Summary
Zhang et al. (2012) and Rozas et al. (2012) find that cysteine string protein α, a protein involved in neurodegeneration, regulates vesicle endocytosis via interaction with dynamin 1, which may participate in regulating synaptic transmission and possibly in maintaining synapses.
The function of the nervous system relies on billions of neurons and their synapses. Loss of neurons and synapses is a feature of neurodegenerative diseases, such as Alzheimer’s and Parkinson’s diseases (Lin and Koleke, 2010). This feature can be replicated in mice lacking cysteine string protein α (CSPα) (Chandra et al., 2005; Fernández-Chacón et al., 2004), a presynaptic vesicle protein that has been implicated in the pathogenesis of neurodegenerative diseases (Nosková et al., 2011). Knockout of CSPα causes activity-dependent synapse loss, progressive defects in neurotransmission, neurodegeneration and early lethality in mice (Chandra et al., 2005; Fernández-Chacón et al., 2004). CSPα KO is therefore a useful tool to study mechanisms underlying synapse loss and neurodegeneration. A thorough understanding on how CSPα works at synapses is a prerequisite to understand the mechanisms underlying synapse loss in CSPα KO mice. In this issue of Neuron, Zhang et al. (2012) and Rozas et al. (2012) found a new role of CSPα – regulation of synaptic vesicle endocytosis via interaction with the vesicle fission protein dynamin 1 (Fig. 1).
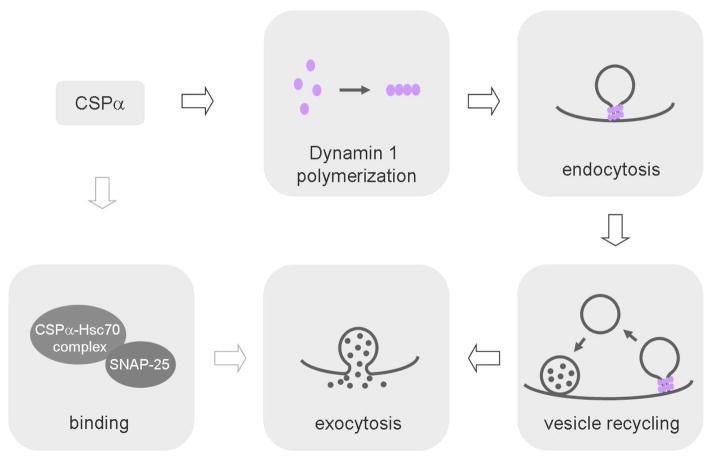
Figure 1. CSPα regulates vesicle endocytosis and exocytosis.
The arrow means regulation. Binding between CSPα-Hsc70 complex and SNAP-25 regulates exocytosis. Binding between CSPα and dynamin 1 facilitates dynamin 1 polymerization and thus endocytosis. Regulation of endocytosis might lead to modulation of exocytosis.
CSPα binds the heat shock protein cognate 70 (Hsc70) and the tetratricopeptide protein SGT to form a chaperone complex on synaptic vesicles (Südhof and Rizo, 2011). One of the substrates of this complex is SNAP-25, a t-SNARE protein critical for exocytosis (Chandra et al., 2005; Sharma et al., 2011b). In CSPα KO mice, SNAP-25 levels are reduced as is exocytosis, contributes to synapse loss (Chandra et al., 2005; Sharma et al., 2011a). However, SNAP-25 heterozygous mice, which have similarly reduced levels of SNAP-25, are phenotypically normal (Washbourne et al., 2002), suggesting that other mechanisms may contribute to synapse loss. To identify these mechanisms, Zhang et al. (2012) searched for CSPα substrates by comparing the protein levels in wild-type and CSPα KO mice using two methods, 2-D fluorescence difference gel electrophoresis and isobaric tagging to obtain relative and absolute quantitative data. Among ~1500 proteins, nearly all of the synaptic proteome in synaptosomes, 37 proteins were decreased, and 22 of them were verified with quantitative immunoblotting and Multiple Reaction Monitoring. These proteins include exocytic proteins like SNAP-25, complexin, and NSF; endocytic proteins like dynamin 1 and Necap, cytoskeletal proteins like Crmp2, BASP1, and GTP binding cytoskeletal proteins like Septin 3, 5, 6, and 7. Since the decrease of these proteins was observed at postnatal day 10 (P10), prior to the onset of synaptic dysfunction and loss in CSPα KO mice (~P20), this may explain the synaptic dysfunction and loss in these mice.
