Abstract
Background
Prostate cancer is one of the most commonly diagnosed solid malignancies among US men. We identified gallic acid (GA) as a major bioactive cytotoxic constituent of a polyherbal Ayurvedic formulation – triphala (TPL). Both TPL and GA were evaluated on (AR)+ LNCaP prostate cancer and normal epithelial cells.
Methods
Total polyphenols in TPL were determined using Folin and Ciocalteu method, followed by GA quantitation by high performance liquid chromatography. Cell toxicity was evaluated by crystal-violet after 24, 48, 72 and 96 h.
Results
TPL contains 40% unidentified polyphenolic acids, of which 2.4% comprised GA. GA induced severe morphological alterations and was about 3-fold more cytotoxic towards cancer cells than TPL. This activity increased further in presence of dihydrotestosterone. GA toxicity on normal cells was low at 72 h. Combination of GA with flutamide caused higher toxicity to cancer cells than either of the compounds alone.
Conclusion
GA appears to have promising anticancer activity.
Keywords: Prostate cancer, cytotoxicity, triphala, gallic acid, Ayurvedic medicine
Prostate cancer is one of the most commonly diagnosed solid malignancies among US men (1). Although surgery and radiation are the initial options available to treat localized disease, different chemotherapeutic modalities are recommended for disseminated tumors, albeit with less success of selective killing. Since several synthetic drugs with low therapeutic indices show severe undesirable side-effects on normal body cells (2), the usage of natural products is seen as an alternate solution to this problem. Recently, we reported the selective cytotoxicity of some new chemical entities under in vitro studies (3). Compounds isolated from natural products also exhibited high selectivity index (4, 5). Paradoxically the utility of natural products is highly neglected in modern therapeutics (6). In an effort to find highly selective anticancer molecules to target tumor cells specifically, we are currently exploring herbal formulations of natural products. Poly herbal drug formulation has been utilized in India for more than 5,000 years as part of the Ayurvedic medical system (7). Triphala (TPL) is one such popular herbal formulation from India, used worldwide for various health benefits (7). It is prepared by mixing equal proportions of three different dry fruit powders, namely of Emblica officinalis, Terminalia bellirica, and Terminalia chebula. The resultant formulation was shown to promote health, immunity, and longevity when used in a chronic manner (8). It was reported that the aqueous extract of TPL selectively killed MCF-7 breast cancer tumor cells leaving MCF-10 normal breast epithelial cells relatively unharmed (9). One of the most abundantly available natural compounds present in the vast majority of plants is gallic acid (GA). It was suggested that amongst the phenolic components of TPL, GA is the major contributor to the anticancer activity (8–10). It is, however, not clear how much of TPL is comprised of polyphenols and GA. The main objective of this study was to determine the total phenolic compounds, and the percentage of GA in TPL, and to evaluate their selective anticancer potential in an in vitro model of androgen-dependent androgen receptor (AR)+ LNCaP prostate cancer cell line. In addition, we also studied whether GA cytotoxicity persisted in the presence of applied androgen dihydrotestosterone (DHT) and/or clinically available AR antagonist flutamide. The selectivity was determined by comparing the results to normal PrEC prostate epithelial cells.
Materials and Methods
Chemicals
RPMI-1640 medium was purchased from the ATCC (Manassas, VA, USA) and PrEC growth medium (PrEGM) was purchased from Clonetics (Walkersville, MD, USA). Phenol red-free RPMI-1640 medium, fetal bovine serum (FBS), penicillin-streptomycin, L-glutamine, 0.25% trypsin-EDTA, dimethylsulfoxide (DMSO), phosphate-buffed saline, Hanks’ balanced salt solution (HBSS), GA (3,4,5-trihydroxybenzoic acid), DHT, flutamide (2-methyl-N-(4-nitro-3-[trifluoromethyl]phenyl)propanamide, 1-1-diphenyl 2-picryl hydrazyl (DPPH), Folin and Ciocalteu’s phenol reagent, crystal violet dye, paraformaldehyde, ethanol, acetonitrile, sodium dihydrogen phosphate, triethylamine, EDTA, phosphoric acid, and perchloric acid were purchased from Sigma-Aldrich Inc. (St. Louis, MO, USA). Bovine pituitary extract (BPE), hydrocortisone, human epidermal growth factor (hEGF), epinephrine, transferrin, insulin, retinoic acid, triiodothyronine (T3), and GA-1000 were purchased from Clonetics (Walkersville, MD, USA). TPL Churna was purchased from Ajanta Pharma Ltd. (Mumbai, India).
