Abstract
We compare social skills in three groups of males with sex chromosome aneuploidies (SCAs) using the Social Responsiveness Scale (SRS). Participants included males with XXY (N=102, M=10.08 years), XYY (N=40, M=9.93 years), and XXYY (N=32, M=11.57 years). XXY had lower (better) SRS scores compared to XYY and XXYY. Scores were not significantly different between XYY and XXYY. In all groups, there were significantly more with SRS scores in the severe range compared to the SRS normative sample. All groups scored lowest (better) on Social Motivation. Relationships between SRS scores and demographic and clinical variables were examined. Results describe the social skills in males with SCA, and suggest that an additional Y chromosome may contribute to increased risk of autistic behaviors.
Keywords: Klinefelter syndrome (KS), autism, social deficits, social skills, XXY, XYY, XXYY
1 INTRODUCTION
Sex chromosome aneuploidy (SCA) describes a group of conditions in which individuals have an atypical number of sex chromosomes. The three most common SCA variations in males include 47,XXY (Klinefelter syndrome), 47,XYY, and 48,XXYY. Klinefelter syndrome (KS) is the most prevalent, occurring in 1 in 650 males, while XYY occurs in 1 in 1000 males (Nielsen, J., 1990). XXYY is less common, occurring in approximately 1 in 18,000 males (Sorensen, K., Nielsen, J., Jacobsen, P., & Rolle, T., 1978). There are shared features in the phenotypes of all three conditions, but there are typically more significant medical problems and psychological features among males with XXYY.
1.1 Medical/Physical Features
The addition of the extra sex chromosome(s) in all three conditions leads to tall stature (Ottesen, A. et al., 2010) and long legs, and features of clinodactyly and pes planus are commonly described. There is not a distinct set of dysmorphic facial features among males with SCA, although hypertelorism has been reported in both KS and in XXYY (Ross, J. L. et al., 2008; Tartaglia, N. et al., 2008). The extra X chromosome in KS and XXYY leads to testicular hyalinization that most often becomes apparent in adolescence with findings of microorchidism, lack of pubertal progression due to testosterone deficiency, and decreased fertility. Testosterone replacement therapy is necessary in most individuals with KS and XXYY. In comparison, testicular size, pubertal progression, and testosterone levels are usually normal in males with XYY. In XXYY syndrome, there are increased risks for additional medical problems such as seizures and other congenital malformations such as cleft palate or congenital heart malformations (Tartaglia, N. et al., 2008).
1.2 Developmental and Behavioral Features
In all three of these SCA conditions, there is an increased risk for developmental delays, speech-language disorders, social-emotional difficulties, and cognitive impairments. In both Klinefelter and XYY syndromes, cognitive abilities are typically in the average to low average range with strengths in visual-perceptual skills and weaknesses in verbal skills (Boada, R., Janusz, J., Hutaff-Lee, C., & Tartaglia, N., 2009). In XXYY syndrome, cognitive abilities are typically lower due to the additional gene dosage effect of having 2 extra sex chromosomes, with mean scores in the borderline range and more significant weaknesses in the verbal domain (Tartaglia, N. et al., 2008). Males with XXYY are also more likely to have complex behavioral or social-emotional difficulties (Tartaglia, N., Ayari, N., Howell, S., D’Epagnier, C., & Zeitler, P., 2011).
1.3 Social-Behavioral Phenotype and Autism
There has been increasing interest in the social development of individuals with SCA over the past decade as research in genetic etiologies of ASDs has grown. Previous studies of children and adolescents with SCA describe a social-behavioral phenotype that includes shyness, social difficulties, and social withdrawal (Bancroft, J., Axworthy, D., & Ratcliffe, S., 1982; Ratcliffe, S. G., Butler, G. E., & Jones, M., 1990; Walzer, S., Bashir, A., & Silbert, A., 1990). Case reports of autism spectrum disorders (ASDs) and autistic behaviors in Klinefelter syndrome, XYY, and XXYY have also been reported (Jha, P., Sheth, D., & Ghaziuddin, M., 2007; Merhar, S. L. & Manning-Courtney, P., 2007; Nicolson, R., Bhalerao, S., & Sloman, L., 1998).
There has been more systematic study of ASD in KS than XYY or XXYY. Bruining et. al administered the Autism Diagnostic Interview–Revised (ADI-R) while evaluating psychiatric characteristics in a group of 51 boys with KS between the ages of 6 and 19, and results showed that 27% met criteria for an ASD (Bruining, H., Swaab, H., Kas, M., & van Engeland, H., 2009). In comparison, Tartaglia et al. administered the Autism Diagnostic Observation Scales (ADOS) and ADI-R to a group of 20 children and adolescents age 6 to 21 with XXY, and found that only 1 of 20 (5%) met criteria for ASD. Of the remaining 19 participants in this study who did not reach diagnostic criteria for ASD, 42% were found to have significant deficits in either communication or reciprocal social interactions (Tartaglia, N., Cordeiro, L., Howell, S., Wilson, R., & Janusz, J., 2010). In another study of KS, Van Rijn et. al (2008) evaluated social abilities and autistic traits in a group of 31 adult males and controls using two standardized self-report questionnaires, the Scale for Interpersonal Behavior (SIB) and Autism Spectrum Quotient (ASQ). Results showed that the KS group reported increased distress during social interactions, and total scores on the ASQ were significantly higher than the control group with 48% scoring above the cut-off for Asperger syndrome (van Rijn, S. et al., 2008).
A study of 26 males diagnosed with XYY syndrome in the postnatal period found that 19% had been previously diagnosed with ASD (Geerts, M., Steyaert, J., & Fryns, J. P., 2003). In the largest descriptive study of XXYY syndrome to date, 28.3% of males six and older had been clinically diagnosed with an ASD (Tartaglia, N. et al., 2008), of which 76.9% had received a diagnosis of PDD-NOS. In one of the few comparison studies of KS and XYY, Bishop et al. (2010) compared autism and language abilities in 19 children with KS (4-15 years) and 58 children (4–16 years) with XYY. In this sample, 11% of the Klinefelter group and 19% of the XYY group had been previously diagnosed with ASD. Even when those with a previous diagnosis of ASD were excluded, children with KS and XYY showed an increased risk for communication deficits similar to those seen in children with ASD (Bishop, D. V. et al., 2010).
