Abstract
Bone marrow mesenchymal stem cells (MSCs) have shown potential to improve treatment of renal failure. The prohealing functions of MSCs have been found to be enhanced by treatment with the lipid mediator, 14S,21R-dihydroxy-docosa4Z,7Z,10Z,12E,16Z,19Z-hexaenoic acid (14S,21R-diHDHA). In this article, using a murine model of renal ischemia/reperfusion (I/R) injury, we found that treatment with 14S,21R-diHDHA enhanced MSC amelioration of renal I/R injury. Treated MSCs more efficiently inhibited I/R-induced elevation of serum creatinine levels, reduced renal tubular cell death, and inhibited infiltration of neutrophils, macrophages, and dendritic cells in kidneys. Conditioned medium from treated MSCs reduced the generation of tumor necrosis factor-α and reactive oxygen species by macrophages under I/R conditions. Infusion of treated MSCs more efficiently reduced I/R-damage to renal histological structures compared with untreated MSCs (injury score: 7.9±0.4 vs. 10.5±0.5). Treated MSCs were resistant to apoptosis in vivo when transplanted under capsules of I/R-injured kidneys (active caspase-3+ MSCs: 4.2%±2.8% vs. 11.7%±2.4% of control) and in vitro when cultured under I/R conditions. Treatment with 14S,21R-diHDHA promoted viability of MSCs through a mechanism involving activation of the phosphoinositide 3-kinase -Akt signaling pathway. Additionally, treatment of MSCs with 14S,21R-diHDHA promoted secretion of renotrophic hepatocyte growth factor and insulin growth factor-1. Similar results were obtained when 14S,21RdiHDHA was used to inhibit apoptosis of human MSCs (hMSCs) and to increase the generation of renotrophic cytokines from hMSCs. These findings provide a lead for new strategies in the treatment of acute kidney injury with MSCs.
Introduction
Bone marrow mesenchymal stem cells (MSCs) have shown potential for improving the treatment of several incurable diseases through their ability to self-renew and differentiate into mesenchymal and non-mesenchymal cells [1–4]. In particular, MSCs can ameliorate renal ischemia/reperfusion (I/R) injury [5–10]. It has been reported that MSCs repair renal damage by differentiating into tubular epithelial cells [11], mesangial cells [12], endothelial cells [13], and podocytes [14], and by producing renotrophic cytokines and growth factors [15–19]. For example, MSCs can produce insulin growth factor-1 (IGF-1) and hepatocyte growth factor (HGF) [15], both of which are renal-protective or renotrophic factors [18,19].
Renal I/R injury induces secretion of inflammatory cytokines, chemokines, and other factors, which recruit neutrophils, macrophages, and dendritic cells (DCs) to the kidneys [20,21]. Inflammatory leukocytes generate more inflammatory cytokines, amplify the renal injury, and contribute to tubular cell apoptosis and necrosis [20]. The renal reparative manifestation of MSCs involves their anti-inflammatory functions [9,15]. Studies show that MSC can suppress macrophage trans-differentiation to inflammatory phenotypes [22], inhibit DC maturation and migration, and halt inflammatory cytokine generation from leukocytes [22–24]. HGF is immunoregulatory and reduces inflammatory cytokine generation from DCs [25]. IGF-1 blocks not only the early onset of apoptosis, but also inflammation and accompanying tissue injury [26]. Although MSCs possess the capability to ameliorate acute kidney injury, the low viability of transplanted cells in I/R injured organs remains an important limitation for MSC therapy [2,27]. Reactive oxygen species (ROS) and inflammatory cytokines generated in I/R are the probable source of damage to the grafted MSCs [8,20]. Several remedial approaches have been recommended to overcome MSC apoptosis [2], including ischemic or cytokine preconditioning and ex vivo genetic modification of stem cells. For example, melatonin increases survival of MSCs and accelerates kidney recovery from acute kidney injury [8]. Kallikrein-modified MSCs exhibit enhanced renotrophic functions [9]. Thus, pretreating MSCs before transplantation has the potential to improve MSC viability and efficacy in the new, potentially inhospitable, niche in the tissue.
Docosahexaenoic acid (DHA) is an essential omega-3 fatty acid present in blood and kidneys [28]. Resolvin D series, neuroprotectin/protectins, and maresins are potent antiinflammatory lipid mediators (LMs) naturally generated from DHA during inflammation and/or resolution of inflammation [29–33]. In particular, resolvin D1 and protectin D1 have been reported to mitigate I/R injury in mice, reduce leukocyte infiltration to kidneys, and reduce generation of tumor necrosis factor-α (TNF-α) [28,34]. Neuroprotectin/protectin D1 has been reported to protect retinal epithelial cells and neuronal-glial cells from apoptosis induced by oxidative stress [35,36]. The discovery of these LMs suggested that the mechanism is responsible for the beneficial effects of DHA, and opened up directions to further delineate the mechanisms.
Recently, we identified a new DHA-derived LM, 14S,21R-dihydroxy-docosa4Z,7Z,10Z,12E,16Z,19Z-hexaenoic acid (14S,21R-diHDHA), which promotes wound healing [37,38]. We also found that this LM stimulates augmented secretion of vascular endothelial growth factors by MSCs and promotes enhancement of wound healing [37]. However, the specific effects of DHA-derived LMs on renotrophic functions of MSCs have not been reported. The objective of this study was to test the possibility that treatment of MSCs with 14S,21R-diHDHA would more efficiently ameliorate renal I/R injury, and if true, to delineate the mechanisms mediating the effects of 14S,21R-diHDHA. The experiments were conducted by using an established murine renal I/R model [28,39] and in vitro cell culture of murine MSCs (mMSCs) and macrophages. To enhance the human relevance of this study and provide preclinical data, we also used human MSCs (hMSCs) in vitro to confirm results obtained with mMSCs.
Materials and Methods
Mice
We used 10-week-old female C57BL/6J mice (Jackson Lab). Animal protocols were approved by the Institutional Animal Care and Use Committee and Institutional Review Board of Louisiana State University Health Sciences Center, New Orleans. Studies were conducted in a blinded fashion.
Cell cultures
mMSCs (from C57BL/6J mice) and hMSCs were provided by the Texas A&M Health Science Center College of Medicine Institute for Regenerative Medicine at Scott & White Hospital through a grant from the National Institutes of Health National Center for Research Resources (Grant No. P40RR017447, Temple, TX). More than 95% of mMSCs were positive for Sca-1 and CD29, and hMSC purity was confirmed by the expression of CD90 and CD105 (>95%). Both mMSCs and hMSCs exhibited adipogenic and chondrogenic differentiation ability and were cultured in Dulbecco's modified Eagle's medium (DMEM) containing 10% mesenchymal supplement (Stem Cell Technologies). Cells in passages 7 through 10 were used for experiments. The murine macrophage cell line, RAW264.7 (American Type Culture Collection) was cultured in RPMI1640 containing 10% fetal bovine serum (FBS).
Generation, separation, and analysis of 14S,21R-diHDHA and its biosynthetic intermediates
14S/R-hydroxy-DHA (14S/R-HDHA) was from Cayman Chemical. 14S-HDHA was separated from 14S/R-HDHA by using chiral liquid chromatography-photodiode array ultra-violet detector-linear ion-trap tandem mass spectrometry (LC-UV-MS/MS) as described next. 14S,21R-diHDHA was prepared by incubating 14S-HDHA (50 μg) with a human cytochrome P450 enzyme kit (BioCatalytics) at 30°C for 12 h [37]. Lipid was extracted for analysis as described [28,37]. For separation or analysis, each sample was subjected to LC-UV-MS/MS on a mass spectrometer (LTQ-XL; Thermo) equipped with a chiral column (Chiralpak-IA, 150 mm×2.1 mm×5 μm; Chiral Technologies). The mobile phase gradient was as previously described [37]. The purified 14S,21R-diHDHA was determined to be ≥95% pure by chiral LC-UV-MS/MS [37].
Renal I/R injury model and MSC transplantation
Renal I/R injury was conducted as previously described [28] with some modifications. Briefly, mice were anesthetized by an intraperitoneal injection of ketamine/xylazine, and body temperature was maintained with a thermostatically controlled heating pad (37°C). A dorsal skin incision was made, and the left renal pedicle was clamped by using an atraumatic vascular micro clamp to occlude artery and vein. After 30 min, the clamp was removed, and the muscle and skin were closed. The right kidney was subjected to the same procedures. For mMSC infusion, cells were cultured in DMEM containing 10% FBS with or without 250 nM 14S,21R-diHDHA for 12 h and collected by trypsinization (0.25% trypsin/0.53 mM ethylenediaminetetraacetic acid; Invitrogen). The cells were labeled with PKH26 red fluorescence cell linker following the company's protocol (Sigma-Aldrich). Labeling efficacy was >98%. Viability, evaluated by trypan blue exclusion, was >98%. The MSCs (106) were washed and suspended in 100 μL DMEM and injected into the tail vein after I/R. I/R control mice were subjected to the same I/R procedures and DMEM injection. Sham-operated control mice underwent the surgical procedures and DMEM injection without clamping of the renal pedicles.