Cell culture
AR+ LNCaP human prostate cancer cells were purchased from the ATCC (Manassas, VA, USA), and were maintained in 75 cm2 flasks with RPMI-1640 growth medium supplemented with 10% FBS and penicillin-streptomycin at 37°C in a humidified atmosphere of 95% air, 5% CO2. The growth medium was replaced every three days and subculture was performed biweekly at a 1:3–1:6 ratio upon 60–80% confluency with 0.25% trypsin-EDTA. Normal (PrEC) prostate epithelial cells were purchased from Clonetics, and maintained in PrEGM supplemented with 2 ml BPE, 0.5 ml hydrocortisone, 0.5 ml hEGF, 0.5 ml epinephrine, 0.5 ml transferrin, 0.5 ml insulin, 0.5 ml retinoic acid, 0.5 ml T3, and 0.5 ml GA-1000 at 37°C in a humidified atmosphere of 95% air, 5% CO2.
Preparation of TPL extracts
Appropriate quantities of TPL powder were accurately weighed out and then extracted via homogenizing the powdered herb in 100% ethanol (500 ml) with stirring at 4°C for 4 days. The extract was then centrifuged (10,000 rpm) at 27°C for 30 min and the supernatant was collected and evaporated to dryness under reduced pressure using a Rotavapor R-210/R-215 rotary evaporator (BUCHI Corp, New Castle, DE, USA). The dried extract was then aliquoted in amber-colored bottles and stored in a desiccator for further use. Prior to analysis, prepared extract was dissolved in either 10 ml HBSS (HPLC analysis) or 0.1% DMSO (cytotoxicity studies) and used in the respective experiments.
Determination of total phenolic content
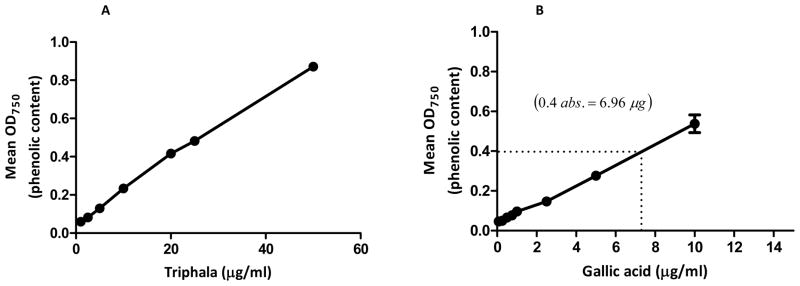
The total polyphenol content of an aqueous extract of TPL was determined using the Folin and Ciocalteu method (11). Briefly, Folin and Ciocalteu phenol reagent reacts with phenols and non-phenolic reducing substances in the presence of aqueous alkali to form blue chromogens that can be detected spectrophotometrically. Folin and Ciocalteu phenol reagent (42 μl) was added to different concentrations of TPL (1–50 μg/ml) in double-distilled water (to give a final volume of 1 ml) and allowed to react for 7 min. To this, 417 μl of 7% sodium carbonate solution was added and the mixture was allowed to stand in a water-bath at 40°C for 1 h, with intermittent vortex. The optical density of the solution was then measured at 750 nm using a Biotek Epoch microplate spectrophotometer (Winooski, VT, USA). TPL absorbance measurements were compared with a standard calibration curve plotted for GA (0.05–25 μg/ml) to calculate for the total polyphenol content of the TPL extracts, which were expressed as percentage GA equivalents.