GST pull-down and co-immunoprecipitation assays of these 22 proteins revealed that dynamin 1 binds to CSPα directly, whereas SNAP-25 binds directly to both CSPα and Hsc70. Further, over-expression of CSPα rescued both the decrease of SNAP-25 and synapse loss in cultured hippocampal neurons derived from CSPα KO mice, consistent with a role of SNAP-25 in maintaining synaptic function and structure. Intriguingly, reduction of dynamin 1 was not observed from the whole neuronal culture derived from CSPα KO mice, most likely because the decrease was limited to the synaptic fraction. The decrease of dynamin 1 in the synaptic fraction was mostly due to reduction in the higher-order dynamin 1 oligomers, but not monomers, suggesting that CSPα facilitates dynamin 1 self-assembly. Since dynamin polymerization is needed to mediate vesicle fission (Schmid and Frolov, 2011), a defect predicts an impairment of endocytosis, consistent with the experimental observation of fewer vesicles in CSPα KO synapses. In a final set of experiments, Zhang et al. (2012) measured CSPα in the frontal cortex of human with Alzheimer’s disease, and found a 40% decrease, which re-emphasizes the clinical importance of studying CSPα KO mice.
In parallel with Zhang et al.’s biochemical and molecular biological study, Rozas et al. (2012) characterized the functional changes of vesicle exocytosis, endocytosis and recycling at the Levator Auris Longus nerve-muscle preparation. They found a decrease of the end-plate potential (EPP) evoked by single nerve stimuli, but not of the miniature EPP that reflects single vesicle fusion, suggesting a decrease in the released vesicle number in CSPα KO neurons. Quantal analysis suggests no decrease in the release probability p, but a decrease in n, which could means either the number of release sites or readily releasable vesicles. The latter possibility seems more probable as activation of protein kinase A by forskolin rescued the EPP decrease in CSPα KO mice, a treatment which seems unlikely to influence the number of release sites. Accordingly, deletion of CSPα was suggested to inhibit vesicle priming for release.
During repetitive stimuli, the EPP was depressed more in CSPα KO mice, implying a defect in vesicle recycling. Vesicle recycling includes at least two steps: endocytosis that retrieves fused vesicles to the recycling vesicle pool and mobilization of vesicles from the recycling pool to the readily releasable pool. To determine which of these steps was affected, Rozas et al. generated synaptopHluorin (spH) expressing CSPα KO mice by crossbreeding CSPα KO mice with spH transgenic mice. The fluorescence of spH is dimmer in an acidic environment inside the vesicle but becomes brighter upon exocytosis due to changes in the vesicle lumen pH to ~7.4. Accordingly, an increase in spH fluorescence reflects exocytosis, whereas a decrease reflects endocytosis. Consistent with the EPP decrease, deletion of CSPα reduced the spH increase induced by a brief train of nerve stimulation, but did not affect the subsequent spH decay, which reflects endocytosis after stimulation. However, endocytosis during stimulation, detected as the difference in the fluorescence increase in the absence and the presence of the vesicle re-acidification blocker folimycin, was significantly inhibited. This inhibition excluded further block of endocytosis by a putative dynamin blocker dynasore, suggesting that CSPα KO blocks dynamin-dependent endocytosis during stimulation. Consistent with these observations, electron microscopy revealed an increase of the clathrin-coated pits at nerve terminals. These results are similar to those observed in dynamin 1 KO mice, where endocytosis during stimulation is more severely impaired (Ferguson et al., 2007). They are also consistent with the decrease of dynamin 1 oligomerization observed in CSPα KO mice (Zhang et al., 2012).
In addition to the endocytosis defect during stimulation, the recycling vesicle pool size, detected as the overall spH increase induced by repeated trains of stimulus (100 Hz, 10 s) in the presence of folimycin, was decreased in CSPα KO mice. Surprisingly, electron microscopy did not reveal a change in the vesicle number at nerve terminals. The apparent discrepancy might be due to the difficulty in mobilizing vesicles from the large reserve vesicle pool to the functional recycling pool. In addition, the recycling rate seems reduced, because when a fluorescent dye FM2-10 was loaded into vesicles, its destaining by nerve stimulation was slower and more incomplete in CSPα KO mice. The functional defects described above were observed as early as P16-20, the age window when nerve terminal degeneration is likely to begin in CSPα KO mice, suggesting that the functional defects may not be secondary to nerve terminal degeneration.
In summary, Rozas et al. (2012) and Zhang et al. (2012) have discovered a regulatory role of CSPα in dynamin 1-mediated synaptic vesicle endocytosis and recycling (Fig. 1). Their findings advance our understanding of the molecular mechanisms regulating synaptic transmission, and may shed light on the study of synapse loss during neurodegeneration. As a new member involved in regulating endocytosis, CSPα binds directly to dynamin 1 and facilitates dynamin 1 polymerization, a conformation critical in mediating vesicle fission (Fig. 1). This mechanism may not only explain the endocytosis defect in CSPα KO mice but also contribute to the observed defects in exocytosis. Recent studies have shown that blocking endocytosis inhibits vesicle mobilization to the readily releasable pool, likely via inhibition of the clearance of the recently exocytosed proteins from the release site (Wu et al., 2009; Hosoi et al., 2009). Consequently, defects in vesicle priming observed in CSPα KO mice may be due to the endocytosis defect (Fig. 1).