While these studies have described an increased rate of social difficulties and autistic behaviors in individual SCA conditions, previous research has not characterized the range of autistic symptoms nor compared the social profiles between individuals with different sex chromosome combinations (i.e. XXY vs. XYY) or with an increasing number of sex chromosomes (i.e. XYY vs. XXYY) using a common standardized measure. In this study, we sought to describe and compare social skills and autistic symptomatology in males with Klinefelter, XYY, and XXYY syndromes by administering the Social Responsiveness Scale (SRS), a parent-report questionnaire that measures 5 domains of social skills including social communication, social cognition, social awareness, social motivation, and autistic mannerisms. We also examined the relationships between SRS scores and factors such as age, socioeconomic status (SES), verbal and nonverbal cognitive abilities, previous clinical diagnosis of ASD, and timing of SCA diagnosis (prenatal vs. postnatal).
2 METHODS
2.1 Design
Participants ages 4–18 years were recruited from national SCA advocacy organizations, and clinics in endocrinology, genetics, and developmental pediatrics for a study of health and development in SCA. Participants were enrolled in the study at either Children’s Hospital Colorado (Denver) (n=102) or Thomas Jefferson University- Philadelphia (TJU) (n=72) and all signed an informed consent as approved by each institution’s research review board. All participants were required to provide results of genetic testing showing the presence of Klinefelter syndrome (XXY), XYY, or XXYY karyotypes. An interview was conducted to review demographic data, age of SCA diagnosis, indication for genetic testing, and previous developmental history including whether the participant had been previously diagnosed with ASD. Age of SCA diagnosis (i.e. prenatal vs. postnatal diagnosis) was obtained for comparisons because previous literature (Linden, M. G. & Bender, B. G., 2002) supports that children with a prenatal diagnosis may have improved outcomes compared to those with postnatal ascertainment.
2.2 Measures
The Social Responsiveness Scale: Parent Report Questionnaire (SRS-P) was completed by the participant’s primary caregiver. The SRS-P contains sixty-five items that measure the severity of autism spectrum symptoms across multiple domains as they occur in natural social settings over the past six months (Constantino, J. N. & Gruber, C. P., 2005). Symptoms are rated on a four-point scale ranging from “not true” to “almost always true”, with some items reverse scored. Standardization is based on a sample of 1,081 children (512 males). The SRS yields five subscale scores in the domains of Social Awareness, Social Cognition, Social Communication, Social Motivation and Autistic Mannerisms. The Social Awareness subscale includes items such as “is aware of what others are thinking or feeling”. The Social Cognition subscale includes items such as “takes things too literally”. The Social Communication subscale includes items such as “has difficulty answering questions directly”. The Social Motivation subscale contains items such as “would rather be alone than with others”. Finally, the Autistic Mannerisms subscale includes items such as “has repetitive, odd behaviors such as hand flapping or rocking”.
Raw scores are converted into T-scores (M=50, SD-10) for each subscale, as well as the SRS Total score. The total T-score can then be classified into one of three categories: normal (0–59), mild to moderate (60–75) or severe (76 or higher), to assist in determining the presence and degree of social deficits and whether symptoms may be consistent with those seen in individuals with ASD. SRS Total T-scores in the ‘mild-to-moderate’ range indicate deficiencies in reciprocal social behavior that are clinically significant and are typical for children with mild or ‘high functioning’ autism spectrum conditions, while SRS Total T-scores in the ‘severe’ range indicate a severe interference in everyday social interactions and are strongly associated with clinical diagnoses of autistic disorder, Asperger’s disorder, or severe cases of PDD-NOS (Constantino, J. N. & Gruber, C. P., 2005). The SRS has excellent inter-rater reliability between mothers and fathers (r= .91), stable test-retest reliability (r= .83), is significantly correlated with ADI-R scores (r= .65- .77, p<.0001) and is not correlated with FSIQ (Constantino, J. N. et al., 2003)
Cognitive testing was conducted as developmentally appropriate by a trained clinician. The Differential Ability Scales–2nd edition (DAS-2) was used at TJU. The DAS-2 yields an overall score (General Conceptual Ability; GCA), as well as verbal, nonverbal and spatial composite scores. At the Denver site, either the Wechsler Abbreviated Scale of Intelligence (WASI), or the Wechsler Intelligence Scale for Children–4th Edition (WISC-IV) was administered. Both the WASI and the WISC-IV include an overall Full Scale IQ score (FSIQ), as well as verbal (VIQ) and performance (PIQ) composite scores. The WASI and the WISC-IV VIQ, PIQ and FSIQ scores are highly correlated (r= .85, .78, and.86, respectively). Also, DAS-2 and WISC-IV verbal and VIQ scores, nonverbal and PIQ, and GCA and FSIQ scores are strongly correlated (r=.71 to .84). The GCA/FSIQ, verbal/VIQ and nonverbal/PIQ scores were used for further analyses. Socioeconomic status (SES) was calculated using the Hollingshead two-factor index of social position (Hollingshead, A. d. B., 1965) based on parent(s) occupation and education.
2.3 Statistical Analysis
One-way ANOVA’s were conducted to assess for significant differences in age, SES, IQ measures (GCA/FSIQ, verbal/VIQ, nonverbal/PIQ), and SRS scores in the three groups. All assumptions for an ANOVA were verified, including the need for larger variance in groups with larger sample size (Field, 2009). For those which achieved significance of p <0.05, post-hoc Tukey analyses was conducted to determine which pairwise differences were statistically significant. The Kendall’s tau-b (τ) correlation was used for correlations including nonparametric variables. Proportion tests (z-tests) (Newcombe, R. G., 1998) were carried out using the SPSS (v17) Custom Tables module (SPSS, 2007a, 2007b) to determine if the percentage of participants in each of the SCA groups whose SRS scores were in the normal, mild-to-moderate and severe ranges in the current study were significantly different from the percentage found in the SRS normative sample of males (N=512). The proportion tests take into account the sample size of each comparison group and the normative sample. Alpha value criterions were adjusted using a Bonferroni correction for multiple comparisons.