Histology
Kidneys were collected at days 1 and 3 post-I/R and perfused with phosphate-buffered saline (PBS) and then with 4% paraformaldehyde. Each kidney was sagittally cut into 2 parts; one part was used to prepare paraffin sections, and the other was used for cryosections. Sections were sagittally cut. The 3 μm paraffin sections were stained with hematoxylin-eosin, and tubular damage was graded as described [40,41]. Briefly, 6 morphologic patterns were analyzed by light microscopy: (1) apical cytoplasm vacuolization; (2) tubular necrosis; (3) tubular dilatation; (4) cell detachment; (5) brush border integrity; and (6) denuded basement membrane. Morphologic changes were scored on a 5-point scale: 1, no abnormality; 2, mild lesions affecting 10% or less of the kidney specimen; 3, lesions affecting 25% of the specimen; 4, lesions affecting 50% of the specimen; and 5, lesions affecting 75% or more of the specimen. Ten nonoverlapping high-power fields (hpfs) were randomly chosen from the cortical and corticomedullary junction areas of each section (400× magnification), and a total score calculated by adding the individual scores of each hpf was used to obtain a mean score per hpf for each mouse.
Immunochemistry
Cryosections (5 μm) of kidneys were blocked with normal goat serum (1% in PBS) for 30 min at room temperature. Sections were then incubated with the following primary antibodies: rat anti-mouse Gr-1 (to detect neutrophils; BD Biosciences), rat anti-mouse F4/80 (to detect macrophages; Santa Cruz Biotechnology), American hamster anti-mouse CD11c (eBioscience) and rabbit anti-mouse MHCII (Santa Cruz Biotechnology) (to detect DCs), and rabbit anti-mouse active caspase-3 (to detect renal cell apoptosis; BD Biosciences). Primary antibodies were followed by incubation with the secondary antibodies: Cy3-labeled goat anti-rat immunoglobulin G (IgG), fluorescein isothiocyanate (FITC)-labeled goat anti-American hamster IgG, Cy5-, or FITC-labeled goat anti-rabbit IgG (Invitrogen). Normal rat IgG, rabbit IgG, and American hamster IgG (Santa Cruz Biotechnology) were used as isotype control. Nuclei were counterstained with Hochest 33342. Positive cells were counted in ten nonoverlapping hpfs (200× magnification) from the cortical and corticomedullary junction areas of each section. The mean number of positive cells per hpf for each mouse was calculated.
Creatinine analysis
Blood was collected from the tail vein or by heart puncture at days 1 and 3 post-I/R. Serum creatinine concentration (a quantitative marker of renal failure) was determined with a creatinine enzymatic assay kit (Fisher Scientific).
Study of MSC apoptosis in vivo
Most grafted MSCs in organs die within a few days [2]. A few intravenously infused MSCs are retained in the kidneys [27,42], which makes it difficult to quantitatively study the effects of 14S,21R-diHDHA on MSC apoptosis in kidneys. Therefore, we transplanted MSCs under the kidney capsule following previously reported procedures [43]. Briefly, C57BL/6J MSCs were treated with 250 nM 14S,21R-diHDHA or vehicle control for 12h and were then labeled with PKH26 red fluorescence cell linker. Renal I/R injury was conducted in C57BL/6J mice as described in “Renal I/R injury model and MSCs transplantation.” A nick was generated on the kidney capsule by scratching with a 23-gauge needle. PKH26-labeled MSCs (2×106) in 5 μL Matrigel (Growth factor reduced, BD Biosciences) were immediately transplanted through the nick underneath the capsule by using a 50 μL syringe with a flat needle. The nick was sealed by low-heat cautery (SouthPointe Surgical). Muscle and skin were closed. Kidneys were collected at days 1 and 3 post-I/R and prepared for cryosections for active caspase-3 immunostaining.
In vitro I/R model
An in vitro I/R model was established as previously described [44–47] with some modifications. MSCs were cultured in DMEM in a hypoxia chamber (95% N2, 5% CO2) for 16 h at 37°C. The cells were then transferred to normal conditions (5% CO2, 95% air) for 4 h. For inducing cell apoptosis, 800 μM H2O2 was added into culture medium during reperfusion procedure in some experiments.
Terminal deoxynucleotidyl transferase 2′-deoxyuridine, 5′-triphosphate nick-end labeling, and DNA fragment analysis
To evaluate apoptosis, MSCs (5×104) were pretreated with 14S,21R-diHDHA for 12 h and subjected to the in vitro I/R procedure (with H2O2). The terminal deoxynucleotidyl transferase 2′-deoxyuridine, 5′-triphosphate nick-end labeling (TUNEL) assay was conducted by using a kit from Roche Applied Science according to the manufacturer's instructions. TUNEL was not used for renal sections obtained from in vivo experiments, because TUNEL staining of renal sections is known to produce false positive apoptosis results [48]. Nuclei were counterstained with Hochest 33342. DNA fragments were analyzed with an enzyme-linked immunosorbent assay (ELISA) kit (Roche Applied Science).
Phosphoinositide 3-kinase—Akt signaling
Phosphoinositide 3-kinase (PI3K) activity was analyzed by ELISA (Echelon Biosciences, Inc.) [45]. Since H2O2 can affect the analysis of PI3K activity, only hypoxia conditions (95% N2, 5% CO2) were used when analyzing PI3K activity. Phosphorylation of Akt was measured by Western-blot as previously described [49]. Briefly, quiescent MSCs (106) were obtained by culturing cells in DMEM for 12 h. Cells were then treated with or without 250 nM 14S,21R-diHDHA under hypoxia conditions for 10 min, 2 h and 12 h, respectively. For analysis of PI3K activity, cells were lysed with NP-40 (Sigma-Aldrich), and PI3K was precipitated by using a PI3K p85α antibody and protein A-agarose (Santa Cruz). PI3K catalysis of the substrate PIP2 into PIP3 was then determined. For Western-blot, cells were lysed with RIPA buffer (Sigma-Aldrich) for analysis of expression of P-Akt (phosphorylated forms) and Akt (nonphosphorylated forms). Western blot was performed as described [37] with minor modifications. Briefly, 30 μg of total proteins from each lysed sample were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis on 4%–15% Tris-HCl gels (Bio-Rad). The electrotransferred protein bands were stained with primary antibody for P-Akt (Ser473) (Cell Signaling Technology), followed by fluorescent-labeled secondary antibody (LI-COR). Bands were quantified by using an Odyssey Imaging System (LI-COR). Blots were stripped and reprobed with antibody for Akt (Cell Signaling Technology). Anti-β Actin antibody (Sigma-Aldrich) was used as an internal control.
Viability assay
After pretreatment with 14S,21R-diHDHA, or with the PI3K inhibitor NVP-BEZ235 (Fisher Scientific), or with both 14S,21R-diHDHA and NVP-BEZ235 for 12 h, MSCs (5×104) were subjected to in vitro I/R procedures (with H2O2) and then incubated with 0.5 mg/mL 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT; Sigma-Aldrich) for 3 h at 37°C. The amount of blue formazan dye formed from MTT was proportional to the number of live cells. The optical densities were measured at 540 nm with a reference wavelength of 630 nm by a microplate reader (Molecular Devices) and used as an index of cell viability.
Cytokine and ROS generation analysis
Quiescent mMSCs or hMSCs (5×104) were treated with 14S,21R-diHDHA (250 nM) in DMEM (under hypoxia condition) or RPMI1640 for 12 h. The culture media were collected and used as MSC-conditioned media. RAW264.7 macrophages were cultured in mMSC-conditioned media (RPMI1640) for 3 h. The media were replaced with fresh RPMI1640 without glucose, and macrophages were subjected to I/R procedure (without H2O2). Supernatants were collected and used for analysis of TNF-α and interleukin-1β (IL-1β) secreted by macrophages. ROS generation in macrophages was assessed by using the probe 2,7-dichlorofluorescein diacetate (DCFH-DA, 10 μM; Sigma). After I/R (without H2O2) treatment, macrophages were cultured with DCFH-DA in RPMI-1640 for 30 min, and DCFH-DA fluorescence was measured by using an excitation wavelength of 480 nm and an emission wavelength of 535 nm [50]. HGF and IGF-1 in MSC conditioned media (DMEM, under hypoxia condition) were analyzed by ELISA kits obtained from RayBiotech and R&D Systems, respectively.
Statistical analysis
Results were analyzed by 1-way analysis of variance (ANOVA) followed by Fisher's least significant difference post-hoc test and expressed as mean±standard error of the mean. 2-way ANOVA was used for the analysis of creatinine, neutrophils, macrophages, and DCs in kidneys, as well as apoptotic MSCs in kidneys. A P value less than 0.05 was considered significant.
