). The medium was changed daily. For feeder-free cultures, hPSCs were maintained on Matrigel® (BD Biosciences, Bedford, MA)-coated culture dishes in STO-conditioned medium (CM) supplemented with 8 ng/mL bFGF as previously described [42].
Differentiation of hPSCs into CD105+ MSCs
Supplementary Figure S1A (Supplementary Data are available online at www.liebertonline.com/scd) depicts the overall procedures for the differentiation of hESCs into functional MSCs. Briefly, hESC clumps were transferred into matrigel-coated dishes in CM containing 8–10 ng/mL bFGF and were then stabilized in an atmosphere of 5% CO2 at 37°C in air for 2 days. To induce mesoderm-lineage cells, hESCs were incubated in UM containing 5 ng/mL Activin A (Peprotech, Rocky Hill, NJ), 2 μM BIO (6-bromoindirubin-3′-oxime) (Sigma), and 20 ng/mL BMP4 (Peprotech) (ABB) for 3 days. The ABB-treated hESCs were cultured further in the MSC-induction medium, which contained 90% alpha minimum essential medium (α-MEM), 10% SR, 10 ng/mL bFGF (Invitrogen), and 10 ng/mL epidermal growth factor (EGF) (Peprotech) for 10 days. To isolate CD105+ cells, hPSC-derived cells were first treated with prewarmed Accutase (Innovative Cell Technologies, San Diego, CA) for 5–10 min at 37°C, dissociated by gentle pipetting, and then passed through 40-μm cell strainers (BD Biosciences). CD105+ cells were isolated from the dissociated cell population by magnetic-activated cell sorting (MACS) sorter using a CD105 microbead-conjugated antibody (Miltenyi Biotech, Bergisch Gladbach, Germany). Sorted cells were maintained and expanded in α-MEM containing 10% fetal bovine serum (FBS).
Differentiation of CD105+ MSCs into adipocytes, osteoblasts, and chondrocytes
The Human Mesenchymal Stem Cell Functional Identification Kit (R&D Systems, Minneapolis, MN) was employed to test the potential of CD105+ cells to differentiate into adipocytes and osteoblasts. Briefly, hPSC-derived CD105+ cells were cultured in basal medium (R&D Systems) containing adipogenic and osteogenic supplements for 2–3 weeks to induce differentiation into adipocytes and osteoblasts, respectively. The medium was exchanged with fresh medium every 3–4 days. The differentiated cells were analyzed by specific staining for adipocytes and osteoblasts. For Oil-Red O staining, differentiated cells were fixed in 10% formalin for at least 1 h, washed with 60% isopropanol, dried completely, and then incubated in Oil-Red O solution (Sigma) for 10 min. For Alizarin Red S staining, differentiated cells were fixed in ice-cold 70% ethanol for 1 h at room temperature, rinsed twice with distilled water, and then incubated with Alizarin Red solution (Sigma) at room temperature for 30 min. For Von Kossa staining, differentiated cells were fixed in 10% formalin for 30 min, stained with 5% silver nitrate under ultraviolet light for 10 min, and then fixed with 5% sodium thiosulfate for 5 min to remove nonreacted silver. After washing with distilled water, stained cells were observed under normal light with a fluorescence microscope (Olympus, Tokyo, Japan).
Chondrogenic differentiation of MSCs was carried out according to the manufacturer's procedure using a StemPro Chondrogenesis Differentiation kit (Invitrogen). Briefly, hPSC-derived CD105+ cells (1.6×107 viable cells/mL) were cultured in 5 μL droplets of cell solution for 3–4 weeks. The hMSC-derived cell pellets were fixed in 4% formaldehyde overnight, cryosectioned using a Leica CM1850 cryostat (Leica Microsystems, Wetzlar, Germany), and then subjected to Alcian Blue staining and immunostaining to detect glycosaminoglycan and aggrecan, respectively.
Total RNA extraction and real-time reverse transcription–polymerase chain reaction
TRIzol (Invitrogen) was used to extract total RNA from cells according to the manufacturer's protocol. Approximately 1–2 μg of total RNA was used to generate first-strand cDNA using Superscript II reverse transcriptase (Invitrogen), and cDNA was amplified using a polymerase chain reaction (PCR) PreMix (Genetbio, Daejeon, Korea). The primers used in this study are listed in Supplementary Table S1. The thermocycling conditions used were as follows: an initial step at 95°C for 5 min, 25–35 cycles of 30 s at 94°C, 30 s at 60°C, and 30 s at 72°C, followed by a final elongation step at 72°C for 5 min. The PCR products were analyzed by electrophoresis on a 1.5% agarose gel. The cDNA from total RNA was also used for real-time PCR using a Prime Q-Master mix (with SYBR Green I; Genetbio). Product amplification was performed on an iCycler iQ5 Real-Time detection system (Bio-Rad Laboratories, Hercules, CA). The reaction parameters for real-time reverse transcription (RT)-PCR analysis were 95°C for 10 min followed by 40 cycles of 95°C for 30 s, 60°C for 30 s, and 72°C for 30 s, with a final elongation step at 72°C for 5 min. Melting curve analysis was employed to verify the absence of nonspecific peaks. All reactions were performed in triplicate.
Immunostaining
The cells were washed with Ca2+/Mg2+-free phosphate-buffered saline (PBS; Invitrogen) and fixed in 4% formaldehyde at room temperature for 20 min. For detection of specific marker proteins, the cells were permeabilized with 0.1% Triton X-100 in PBS for 30 min, blocked with 4% normal donkey serum for 1 h at room temperature, and incubated with the respective primary antibodies at 4°C overnight. In this study, primary antibodies against octamer-binding transcription factor 4 (OCT4) (1:200), T (1:200), fat acid-binding protein (FABP-4) (1:30), osteocalcin (1:30), and aggrecan (1:30) were properly diluted with blocking solution. Finally, the cells were washed several times with 0.1% Tween-20 in PBS and incubated with Alexa-488- or Alexa-594-conjugated secondary antibodies (Invitrogen). Immunostained cells were observed on a fluorescence microscope (Olympus).