Like many pioneering studies, the studies by Rozas et al. (2012) and Zhang et al. (2012) raise many important questions and unsettled issues. For example, we do not know how CSPα facilitates dynamin 1 polymerization. The form of endocytosis regulated by CSPα also remains unclear, considering that there are at least three forms of endocytosis: the classical clathrin-dependent slow endocytosis, rapid, clathrin-independent endocytosis, and bulk endocytosis that generates large endosome-like structures (Wu et al., 2007). Although impaired dynamin 1 polymerization seems the obvious cause of inhibition in endocytosis, whether it is also responsible for the decrease in the recycling pool and the difficulty in re-releasing recently endocytosed vesicles in CSPα KO mice is unclear. The evidence supporting a defect in vesicle priming in CSPα KO mice is indirect. Direct evidence showing a decrease in the docked vesicle number, the readily releasable vesicle pool size, and/or the rate of vesicle mobilization to the readily releasable pool awaits further study. It also remains untested whether the defects in dynamin 1 polymerization and vesicle recycling cause synapse loss. This possibility has been challenged by a recent study showing that SNAP-25 overexpression is sufficient to rescue synapse loss and degeneration in cultured neurons derived from CSPα KO mice (Sharma et al., 2011a). In addition to SNAP-25 and dynamin 1, there are around 20 other proteins that are reduced in CSPα KO mice (Zhang et al., 2012). Further investigation is needed to understand how these other proteins are regulated by CSPα and whether their decrease contributes to synaptic disfunction and loss observed in CSPα KO mice. The studies by Rozas et al. (2012) and Zhang et al. (2012) have laid a foundation for future studies that will aim to resolve aforementioned questions.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Chandra S, Gallardo G, Fernández-Chacón R, Schlüter OM, Südhof TC. Cell. 2005;123:383–396. doi: 10.1016/j.cell.2005.09.028. [DOI] [PubMed] [Google Scholar]
- Ferguson SM, Brasnjo G, Hayashi M, Wölfel M, Collesi C, Giovedi S, Raimondi A, Gong LW, Ariel P, Paradise S, et al. Science. 2007;316:570–574. doi: 10.1126/science.1140621. [DOI] [PubMed] [Google Scholar]
- Fernández-Chacón R, Wölfel M, Nishimune H, Tabares L, Schmitz F, Castellano-Muñoz M, Rosenmund C, Montesinos ML, Sanes JR, Schneggenburger R, Südhof TC. Neuron. 2004;42:237–251. doi: 10.1016/s0896-6273(04)00190-4. [DOI] [PubMed] [Google Scholar]
- Hosoi N, Holt M, Sakaba T. Neuron. 2009;63:216–229. doi: 10.1016/j.neuron.2009.06.010. [DOI] [PubMed] [Google Scholar]
- Lin YC, Koleske AJ. Annu Rev Neurosci. 2010;33:349–378. doi: 10.1146/annurev-neuro-060909-153204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nosková L, Stránecký V, Hartmannová H, Přistoupilová A, Barešová V, Ivánek R, Hůlková H, Jahnová H, van der Zee J, Staropoli JF, et al. Am J Hum Genet. 2011;89:241–252. doi: 10.1016/j.ajhg.2011.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozas JL, Gómez-Sánchez L, Mircheski J, Linares-Clemente P, Nieto-González JL, Vázquez ME, Luján R, Fernández-Chancón R. Neuron. 2012 doi: 10.1016/j.neuron.2012.02.019. this issue. [DOI] [PubMed] [Google Scholar]
- Schmid SL, Frolov VA. Annu Rev Cell Dev Biol. 2011;27:79–105. doi: 10.1146/annurev-cellbio-100109-104016. [DOI] [PubMed] [Google Scholar]
- Sharma M, Burré J, Bronk P, Zhang Y, Xu W, Südhof TC. EMBO J. 2011a;31:829–841. doi: 10.1038/emboj.2011.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma M, Burré J, Südhof TC. Nat Cell Biol. 2011b;13:30–39. doi: 10.1038/ncb2131. [DOI] [PubMed] [Google Scholar]
- Südhof TC, Rizo J. Cold Spring Harb Perspect Biol. 2011:3. doi: 10.1101/cshperspect.a005637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Washbourne P, Thompson PM, Carta M, Costa ET, Mathews JR, Lopez-Benditó G, Molnár Z, Becher MW, Valenzuela CF, Partridge LD, Wilson MC. Nat Neurosci. 2002;5:19–26. doi: 10.1038/nn783. [DOI] [PubMed] [Google Scholar]
- Wu LG, Ryan TA, Lagnado L. J Neurosci. 2007;27:11793–11802. doi: 10.1523/JNEUROSCI.3471-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu XS, McNeil BD, Xu J, Fan J, Xue L, Melicoff E, Adachi R, Bai L, Wu LG. Nat Neurosci. 2009;12:1003–1010. doi: 10.1038/nn.2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang YQ, Henderson MX, Colangelo CM, Ginsberg SD, Bruce C, Wu T, Chandra S. Neuron. 2012 doi: 10.1016/j.neuron.2012.01.029. this issue. [DOI] [PMC free article] [PubMed] [Google Scholar]