Results
14S,21R-diHDHA treatment promotes the capacity of mMSCs to mitigate loss of kidney function and histological structures impaired by I/R injury
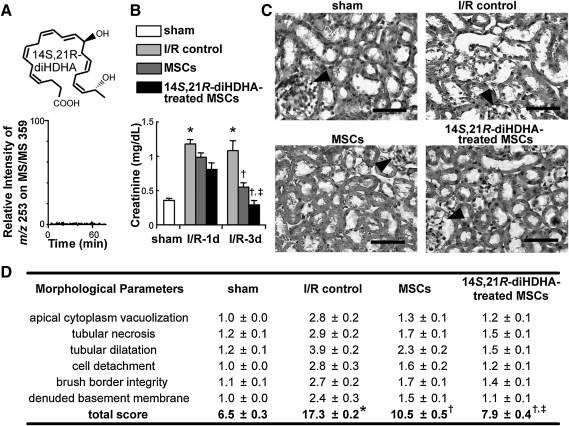
To determine whether 14S,21R-diHDHA-treated MSCs carry this compound when infused, we analyzed its level in MSCs by LC-MS/MS. 14S,21R-diHDHA was not detectable in MSC pellets after its treatment of MSCs for 12 h (Fig. 1A). To determine the effect of 14S,21R-diHDHA treatment on mMSC amelioration of I/R injury, mice were subjected to I/R injury followed by mMSC infusion, and renal function was assessed by serum creatinine level. We found that I/R injury increased serum creatinine compared with sham at day 1 (1.2±0.06 vs. 0.35±0.04 mg/dL, P<0.01) and day 3 (1.08±0.14 vs. 0.35±0.04 mg/dL, P<0.05) post-I/R (Fig. 1B), which is in agreement with previous reports [8,51]. Mice transplanted with mMSCs exhibited lower serum creatinine levels than the I/R control at day 1 (0.98±0.06 mg/dL, P=0.08) and day 3 (0.6±0.05 mg/dL, P<0.05) post-I/R, which is also consistent with a previous report [8]. However, 14S,21R-diHDHA-treated mMSCs were more effective than untreated mMSCs in reducing serum creatinine (0.8±0.09 mg/dL, P=0.07 at day 1 and 0.3±0.05 mg/dL, P<0.01 at day 3 post-I/R (Fig. 1B).
FIG. 1.
14S,21R-diHDHA-treated mMSCs mitigate deterioration of renal function and histological structures after renal ischemia/reperfusion injury. After I/R, mice received Dulbecco's modified Eagle's medium, PKH26-labeled mMSCs, or 14S,21R-diHDHA-treated PKH26-labeled mMSCs. (A) 14S,21R-diHDHA was not detectable in the MSC pellets after its treatment of MSCs, because there was no peak of this compound in the liquid chromatography-linear ion-trap tandem mass spectrometry selective reaction monitoring chromatogram (m/z 359>253). (B) Serum creatinine (days 1 and 3; n=6). (C) Hematoxylineosin–stained kidney (day 3; magnification, ×400; scale bar=50 μm). Arrowheads show glomeruli. (D) Tubular damage (n=6). Results are expressed as mean±SEM. *P<0.05 versus sham, †P<0.05 versus I/R control, ‡P<0.05 versus MSCs. 14S,21R-diHDHA, 14S,21R-dihydroxy-docosa-4Z,7Z,10Z,12E,16Z,19Z-hexaenoic acid; I/R, ischemia/reperfusion; mMSCs, murine mesenchymal stem cells; SEM, standard error of the mean.
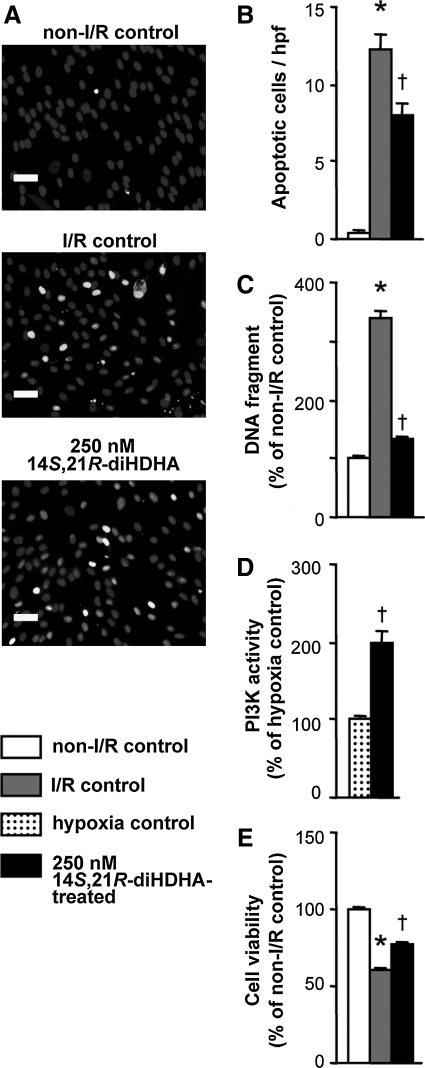
Histologic analysis of the kidneys at day 3 post-I/R revealed considerable tubular damage of the cortical and corticomedullary junction areas with apical cytoplasm vacuolization, tubular necrosis, tubular dilatation, cell detachment, loss of brush border, and denuded basement membrane, thus resulting in a higher injury score (17.3±0.2) than in sham controls (6.5±0.3, P<0.001; Fig. 1C, D). Infusion of untreated MSCs after I/R reduced the injury score to 10.5±0.5 (P<0.001), but infusion of 14S,21R-diHDHA-treated MSCs further reduced the tubular damage score compared with the untreated MSC group (7.9±0.4, P<0.001; Fig. 1C, D), thus demonstrating that 14S,21R-diHDHA improved MSC amelioration of I/R-induced tubular damage.
14S,21R-diHDHA treatment of mMSCs ameliorates kidney I/R injury through anti-apoptotic and anti-inflammatory mechanisms
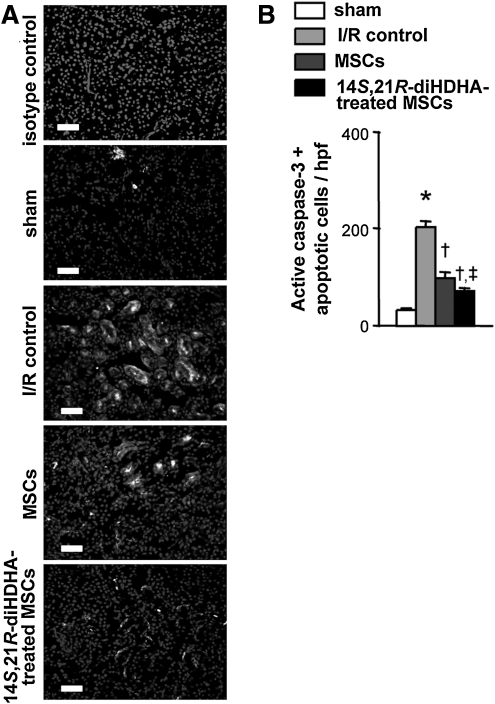
Apoptosis is a critical mechanism for renal lesions in I/R injury [5,8,9]. MSC infusion attenuates renal cell apoptosis, thereby protecting kidneys [5,8,9]. Thus, we evaluated whether 14S,21R-diHDHA treatment increased MSC renal protection by enhancing MSC anti-apoptotic actions. Consistent with a previous report [8], more apoptotic (active caspase-3+) cells were detected in I/R-injured kidneys than in kidneys of sham controls (200.6±12.8 vs. 30.4±2.5 active caspase-3+ cells/hpf, P<0.001; Fig. 2). As expected, MSC treatment reduced the number of apoptotic cells (100.8±8.8 active caspase-3+ cells/hpf, P<0.001; Fig. 2); moreover, 14S,21R-diHDHA-treated MSCs reduced the number of apoptotic cells in the kidneys (72.2±6.8 active caspase-3+ cells/hpf, P<0.01) compared with non-treated MSCs (Fig. 2), thus demonstrating that 14S,21R-diHDHA treatment enhanced the anti-apoptotic action of MSCs.
FIG. 2.
14S,21R-diHDHA treatment enhances anti-apoptosis action of mMSCs in kidneys after renal ischemia/reperfusion injury. Immunostaining against active caspase-3 was performed at day 3 post-I/R. (A) Representative kidney sections (magnification, ×200; scale bar=50 μm). (B) Active caspase-3+ apoptotic cells (cells/hpf ). Results are expressed as mean±SEM, n=6. *P<0.05 versus sham, †P<0.05 versus I/R control, ‡P<0 .05 versus MSCs.