Western blotting
Total proteins were extracted from cells using the PRO-PREP™ protein extraction kit (Intronbio, Seong-Nam, Korea) and were quantified by the Bradford assay. Total proteins (20–30 μg) were separated by 10% SDS-PAGE and transferred to a nitrocellulose membrane (Bio-Rad). The blotted membranes were blocked with 5% skim milk dissolved in TBST [10 mM Tris-HCl (pH 7.5), 150 nM NaCl, and 0.1% Tween-20] for 1 h at room temperature. The membranes were then incubated at 4°C overnight with primary antibodies for OCT3/4 (Santa Cruz Biotechnology, Santa Cruz, CA; 1:1,000), T (R&D; 1:500), phospho-SMAD1/5/8 (Cell Signaling Technologies, Boston, MA; 1:1,000), SMAD1 (Abcam, Cambridge, MA; 1:1,000), β-catenin (Santa Cruz; 1:300), phospho-β-catenin (Santa Cruz; 1:1,000) and α-tubulin (Sigma; 1:3,000), respectively. After washing 3 times with TBST, the samples were treated with the appropriate HRP-conjugated secondary antibody (Santa Cruz Biotechnology; 1:3,000) in blocking solution at room temperature for 1 h. The membrane was then washed and signals were detected using an ECL system (Pierce, Rockford, IL) as recommended by the manufacturer's protocol.
Flow cytometry
The dissociated cells were suspended in PBS containing 2% FBS and were then labeled with antibodies against CD73-phycoerythrin (PE), CD34-allophycocyanin (APC), CD45-fluorescein isothiocyanate (FITC), PE-CD31, PE-CD29, APC-CD 90, PE-HLA-ABC, PE-CD44 (BD PharMingen, Minneapolis, MN), APC-HLA-DR, and CD105-APC (R&D Systems) at 4°C for 30–45 min. After washing 3 times with PBS containing 2% FBS, the antibody-labeled cells were analyzed using an LSRII flow cytometer (Becton Dickson, San Jose, CA) according to the manufacturer's instructions. Data were analyzed using FlowJo software (Tree Star, Inc., Ashland, OR).
Statistical analysis
The relative gene expression level measured by real-time RT-PCR was expressed as the mean±SD. The statistical significance of the real-time RT-PCR data was evaluated using the 1-way analysis of variance and paired t-tests. Values of P<0.05 were considered as significant.
Results
Synergistic effects of BIO, Activin A, and BMP4 on the induction of hESCs to the mesodermal lineage
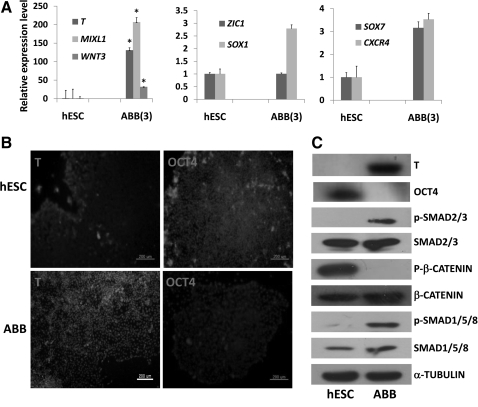
Here, we suggest that the combined modulation of 3 signaling pathways such as WNT, BMP, and Activin A could enhance the expression of mesoderm-lineage genes such as T, MIXL1, and WNT3, indicating synergistic effects on a specialized hESC-lineage differentiation pathway. As shown in Fig. 1A, transcriptional expression levels of mesoderm-lineage genes (T, MIXL1, and WNT3) increased significantly in hESCs treated with ABB for 3 days, compared with nontreated hESCs. Intriguingly, ABB treatment did not enhance the transcript levels of ectoderm-lineage genes (ZIC1 and SOX1) and definitive endoderm-lineage genes (SOX17 and CXCR4) significantly in hESCs. Maximum expression of mesoderm-lineage genes was shown in hESCs on day 3 after the chemical treatment (Supplementary Fig. S1B). Also, ABB treatment activated expression of the mesodermal marker, T, and downregulated OCT4 expression at the protein level (Fig. 1B, C). Thus, the dynamics of lineage-specific molecules such as T and OCT4 might be modulated indirectly in hESCs by the interplay of intracellular molecules downstream of 3 signaling pathways. As shown in Fig. 1C, Activin A activated phosphorylated SMAD2/3, which is an intracellular molecule in the TGF-β signaling pathway; BIO treatment suppressed phosphorylation of β-catenin, which is an intracellular molecule in the WNT signaling pathway, and the activity of phosphorylated SMAD1/5/8, which is an intracellular molecule in the BMP4 signaling pathway, was increased in hESCs by the activation of BMP4 signaling. The results indicated that mesodermal lineage cells might be induced in hESCs by the indirect interplay of intracellular molecules downstream of 3 signaling pathways.
FIG. 1.
Induction of hESCs to the mesodermal lineage by combined treatments with BIO, Activin A, and BMP4. (A) Expression patterns of mesoderm-lineage genes (T, MIXL1, and WNT3), ectoderm-lineage genes (SOX1 and ZIC1), and endoderm-lineage genes (SOX17 and CXCR4) in ABB-treated hESCs (ABB stands for a combination of Activin A, BIO, and BMP4). Real-time PCR was used to estimate and compare the expression levels of early developmental genes in untreated hESCs (control) and ABB-treated hESCs. Statistical significance between samples was evaluated by paired t-test (*P<0.05). (B) Protein level expression of the mesoderm-lineage gene (T) and pluripotent gene (OCT4) in ABB-treated hESCs (bottom) and untreated hESCs (top). In this figure, immunostaining was used to detect the presence of T and OCT4 in hESCs and ABB-treated hESCs (scale bar is 200 μm). (C) Activation status of signaling pathways: WNT, bone morphogenetic protein (BMP), and TGF-β in ABB-treated hESCs. In this figure, the increases in phosphorylation of SMAD1/5/8 and SMAD2/3 represent the activation of BMP and TGF-β, respectively; the decrease in phosphorylation of β-catenin represents the activation of the WNT signaling pathway; α-tubulin was used as housekeeping gene. The expression of T and OCT4 at the protein level in ABB-treated cells and untreated hESCs was confirmed by western blotting. hESCs, human embryonic stem cells; BIO, 6-bromoindirubin-3′-oxime; BMP4, bone morphogenetic protein 4; PCR, polymerase chain reaction; TGF-β, transforming growth factor β.