3 RESULTS
3.1 Participants
There were 174 participants overall, including 102 with KS (M age=10.08 years, SD=3.21), 40 with XYY (M age=9.93 years, SD=3.06) and 32 with XXYY (M age=11.57 years, SD=3.97). There were no significant differences in age (F (2, 171)=2.82, p=.063) or SES (F (2, 153)=1.69, p=.187) across the three groups.
3.2 Cognitive Abilities
Cognitive testing results were obtained on 86% of the total sample (149/174). Verbal, performance/non-verbal and full scale/GCA scores from the Wechsler and DAS-2 reflected a wide range of cognitive abilities, with the least variation in the XXYY group (range of XXY scores= 46–146, XYY= 46–139, XXYY= 71–88). DAS-2 and Wechsler scores in the KS and XYY groups were not significantly different (all p>.096). However, the FSIQ, VIQ and PIQ scores were significantly lower in the XXYY group compared to the KS group (M difference FSIQ= −18.32, VIQ= −14.95, and PIQ= −11.56). The mean FSIQ, VIQ, and PIQ of the XXYY group was 10 or more points lower than XYY group, however these differences did not reach statistical significance. VIQ was significantly less than PIQ in each of the three groups of participants, which is the typical cognitive profile in these SCA conditions.
3.3 Prenatal versus Postnatal Diagnosis
In the KS group, an almost equal percentage of participants were identified in the prenatal versus postnatal periods (55.9% and 44.1%, respectively). Fewer males with XYY were diagnosed in the prenatal versus postnatal period (32.5% and 67.5%, respectively). Prenatally diagnosed cases of XXYY syndrome in the population are rare, and none of the XXYY participants in our sample were diagnosed in the prenatal period. Developmental-behavioral concerns were the primary ndication for postnatal testing in all three groups.
3.4 Autism Spectrum Disorders
Lifetime ASD diagnosis information was collected on all participants. The lowest rate of ASD was reported in the XXY group (11.8%). A larger percentage of the XYY group (50%) had a previous ASD diagnosis compared to the XXYY group (37.5%). The rate of ASD was significantly different across the three groups (X2(2)= 25.18, p=.000).
3.5 Social Responsiveness Scale
The subscale and Total SRS T-scores are summarized for each group in Table 2. Other than the Social Awareness subscale in the KS group, the mean T-scores in all three groups fell in either the mild to moderate or severe range. The standard deviation of all scores in all groups ranged between 11.34 to 15.8 points, suggesting greater variability in scores than the normative sample (SD=10).
Table 2.
Mean SRS T-scores with One-way ANOVA Results and Classification of SRS T-scores by Group
| SRS T-score Classification |
||||||
|---|---|---|---|---|---|---|
| SRS Subscale | Group | Mean (SD) | ANOVA Results | Normal | Mild-to- Moderate |
Severe |
| Social Awareness |
XXY | 56.31 (11.34) | F (2, 171)=12.8 p=.000a |
65.7% | 29.4% | 4.9% |
| XYY | 65.70 (13.09) | 32.5% | 47.5% | 20.0% | ||
| XXYY | 65.59 (12.61) | 34.4% | 43.8% | 21.9% | ||
| Social Cognition |
XXY | 61.99 (15.36) | F (2, 171)=9.51 p=.000a |
47.1% | 29.4% | 23.5% |
| XYY | 71.68 (13.77) | 25.0% | 25.0% | 50.0% | ||
| XXYY | 71.91 (12.86) | 15.6% | 40.6% | 43.8% | ||
| Social Communication |
XXY | 60.33 (14.61) | F (2, 171)=9.99 p=.000a |
53.9% | 31.4% | 14.7% |
| XYY | 70.93 (14.75) | 20.0% | 35.0% | 45.0% | ||
| XXYY | 69.31 (13.42) | 25.0% | 43.8% | 31.3% | ||
| Social Motivation |
XXY | 60.10 (13.74) | F (2, 171)=.699 p=.499 |
52.0% | 31.4% | 16.7% |
| XYY | 62.65 (13.67) | 40.0% | 42.5% | 17.5% | ||
| XXYY | 62.56 (14.24) | 50.0% | 25.0% | 25.0% | ||
| Autistic Mannerisms |
XXY | 61.20 (14.12) | F (2, 171)=14.39 p=.000a |
54.9% | 25.5% | 19.6% |
| XYY | 73.45 (14.53) | 20.0% | 30.0% | 50.0% | ||
| XXYY | 72.22 (14.76) | 25.0% | 21.9% | 53.1% | ||
| Total Score |
XXY | 62.00 (15.44) | F (2, 171)=9.92 p=.000a |
52.9% | 27.5% | 19.6% |
| XYY | 72.83 (15.80) | 15.0% | 35.0% | 50.0% | ||
| XXYY | 72.22 (14.76) | 21.9% | 34.4% | 43.8% | ||
Tukey post-hoc analysis shows that XXY was significantly < XYY and significantly <XXYY in these domains. There were no significant differences between XYY and XXYY on post-hoc analysis
A one-way ANOVA was conducted to test for differences across the three groups on all SRS T-scores, as shown in Table 2. SRS Total T-scores differed significantly across the three groups, F (2, 171) = 9.92, p=.000. Tukey post-hoc comparisons revealed that the KS group (M=62.00, 95% CI (58.97, 65.03)) had significantly lower SRS Total T-scores than both the XYY (M=72.83, 95% CI (67.77, 77.88)) and XXYY groups (M=72.22, 95% CI (66.90, 77.54)), p=.000. Therefore, the parents of children with KS reported less severe problems with social impairments, compared to both XYY and XXYY. There was no difference between the XYY and XXYY groups, such that they exhibited a similar degree of social impairment. The distributions of SRS Total T-scores in the three groups compared to the distribution of the males in the normative sample of the SRS are shown in Figure 1. There is a visible shift to higher scores (more autism symptomatology) in all three SCA groups, with an almost identical trend in the XYY and XXYY groups.
Figure 1.