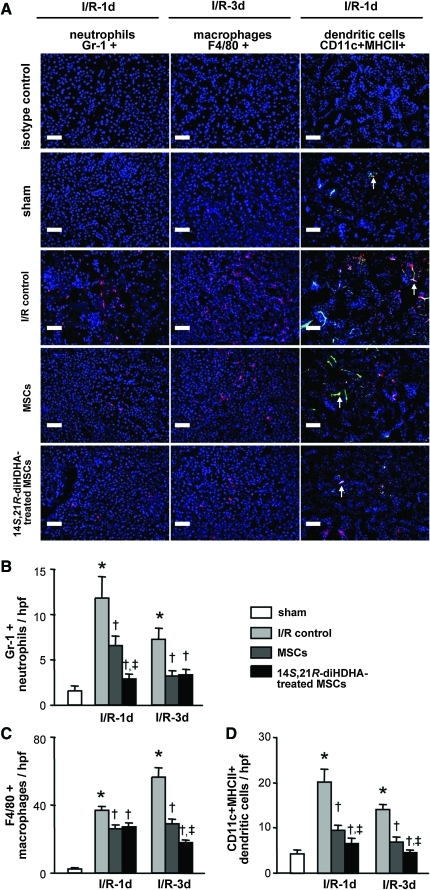
Inflammation caused by leukocytes plays an important role in I/R injury [20]. To investigate whether anti-inflammatory mechanisms were involved in the enhancement of MSC actions, we assessed neutrophil, macrophage, and DC infiltration of kidneys at days 1 and 3 post-I/R. The number of infiltrated neutrophils was clearly higher in the cortical and corticomedullary junction areas of I/R-injured kidneys at day 1 post-I/R than in kidneys of sham controls (11.8±2.4 vs. 1.6±0.5 neutrophils/hpf, P<0.001; Fig. 3A, B). Transplanted MSCs attenuated neutrophil infiltration (6.7±1.0 neutrophils/hpf, P<0.05) compared with the I/R control. More strikingly, treatment with 14S,21R-diHDHA increased the effect of MSCs on neutrophil infiltration at day 1 post-I/R (3.0±0.4 neutrophils/hpf, P<0.001; Fig. 3A, B). Similarly, massive macrophage infiltration was observed in the cortical and corticomedullary junction regions of I/R-injured kidneys at day 3 post-I/R, whereas a few macrophages were observed in the sham controls (57.1±4.4 vs. 2.5±0.8 macrophages/hpf, P<0.001; Fig. 3A, C). In MSC-treated mice, the number of macrophages in I/R-injured kidneys was clearly reduced compared with I/R controls (29.0±2.3 macrophages/hpf, P<0.001; Fig. 3A, C). Treatment with 14S,21RdiHDHA improved the anti-inflammatory action of MSCs at day 3 post-I/R (17.8±2.1 macrophages/hpf, P<0.001; Fig 3A, C), although no difference was found between mice transplanted with 14S,21R-diHDHA-treated MSCs and nontreated MSC controls at day 1 post-I/R (P=0.34; Fig. 3C). During I/R injury, TNF-α causes an influx of pro-inflammatory DCs into kidneys and contributes to parenchymal damage [52]. CD11c+MHCII+ DCs were increased in I/R injured kidneys (Fig. 3A, D). However, MSC infusion considerably decreased DC influx compared with I/R control at day 1 post-I/R (9.6±1.1 vs. 20.1±2.9 DCs/hpf, P<0.001) and day 3 post-I/R (7.0±0.9 vs. 14.0±1.2 DCs/hpf, P<0.001) (Fig. 3A, D). MSCs treated with 14S,21R-diHDHA further decreased infiltrated DCs in kidneys compared with nontreated MSCs at day 1 (P<0.05) and day 3 (P<0.05) post-I/R, thus suggesting that 14S,21R-diHDHA treatment improved the anti-inflammatory action of MSCs (Fig. 3A, D).
FIG. 3.
14S,21R-diHDHA treatment promotes anti-inflammatory action of mMSCs after renal ischemia/reperfusion injury. Immunostaining against Gr-1, F4/80, CD11c, and MHCII were performed on kidney sections collected at days 1 and 3 post-I/R. (A) Representative kidney sections showing Gr-1+ neutrophils, F4/80+ macrophages, and CD11c+MHCII+ DCs (arrows) at day 1 or 3 post-I/R (magnification, ×200; scale bar=50 μm). Quantification of Gr 1+ neutrophils (B), F4/80+ macrophages (C), and CD11c+ MHCII+ DCs (D) in kidney. Results are expressed as mean±SEM, n=6. *P<0.05 versus sham, †P<0.05 versus I/R control, ‡P<0.05 versus MSCs. Color images available online at www.liebertonline.com/scd
14S,21R-diHDHA treatment inhibits apoptosis of MSCs in vivo
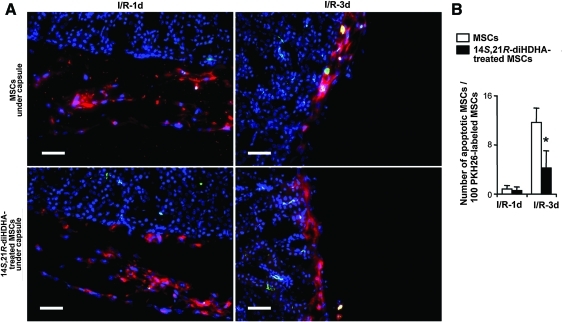
Since treatment with 14S,21R-diHDHA enhanced the ability of MSCs to ameliorate I/R injury (Fig. 1), coupled with the fact that increased MSC survival is associated with enhanced MSC promotion of kidney recovery from I/R injury [8,9], we hypothesized that treatment with 14S,21R-diHDHA inhibited apoptosis of MSCs. To test this hypothesis, we assessed apoptosis of PKH26-labeled MSCs transplanted under kidney capsule. Nearly 11.7%±2.4% of grafted MSCs at day 3 post-I/R were active caspase-3+ and underwent apoptosis (Fig. 4). However, treatment with 14S,21R-diHDHA decreased apoptosis of transplanted MSCs as shown by decreased active caspase-3+ cells (4.2%±2.8%, P<0.05; Fig. 4), thus suggesting that 14S,21R-diHDHA inhibits apoptosis.
FIG. 4.
14S,21R-diHDHA treatment decreases apoptosis of mMSCs in kidneys after renal ischemia/reperfusion injury. PKH26-labeled mMSCs in matrigel were transferred under kidney capsules of mice immediately after I/R. Kidneys were collected at days 1 and 3 post-I/R and prepared for cryosections that were then subjected to active caspase-3 staining. (A) Apoptotic PKH26-labeled mMSCs (yellow stained appearance resulting from the overlap of red PKH26+ labeling of MSCs and green apoptosis marker of active caspase-3+) in cryosections of kidney. (B) Quantification of apoptotic PKH26-lableled mMSCs in kidney: number of active caspase-3+ PKH26-labeled mMSCs/100 PKH26-labeled mMSCs (n=15). Magnification, ×200. Scale bar=50 μm. Results are expressed as mean±SEM. *P<0.05 compared with MSC control without treatment. Color images available online at www.liebertonline.com/scd
Enhanced MSC survival after 14S,21R-diHDHA treatment involves PI3K activation
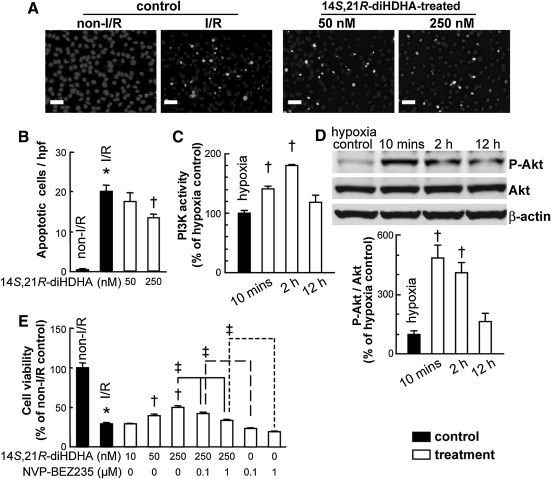
To confirm the action of 14S,21R-diHDHA on viability and apoptosis of MSCs in vivo in kidneys, and to explore possible mechanisms, we subjected mMSCs to the in vitro I/R procedure, that is, hypoxia followed by H2O2 [8,45]. Results of TUNEL analysis revealed that apoptosis induced by in vitro I/R was reduced by 14S,21R-diHDHA treatment (non-I/R control: 0.4±0.2 TUNEL+ cells/hpf; I/R control: 20±1.8 TUNEL+ cells/hpf; 250 nM 14S,21R-diHDHA: 13.4±0.9 TUNEL+ cells/hpf, P<0.01 compared with I/R control; Fig. 5A, B).
FIG. 5.
14S,21R-diHDHA treatment reduces apoptosis of mMSCs and promotes viability of mMSCs via a mechanism involving activation of PI3K-Akt. After in vitro I/R treatment (Ischemia: 95% N2, 5% CO2, reperfusion: 5% CO2, 95% air and treated with 800 μM H2O2), mMSC apoptosis, viability, or PI3K-Akt signaling (under hypoxia: 95% N2, 5%CO2) was analyzed. (A) Representative micrographs of TUNEL stained apoptotic mMSCs. (B) Quantification mMSC apoptosis: the number of TUNEL+ apoptotic cells/hpf (n=15; magnification, ×200; scale bar=50 μm). (C) PI3K activity of mMSCs (n=3). (D) mMSC lysates were analyzed by western blot. P-Akt quantities relative to Akt were measured based on optical densities of the gel bands, and normalized to that of hypoxia control (n=4). (E) mMSC viability was decreased by PI3K inhibition. The mMSCs were pretreated with 14S,21R-diHDHA, PI3K inhibitor NVP-BEZ235, or 14S,21R-diHDHA+NVP-BEZ235 and then subjected to in vitro simulated I/R treatment. Cell viability was analyzed by MTT (n=6). Results are expressed as mean±SEM. *P<0.05 versus non-I/R. †P<0.05 versus I/R or hypoxia controls. ‡P<0.05 for the marked comparison. TUNEL, terminal deoxynucleotidyl transferase 2′-deoxyuridine, 5′-triphosphate nick-end labeling; PI3K, phosphoinositide 3-kinase; MTT, 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide.