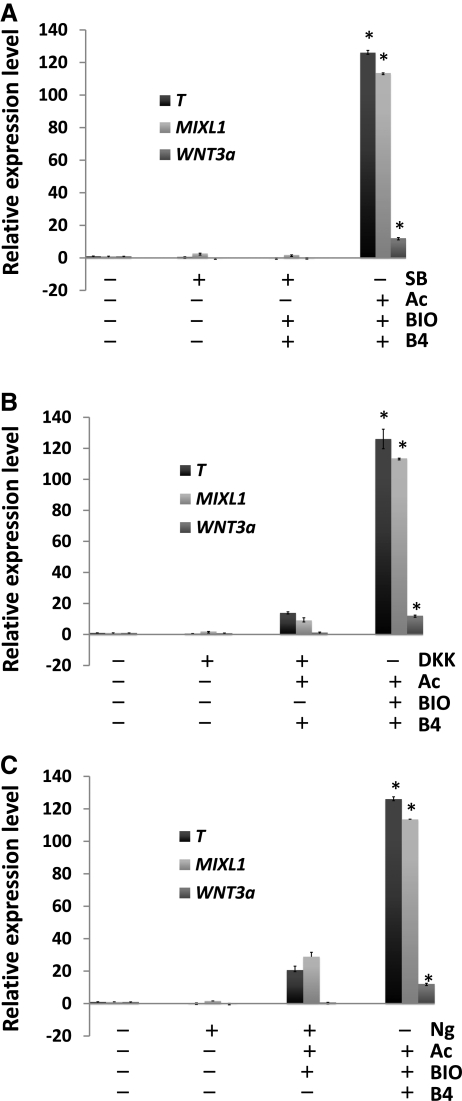
Effect of inhibition of each signaling pathway on the mesodermal induction of hESCs
Our next question was whether or not the combined interplay of the 3 signaling pathways is required for the induction of hESCs to the mesodermal lineage. To address this, hESCs were treated with an inhibitor specific to each signaling pathway on a feeder-free system for 3 days. As shown in Fig. 2, inhibition of each signaling pathway did not activate the transcription of mesoderm-lineage genes such as T, MIXL1, and WNT3a in hESCs. In particular, treatment with SB431542, an inhibitor of TGF-β signaling, suppressed the activation of mesoderm-lineage genes in hESCs (BBS group in Fig. 2A), whereas inhibition of the WNT and BMP signaling pathways did not entirely suppress the expression of mesoderm-lineage genes (ABD group in Fig. 2B and ABN group in Fig. 2C). Thus, TGF-β signaling does not seem to be associated with the activation of mesoderm-lineage genes in hESCs.
FIG. 2.
Effect of inhibition of each signaling pathway on the activation of mesoderm-lineage genes in hESCs. Effects of (A) TGF-β signaling inhibitor (SB431542, SB), (B) WNT signaling inhibitor [dickkopf-related protein 1 (DKK1), DKK], and (C) BMP signaling inhibitor (Noggin, Ng) on the expression patterns of mesoderm-lineage genes in hESCs. After 3 days of treatment, real-time PCR was used to measure the expression levels of mesoderm-lineage genes (T, MIXL1, and WNT3a) in the chemical-treated hESCs. Untreated hESCs and ABB-treated hESCs were used as the negative control and positive control, respectively. Statistical significance among samples was evaluated by ANOVA (*P<0.05). ANOVA, analysis of variance.
Next, to test whether mesodermal induction of hESCs could result from the sequential or simultaneous interaction of the 3 signaling pathways, hESCs were grown under culture conditions with different combinations of signaling-modulating chemicals at varying intervals for 3 days. The results show that the combined treatment of Activin A, BIO, and BMP4 significantly enhanced the expression of the mesoderm-lineage gene, T, in hESCs compared with other treatment combinations (Supplementary Fig. S2). In addition, single chemical treatments were not effective on the transcription of mesoderm-lineage genes in hESCs compared with the ABB-treated group (Supplementary Fig. S3A). Treatment with BIO and BMP4 showed lower transcriptional levels of mesoderm-lineage genes than the ABB-treated group (Supplementary Fig. S3B). The results demonstrate that the combined interplay of 3 signaling pathways synergistically activated the transcription of mesoderm-lineage genes in hESCs.
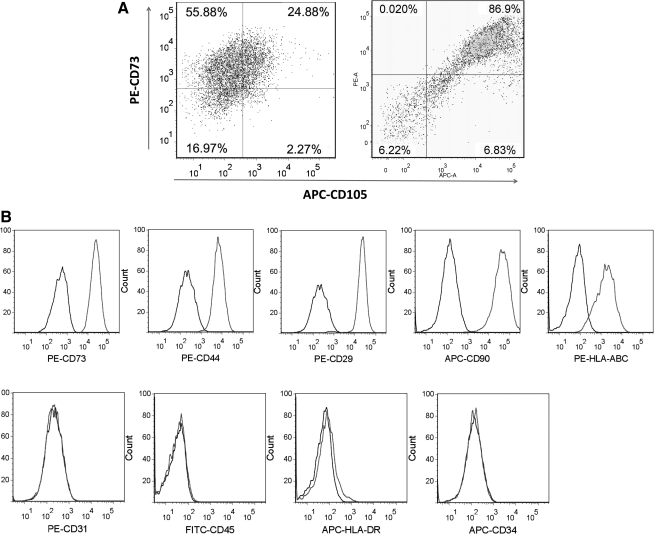
Differentiation of ABB-treated hESCs into CD105+ MSCs
CD105 has been widely used for isolating hPSC-derived MSCs [39,40]. In this study, approximately 24% of ABB-treated hESCs developed into CD105+ cells when cultured in MSC-induction medium for 10 days, and the efficiency of isolation by using a MACS sorter was approximately 90% (Fig. 3A). Isolated CD105+ cells also expressed MSC-positive markers such as CD29, CD44, CD73, CD90, CD105, and HLA-ABC but not MSC-negative markers such as CD34, CD45, CD31, and HLA-DR (Fig. 3B). The surface-antigen profiles were the same as for bone marrow-derived MSCs (Supplementary Fig. S4). These results indicated that the molecular features of hESC-derived MSCs might be similar to those of in vivo derived MSCs.
FIG. 3.
Generation of CD105+ cells from ABB-treated hESCs. (A) The proportion of CD105+ and CD73+ cells before sorting (left) and after sorting (right) by magnetic-activated cell sorting (MACS). (B) Surface-antigen profiles of sorted cells were characterized by fluorescence-activated cell sorting (FACS) using the following antibodies: PE-CD29, PE-CD44, APC-CD90, PE-CD73, PE-HLA-ABC, PE-CD31, FITC-CD45, APC-HLA-DR, and APC-CD34.