HPLC quantification of GA in TPL extract
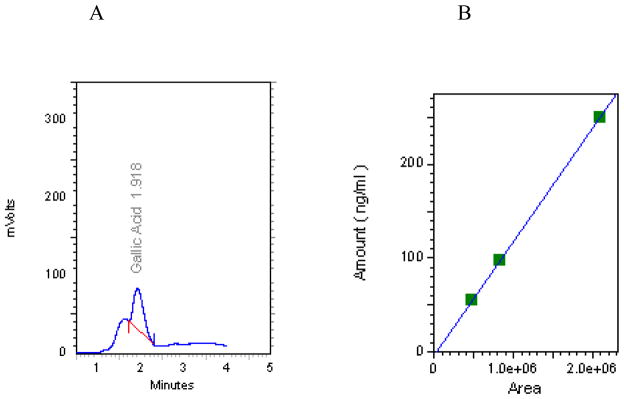
GA concentrations in ethanolic TPL extracts were determined using a Waters 717 Plus autosampler, Waters 510 HPLC pump, and an ESA Coulochem II electrochemical detector purchased from Dionex-ESA (Chelmsford, MA, USA) with settings: guard: 350 mV, E1: 175/100 μA, E2: 325 mV/5 μA, an ESA C-18 column with a particle size of 3 μm, 80 × 4.6 mm and a mobile phase consisting of 10% acetonitrile/water, 75 mM sodium dihydrogen phosphate, 1.7 mM 1-octanefulfonic acid, 100 μl/l triethylamine, and 25 μM EDTA, at a pH of 3.0 adjusted with phosphoric acid. Prior to analysis, TPL extract was diluted to 10 ml with HBSS. TPL samples (3750 ng/ml) and GA standards (55–250 ng/ml) were prepared in 0.01 N perchloric acid and measured in triplicate. The flow rate was set at 1.2 ml/min. and analysis was performed using EZ Start analytical chromatography software.
Cytotoxicity assays
AR+ LNCaP cells were seeded in 96-well tissue culture plates in 190 μl RPMI-1640 growth medium at a density of 1 × 104 and were incubated at 37°C in humidified atmosphere of 95% air, 5% CO2 for 24 h. Growth medium was replaced with phenol red-free experimental medium and 2.5% FBS containing 10 μl of TPL (25–500 μg/ml), or GA (5–80 μg/ml), or DMSO (0.1%, vehicle control). Treatments were continued for 24, 48, 72 and 96 h. In some studies, AR+ LNCaP cells were additionally pretreated (3 h) with 1 nM DHT or co-treated with 25 μM of flutamide, followed by addition of GA (5–80 μg/ml) at the time points mentioned above. PrEC cells were seeded in 96-well tissue culture plates in 190 μl PrEGM at a density of 1 × 104 and were incubated as above for 24 h. Growth medium was replaced with unsupplemented PrEGM medium containing GA (5–80 μg/ml), or DMSO (0.1%, vehicle control) for 72 h. At the end of incubations, the cytotoxicity of the compounds was evaluated by dye uptake assay using crystal violet as described previously (12). The optical density measurements were obtained at 540 nm using a microplate reader. The average absorbance values of controls were taken as 100% cell viability.
Morphological alterations
AR+ LNCaP cells were seeded in 60 mm2 tissue culture dishes in 7 ml of RPMI-1640 growth medium at a density of 2 × 106 and were incubated as above for 24 h. Growth medium was replaced with phenol red-free and 2.5% FBS experimental medium containing either 30 or 80 μg/ml GA, or DMSO (0.1%, vehicle control) for 24–96 h. Morphological changes were recorded under an inverted phase-contrast microscope (Nikon TS100; Melville, NY, USA) with 10X objective.
Statistical analysis
Results are presented as the mean±standard error of the mean (SEM). The data were analyzed for significance by one-way analysis of variance (ANOVA) and compared by Bonferroni’s multiple comparison test using GraphPad Prism Software, version 5.00 (San Diego, CA, USA). A test value of p<0.05 was considered significant.
Results
Total phenolic content of TPL
Previous studies (13–15) have reported that bioactive phenolic constituents are primarily responsible for the pharmacological activities of many Ayurvedic herbs. Therefore, we estimated the total polyphenol content in the aqueous extract of TPL by Folin and Ciocalteu method. Absorbance readings of aqueous TPL extract (F1) were compared with the standard readings of the polyphenol GA (F1) and expressed as percentage GA equivalents. Our results suggest that TPL contains 40±2% of polyphenolic acids.