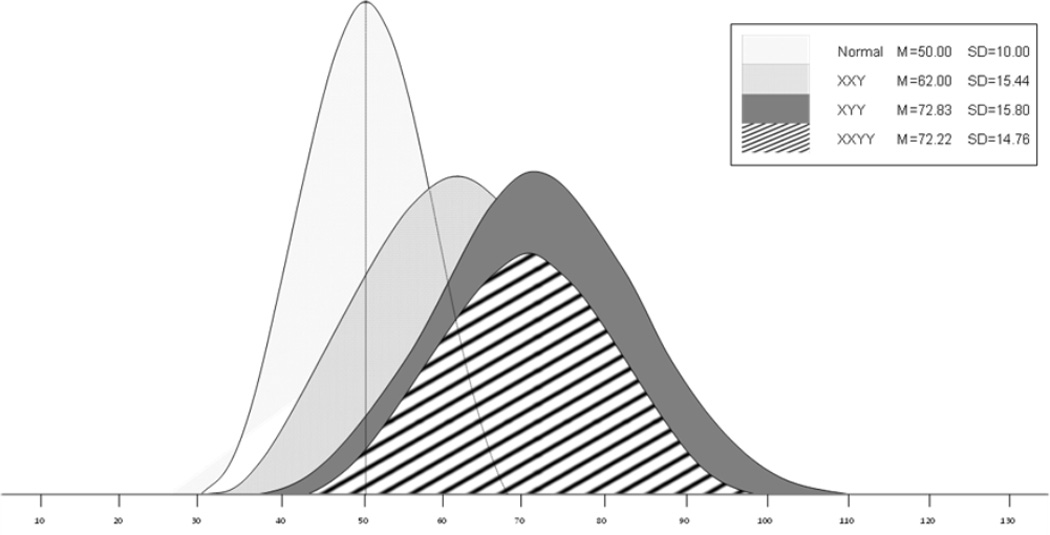
Social responsiveness scale total t-score distributions in the normative sample, XXY, XYY and XXYY groups.
When comparing each of the SRS subscale scores between the three groups, the Klinefelter group’s scores continued to be significantly lower compared to XYY and XXYY, with the exception of the social motivation subscale. Similar to the pattern found for the SRS Total T-score, there were no differences between the XYY and XXYY groups on any of the subscale T-scores. Interestingly, in the social motivation domain, the mean scores for all three SCA groups did not differ and fell just above the normal range cutoff.
3.5.1 Classification of Social Responsiveness Scale Scores
Due to the variability in SRS scores within the SCA groups, means scores alone did not completely represent the performance of each group on the SRS. Therefore, SRS T-scores were also categorized into the normal (0–59), mild-to-moderate (60–75) or severe (76 or higher) classification as determined by the scoring criteria of the SRS. The proportions of individuals with SRS T-scores falling into each classification are reported in Table 2. A z-test of proportions was used to compare the Total T-score classification of the SRS normative sample of males to the three study groups. In the KS group, the numbers of participants with SRS Total T-scores in the normal range was similar to the normative sample. However, the XYY and XXYY groups had significantly fewer participants with SRS Total T-scores in the normal range (p<.00001) compared to the normative sample. Further, in all three SCA groups, significantly more participants had SRS Total T-scores in the severe range (p<.003) compared to the normative sample.
The proportions of each study group that fell into the normal, mild-to-moderate or severe ranges for each of the SRS subscales are shown in Table 2. Overall, more of the participants in the KS group (roughly half or more) had SRS subscale scores in the normal range compared to the other two groups. In the XYY group, roughly a third or less fell in the normal range on all the SRS subscales, except in the Social Motivation domain where 40% were in the normal range. In the XXYY group, while 35% or less fell in the normal range in all domains, on the Social Motivation subscale, 50% were in the normal range.
3.5.2 Relationship between Social Responsiveness Scale and other variables
Next, we wanted to further explore other variables that may relate to SRS results, including age, verbal and nonverbal cognitive abilities, previous clinical diagnosis of ASD, timing of SCA diagnosis (prenatal versus postnatal), and SES. Neither age (all p>.11) nor SES (all p>.37) were significantly correlated with SRS Total scores in any of the three study groups.
Since cognitive deficits and language disorders are commonly seen in males with SCA, we evaluated the relationship between verbal/VIQ and nonverbal/PIQ cognitive abilities and SRS scores. Results showed that in the KS and XXYY groups, there were weak but significant correlations between SRS Total T-score and verbal cognitive abilities on the DAS-2 (Klinefelter Kendall’s tau-b=−.21, p=.027) and the VIQ scores on the Wechsler tests (Klinefelter Kendall’s tau-b=−.32, p=.004, XXYY Kendall’s tau-b=−.34, p=.031). In comparison, there was not a significant correlation between verbal/VIQ scores and SRS Total T-scores in the XYY group (verbal p=.151 and VIQ p=.713). There was no significant correlation between nonverbal cognitive abilities and SRS Total T-scores in any of the three study groups.
In all SCA groups, those who had a previous diagnosis of an ASD had significantly higher SRS Total T-scores than those without a previous ASD diagnosis (all p>.05). Even among those without a previous clinical diagnosis of ASD, the mean SRS Total T-score remained elevated (M(SD) XXY=59.88(13.42), XYY=65.80(14.91), XXYY=66.60(13.84)), at 1 or more SD above the mean. Of the 90 participants with KS without a previous clinical diagnosis of ASD, 28.9% (26/90) had SRS Total T-scores in the mild-to-moderate range and 14.4% (13/90) had SRS Total T-scores in the severe range. Similarly, a large percentage of the XYY and XXYY participants without a previous clinical diagnosis of ASD had elevated SRS Total T-scores in the mild-to-moderate range (XYY 45% (9/20); XXYY 40% (8/20)) and the severe range (XYY 30 (6/20); XXYY 25% (5/20)).
Due to concerns about ascertainment bias when describing the behavioral phenotype of SCA groups, and because previous literature supports that individuals with SCAs identified in the prenatal period may have improved outcomes compared to those diagnosed after birth (Linden, M. G. & Bender, B. G., 2002), we also compared SRS Total T-scores for those diagnosed prenatally versus postnatally in the KS and XYY groups. There were no prenatally diagnosed cases of XXYY included in this study. T-test comparisons of SRS scores within the KS (prenatal M(SD)=58.63(14.15), postnatal M(SD)=66.27(16.10)) and XYY (prenatal M(SD)= 63.46(15.60), postnatal M(SD)=77.33(14.05)) groups revealed that those who were postnatally diagnosed had significantly higher SRS Total T-scores (p<.01). Further, SES was significantly higher among those prenatally versus postnatally diagnosed in the KS group (p=.002).