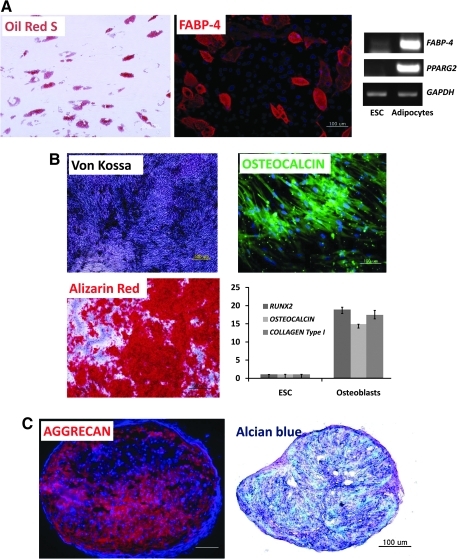
Differentiation potentials of hESC-derived CD105+CD73+ cells
The next experiment was performed to investigate the differentiation potentials of hESC-derived MSCs into specialized cell types. As the results indicated, hESC-derived MSCs could differentiate into adipocytes, which stained positive with Oil-Red S and the FABP-4 antibody. Adipocyte-specific genes such as FABP-4 and PPARG2 were also transcribed in hESC-derived adipocytes (Fig. 4A). As shown in Fig. 4B, osteoblasts derived from hESC-derived MSCs stained positive with the Von Kossa stain, Alizarin Red, and an osteocalcin antibody and demonstrated high levels of expression of osteoblast-specific genes such as RUNX2, OSTEOCALCIN, and COLLAGEN Type I. Further, hESC-derived MSCs formed a cell pellet of chondrocytes that expressed aggrecan and glycosaminoglycan (Fig. 4C). Thus, hESC-derived MSCs appear to be functional in that they retain the potential to differentiate into chondrocytes, adipocytes, and osteoblasts.
FIG. 4.
Differentiation potential of hESC-derived MSCs. (A) Differentiation of hESC-derived CD105+ cells into adipocytes: Oil-Red S staining and immunostaining with FABP-4 antibody were used to detect lipid drops (red color) and FABP-4 (red color) in adipocytes, respectively. Scale bar is 100 μm. Transcripts of FABP-4 and PPARG2 were detected by RT-PCR. (B) Differentiation of hESC-derived CD105+ cells into osteoblasts; Von Kossa staining (black color) and Alizarin Red staining (red color) were used to detect the calcium deposits in osteoblast cells (scale bar is 500 μm). Osteocalcin, a typical osteoblast marker, was also detected by immunostaining (scale bar is 100 μm). RUNX2, OSTEOCALCIN, and COLLAGEN Type I transcripts were analyzed by real-time PCR. (C) Differentiation of hESC-derived CD105+ cells into chondrocytes; the 5–10-μm pellet sections were stained with Alcian Blue and immunostained with aggrecan antibody to detect glycosaminoglycans (blue color) and aggrecan (red color) in chondrocytes, respectively. Scale bar is 100 μm. RT-PCR, reverse transcription–polymerase chain reaction; FABP, fatty acid-binding protein.
Differentiation of hiPSCs into CD105+ MSCs
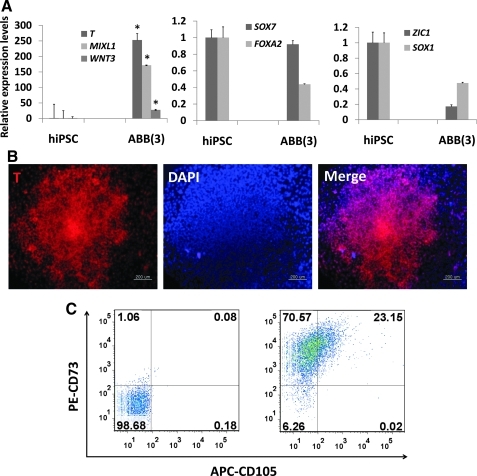
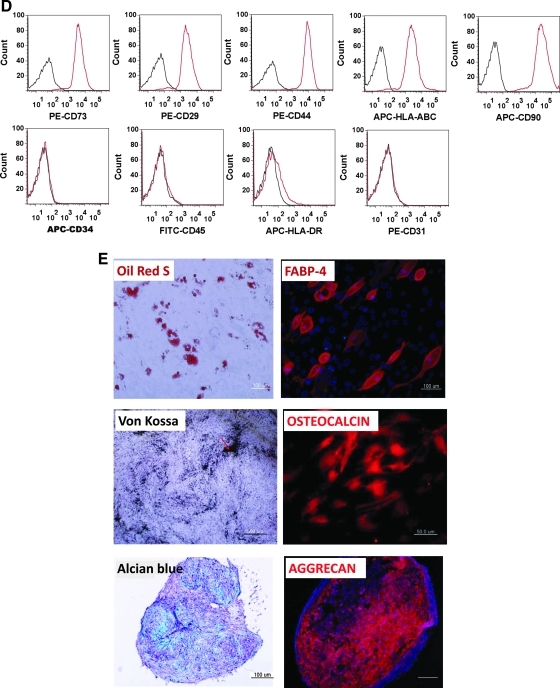
Finally, it was decided to investigate whether our protocol for the induction of MSCs from hESCs could be applied to hiPSCs. As the results show, ABB treatment led to enhanced expression of mesodermal genes at both the transcriptional and protein level in hiPSCs, but not of endodermal and ectodermal genes (Fig. 5A, B). ABB-treated hiPSCs could develop further into MSCs with a high efficiency of approximately 23% (Fig. 5C). Like hESC-derived MSCs, hiPSC-derived MSCs were positive for CD73, CD29, CD44, CD90, and HLA-ABC, but were negative for CD34, CD45, CD31, and HLA-DR (Fig. 5D). Also, they could differentiate further into adipocytes, osteoblasts, and chondrocytes (Fig. 5E). Taken together, the results indicated that the combined regulation of the WNT, BMP, and TGF-β signaling pathways efficiently induced hESCs and hiPSCs to mesodermal-lineage cells that in turn have the ability to differentiate into functional MSCs in vitro.
FIG. 5.