PI3K-Akt signaling regulates cell survival; therefore, its activation may enhance MSC viability and protect against apoptosis [53]. For this reason, we investigated the effect of 14S,21R-diHDHA on PI3K activation in MSCs. We found that PI3K activity was rapidly induced in mMSCs by 14S,21R-diHDHA treatment (P<0.01; Fig. 5C). Akt plays an important role in controlling cell survival and apoptosis [53,54] through phosphorylating and inactivating several targets, including Bcl-2 homology domain 3-only protein (BAD), Forkhead box transcription factor, class O (FOXO), and caspase-9 [55]. Treatment with 14S,21R-diHDHA triggered phosphorylation of Akt (Fig. 5D). Results of MTT analysis showed that in vitro I/R reduced MSC viability, but 14S,21R-diHDHA treatment restored viability in a dose-dependent manner (Fig. 5E). Conversely, the increase in MSC viability mediated by 14S,21R-diHDHA was diminished by blocking PI3K activation (Fig. 5E). Taken together, these results indicate that treatment with 14S,21R-diHDHA enhances viability and inhibits apoptosis of MSCs, at least in part, by activating the PI3K-Akt signaling pathway.
To determine whether these findings are applicable to hMSCs, we evaluated the effect of 14S,21R-diHDHA treatment on hMSC viability and apoptosis under the same in vitro I/R conditions. As predicted, 14S,21R-diHDHA treatment reduced hMSC apoptosis (Fig. 6A, B) and DNA fragmentation (Fig. 6C). Additionally, 14S,21R-diHDHA activated the PI3K (Fig. 6D) and increased hMSC viability (Fig. 6E).
FIG. 6.
14S,21R-diHDHA treatment decreases apoptosis of hMSCs, increases their viability, and increases activation of PI3K. After 14S,21R-diHDHA pretreatment, hMSCs were subjected to simulated I/R (with H2O2) or hypoxia. (A) Apoptotic cells were stained by TUNEL and (B) quantified: number of TUNEL+ apoptotic cells/hpf (n=15, magnification, ×200. Scale bar=50 μm.). (C) DNA fragment was analyzed by ELISA kit (n=4). (D) PI3K activity. Cells were treated with 14S,21R-diHDHA under hypoxia conditions. Activity of PI3K was analyzed by ELISA (n=3). (E) Cell viability was analyzed by MTT (n=3). Results are presented as mean±SEM. *P<0.05 versus non-I/R control. †P<0.05 versus I/R control or hypoxia control. hMSCs, human MSCs; ELISA, enzyme-linked immunosorbent assay.
14S,21R-diHDHA treatment enhances mMSC anti-inflammatory function in vitro by suppressing the generation of inflammatory cytokines and ROS in RAW264.7 macrophages
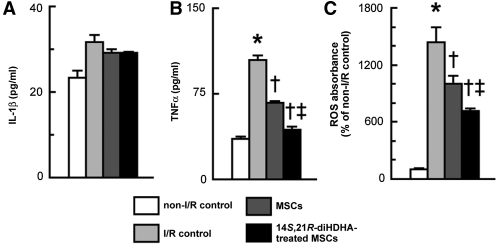
Acute kidney injury has been shown to induce macrophages to produce inflammatory cytokines and ROS that play crucial roles in inflammation and injury processes of kidneys [20,28]. In order to determine whether 14S,21R-diHDHA treatment enhances MSC anti-inflammatory function, we studied the effect of 14S,21R-diHDHA-treated MSCs on the generation of inflammatory cytokines and ROS by RAW264.7 macrophages under I/R conditions. Although 14S,21R-diHDHA-treated MSCs had no effect on IL-1β generation (Fig. 7A), MSC conditioned media reduced the generation of TNF-α (67.1±1.4 vs. 104.0±3.8 pg/mL, P<0.01) from RAW264.7 macrophages compared with I/R controls (Fig. 7B). When 14S,21R-diHDHA-treated MSC conditioned medium was used, the level of TNF-α was further decreased to 43.1±2.8 pg/mL (P<0.01 compared with MSC conditioned medium; Fig. 7B). ROS generation in RAW264.7 macrophages can be induced by I/R treatment. However, MSCs reduced ROS levels in RAW264.7 cells (P<0.05), and 14S,21R-diHDHA treated MSCs further decreased ROS induction by I/R treatment (P<0.01; Fig. 7C). These results suggest that 14S,21R-diHDHA treatment enhances MSC anti-inflammatory function by reducing the capacity of macrophages to generate inflammatory cytokines and ROS.
FIG. 7.
14S,21R-diHDHA treatment enhances anti-inflammatory functions of mMSC in vitro by suppressing the generation of TNF-α and ROS from RAW264.7 macrophages. RAW264.7 macrophages were treated with mMSC conditioned medium for 3 h. Medium was replaced with fresh RPMI1640 without glucose, and macrophages were subjected to I/R treatment (Ischemia: 95% N2, 5% CO2, Reperfusion: 5% CO2, 95% air without H2O2). (A) IL-1β and (B) TNF-α secretion from RAW264.7 cells (n=4). (C) ROS generation in cells was assessed by using the probe, 2,7-dichlorofluorescein diacetate (n=4). Results are expressed as mean±SEM. *P<0.05 versus normal control, †P<0.05 versus I/R control, ‡P<0.05 versus MSCs. IL-1β, interleukin-1β; TNF-α, tumor necrosis factor-α; ROS, reactive oxygen species.
14S,21R-diHDHA treatment enhances mMSC and hMSC secretion of renotrophic growth factors
MSCs generate an array of cytokines and growth factors [15,56] that protect kidneys from injuries [15–17,57]. Therefore, we postulated that 14S,21R-diHDHA treatment enhanced the beneficial effects of MSCs by augmenting their secretion of renotrophic factors. We found that 14S,21R-diHDHA treatment increased the amount of the renotrophic growth factors IGF-1 and HGF secreted by mMSCs into the cell culture medium (IGF-1: 15.3±1.0 vs. 10.7±1.5 pg/mL, P<0.05; HGF: 405.3±10.6 vs. 277.5±2.9 pg/mL, P<0.01; Fig. 8A, B). Treatment with 14S,21R-diHDHA also augmented the generation of HGF and IGF-1 by hMSCs (IGF-1: 1982.7±194.3 vs. 1208.0±115.4 pg/mL, P<0.05; HGF: 36.5±2.6 vs. 18.7±1.7 pg/mL, P<0.001; Fig. 8C, D).
FIG. 8.
14S,21R-diHDHA treatment increases the production of IGF-1 and HGF by mMSCs and hMSCs in vitro. mMSCs or hMSCs (5×104) were treated with 4S,21R-diHDHA (250 nM) under hypoxia condition (95% N2, 5% CO2) for 12 h. The level of IGF-1 or HGF in 2 culture supernatants was analyzed by ELISA. (A) IGF-1 and (B) HGF secretion from mMSCs. (C) IGF-1 and (D) HGF secretion from hMSCs (n=4). Results are presented as mean±SEM. *P<0.05 versus control. IGF-1, insulin growth factor-1; HGF, hepatocyte growth factor.
Discussion
Compared with untreated MSCs, 14S,21R-diHDHA-treated MSCs more efficiently improved renal function by suppressing I/R-induced elevation of serum creatinine (Fig. 1), restoring I/R-damaged renal histological structures, and decreasing tubular cell apoptosis (Fig. 2).
Tubular cell damage is an important characteristic of acute kidney injury, and the elevation of serum creatinine indicates impaired renal functions [6–9,15]. Apoptosis is an early event in renal I/R injury that interacts with the subsequent inflammation and tissue injury. Our data demonstrate that 14S,21R-diHDHA treatment improves the capacity of transplanted MSCs to ameliorate I/R injury. The observed effects were not likely mediated by direct transfer of free 14S,21R-diHDHA, because this LM was not detectable in MSC pellets after treatment for 12 h (Fig. 1A), and MSCs were resuspended in fresh 14S,21R-diHDHA-free DMEM before infusion. Although 14S,21R-diHDHA was not detectable in renal tissue by LC-MS/MS, it is known to promote wound healing [37,38,58], and its potential use as a therapeutic alone in treating I/R injury should be assessed in addition to being used as a promoter of MSC renotrophic functions under the scope of this report.
Both animal studies and clinical studies have demonstrated the safety, feasibility, and efficacy of MSC-based therapies for repair of acute kidney injury [6–9,15]. However, the mechanisms by which MSCs ameliorate I/R injury are not clear. Several studies have demonstrated the capacity of MSC to differentiate into kidney cells [11–14]. Controversial studies indicated that the grafted MSCs were not the predominant cellular components involved in the regeneration of injured tubuli [7,59], and it has been postulated that, rather than differentiation, cell fusion accounts for these cells [7,59–61]. Recent studies have ascribed reparative effects of MSCs to their endocrine/paracrine functions [15–19]. MSCs can generate different cytokines and growth factors that are important to injury recovery [17,57,62, 63], including renotrophic factors IGF-1 and HGF (Fig. 8). We found that 14S,21R-diHDHA promoted generation of these two growth factors by MSCs (Fig. 8).
Renal ischemia and reperfusion results in leukocyte infiltration and activation, which play crucial roles in kidney inflammation and injury [28]. Treatment with 14S,21R-diHDHA enhanced the capacity of MSCs to suppress neutrophil, macrophage, and DC infiltration in kidneys post I/R (Fig. 3), which parallels the enhanced capacity of 14S,21R-diHDHA-treated MSCs to inhibit renal tubular cell death in kidneys (Figs. 1 and 2). Depletion of macrophages during acute kidney injury reduces renal inflammation and protects kidneys against ischemic injury [28,64]. Conditioned medium from 14S,21R-diHDHA-treated MSCs is more efficient at inhibiting the generation of TNF-α and ROS by macrophages than is conditioned medium from untreated MSCs (Fig. 7), which suggests that 14S,21R-diHDHA treatment promotes production of anti-inflammatory mediators by MSCs in I/R injury.