4 DISCUSSION
This study characterizes and compares the profile of social skills and autism symptomatology in a large cohort of three most common SCA conditions in males, including Klinefelter/XXY, XYY, and XXYY syndromes at two study sites. Overall, the KS group had fewer reports of social difficulties and fewer significant differences from the normative sample compared to the XYY and XXYY groups. However, even in the KS group, the mean SRS subscale results were in low end of the mild-to-moderate range with wide variability in scores, and there was a significantly higher percentage (almost 20%) of participants with scores in the severe range compared to the normative sample. These findings are consistent with other studies showing higher rates of social difficulties and autistic behaviors in males with SCA (Bishop, D. V. et al., 2010; Geerts, M. et al., 2003; Tartaglia, N. et al., 2010; van Rijn, S., Aleman, A., De Sonneville, L., & Swaab, H., 2009; van Rijn, S., Swaab, H., Aleman, A., & Kahn, R., 2006; van Rijn, S. et al., 2008).
When we examined factors within the SCA study groups that may be related to SRS scores, we found that lower verbal cognitive abilities (in the KS and XXYY groups), a postnatal diagnosis of SCA (in the KS and XYY groups), and a previous clinical diagnosis of ASD (in all three groups) were all associated with greater impairment in social responsiveness. There were no significant correlations between age, nonverbal cognitive abilities, or SES and SRS scores in any of the study groups.
4.1 Social Responsiveness and Verbal Abilities
When considering correlations between cognitive abilities and SRS scores in the three study groups, there was a relatively weak but significant correlation between verbal cognitive scores and SRS scores (τ=−.21 to −.34) in KS and XXYY groups, but not the XYY group. Thus, the poorer language abilities among individuals with KS and XXYY may be contributing to their social difficulties. This finding is similar to the 2009 study by Bruining et. al (2009) where children with KS who had an ASD were more likely to have a comorbid language disorder (Bruining, H. et al., 2009). From a clinical perspective, these results suggest that when individuals with SCA are presenting with concerns related to social functioning and possible ASD diagnosis, evaluation of verbal cognitive abilities and speech-language skills are important since they may be a factor contributing to social difficulties that can be targeted in an intervention plan.
In the XYY group, our results suggest that the social difficulties and autism symptomatology are more independent of their verbal abilities since verbal/VIQ scores were not significantly correlated with deficits in social responsiveness. This finding may be partly related to the more significant social and behavioral difficulties in XYY compared to KS (Ross, J. et al., in press). Thus, individuals with XYY with social and behavioral disorders but normal verbal cognitive abilities would be more likely to be ascertained compared to KS.
4.2 Social Responsiveness and Timing of SCA Diagnosis
When comparing subgroups of participants with KS and XYY diagnosed in the prenatal period to those in the postnatal period, the subgroups with a prenatal diagnosis had fewer social difficulties compared to those diagnosed in the postnatal period. This is consistent with previous literature of improved outcomes in individuals with a prenatal diagnosis.(Linden, M. G., Bender, B., & Robinson, A., 1996; Linden, M. G. & Bender, B. G., 2002) These differences are likely due to a variety of factors including an increased likelihood of prenatal care, increased family supports, increased awareness of possible neurodevelopmental problems, and earlier initiation of early intervention therapies and supports in the prenatally diagnosed groups. In this study, SES was not significantly different between those with a prenatal or postnatal diagnosis of XYY, nor was SES significantly correlated to SRS scores for any of 3 the study groups.
In individuals with a postnatal diagnosis, the SCA condition was ascertained due to the presence of features that were concerning to medical professionals. In most cases, the indications for genetic testing are related to moderate to severe developmental delays or cognitive impairments, behavioral disorders, or the presence of autism spectrum disorders, all of which would contribute to the higher SRS scores in this subgroup. These study results are important in genetic counseling of individuals with a prenatal diagnosis of SCA. It is important to point out that while the risk for delays and social difficulties is increased compared to the general population, there is a wide spectrum of involvement and not all individuals have significant delay, social deficits, or autism spectrum disorders.
4.3 Social Responsiveness and Previous Clinical Diagnoses of ASD
While it makes sense that those with a previous clinical diagnosis of ASD would have higher SRS scores than those without a previous diagnosis, it was interesting to find that a large percentage of individuals with SCA without a previous diagnosis of ASD also had elevations in SRS scores compared to what is expected in the general population (over 40% in the KS group, and over 70% in the XYY and XXYY groups). These results suggest that many of these participants have social skills difficulties and autistic symptomatology that are subthreshold for a classification of ASD or who may indeed meet criteria for ASD upon further evaluation.
4.4 Social Motivation in SCA
Another interesting finding was the general preservation of skills in the Social Motivation subscale compared to the other subscales of the SRS in the three SCA study groups. The Social Motivation subscale of the SRS includes behaviors such as desire for social interactions, withdrawal or avoidance of social settings, and confidence in social situations. This finding suggests that most children and adolescents with SCA have an interest in and are motivated by social interactions, however their deficits in social cognition, social communication, and autistic mannerisms are stronger contributors to their overall social difficulties.
This finding is consistent with a series of previous reports in an adult cohort with KS where van Rijn et al. reported that the frequency of social interactions (i.e. social motivation) did not differ between a KS group and control group (van Rijn, S. et al., 2008). However, the study did find that the KS group exhibited social cognitive deficits in multiple other areas such as recognition of facial expressions, processing of social cues (van Rijn, S. et al., 2006), and understanding of affective prosodic language cues (tone of voice) (van Rijn, S. et al., 2007). Similar studies that deconstruct different aspects of social skills in XYY and XXYY have not been reported, and would be an important contribution since social difficulties are more common in these groups.