Differentiation of hiPSCs into functional CD105+ MSCs. (A) Expression patterns of mesoderm-lineage genes (T, MIXL1, and WNT3), ectoderm-lineage genes (SOX1 and ZIC1), and endoderm-lineage genes (SOX17 and CXCR4) in ABB-treated hiPSCs. Real-time PCR was used to estimate and compare the expression levels of early developmental genes in untreated hiPSCs (control) and ABB-treated hiPSCs. Statistical significance among samples was evaluated by paired t-test (*P<0.05). (B) Protein level expression of the mesoderm-lineage gene T in ABB-treated hiPSCs. Scale bar is 100 μm. (C) Proportion of the CD105+CD73+ cell population after culturing in MSC-induction medium for 10 days. In this figure, PE-CD73 and APC-CD105 were used for FACS and isotope controls (left figure) were used as a negative control. (D) FACS was used to characterize surface-antigen profiles of sorted cells using the following antibodies: PE-CD29, PE-CD44, APC-CD90, PE-CD73, PE-HLA-ABC, PE-CD31, FITC-CD45, APC-HLA-DR, and APC-CD34. (E) Differentiation potentials of hiPSC-derived MSCs. Oil-Red S staining and immunostaining with FABP-4 antibody were used to detect lipid drops (red) and FABP-4 (red) in adipocytes, respectively (top figures). Scale bar is 100 μm. Von Kossa staining and immunostaining with osteocalcin antibody were used to detect calcium deposits (black color; scale bar is 100 μm) and osteocalcin (red color; scale bar is 50 μm) in osteoblasts, respectively (middle figures). Alcian blue staining and immunostaining with aggrecan antibody were used to detect glycosaminoglycan (blue) and aggrecan (red) in chondrocyte pellets, respectively (bottom figures). Scale bar is 100 μm. hiPSCs, human induced pluripotent stem cells; MSC, mesenchymal stem cell; DAPI, 4',6-diamidino-2-phenylindole (nuclear staining solution).
Discussion
The efficient derivation of hPSCs into specialized cell types will be very important in transplantation therapy using hPSCs. In this study, we developed an efficient protocol to generate functional MSCs from hPSCs under a feeder-free culture system by modulating signaling pathways. To date, it has been reported that signaling pathways play important roles in the regulation of the self-renewal and/or differentiation in hESCs [43–48]. For example, the TGF-β signaling pathway has 2 functions in hESCs via interactions with other signaling pathways: the maintenance of pluripotency [22,25,35,49] and the induction to definitive endoderm [28,50,51]. The canonical WNT/β-catenin signaling pathway is known to be associated with both the maintenance of pluripotency in ESCs [24,52] and the induction of ESCs to the mesodermal lineage [53–57]. BMPs, which are involved in multiple cellular functions such as osteogenesis, cell differentiation, and growth are known to be associated with differentiation in hESCs. hESCs treated with BMP4 for a long term (up to 7 days) differentiate into trophoblasts [26], whereas short-term treatment (1 day) initiates mesodermal induction [27]. Thus, BMP signaling activity may direct the cell fate of hESCs toward different lineages in a time-dependent manner.
Primitive streak (PS)/mesodermal progenitors were induced from hESCs by the cooperative action of beta-catenin signaling together with Activin and BMP signaling pathways [56]; however, only the differentiation capacity of the PS to endothelial-like cells was examined. Cardiac mesodermal cells have been derived from hPSCs by treatments with Activin A and BMP4 [32]. Multipotent mesoderm-committed CD326−CD56+ progenitors were generated from hESCs by treatment with Activin A for 1 day followed by treatments with BMP4, VEGF, and bFGF for 2.5 days [31]. In this study, we found that combined treatments with Activin A, BIO, and BMP4 together could direct the fate of hPSCs to the mesodermal lineage, but not the endodermal or ectodermal lineages (Figs. 1 and 5). Mesoderm-lineage genes were highly activated in hESCs via the simultaneous interplay between 3 signaling pathways, whereas these genes were strongly suppressed by the inhibition of each signaling pathway (Fig. 2 and Supplementary Figs. S2 and S3). Our results indicated that interactions between the 3 signaling pathways might promote the direction of the hPSC fate to the mesodermal lineage.
In this study, we investigated whether hPSC-derived MSCs could retain their differentiation competence toward subtype cells. To achieve this, CD105+ cells were isolated from ABB-treated hPSCs using a MACS sorter. The surface antigens, CD105 and CD73, have been used as specific markers for isolation of MSCs in hPSC-derived cells [35,39,40]. In our preliminary experiments, when ABB-treated hESCs were cultured in DMEM/F12 medium supplemented with bFGF and PDGF-BB for 6 days, only a small number of CD105+ cells (4.45%) expressed CD73, thereby probably leading to low differentiation potentials to adipocytes and osteoblasts (Supplementary Fig. S5B) and a paucity of chondrogenic development (data not shown). After many trials and failures, CD105+CD73+ cells with chondrogenic activity were finally derived with high efficiency (>20%) by culturing ABB-treated hESCs in MSC-induction medium, which was newly modified for this study (Fig. 3A). The hPSC-derived MSCs exhibited similar surface-antigen profiles compared with adult MSCs (Figs. 3B and 5D). Moreover, MSCs derived from hPSCs in this study were multipotent in that they could differentiate into adipocytes, osteoblasts, and chondrocytes (Figs. 4 and 5E), although their functions in vivo remain to be evaluated.
This study may help to overcome several limitations of adult MSCs intended for the cell therapy. One of the main difficulties is securing a large number of MSCs, because their presence in tissues is extremely rare (eg, 10 MSCs per million of all bone marrow cells) and in vitro expansion of the cells is also challenging. In contrast, hPSC-derived MSCs have several advantages compared with adult MSCs: hPSCs can self-renew indefinitely in vitro, which can provide enough of a cell source for the generation of MSCs. Another advantage of the protocol developed in this study is its high efficiency in that a large number of MSCs can be derived from hPSCs in an experiment. Also, all the differentiation processes for the production of hPSC-derived MSCs were performed under a feeder- and serum-free system, which diminishes the side effects of unknown factors when hPSC-derived MSCs are used in transplantation therapy. In addition, our protocol may be useful for exploring underlying developmental events that occur during the transition from the pluripotent state to the multipotency of lineage progenitors.
In summary, this study demonstrated that functional CD105+ MSCs could be generated efficiently from hPSCs in an animal serum- and feeder-free system by modulating signaling pathways. These hPSC-derived MSCs can be employed as an alternative cell source for tissue engineering and MSC-based therapies.