The low viability of transplanted MSCs in I/R injured organs remains an important limitation for MSC therapy [2,27]. The enhancement of MSC renotrophic functions after 14S,21R-diHDHA treatment is likely mediated in part by the promotion of MSC survival, as suggested by the reduced rate of apoptosis of MSCs grafted into I/R injured kidneys, an inhospitable environment for MSC survival (Fig. 4). This notion was further supported by in vitro experiments showing that 14S,21R-diHDHA treatment increased viability and reduced apoptosis of MSCs (Fig. 5).
Activation of PI3K-Akt signaling pathway is crucial to many aspects of cell growth and survival in adverse physiologic and pathologic conditions. PI3K activates Akt kinase, which directly binds and inactivates BAD and subsequently blocks the functions of proapoptotic proteins [65]. Akt can directly phosphorylate FOXOs and inhibit their ability to induce the expression of death genes, subsequently resulting in the inhibition of the activation of caspase-9 [55]. Many approaches to enhancing MSC viability involve activating the PI3K-Akt signaling pathway [2]. Overexpression of PI3K in cardiomyocytes significantly enhanced their survival on subsequent exposure to hypoxia [66]. Akt gene modified stem cells exhibited enhanced survival after engraftment into the infarcted heart [67]. We found that 14S,21R-diHDHA treatment activated the PI3K-Akt signaling pathway in MSCs, and that inhibition of PI3K activity in MSCs suppressed the capacity of 14S,21R-diHDHA treatment to promote MSC viability (Figs. 5 and 6), thus suggesting that the mechanism by which 14S,21R-diHDHA treatment promotes MSC survival involves, or is mediated through, the PI3K-Akt signaling pathway.
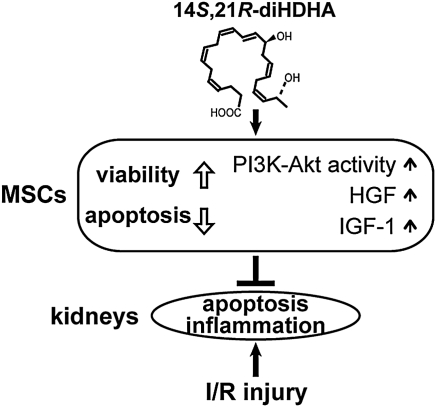
Differences between hMSCs and mMSCs were previously reported. For example, hMSCs, but not mMSCs, possess antimicrobial properties against a range of clinically relevant bacteria through their ability to up-regulate the expression of indoleamine 2,3-dioxygenase [68]. Additionally, mMSCs, but not hMSCs, express inducible nitric oxide synthase [68], and it has been shown that hMSCs and mMSCs express different chemokine receptors [69]. In view of these known differences between hMSCs and mMSCs, we intended to determine whether the results obtained from 14S,21R-diHDHA-treated mMSCs could be confirmed in hMSCs. In this regard, our results demonstrating that 14S,21R-diHDHA treatment inhibited apoptosis of hMSCs (Fig. 6) and promoted renotrophic cytokine generation (Fig. 8) enhanced the potential of using 14S,21RdiHDHA-treated hMSCs in treatment of human acute kidney injury. As summarized in Fig. 9, the LM 14S,21R-diHDHA improves MSC mitigation of renal I/R injury in both renal functions and histological structures. This improvement includes inhibiting renal tubular cell apoptosis and leukocyte infiltration and inflammatory cytokine generation. The mechanisms by which 14S,21R-diHDHA improves MSC renal protective functions involve promoting MSC survival and generation of renotrophic factors. The PI3K-Akt signaling pathway plays a role in 14S,21R-diHDHA promotion of MSC survival. Pretreatment of MSCs with 14S,21R-diHDHA, or related LMs or structural mimics, may offer a new clinical strategy for improved treatment of acute kidney injury.
FIG. 9.
Mechanisms involved in 14S,21R-diHDHA-induced improvement in the capacity of MSCs to mitigate I/R injury. This improvement includes inhibiting renal tubular cell apoptosis and leukocyte infiltration and inflammatory cytokine generation. The mechanisms by which 14S,21R-diHDHA improve MSC renal protective functions involve promotion of MSC survival, and generation of renotrophic factors. PI3K-Akt signaling participates in the enhancement of MSC survival.
Acknowledgments
The authors thank the Institute for Regenerative Medicine at Scott & White Hospital, Texas A&M Health Science Center for providing MSCs through a grant from the National Institutes of Health National Center for Research Resources (Grant No. P40RR017447). They also thank Dr. Qiang Wu and Mr. Jose A. Galindo for technical support, and Mr. Ryan R. Labadens and Dr. Eric C. B. Milner for editing. This work was sponsored by a Bridge Grant (to S.H.) from Louisiana State University Health Sciences Center, New Orleans.
Author Disclosure Statement
No conflicts of interest, financial or otherwise, are declared by the authors.
References
- 1.Togel FE. Westenfelder C. Mesenchymal stem cells: a new therapeutic tool for AKI. Nat Rev Nephrol. 2010;6:179–183. doi: 10.1038/nrneph.2009.229. [DOI] [PubMed] [Google Scholar]
- 2.Haider H. Ashraf M. Strategies to promote donor cell survival: combining preconditioning approach with stem cell transplantation. J Mol Cell Cardiol. 2008;45:554–566. doi: 10.1016/j.yjmcc.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Prockop DJ. Kota DJ. Bazhanov N. Reger RL. Evolving paradigms for repair of tissues by adult stem/progenitor cells (MSCs) J Cell Mol Med. 2010;14:2190–2199. doi: 10.1111/j.1582-4934.2010.01151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang G. Bunnell BA. Painter RG. Quiniones BC. Tom S. Lanson NA., Jr. Spees JL. Bertucci D. Peister A, et al. Adult stem cells from bone marrow stroma differentiate into airway epithelial cells: potential therapy for cystic fibrosis. Proc Natl Acad Sci U S A. 2005;102:186–191. doi: 10.1073/pnas.0406266102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lange C. Togel F. Ittrich H. Clayton F. Nolte-Ernsting C. Zander AR. Westenfelder C. Administered mesenchymal stem cells enhance recovery from ischemia/reperfusion-induced acute renal failure in rats. Kidney Int. 2005;68:1613–1617. doi: 10.1111/j.1523-1755.2005.00573.x. [DOI] [PubMed] [Google Scholar]
- 6.Togel F. Cohen A. Zhang P. Yang Y. Hu Z. Westenfelder C. Autologous and allogeneic marrow stromal cells are safe and effective for the treatment of acute kidney injury. Stem Cells Dev. 2009;18:475–485. doi: 10.1089/scd.2008.0092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Duffield JS. Park KM. Hsiao LL. Kelley VR. Scadden DT. Ichimura T. Bonventre JV. Restoration of tubular epithelial cells during repair of the postischemic kidney occurs independently of bone marrow-derived stem cells. J Clin Invest. 2005;115:1743–1755. doi: 10.1172/JCI22593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mias C. Trouche E. Seguelas MH. Calcagno F. Dignat-George F. Sabatier F. Piercecchi-Marti MD. Daniel L. Bianchi P, et al. Ex vivo pretreatment with melatonin improves survival, proangiogenic/mitogenic activity, and efficiency of mesenchymal stem cells injected into ischemic kidney. Stem Cells. 2008;26:1749–1757. doi: 10.1634/stemcells.2007-1000. [DOI] [PubMed] [Google Scholar]
- 9.Hagiwara M. Shen B. Chao L. Chao J. Kallikrein-modified mesenchymal stem cell implantation provides enhanced protection against acute ischemic kidney injury by inhibiting apoptosis and inflammation. Hum Gene Ther. 2008;19:807–819. doi: 10.1089/hum.2008.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee RH. Seo MJ. Reger RL. Spees JL. Pulin AA. Olson SD. Prockop DJ. Multipotent stromal cells from human marrow home to and promote repair of pancreatic islets and renal glomeruli in diabetic NOD/scid mice. Proc Natl Acad Sci U S A. 2006;103:17438–17443. doi: 10.1073/pnas.0608249103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Poulsom R. Forbes SJ. Hodivala-Dilke K. Ryan E. Wyles S. Navaratnarasah S. Jeffery R. Hunt T. Alison M, et al. Bone marrow contributes to renal parenchymal turnover and regeneration. J Pathol. 2001;195:229–235. doi: 10.1002/path.976. [DOI] [PubMed] [Google Scholar]
- 12.Imasawa T. Utsunomiya Y. Kawamura T. Zhong Y. Nagasawa R. Okabe M. Maruyama N. Hosoya T. Ohno T. The potential of bone marrow-derived cells to differentiate to glomerular mesangial cells. J Am Soc Nephrol. 2001;12:1401–1409. doi: 10.1681/ASN.V1271401. [DOI] [PubMed] [Google Scholar]