The apparent relative strengths and preservation of social motivation is important for two other reasons. First, an interest in reciprocal social interactions does not exclude diagnosis of an ASD. Children and adolescents with SCA, despite typical social motivation and a desire for friendships and social interactions, may still have deficits in social awareness, social cognition, communication, and other autistic behaviors that are still causing enough impairment to meet criteria for diagnosis of an ASD. Thus, the presence of social motivation in the SCA population should not be used by clinicians as a reason to dismiss or not pursue further ASD evaluation in children with SCA who have social difficulties. This would result in under diagnosis of ASDs in SCA, and those undiagnosed may then not receive or qualify for services and interventions targeting the social and communication deficits. Second, these results show that children with SCA may be more socially motivated than would be expected in the typical profile of children with social deficits or ASD, and this positive interest in social engagement can be used in developing intervention strategies for the SCA group.
4.5 The Effect of Extra “X” or “Y” Chromosomes
When comparing SCA groups and phenotypic features, questions arise around whether differences between the groups are due to gene dosage effects from the extra X and/or Y chromosomes, or whether differences in exposure to androgens may be contributing to differences. In both XXY/KS and XXYY, the extra X chromosome leads to testicular dysfunction and decreased overall testosterone levels compared to typical males with XY and to males with XYY who have normal testosterone levels. These differences in testosterone levels are usually present by mid-puberty and throughout adulthood, when the majority of individuals with KS and XXYY are treated with testosterone replacement therapy. There is also some evidence that prepubertal testosterone levels are lower in male children with KS (Lahlou, N., Fennoy, I., Carel, J. C., & Roger, M., 2004; Ross, J. L. et al., 2005), which may play a role in neurodevelopmental differences. While it could be proposed that some of the social difficulties and language deficits in KS and XXYY may be associated with neurodevelopmental effects of lower androgen levels, our results suggest that the effect of the extra Y chromosome in the XYY and XXYY groups is more strongly associated with social deficits and ASD symptoms compared to the extra X chromosome and androgen deficiency effects.
To further consider the effects from the extra X and/or Y chromosomes on social functioning, the most interesting comparisons are between the XXY/KS and XYY groups (two sex chromosome trisomy groups with similar cognitive abilities), and the comparison between the XYY and XXYY groups (where the extra sex chromosome in the XXYY group leads to significantly lower cognitive scores of 10 or more points in both verbal and nonverbal domains compared to XYY). First, for age-matched groups with similar verbal and nonverbal cognitive abilities (KS versus XYY), the XYY group presents with significantly more social deficits and autistic symptomatology compared to the KS group. This, combined with the lack of relationship between all IQ measures and SRS scores in XYY, provides evidence that the socialdeficits in XYY may be independent of cognitive abilities.
In the second comparison (XYY vs. XXYY), despite greater clinical involvement characteristic of XXYY, our study found SRS Total and subscale scores (averages, score distributions and category proportions) are nearly identical in XYY and XXYY. This finding suggests that the effect of the extra Y chromosome on social skills and autistic behaviors is more significant than the effect of the extra X chromosome, and, again, is also relatively independent of cognitive abilities.
The phenotype of the SCA conditions results from overexpression of genes on the sex chromosomes, however the specific genes associated with the behavioral phenotype of SCAs are not yet known. There are several genes on the sex chromosomes important in neuronal development and neurotransmission, and which have been associated with ASD. For example, the ASMT gene codes for the enzyme which catalyzes the final step of melatonin synthesis, and both polymorphisms and duplications in the ASMT gene have been associated with an increased risk for ASD.(Cai, G. et al., 2008; Jonsson, L. et al., 2010; Melke, J. et al., 2008) Neuroligins are cell adhesion molecules involved in synapse formation and function, and neuroligin 4 (NLGN4) gene mutations have also been associated with autism (Jamain, S. et al., 2003; Laumonnier, F. et al., 2004; Yan, J. et al., 2005), and have been proposed as being important in the language disorders in children with SCAs (Bishop, D. V. & Scerif, G., 2011). The protocadherin 11 (PCDH11Y) gene on the Y chromosome is expressed primarily in brain tissue during synaptogenesis (Durand, C. M. et al., 2006), and abnormal expression of protocadherin proteins may result in abnormal synapse formation related to ASD. Further studies of how these and other X&Y chromosome genes contribute to the neurodevelopmental phenotype of SCA is an important next step in this research, and results may also help us to better understand the biological basis for the male bias in ASD.
There are some important limitations of this study. Our study samples were largely clinically referred and therefore, may represent a group of males with SCAs with more clinical involvement. However, not all participants were clinically referred for behavioral or social issues, as many were ascertained by endocrinology clinics. Also, though the SRS is psychometrically sound and able to measure a range of autistic symptomatology without floor effects, it is not able to diagnose ASDs. Additional studies of autism in SCAs are needed and should include other well-validated measures and not rely solely upon parent-report questionnaire and historical autism diagnosis information.
In summary, our results show that males with KS, XYY and XXYY are at increased risk for social deficits and do demonstrate a range of autistic behaviors. While in the cognitive domains males with XYY often fare better than XXYY, there was an almost identical profile of social deficits in the XYY and XXYY groups. These results point to the need for further genetic studies of the relationships between genes on the Y chromosome and autistic behaviors.
Highlights.
This study compares social skills in three groups with sex chromosome aneuploidies.
In XXY, XYY and XXYY a significant proportion of participants had social deficits.
Lower verbal abilities (in XXY and XXYY) were significantly related to social skills.
Findings in XYY and XXYY suggest the Y chromosome may contribute to social deficits.
Figure 2.