Supplementary Material
Acknowledgments
The authors thank Ms. M.J. Jang for maintaining the hESCs. This research was supported by a grant (SC-2210) from the Stem Cell Research Center, a grant (2011-0019509) from the NRF funded by the MEST, and a grant (A084697) from the Korea Healthcare Technology R&D Project funded by the MW, Republic of Korea.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Friedenstein AJ. Gorskaja JF. Kulagina NN. Fibroblast precursors in normal and irradiated mouse hematopoietic organs. Exp Hematol. 1976;4:267–274. [PubMed] [Google Scholar]
- 2.Fukuchi Y. Nakajima H. Sugiyama D. Hirose I. Kitamura T. Tsuji K. Human placenta-derived cells have mesenchymal stem/progenitor cell potential. Stem Cells. 2004;22:649–658. doi: 10.1634/stemcells.22-5-649. [DOI] [PubMed] [Google Scholar]
- 3.Javazon EH. Beggs KJ. Flake AW. Mesenchymal stem cells: paradoxes of passaging. Exp Hematol. 2004;32:414–425. doi: 10.1016/j.exphem.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 4.Kassis I. Zangi L. Rivkin R. Levdansky L. Samuel S. Marx G. Gorodetsky R. Isolation of mesenchymal stem cells from G-CSF-mobilized human peripheral blood using fibrin microbeads. Bone Marrow Transplant. 2006;37:967–976. doi: 10.1038/sj.bmt.1705358. [DOI] [PubMed] [Google Scholar]
- 5.Wang HS. Hung SC. Peng ST. Huang CC. Wei HM. Guo YJ. Fu YS. Lai MC. Chen CC. Mesenchymal stem cells in the Wharton's jelly of the human umbilical cord. Stem Cells. 2004;22:1330–1337. doi: 10.1634/stemcells.2004-0013. [DOI] [PubMed] [Google Scholar]
- 6.Hung SC. Cheng H. Pan CY. Tsai MJ. Kao LS. Ma HL. In vitro differentiation of size-sieved stem cells into electrically active neural cells. Stem Cells. 2002;20:522–529. doi: 10.1634/stemcells.20-6-522. [DOI] [PubMed] [Google Scholar]
- 7.Sanchez-Ramos J. Song S. Cardozo-Pelaez F. Hazzi C. Stedeford T. Willing A. Freeman TB. Saporta S. Janssen W, et al. Adult bone marrow stromal cells differentiate into neural cells in vitro. Exp Neurol. 2000;164:247–256. doi: 10.1006/exnr.2000.7389. [DOI] [PubMed] [Google Scholar]
- 8.Kadivar M. Khatami S. Mortazavi Y. Shokrgozar MA. Taghikhani M. Soleimani M. In vitro cardiomyogenic potential of human umbilical vein-derived mesenchymal stem cells. Biochem Biophys Res Commun. 2006;340:639–647. doi: 10.1016/j.bbrc.2005.12.047. [DOI] [PubMed] [Google Scholar]
- 9.Reyes M. Lund T. Lenvik T. Aguiar D. Koodie L. Verfaillie CM. Purification and ex vivo expansion of postnatal human marrow mesodermal progenitor cells. Blood. 2001;98:2615–2625. doi: 10.1182/blood.v98.9.2615. [DOI] [PubMed] [Google Scholar]
- 10.Kang XQ. Zang WJ. Bao LJ. Li DL. Song TS. Xu XL. Yu XJ. Fibroblast growth factor-4 and hepatocyte growth factor induce differentiation of human umbilical cord blood-derived mesenchymal stem cells into hepatocytes. World J Gastroenterol. 2005;11:7461–7465. doi: 10.3748/wjg.v11.i47.7461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Klyushnenkova E. Mosca JD. Zernetkina V. Majumdar MK. Beggs KJ. Simonetti DW. Deans RJ. McIntosh KR. T cell responses to allogeneic human mesenchymal stem cells: immunogenicity, tolerance, and suppression. J Biomed Sci. 2005;12:47–57. doi: 10.1007/s11373-004-8183-7. [DOI] [PubMed] [Google Scholar]
- 12.Lee RH. Kim B. Choi I. Kim H. Choi HS. Suh K. Bae YC. Jung JS. Characterization and expression analysis of mesenchymal stem cells from human bone marrow and adipose tissue. Cell Physiol Biochem. 2004;14:311–324. doi: 10.1159/000080341. [DOI] [PubMed] [Google Scholar]
- 13.Madonna R. Geng YJ. De Caterina R. Adipose tissue-derived stem cells: characterization and potential for cardiovascular repair. Arterioscler Thromb Vasc Biol. 2009;29:1723–1729. doi: 10.1161/ATVBAHA.109.187179. [DOI] [PubMed] [Google Scholar]
- 14.Le Blanc K. Rasmusson I. Sundberg B. Gotherstrom C. Hassan M. Uzunel M. Ringden O. Treatment of severe acute graft-versus-host disease with third party haploidentical mesenchymal stem cells. Lancet. 2004;363:1439–1441. doi: 10.1016/S0140-6736(04)16104-7. [DOI] [PubMed] [Google Scholar]
- 15.Baksh D. Song L. Tuan RS. Adult mesenchymal stem cells: characterization, differentiation, and application in cell and gene therapy. J Cell Mol Med. 2004;8:301–316. doi: 10.1111/j.1582-4934.2004.tb00320.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Digirolamo CM. Stokes D. Colter D. Phinney DG. Class R. Prockop DJ. Propagation and senescence of human marrow stromal cells in culture: a simple colony-forming assay identifies samples with the greatest potential to propagate and differentiate. Br J Haematol. 1999;107:275–281. doi: 10.1046/j.1365-2141.1999.01715.x. [DOI] [PubMed] [Google Scholar]
- 17.Kretlow JD. Jin YQ. Liu W. Zhang WJ. Hong TH. Zhou G. Baggett LS. Mikos AG. Cao Y. Donor age and cell passage affects differentiation potential of murine bone marrow-derived stem cells. BMC Cell Biol. 2008;9:60. doi: 10.1186/1471-2121-9-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pittenger MF. Mackay AM. Beck SC. Jaiswal RK. Douglas R. Mosca JD. Moorman MA. Simonetti DW. Craig S. Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 19.Stenderup K. Justesen J. Clausen C. Kassem M. Aging is associated with decreased maximal life span and accelerated senescence of bone marrow stromal cells. Bone. 2003;33:919–926. doi: 10.1016/j.bone.2003.07.005. [DOI] [PubMed] [Google Scholar]