- 13.Rookmaaker MB. Smits AM. Tolboom H. Van 't Wout K. Martens AC. Goldschmeding R. Joles JA. Van Zonneveld AJ. Grone HJ. Rabelink TJ. Verhaar MC. Bone-marrow-derived cells contribute to glomerular endothelial repair in experimental glomerulonephritis. Am J Pathol. 2003;163:553–562. doi: 10.1016/S0002-9440(10)63683-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Prodromidi EI. Poulsom R. Jeffery R. Roufosse CA. Pollard PJ. Pusey CD. Cook HT. Bone marrow-derived cells contribute to podocyte regeneration and amelioration of renal disease in a mouse model of Alport syndrome. Stem Cells. 2006;24:2448–2455. doi: 10.1634/stemcells.2006-0201. [DOI] [PubMed] [Google Scholar]
- 15.Togel F. Hu Z. Weiss K. Isaac J. Lange C. Westenfelder C. Administered mesenchymal stem cells protect against ischemic acute renal failure through differentiation-independent mechanisms. Am J Physiol Renal Physiol. 2005;289:F31–F42. doi: 10.1152/ajprenal.00007.2005. [DOI] [PubMed] [Google Scholar]
- 16.Horwitz EM. Prather WR. Cytokines as the major mechanism of mesenchymal stem cell clinical activity: expanding the spectrum of cell therapy. Isr Med Assoc J. 2009;11:209–211. [PubMed] [Google Scholar]
- 17.Iwatani H. Imai E. Kidney repair using stem cells: myth or reality as a therapeutic option? J Nephrol. 2010;23:143–146. [PubMed] [Google Scholar]
- 18.Imberti B. Morigi M. Tomasoni S. Rota C. Corna D. Longaretti L. Rottoli D. Valsecchi F. Benigni A, et al. Insulin-like growth factor-1 sustains stem cell mediated renal repair. J Am Soc Nephrol. 2007;18:2921–2928. doi: 10.1681/ASN.2006121318. [DOI] [PubMed] [Google Scholar]
- 19.Chen Y. Qian H. Zhu W. Zhang X. Yan Y. Ye S. Peng X. Li W. Xu W. Hepatocyte growth factor modification promotes the amelioration effects of human umbilical cord mesenchymal stem cells on rat acute kidney injury. Stem Cells Dev. 2011;20:103–113. doi: 10.1089/scd.2009.0495. [DOI] [PubMed] [Google Scholar]
- 20.Akcay A. Nguyen Q. Edelstein CL. Mediators of inflammation in acute kidney injury. Mediators Inflamm. 2009;2009:137072. doi: 10.1155/2009/137072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kinsey GR. Li L. Okusa MD. Inflammation in acute kidney injury. Nephron Exp Nephrol. 2008;109:e102–e107. doi: 10.1159/000142934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maggini J. Mirkin G. Bognanni I. Holmberg J. Piazzon IM. Nepomnaschy I. Costa H. Canones C. Raiden S. Vermeulen M. Geffner JR. Mouse bone marrow-derived mesenchymal stromal cells turn activated macrophages into a regulatory-like profile. PLoS One. 2010;5:e9252. doi: 10.1371/journal.pone.0009252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.English K. Barry FP. Mahon BP. Murine mesenchymal stem cells suppress dendritic cell migration, maturation and antigen presentation. Immunol Lett. 2008;115:50–58. doi: 10.1016/j.imlet.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 24.Kang JW. Kang KS. Koo HC. Park JR. Choi EW. Park YH. Soluble factors-mediated immunomodulatory effects of canine adipose tissue-derived mesenchymal stem cells. Stem Cells Dev. 2008;17:681–693. doi: 10.1089/scd.2007.0153. [DOI] [PubMed] [Google Scholar]
- 25.Okunishi K. Dohi M. Nakagome K. Tanaka R. Mizuno S. Matsumoto K. Miyazaki J. Nakamura T. Yamamoto K. A novel role of hepatocyte growth factor as an immune regulator through suppressing dendritic cell function. J Immunol. 2005;175:4745–4753. doi: 10.4049/jimmunol.175.7.4745. [DOI] [PubMed] [Google Scholar]
- 26.Daemen MA. van 't Veer C. Denecker G. Heemskerk VH. Wolfs TG. Clauss M. Vandenabeele P. Buurman WA. Inhibition of apoptosis induced by ischemia-reperfusion prevents inflammation. J Clin Invest. 1999;104:541–549. doi: 10.1172/JCI6974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Burst VR. Gillis M. Putsch F. Herzog R. Fischer JH. Heid P. Muller-Ehmsen J. Schenk K. Fries JW. Baldamus CA. Benzing T. Poor cell survival limits the beneficial impact of mesenchymal stem cell transplantation on acute kidney injury. Nephron Exp Nephrol. 2010;114:e107–e116. doi: 10.1159/000262318. [DOI] [PubMed] [Google Scholar]
- 28.Duffield JS. Hong S. Vaidya VS. Lu Y. Fredman G. Serhan CN. Bonventre JV. Resolvin D series and protectin D1 mitigate acute kidney injury. J Immunol. 2006;177:5902–5911. doi: 10.4049/jimmunol.177.9.5902. [DOI] [PubMed] [Google Scholar]
- 29.Marcheselli VL. Mukherjee PK. Arita M. Hong S. Antony R. Sheets K. Winkler JW. Petasis NA. Serhan CN. Bazan NG. Neuroprotectin D1/protectin D1 stereoselective and specific binding with human retinal pigment epithelial cells and neutrophils. Prostaglandins Leukot Essent Fatty Acids. 2010;82:27–34. doi: 10.1016/j.plefa.2009.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marcheselli VL. Hong S. Lukiw WJ. Tian XH. Gronert K. Musto A. Hardy M. Gimenez JM. Chiang N. Serhan CN. Bazan NG. Novel docosanoids inhibit brain ischemia-reperfusion-mediated leukocyte infiltration and pro-inflammatory gene expression. J Biol Chem. 2003;278:43807–43817. doi: 10.1074/jbc.M305841200. [DOI] [PubMed] [Google Scholar]
- 31.Serhan CN. Clish CB. Brannon J. Colgan SP. Chiang N. Gronert K. Novel functional sets of lipid-derived mediators with antiinflammatory actions generated from omega-3 fatty acids via cyclooxygenase 2-nonsteroidal antiinflammatory drugs and transcellular processing. J Exp Med. 2000;192:1197–1204. doi: 10.1084/jem.192.8.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hong S. Tjonahen E. Morgan EL. Lu Y. Serhan CN. Rowley AF. Rainbow trout (Oncorhynchus mykiss) brain cells biosynthesize novel docosahexaenoic acid-derived resolvins and protectins-Mediator lipidomic analysis. Prostaglandins Other Lipid Mediat. 2005;78:107–116. doi: 10.1016/j.prostaglandins.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 33.Bazan HA. Lu Y. Thoppil D. Fitzgerald TN. Hong S. Dardik A. Diminished omega-3 fatty acids are associated with carotid plaques from neurologically symptomatic patients: implications for carotid interventions. Vascul Pharmacol. 2009;51:331–336. doi: 10.1016/j.vph.2009.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hassan IR. Gronert K. Acute changes in dietary omega-3 and omega-6 polyunsaturated fatty acids have a pronounced impact on survival following ischemic renal injury and formation of renoprotective docosahexaenoic acid-derived protectin D1. J Immunol. 2009;182:3223–3232. doi: 10.4049/jimmunol.0802064. [DOI] [PubMed] [Google Scholar]
- 35.Mukherjee PK. Marcheselli VL. Serhan CN. Bazan NG. Neuroprotectin D1: a docosahexaenoic acid-derived docosatriene protects human retinal pigment epithelial cells from oxidative stress. Proc Natl Acad Sci U S A. 2004;101:8491–8496. doi: 10.1073/pnas.0402531101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhao Y. Calon F. Julien C. Winkler JW. Petasis NA. Lukiw WJ. Bazan NG. Docosahexaenoic acid-derived neuroprotectin D1 induces neuronal survival via secretase- and PPARgamma-mediated mechanisms in Alzheimer's disease models. PLoS One. 2011;6:e15816. doi: 10.1371/journal.pone.0015816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tian H. Lu Y. Shah SP. Hong S. 14S,21RDihydroxydocosahexaenoic acid remedies impaired healing and mesenchymal stem cell functions in diabetic wounds. J Biol Chem. 2011;286:4443–4453. doi: 10.1074/jbc.M110.100388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lu Y. Tian H. Hong S. Novel 14,21-dihydroxy-docosahexaenoic acids: structures, formation pathways, and enhancement of wound healing. J Lipid Res. 2010;51:923–932. doi: 10.1194/jlr.M000059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Linden T. Katschinski DM. Eckhardt K. Scheid A. Pagel H. Wenger RH. The antimycotic ciclopirox olamine induces HIF-1alpha stability, VEGF expression, and angiogenesis. Faseb J. 2003;17:761–763. doi: 10.1096/fj.02-0586fje. [DOI] [PubMed] [Google Scholar]
- 40.Goujon JM. Hauet T. Menet E. Levillain P. Babin P. Carretier M. Histological evaluation of proximal tubule cell injury in isolated perfused pig kidneys exposed to cold ischemia. J Surg Res. 1999;82:228–233. doi: 10.1006/jsre.1998.5526. [DOI] [PubMed] [Google Scholar]