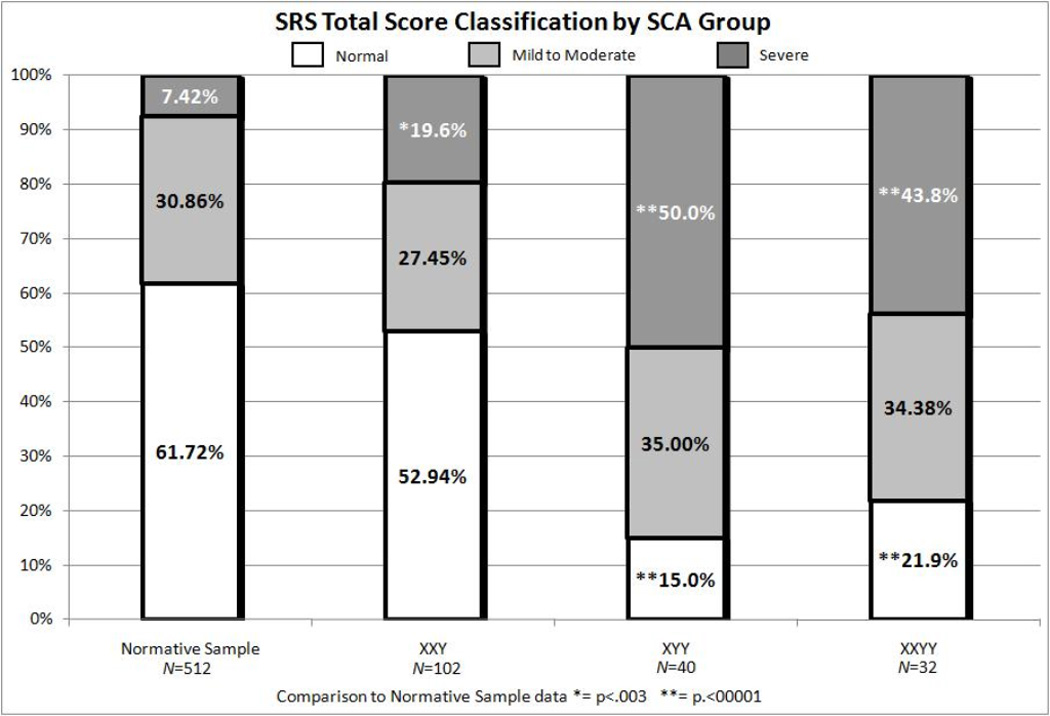
Proportions of XXY, XYY and XXYY groups with social responsiveness scale total tscores in the normal, mild to moderate, and severe ranges compared to the normative sample.
Table 1.
Description of Participants
| XXY N=102 |
XYY N=40 |
XXYY N=32 |
Group Comparison Resultsh |
|
|---|---|---|---|---|
| Age | ||||
| M (SD) | 10.08 (3.21) | 9.93 (3.06) | 11.57 (3.97) | F (2, 171)=2.82 |
| Range | 4.6–18.68 | 4.3–17.48 | 4.43–18.94 | p=.063 |
| IQ (DAS-2) TJU, Philadelphia N | 55 | 15 | 2 | |
| Verbal- M (SD) | 90.58 (16.98) | 92.20 (15.34) | 71.00 (9.90) | t (2,68)= −.334 |
| Verbal- Range | 53–146 | 51–115 | 64–78 | p=.740d |
| Nonverbal- M (SD) | 95.13 (15.32) | 92.53 (15.74) | 83.5 (19.09) | t (2,68)=.578 |
| Nonverbal-Range | 56–125 | 61–124 | 70–97 | p=.565d |
| GCA- M (SD) | 90.73 (14.67) | 92.27 (15.76) | 73.50 (4.95) | t (2,68)= −.340 |
| GCA- Range | 56–121 | 52–124 | 70–77 | p=.737d |
| IQ (Wechsler) TCH, Denver N | 40 | 14 | 23 | |
| Verbal- M (SD) | 92.95 (18.66) | 89.31 (19.34) | 78.00 (15.08) | F (2,73)=5.08 |
| Verbal- Range | 53–119 | 46–113 | 59–99 | p=.009e |
| Performance- M (SD) | 99.93 (15.50) | 98.00 (19.76) | 88.36 (15.19) | F (2,73)=3.74 |
| Performance- Range | 61–129 | 73–139 | 64–112 | p=.028e |
| Full Scale- M (SD) | 97.75 (18.51) | 92.36 (19.39) | 79.43 (15.90) | F (2,74)=7.64 |
| Full Scale- Range | 46–141 | 56–127 | 48–102 | p=.001e |
| Lifetime ASDa Diagnosis N (%) | ||||
| ASD | 12 (11.8) | 20 (50.0) | 12 (37.5) | X2(2)= 25.18, p<.02f |
| SCAb Diagnosis N (%) | ||||
| Prenatal | 57 (55.9) | 13 (32.5) | 0 (0.0) | X2(2)= 32.74, |
| Postnatal | 45 (44.1) | 27 (67.5) | 32 (100) | p=.000g |
| Developmental-Behavioral Indication | 28 (62.2) | 22 (81.5) | 28 (87.5) | X2(2)= 7.19, |
| Medical Indication | 17 (37.8) | 5 (18.5) | 4 (12.5) | p=.449 |
| SEScN | 93 | 36 | 27 | F (2, 153)=1.69, |
| M (SD) | 46.64 (11.54) | 46.93 (12.13) | 42.15 (12.07) | p=.187 |
ASD= any Autism Spectrum Disorder;
SCA= Sex Chromosome Aneuploidy;
SES= Socioeconomic status;
Due to N=2 in XXYY with DAS-2 scores, t-tests were used for XXY vs. XYY at TJU;
Post-hoc Tukey results XXY > XXYY;
Post-hoc z-test of autism rates results XXY<XYY and XXY<XXYY after Bonferroni correction;
Post-hoc z-test of postnatal rates results XXY<XYY, XXY<XXYY, and XYY<XXYY after Bonferroni correction;
All z-test significant results were p<.016 after Bonferroni correction
ACKNOWLEDGEMENTS
The authors thank Cheryl D’Epagnier and Susan Howell for their assistance with data collection. This work was supported by The Children’s Hospital Research Institute and the University of Colorado School of Medicine Department of Pediatrics, IDDRC, and CCTSI (NIH/NCRR Colorado CTSI Grant Number UL1 RR025780) and National Institutes of Health (NIH NS050597 (JR), NIH/NINDS 1K23NS070337-01A1 (NT)). Its contents are the authors’ sole responsibility and do not necessarily represent official NIH views. We appreciate recruitment efforts by KS&A, AAKSIS, and The XXYY Project. We are especially thankful for the participants and their families and their contribution to the understanding of SCA conditions.