- 20.Wagner W. Bork S. Horn P. Krunic D. Walenda T. Diehlmann A. Benes V. Blake J. Huber FX, et al. Aging and replicative senescence have related effects on human stem and progenitor cells. PLoS One. 2009;4:e5846. doi: 10.1371/journal.pone.0005846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Greber B. Lehrach H. Adjaye J. Fibroblast growth factor 2 modulates transforming growth factor beta signaling in mouse embryonic fibroblasts and human ESCs (hESCs) to support hESC self-renewal. Stem Cells. 2007;25:455–464. doi: 10.1634/stemcells.2006-0476. [DOI] [PubMed] [Google Scholar]
- 22.James D. Levine AJ. Besser D. Hemmati-Brivanlou A. TGFbeta/activin/nodal signaling is necessary for the maintenance of pluripotency in human embryonic stem cells. Development. 2005;132:1273–1282. doi: 10.1242/dev.01706. [DOI] [PubMed] [Google Scholar]
- 23.Rho JY. Yu K. Han JS. Chae JI. Koo DB. Yoon HS. Moon SY. Lee KK. Han YM. Transcriptional profiling of the developmentally important signalling pathways in human embryonic stem cells. Hum Reprod. 2006;21:405–412. doi: 10.1093/humrep/dei328. [DOI] [PubMed] [Google Scholar]
- 24.Sato N. Meijer L. Skaltsounis L. Greengard P. Brivanlou AH. Maintenance of pluripotency in human and mouse embryonic stem cells through activation of Wnt signaling by a pharmacological GSK-3-specific inhibitor. Nat Med. 2004;10:55–63. doi: 10.1038/nm979. [DOI] [PubMed] [Google Scholar]
- 25.Vallier L. Reynolds D. Pedersen RA. Nodal inhibits differentiation of human embryonic stem cells along the neuroectodermal default pathway. Dev Biol. 2004;275:403–421. doi: 10.1016/j.ydbio.2004.08.031. [DOI] [PubMed] [Google Scholar]
- 26.Xu RH. Chen X. Li DS. Li R. Addicks GC. Glennon C. Zwaka TP. Thomson JA. BMP4 initiates human embryonic stem cell differentiation to trophoblast. Nat Biotechnol. 2002;20:1261–1264. doi: 10.1038/nbt761. [DOI] [PubMed] [Google Scholar]
- 27.Zhang P. Li J. Tan Z. Wang C. Liu T. Chen L. Yong J. Jiang W. Sun X, et al. Short-term BMP-4 treatment initiates mesoderm induction in human embryonic stem cells. Blood. 2008;111:1933–1941. doi: 10.1182/blood-2007-02-074120. [DOI] [PubMed] [Google Scholar]
- 28.McLean AB. D'Amour KA. Jones KL. Krishnamoorthy M. Kulik MJ. Reynolds DM. Sheppard AM. Liu H. Xu Y. Baetge EE. Dalton S. Activin a efficiently specifies definitive endoderm from human embryonic stem cells only when phosphatidylinositol 3-kinase signaling is suppressed. Stem Cells. 2007;25:29–38. doi: 10.1634/stemcells.2006-0219. [DOI] [PubMed] [Google Scholar]
- 29.Chambers SM. Fasano CA. Papapetrou EP. Tomishima M. Sadelain M. Studer L. Highly efficient neural conversion of human ES and iPS cells by dual inhibition of SMAD signaling. Nat Biotechnol. 2009;27:275–280. doi: 10.1038/nbt.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Park SW. Jun Koh Y. Jeon J. Cho YH. Jang MJ. Kang Y. Kim MJ. Choi C. Sook Cho Y, et al. Efficient differentiation of human pluripotent stem cells into functional CD34+ progenitor cells by combined modulation of the MEK/ERK and BMP4 signaling pathways. Blood. 2010;116:5762–5772. doi: 10.1182/blood-2010-04-280719. [DOI] [PubMed] [Google Scholar]
- 31.Evseenko D. Zhu Y. Schenke-Layland K. Kuo J. Latour B. Ge S. Scholes J. Dravid G. Li X. MacLellan WR. Crooks GM. Mapping the first stages of mesoderm commitment during differentiation of human embryonic stem cells. Proc Natl Acad Sci U S A. 2010;107:13742–13747. doi: 10.1073/pnas.1002077107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kattman SJ. Witty AD. Gagliardi M. Dubois NC. Niapour M. Hotta A. Ellis J. Keller G. Stage-specific optimization of activin/nodal and BMP signaling promotes cardiac differentiation of mouse and human pluripotent stem cell lines. Cell Stem Cell. 2011;8:228–240. doi: 10.1016/j.stem.2010.12.008. [DOI] [PubMed] [Google Scholar]
- 33.Ferreira LS. Gerecht S. Shieh HF. Watson N. Rupnick MA. Dallabrida SM. Vunjak-Novakovic G. Langer R. Vascular progenitor cells isolated from human embryonic stem cells give rise to endothelial and smooth muscle like cells and form vascular networks in vivo. Circ Res. 2007;101:286–294. doi: 10.1161/CIRCRESAHA.107.150201. [DOI] [PubMed] [Google Scholar]
- 34.Pick M. Azzola L. Mossman A. Stanley EG. Elefanty AG. Differentiation of human embryonic stem cells in serum-free medium reveals distinct roles for bone morphogenetic protein 4, vascular endothelial growth factor, stem cell factor, and fibroblast growth factor 2 in hematopoiesis. 2007;25:2206–2214. doi: 10.1634/stemcells.2006-0713. [DOI] [PubMed] [Google Scholar]
- 35.Barberi T. Willis LM. Socci ND. Studer L. Derivation of multipotent mesenchymal precursors from human embryonic stem cells. PLoS Med. 2005;2:e161. doi: 10.1371/journal.pmed.0020161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Olivier EN. Rybicki AC. Bouhassira EE. Differentiation of human embryonic stem cells into bipotent mesenchymal stem cells. Stem Cells. 2006;24:1914–1922. doi: 10.1634/stemcells.2005-0648. [DOI] [PubMed] [Google Scholar]