HPLC quantification
The exact amount of GA in the Ayurvedic formulation was quantified by HPLC. The chromatogram for TPL shows a prominent peak at a retention time of 1.918 min (F2), which corresponds to GA as determined by the standard (55–250 ng/ml). Under our chromatographic conditions, the amount of GA in the injected ethanolic extract was calculated from a calibration curve. The results indicate that the concentration of GA in 3,750 ng/ml of TPL extract was 89.02±5.67 ng/ml. This is equivalent to 2.4% (w/w) of the total phenolic acid (40%) content previously estimated by the Folin and Ciocalteu method (F1).
Cytotoxicity studies
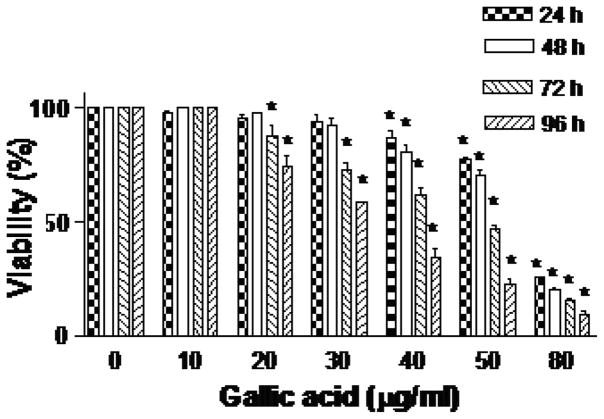
TPL extract showed significant time and dose-dependent cytotoxicity towards AR+ LNCaP cells compared to untreated DMSO (0.1%) control. The lethal concentration needed to kill 50% cells (LC50) was found to be >500, 400, 130, and 90 μg/ml at 24, 48, 72 and 96 h, respectively (F3). GA treatment of AR+ LNCaP cells also demonstrated significant time and dose-dependent cytotoxicity. The LC50 was found to be 63.6, 60, 48.1 and 33.3 μg/ml at 24, 48, 72 and 96 h, respectively (F4). The results clearly indicate that TPL and GA samples have chemotherapeutic potential against prostate AR+ LNCaP cells, although the synthetic preparation of GA demonstrates significantly higher cytotoxicity (~3 or >3 fold), as compared to the TPL extract. Phase-contrast micrographs of AR+ LNCaP cells treated with 30 and 80 μg/ml GA at 24–96 h (F5) substantiated these observations, as well as revealing the individual potential of GA to induce the morphological characteristics indicative of apoptosis, such as surface blebbing.
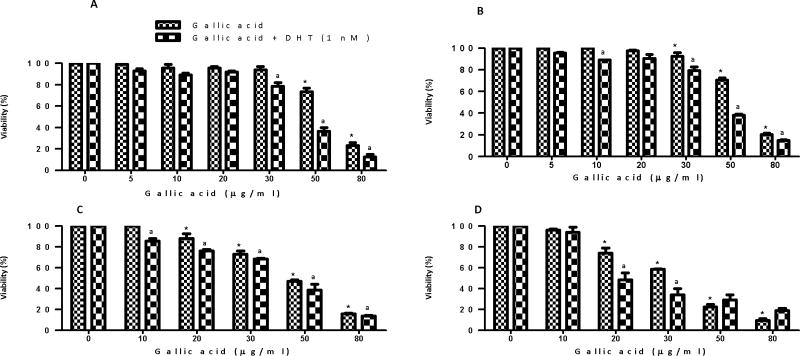
These observations prompted us to explore further the individual anticancer activity of a synthetic preparation of GA in early-stage androgen-dependent prostate cancer via measuring its cytotoxic potential on AR+ LNCaP cells in the presence of a physiological dose of DHT (1 nM), the primary ligand for the AR at the level of the prostatic epithelium (16). AR+ LNCaP cells were pretreated (3 h) with DHT and then treated with different GA concentrations (5–80 μg/ml). Pretreatment with DHT increased GA cytotoxicity in comparison to treatments without DHT (F6) at all time points. For instance, at 24 h study, the LC50 of GA without DHT was found to be 63.6 μg/ml, while on pretreatment with DHT, the value decreased to 45 μg/ml.