- 41.Tirapelli LF. Barione DF. Trazzi BF. Tirapelli DP. Novas PC. Silva CS. Martinez M. Costa RS. Tucci S, Jr., et al. Comparison of two models for evaluation histopathology of experimental renal ischemia. Transplant Proc. 2009;41:4083–4087. doi: 10.1016/j.transproceed.2009.09.061. [DOI] [PubMed] [Google Scholar]
- 42.Nemeth K. Leelahavanichkul A. Yuen PS. Mayer B. Parmelee A. Doi K. Robey PG. Leelahavanichkul K. Koller BH, et al. Bone marrow stromal cells attenuate sepsis via prostaglandin E(2)-dependent reprogramming of host macrophages to increase their interleukin-10 production. Nat Med. 2009;15:42–49. doi: 10.1038/nm.1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Curtis LM. Chen S. Chen B. Agarwal A. Klug CA. Sanders PW. Contribution of intrarenal cells to cellular repair after acute kidney injury: subcapsular implantation technique. Am J Physiol Renal Physiol. 2008;295:F310–F314. doi: 10.1152/ajprenal.90205.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Luo L. Lu J. Wei L. Long D. Guo JY. Shan J. Li FS. Lu PY. Li PY. Feng L. The role of HIF-1 in up-regulating MICA expression on human renal proximal tubular epithelial cells during hypoxia/reoxygenation. BMC Cell Biol. 2010;11:91. doi: 10.1186/1471-2121-11-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Keyes KT. Ye Y. Lin Y. Zhang C. Perez-Polo JR. Gjorstrup P. Birnbaum Y. Resolvin E1 protects the rat heart against reperfusion injury. Am J Physiol Heart Circ Physiol. 2010;299:H153–H164. doi: 10.1152/ajpheart.01057.2009. [DOI] [PubMed] [Google Scholar]
- 46.Li M. Khan AM. Maderdrut JL. Simon EE. Batuman V. The effect of PACAP38 on MyD88-mediated signal transduction in ischemia-/hypoxia-induced acute kidney injury. Am J Nephrol. 2010;32:522–532. doi: 10.1159/000321491. [DOI] [PubMed] [Google Scholar]
- 47.Rusai K. Wagner B. Roos M. Schmaderer C. Strobl M. Boini KM. Grenz A. Kuhl D. Heemann U. Lang F. Lutz J. The serum and glucocorticoid-regulated kinase 1 in hypoxic renal injury. Cell Physiol Biochem. 2009;24:577–584. doi: 10.1159/000257527. [DOI] [PubMed] [Google Scholar]
- 48.Pulkkanen KJ. Laukkanen MO. Naarala J. Yla-Herttuala S. False-positive apoptosis signal in mouse kidney and liver detected with TUNEL assay. Apoptosis. 2000;5:329–333. doi: 10.1023/a:1009631424351. [DOI] [PubMed] [Google Scholar]
- 49.Pasha Z. Wang Y. Sheikh R. Zhang D. Zhao T. Ashraf M. Preconditioning enhances cell survival and differentiation of stem cells during transplantation in infarcted myocardium. Cardiovasc Res. 2008;77:134–142. doi: 10.1093/cvr/cvm025. [DOI] [PubMed] [Google Scholar]
- 50.Tian H. Lu Y. Shah SP. Hong S. Autacoid 14S,21R-dihydroxydocosahexaenoic acid counteracts diabetic impairment on macrophage pro-healing functions. Am J Pathol. 2011 doi: 10.1016/j.ajpath.2011.06.026. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sadis C. Teske G. Stokman G. Kubjak C. Claessen N. Moore F. Loi P. Diallo B. Barvais L. Goldman M. Florquin S. Le Moine A. Nicotine protects kidney from renal ischemia/reperfusion injury through the cholinergic antiinflammatory pathway. PLoS One. 2007;2:e469. doi: 10.1371/journal.pone.0000469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hochheiser K. Tittel A. Kurts C. Kidney dendritic cells in acute and chronic renal disease. Int J Exp Pathol. 2011;92:193–201. doi: 10.1111/j.1365-2613.2010.00728.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Eun LY. Song BW. Cha MJ. Song H. Kim IK. Choi E. Chang W. Lim S. Choi EJ, et al. Overexpression of phosphoinositide-3-kinase class II alpha enhances mesenchymal stem cell survival in infarcted myocardium. Biochem Biophys Res Commun. 2010;402:272–279. doi: 10.1016/j.bbrc.2010.10.013. [DOI] [PubMed] [Google Scholar]
- 54.Franke TF. Kaplan DR. Cantley LC. PI3K: downstream AKTion blocks apoptosis. Cell. 1997;88:435–437. doi: 10.1016/s0092-8674(00)81883-8. [DOI] [PubMed] [Google Scholar]
- 55.Brunet A. Datta SR. Greenberg ME. Transcription-dependent and independent control of neuronal survival by the PI3K-Akt signaling pathway. Curr Opin Neurobiol. 2001;11:297–305. doi: 10.1016/s0959-4388(00)00211-7. [DOI] [PubMed] [Google Scholar]
- 56.Chen L. Tredget EE. Wu PY. Wu Y. Paracrine factors of mesenchymal stem cells recruit macrophages and endothelial lineage cells and enhance wound healing. PLoS One. 2008;3:e1886. doi: 10.1371/journal.pone.0001886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bussolati B. Tetta C. Camussi G. Contribution of stem cells to kidney repair. Am J Nephrol. 2008;28:813–822. doi: 10.1159/000137681. [DOI] [PubMed] [Google Scholar]
- 58.Tian H. Lu Y. Shah SP. Hong S. Novel 14S,21-dihydroxydocosahexaenoic acid rescues wound healing and associated angiogenesis impaired by acute ethanol intoxication/exposure. J Cell Biochem. 2010;111:266–273. doi: 10.1002/jcb.22709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Humphreys BD. Valerius MT. Kobayashi A. Mugford JW. Soeung S. Duffield JS. McMahon AP. Bonventre JV. Intrinsic epithelial cells repair the kidney after injury. Cell Stem Cell. 2008;2:284–291. doi: 10.1016/j.stem.2008.01.014. [DOI] [PubMed] [Google Scholar]
- 60.Fang TC. Alison MR. Cook HT. Jeffery R. Wright NA. Poulsom R. Proliferation of bone marrow-derived cells contributes to regeneration after folic acid-induced acute tubular injury. J Am Soc Nephrol. 2005;16:1723–1732. doi: 10.1681/ASN.2004121089. [DOI] [PubMed] [Google Scholar]
- 61.Held PK. Al-Dhalimy M. Willenbring H. Akkari Y. Jiang S. Torimaru Y. Olson S. Fleming WH. Finegold M. Grompe M. In vivo genetic selection of renal proximal tubules. Mol Ther. 2006;13:49–58. doi: 10.1016/j.ymthe.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 62.Ohtaki H. Ylostalo JH. Foraker JE. Robinson AP. Reger RL. Shioda S. Prockop DJ. Stem/progenitor cells from bone marrow decrease neuronal death in global ischemia by modulation of inflammatory/immune responses. Proc Natl Acad Sci U S A. 2008;105:14638–14643. doi: 10.1073/pnas.0803670105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Choi H. Lee RH. Bazhanov N. Oh JY. Prockop DJ. Anti-inflammatory protein TSG-6 secreted by activated MSCs attenuates zymosan-induced mouse peritonitis by decreasing TLR2/NF-{kappa}B signaling in resident macrophages. Blood. 2011;118:330–338. doi: 10.1182/blood-2010-12-327353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jo SK. Sung SA. Cho WY. Go KJ. Kim HK. Macrophages contribute to the initiation of ischaemic acute renal failure in rats. Nephrol Dial Transplant. 2006;21:1231–1239. doi: 10.1093/ndt/gfk047. [DOI] [PubMed] [Google Scholar]
- 65.Manning BD. Cantley LC. AKT/PKB signaling: navigating downstream. Cell. 2007;129:1261–1274. doi: 10.1016/j.cell.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Matsui T. Tao J. del Monte F. Lee KH. Li L. Picard M. Force TL. Franke TF. Hajjar RJ. Rosenzweig A. Akt activation preserves cardiac function and prevents injury after transient cardiac ischemia in vivo. Circulation. 2001;104:330–335. doi: 10.1161/01.cir.104.3.330. [DOI] [PubMed] [Google Scholar]
- 67.Mangi AA. Noiseux N. Kong D. He H. Rezvani M. Ingwall JS. Dzau VJ. Mesenchymal stem cells modified with Akt prevent remodeling and restore performance of infarcted hearts. Nat Med. 2003;9:1195–1201. doi: 10.1038/nm912. [DOI] [PubMed] [Google Scholar]
- 68.Meisel R. Brockers S. Heseler K. Degistirici O. Bulle H. Woite C. Stuhlsatz S. Schwippert W. Jager M, et al. Human but not murine multipotent mesenchymal stromal cells exhibit broad-spectrum antimicrobial effector function mediated by indoleamine 2,3dioxygenase. Leukemia. 2011;25:648–654. doi: 10.1038/leu.2010.310. [DOI] [PubMed] [Google Scholar]
- 69.Chamberlain G. Wright K. Rot A. Ashton B. Middleton J. Murine mesenchymal stem cells exhibit a restricted repertoire of functional chemokine receptors: comparison with human. PLoS One. 2008;3:e2934. doi: 10.1371/journal.pone.0002934. [DOI] [PMC free article] [PubMed] [Google Scholar]