- 37.Trivedi P. Hematti P. Derivation and immunological characterization of mesenchymal stromal cells from human embryonic stem cells. Exp Hematol. 2008;36:350–359. doi: 10.1016/j.exphem.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vodyanik MA. Yu J. Zhang X. Tian S. Stewart R. Thomson JA. Slukvin II. A mesoderm-derived precursor for mesenchymal stem and endothelial cells. Cell Stem Cell. 2010;7:718–729. doi: 10.1016/j.stem.2010.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lian Q. Lye E. Suan Yeo K. Khia Way Tan E. Salto-Tellez M. Liu TM. Palanisamy N. El Oakley RM. Lee EH. Lim B. Lim SK. Derivation of clinically compliant MSCs from CD105+, CD24- differentiated human ESCs. Stem Cells. 2007;25:425–436. doi: 10.1634/stemcells.2006-0420. [DOI] [PubMed] [Google Scholar]
- 40.Lian Q. Zhang Y. Zhang J. Zhang HK. Wu X. Zhang Y. Lam FF. Kang S. Xia JC, et al. Functional mesenchymal stem cells derived from human induced pluripotent stem cells attenuate limb ischemia in mice. Circulation. 2010;121:1113–1123. doi: 10.1161/CIRCULATIONAHA.109.898312. [DOI] [PubMed] [Google Scholar]
- 41.Lee JE. Kang MS. Park MH. Shim SH. Yoon TK. Chung HM. Lee DR. Evaluation of 28 human embryonic stem cell lines for use as unrelated donors in stem cell therapy: implications of HLA and ABO genotypes. Cell Transplant. 2010;19:1383–1395. doi: 10.3727/096368910X513991. [DOI] [PubMed] [Google Scholar]
- 42.Xu C. Inokuma MS. Denham J. Golds K. Kundu P. Gold JD. Carpenter MK. Feeder-free growth of undifferentiated human embryonic stem cells. Nat Biotechnol. 2001;19:971–974. doi: 10.1038/nbt1001-971. [DOI] [PubMed] [Google Scholar]
- 43.Dreesen O. Brivanlou AH. Signaling pathways in cancer and embryonic stem cells. Stem Cell Rev. 2007;3:7–17. doi: 10.1007/s12015-007-0004-8. [DOI] [PubMed] [Google Scholar]
- 44.Heasley LE. Petersen BE. Signalling in stem cells: meeting on signal transduction determining the fate of stem cells. EMBO Rep. 2004;5:241–244. doi: 10.1038/sj.embor.7400098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Katoh M. Katoh M. WNT signaling pathway and stem cell signaling network. Clin Cancer Res. 2007;13:4042–4045. doi: 10.1158/1078-0432.CCR-06-2316. [DOI] [PubMed] [Google Scholar]
- 46.Nusse R. Fuerer C. Ching W. Harnish K. Logan C. Zeng A. ten Berge D. Kalani Y. Wnt signaling and stem cell control. Cold Spring Harb Symp Quant Biol. 2008;73:59–66. doi: 10.1101/sqb.2008.73.035. [DOI] [PubMed] [Google Scholar]
- 47.Okita K. Yamanaka S. Intracellular signaling pathways regulating pluripotency of embryonic stem cells. Curr Stem Cell Res Ther. 2006;1:103–111. doi: 10.2174/157488806775269061. [DOI] [PubMed] [Google Scholar]
- 48.Valdimarsdottir G. Mummery C. Functions of the TGFbeta superfamily in human embryonic stem cells. APMIS. 2005;113:773–789. doi: 10.1111/j.1600-0463.2005.apm_3181.x. [DOI] [PubMed] [Google Scholar]
- 49.Amit M. Shariki C. Margulets V. Itskovitz-Eldor J. Feeder layer- and serum-free culture of human embryonic stem cells. Biol Reprod. 2004;70:837–845. doi: 10.1095/biolreprod.103.021147. [DOI] [PubMed] [Google Scholar]
- 50.D'Amour KA. Agulnick AD. Eliazer S. Kelly OG. Kroon E. Baetge EE. Efficient differentiation of human embryonic stem cells to definitive endoderm. Nat Biotechnol. 2005;23:1534–1541. doi: 10.1038/nbt1163. [DOI] [PubMed] [Google Scholar]
- 51.Parashurama N. Nahmias Y. Cho CH. van Poll D. Tilles AW. Berthiaume F. Yarmush ML. Activin alters the kinetics of endoderm induction in embryonic stem cells cultured on collagen gels. Stem Cells. 2008;26:474–484. doi: 10.1634/stemcells.2007-0303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hao J. Li TG. Qi X. Zhao DF. Zhao GQ. Wnt/beta-catenin pathway up-regulates Stat3 and converges on LIF to prevent differentiation of mouse embryonic stem cells. Dev Biol. 2006;290:81–91. doi: 10.1016/j.ydbio.2005.11.011. [DOI] [PubMed] [Google Scholar]
- 53.Gadue P. Huber TL. Paddison PJ. Keller GM. Wnt and TGF-beta signaling are required for the induction of an in vitro model of primitive streak formation using embryonic stem cells. Proc Natl Acad Sci U S A. 2006;103:16806–16811. doi: 10.1073/pnas.0603916103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lindsley RC. Gill JG. Kyba M. Murphy TL. Murphy KM. Canonical Wnt signaling is required for development of embryonic stem cell-derived mesoderm. Development. 2006;133:3787–3796. doi: 10.1242/dev.02551. [DOI] [PubMed] [Google Scholar]
- 55.Oldershaw RA. Baxter MA. Lowe ET. Bates N. Grady LM. Soncin F. Brison DR. Hardingham TE. Kimber SJ. Directed differentiation of human embryonic stem cells toward chondrocytes. Nat Biotechnol. 2010;28:1187–1194. doi: 10.1038/nbt.1683. [DOI] [PubMed] [Google Scholar]
- 56.Sumi T. Tsuneyoshi N. Nakatsuji N. Suemori H. Defining early lineage specification of human embryonic stem cells by the orchestrated balance of canonical Wnt/beta-catenin, Activin/Nodal and BMP signaling. Development. 2008;135:2969–2979. doi: 10.1242/dev.021121. [DOI] [PubMed] [Google Scholar]
- 57.Tada S. Era T. Furusawa C. Sakurai H. Nishikawa S. Kinoshita M. Nakao K. Chiba T. Nishikawa S. Characterization of mesendoderm: a diverging point of the definitive endoderm and mesoderm in embryonic stem cell differentiation culture. Development. 2005;132:4363–4374. doi: 10.1242/dev.02005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.