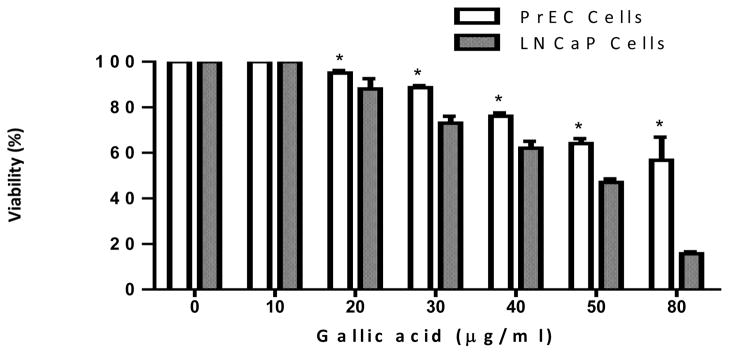
We then determined the selectivity of GA action. For this purpose, the cytotoxicity assay was performed on normal PrEC prostate epithelial cells after treatment with GA for 72 h. It was found that GA did not show significant cytotoxicity towards normal cells. Cell death at the highest concentration (80 μg/ml) after 72 h was found to be only 48% compared to the untreated DMSO (0.1%) vehicle control (F7). The estimated LC50 was found to be >80 μg/ml.
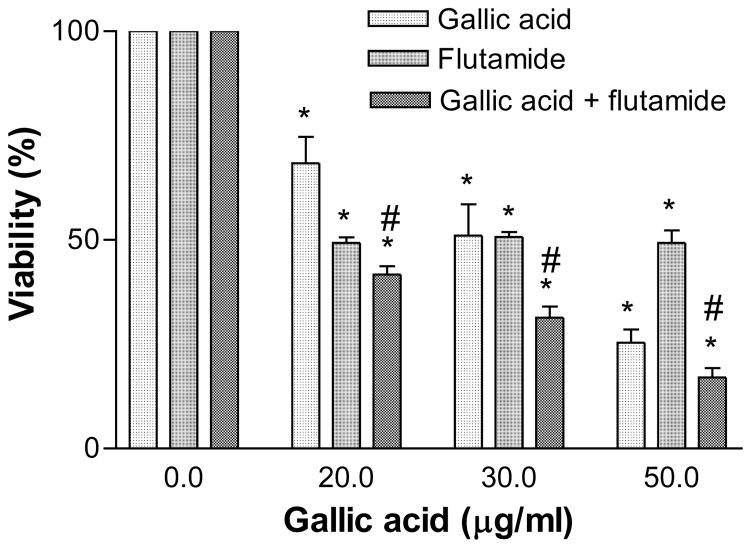
Next we determined the effectiveness of cytotoxicity by co-treating the AR+ LNCaP cells with GA and flutamide. It was observed that co-treatment caused higher cytotoxicity compared to GA alone, with an LC50 of 14 μg/ml at 96 h (F8).
Discussion
The present study was undertaken to determine the amount of GA in total polyphenols of TPL. We found that TPL contained 400 mg of unidentified phenolic acids for every 1 g of extract, which is equal to 40±2% phenolic content (Figure 1). The concentration of GA in the total phenolic content of TPL was determined by HPLC (Figure 2), and was found to be 2.4% (w/w). Earlier reports showed these to be about 14% of GA in TPL extracts (14), while others showed its concentration to be 30% (9). The significant variability observed between these similar measurements suggests that the particular extraction method may ultimately determine the amount of GA detected. GA is a secondary metabolite of higher plants, and present in fruits and vegetables or plant-derived products in either the free form, such as methylated GA molecules, or bound to larger tannin molecules, such as gallotannins and ellagotannins (17). As a consequence, the amount of GA present in any HPLC measurements will depend heavily on the thoroughness of the extraction process, as tannins become hydrolyzed to give free GA molecules in the resultant extract, or alternatively remain in an undetectable bound form (17).
Figure 1.
Total phenolic content of an aqueous extract of Triphala. (A) Triphala absorbance at various concentrations (1–50 μg/ml) was compared with the standard calibration curve (B) plotted for the known polyphenol gallic acid (0.05–25 μg/ml). Total phenolic content in Triphala was calculated and expressed as percentage gallic acid equivalents. Results represent the mean of three independent experiments.
Figure 2.
HPLC chromatogram of gallic acid in Triphala (A) and standard curve (B). A 100% ethanol extract of Triphala (3750 ng/ml) and gallic acid standards (55–250 ng/ml) were both prepared in 0.01 N perchloric acid and analyzed by HPLC.
From the perspective of GA being a major phenolic constituent responsible for in vitro bioactivity (8–10), we determined the extent of its contribution to TPL anticancer activity by comparing the cytotoxicity of an ethanolic extract of TPL to that given by a synthetic preparation of GA in early-stage androgen-dependent prostate cancer AR+ LNCaP cells and normal PrEC prostate epithelial cells. We found that GA was about three times more potent than the parent TPL extract (Figure 3 and 4).
Figure 3.

Cytotoxic effect of Triphala (50–500 μg/ml) on AR+ LNCaP cells. Results represent the average of three independent experiments. *p<0.05, significantly different from the control.
Figure 4.
Cytotoxic effect of gallic acid (10–80 μg/ml) on AR+ LNCaP cells. Results represent the average of three independent experiments. *p<0.05, significantly different from the control.
To better demonstrate the usefulness of the GA for the treatment of hormone-responsive prostate cancer, we measured the cytotoxicity associated with dosing AR+ LNCaP cells with various concentrations of GA in the presence of a physiological dose of DHT (1 nM), the primary ligand for the AR (18). This is a very important consideration since circulating androgens are encountered in vivo and increased levels are a well documented risk factor in prostate cancer development (16). Thus, this added variable may very well offer important insight into the phytochemical’s target site response in actual patients. Our studies showed that cytotoxicity actually increased in the presence of a mitogenic DHT pretreatment, suggesting a molecular target other than the AR site. It is likely that GA may be a potential anticancer candidate for further preclinical studies. In terms of hormone-responsive disease, the ability of any anticancer agent to kill the transformed cells through a mechanism other than blockade of AR sites may possibly carry a benefit because it can bypass the signal transduction pathway, which otherwise encourages a shift towards the incurable androgen-independent stage. Another significant observation of our study was that GA exhibited selective cytotoxicity towards AR+ LNCaP cells compared to normal PrEC prostate epithelial cells (Figure 7). Therefore, GA appears to offer potential as a safe targeted therapy against prostate cancer in patients without many undesirable effects on normal cells. This premise, however, needs further exploration in preclinical studies.
Figure 7.
The differential cytotoxic effect of gallic acid (10–80 μg/ml) on normal PrEC prostate epithelial cells vs. androgen-dependent LNCaP prostate cancer cells at 72 h. Results represent the averages (±SEM) from three independent experiments. Significantly different *(p<0.05) from gallic acid-treated LNCaP cells.
GA also demonstrated an additive cytotoxic effect in AR+ LNCaP cells when combined with the synthetic anti-androgen flutamide (Figure 8). The combinatory effect of GA and flutamide produced a much higher cytotoxic effect, which can be described as non-interaction or additive (19). Hence, GA may be co-administered with flutamide at much lower concentrations, which further reduces the appearance of adverse effects on normal cells without sacrificing cytotoxic potency against transformed cells. These observations validate the continued study of GA as a possible therapeutic candidate in the treatment of early-stage androgen-dependent prostate cancer.
Figure 8.
Cytotoxic effect of gallic acid (5–50 μg/ml) in the presence of flutamide (25 μM) vs. that of gallic acid alone in AR+ LNCaP cells at 96 h. Results represent the averages (±SEM) from three independent experiments. Significantly different *(p<0.05) from the respective controls. #Significant when compared between combined treatment with gallic acid or flutamide-treated LNCaP cells.
In conclusion, this is the first combination study to demonstrate the feasibility of employing GA with flutamide as a selective potential anticancer agent against prostate cancer cells. Further studies in animal models will test the efficacy of the combination therapy; such studies are currently underway.
Figure 5.
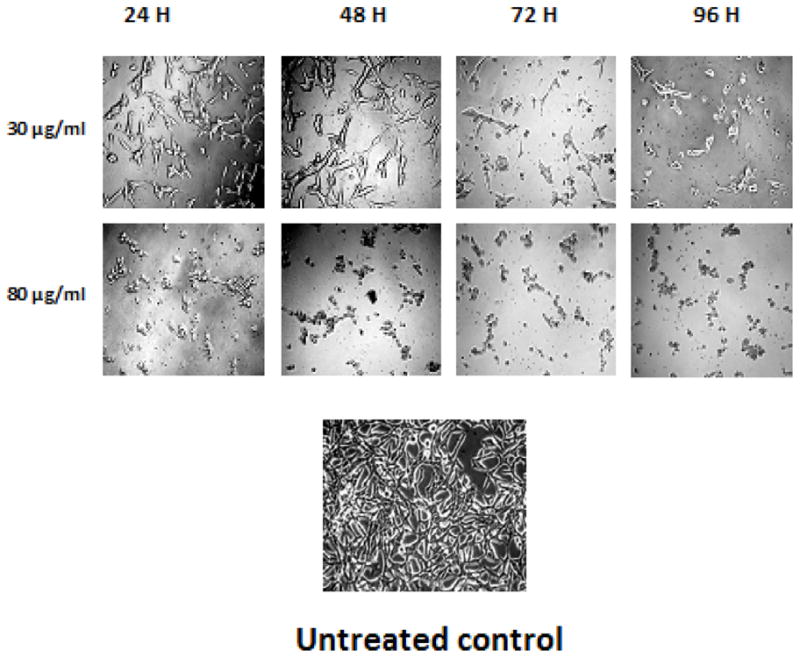
Morphological alterations of AR+ LNCaP cells induced by gallic acid. The cells were seeded in 60 mm plates and treated with either 30 or 80 μg/ml gallic acid or DMSO (0.1%, vehicle control) treatment for 24–96 h. Photographs were taken directly from culture plates with a phase-microscope with x10 objective.
Figure 6.
Cytotoxic effect of gallic acid (10–80 μg/ml) in the presence of 1 nM DHT vs. the cytotoxic effect of gallic acid alone in AR+ LNCaP cells at (A) 24 h, (B) 48 h, (C) 72 h, and (D) 96 h. Results represent the mean (±SEM) from three independent experiments. Significantly different *(p<0.05) from the control, and non-DHT treatment.
Acknowledgments
This research was supported by NCRR/RCMI G12 RR03020, NIGMS/MBRS/SCORE GM08111, and HRSA SD34HP0 4018.
References
- 1.Siegel R, Ward E, Brawley O, Jemal A. Cancer statistics, 2011: The impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J Clin. 2011;61:212–236. doi: 10.3322/caac.20121. [DOI] [PubMed] [Google Scholar]
- 2.Kamil N, Kamil S, Ahmed SP, Ashraf R, Khurram M, Ali MO. Toxic effects of multiple a anticancer drugs on skin. Pak J Pharm Sci. 2010;23:7–14. [PubMed] [Google Scholar]
- 3.Badisa RB, Darling-Reed SF, Patrick J, Cooperwood JS, Latinwo LM, Goodman CB. Selective cytotoxic activities of two novel synthetic drugs on human breast carcinoma MCF-7 cells. Anticancer Res. 2009;29:2993–2996. [PMC free article] [PubMed] [Google Scholar]
- 4.Chaudhuri SK, Badisa RB, Pilarinou E, Walker EH. Licamichauxii A and B acids Two – Ent-kaurene diterpenoids from Licania michauxii. Nat Prod Lett. 2002;16:39–45. doi: 10.1080/1057563029001/4836. [DOI] [PubMed] [Google Scholar]
- 5.Badisa RB, Lambert AT, Ikediobi CO, Walker EH. Selective anticancer activity of pure licamichauxiioic-B acid in cultured cell lines. Pharm Biol. 2006;44:141–145. [Google Scholar]
- 6.de Bono JS, Ashworth A. Translating cancer research into targeted therapeutics. Nature. 2010;467:543–549. doi: 10.1038/nature09339. [DOI] [PubMed] [Google Scholar]
- 7.Jagetia GC, Baliga MS, Malagi KJ, Kamath MS. The evaluation of the radioprotective effect of Triphala (an Ayurvedic rejuvenating drug) in the mice exposed to gamma-radiation. Phytomedicine. 2002;9:99–108. doi: 10.1078/0944-7113-00095. [DOI] [PubMed] [Google Scholar]
- 8.Sandhya T, Lathika KM, Pandey BN, Mishra KP. Potential of traditional Ayurvedic formulation, triphala, as a novel anticancer drug. Cancer Lett. 2006;231:206–214. doi: 10.1016/j.canlet.2005.01.035. [DOI] [PubMed] [Google Scholar]
- 9.Sandhya T, Mishra KP. Cytotoxic response of breast cancer cell lines, MCF7 and T47D to Triphala and its modification by antioxidants. Cancer Lett. 2006;238:304–313. doi: 10.1016/j.canlet.2005.07.013. [DOI] [PubMed] [Google Scholar]
- 10.Kaur S, Arora S, Kaur K, Kumar S. The in vitro antimutagenic activity of Triphala an – Indian herbal drug. Food Chem Toxicol. 2002;40:527–534. doi: 10.1016/s0278-6915(01)00101-6. [DOI] [PubMed] [Google Scholar]
- 11.Singleton VL, Rossi JA., Jr Colorimetry of total phenolics with phosphomolybdicphosphotungstic acid reagents. Am J Enol Viticul. 1965;16:144–153. [Google Scholar]
- 12.Badisa RB, Tzakou O, Couladis M, Pilarinou E. Cytotoxic activities of some Greek Labiatae herbs. Phytother Res. 2003;17:472–476. doi: 10.1002/ptr.1175. [DOI] [PubMed] [Google Scholar]
- 13.Yen GC, Duh PD, Tsai LH. Antioxidant and pro-oxidant properties of ascorbic acid and gallic acid. Food Chem. 2002;79:307–313. [Google Scholar]
- 14.Naik GH, Priyadarsini KI, Bhagirathi RG, Mishra B, Mishra KP, Banavalikar MM, Mohan H. In vitro antioxidant studies and free radical reactions of Triphala, an Ayurvedic formulation and its constituents. Phytother Res. 2005;19:582–586. doi: 10.1002/ptr.1515. [DOI] [PubMed] [Google Scholar]
- 15.Pfundstein B, El Desouky SK, Hull WE, Haubner R, Erben G, Owen RW. Polyphenolic compounds in the fruits of Egyptian medicinal plants (Terminalia bellerica, Terminalia chebula and Terminalia horrid): Characterization, quantitation and determination of antioxidant capacities. Phytochemistry. 2010;71:1132–1148. doi: 10.1016/j.phytochem.2010.03.018. [DOI] [PubMed] [Google Scholar]
- 16.Mukherjee B, Mayer D. Dihydrotestosterone interacts with EGFR/MAPK signaling and modulates EGFR levels in androgen receptor-positive LNCaP prostate cancer cells. Int J Oncol. 2008;33:623–629. [PubMed] [Google Scholar]
- 17.Lu Z, Nie G, Belton PS, Tang H, Zhao B. Structure–activity relationship analysis of antioxidant ability and neuroprotective effect of gallic acid derivatives. Neurochem Int. 2006;48:263–274. doi: 10.1016/j.neuint.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 18.Horoszewicz JS, Leong SS, Kawinski E, Karr JP, Rosenthal H, Chu TM, Mirand EA, Murphy GP. LNCaP model of human prostatic carcinoma. Cancer Res. 1983;43:1809–1818. [PubMed] [Google Scholar]
- 19.Chou TC. Drug combination studies and their synergy quantification using the Chou-Talalay method. Cancer Res. 2010;70:440. doi: 10.1158/0008-5472.CAN-09-1947. [DOI] [PubMed] [Google Scholar]