Abbreviations
- (SCA)
sex chromosome aneuploidy
- (KS)
Klinefelter syndrome
- (ASD)
autism spectrum disorder
- (SRS)
Social Responsiveness Scale
- (DAS-2)
Differential Ability Scales – 2nd edition
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Bancroft J, Axworthy D, Ratcliffe S. The personality and psycho-sexual development of boys with 47 XXY chromosome constitution. Journal of Child Psychology and Psychiatry and Allied Disciplines. 1982;23(2):169–180. doi: 10.1111/j.1469-7610.1982.tb00061.x. [DOI] [PubMed] [Google Scholar]
- Bishop DV, Jacobs PA, Lachlan K, Wellesley D, Barnicoat A, Boyd PA, Scerif G. Autism, language and communication in children with sex chromosome trisomies. Archives of Disease in Childhood. 2010;96(10):954–959. doi: 10.1136/adc.2009.179747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop DV, Scerif G. Klinefelter syndrome as a window on the aetiology of language and communication impairments in children: the neuroligin-neurexin hypothesis. Acta Paediatr. 2011;100(6):903–907. doi: 10.1111/j.1651-2227.2011.02150.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boada R, Janusz J, Hutaff-Lee C, Tartaglia N. The cognitive phenotype in Klinefelter syndrome: A review of the literature including genetic and hormonal factors. Developmental Disabilities Research Reviews. 2009;15(4):284–294. doi: 10.1002/ddrr.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruining H, Swaab H, Kas M, van Engeland H. Psychiatric characteristics in a self-selected sample of boys with Klinefelter syndrome. Pediatrics. 2009;123(5):e865–e870. doi: 10.1542/peds.2008-1954. [DOI] [PubMed] [Google Scholar]
- Cai G, Edelmann L, Goldsmith JE, Cohen N, Nakamine A, Reichert JG, Buxbaum JD. Multiplex ligation-dependent probe amplification for genetic screening in autism spectrum disorders: efficient identification of known microduplications and identification of a novel microduplication in ASMT. BMC Med Genomics. 2008;1:50. doi: 10.1186/1755-8794-1-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantino JN, Davis SA, Todd RD, Schindler MK, Gross MM, Brophy SL, Reich W. Validation of a brief quantitative measure of autistic traits: comparison of the social responsiveness scale with the autism diagnostic interview-revised. Journal of Autism and Developmental Disorders. 2003;33(4):427–433. doi: 10.1023/a:1025014929212. [DOI] [PubMed] [Google Scholar]
- Constantino JN, Gruber CP. The Social Responsiveness Scale (SRS) Manual. Los Angeles: Western Psychological Services; 2005. [Google Scholar]
- Durand CM, Kappeler C, Betancur C, Delorme R, Quach H, Goubran-Botros H, Bourgeron T. Expression and genetic variability of PCDH11Y, a gene specific to Homo sapiens and candidate for susceptibility to psychiatric disorders. American Journal of Medical Genetics B Neuropsychiatric Genetics. 2006;141B(1):67–70. doi: 10.1002/ajmg.b.30229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geerts M, Steyaert J, Fryns JP. The XYY syndrome: a follow-up study on 38 boys. Genetic Counseling. 2003;14(3):267–279. [PubMed] [Google Scholar]
- Hollingshead AdB. Two-factor index of social position. New Haven, CT: 1965. [Google Scholar]
- Jamain S, Quach H, Betancur C, Rastam M, Colineaux C, Gillberg IC, Bourgeron T. Mutations of the X-linked genes encoding neuroligins NLGN3 and NLGN4 are associated with autism. Nature Genetics. 2003;34(1):27–29. doi: 10.1038/ng1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jha P, Sheth D, Ghaziuddin M. Autism spectrum disorder and Klinefelter syndrome. European Child and Adolescent Psychiatry. 2007;16(5):305–308. doi: 10.1007/s00787-007-0601-8. [DOI] [PubMed] [Google Scholar]
- Jonsson L, Ljunggren E, Bremer A, Pedersen C, Landen M, Thuresson K, Melke J. Mutation screening of melatonin-related genes in patients with autism spectrum disorders. BMC Med Genomics. 2010;3:10. doi: 10.1186/1755-8794-3-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahlou N, Fennoy I, Carel JC, Roger M. Inhibin B and anti-Mullerian hormone, but not testosterone levels, are normal in infants with nonmosaic Klinefelter syndrome. Journal of Clinical Endocrinology and Metabolism. 2004;89(4):1864–1868. doi: 10.1210/jc.2003-031624. [DOI] [PubMed] [Google Scholar]
- Laumonnier F, Bonnet-Brilhault F, Gomot M, Blanc R, David A, Moizard MP, Briault S. X-linked mental retardation and autism are associated with a mutation in the NLGN4 gene, a member of the neuroligin family. American Journal of Human Genetics. 2004;74(3):552–557. doi: 10.1086/382137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linden MG, Bender B, Robinson A. Intrauterine diagnosis of sex chromosome aneuploidy. Obstetrics and Gynecology. 1996;87(3):468–475. doi: 10.1016/0029-7844(95)00419-x. [DOI] [PubMed] [Google Scholar]
- Linden MG, Bender BG. Fifty-one prenatally diagnosed children and adolescents with sex chromosome abnormalities. American Journal of Medical Genetics. 2002;110(1):11–18. doi: 10.1002/ajmg.10394. [DOI] [PubMed] [Google Scholar]
- Melke J, Goubran Botros H, Chaste P, Betancur C, Nygren G, Anckarsater H, Bourgeron T. Abnormal melatonin synthesis in autism spectrum disorders. Molecular Psychiatry. 2008;13(1):90–98. doi: 10.1038/sj.mp.4002016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merhar SL, Manning-Courtney P. Two boys with 47, XXY and autism. Journal of Autism and Developmental Disorders. 2007;37(5):840–846. doi: 10.1007/s10803-006-0211-1. [DOI] [PubMed] [Google Scholar]
- Newcombe RG. Two-sided confidence intervals for the single proportion: comparison of seven methods. Statistics in Medicine. 1998;17(8):857–872. doi: 10.1002/(sici)1097-0258(19980430)17:8<857::aid-sim777>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Nicolson R, Bhalerao S, Sloman L. 47,XYY karyotypes and pervasive developmental disorders. Canadian Journal of Psychiatry. Revue Canadienne de Psychiatrie. 1998;43(6):619–622. doi: 10.1177/070674379804300611. [DOI] [PubMed] [Google Scholar]
- Nielsen J. Sex Chromosome Abnormalities found among 34,910 newborn children: results from a 13-year incidence study in Arhus, Denmark. Birth Defects Original Article Series. 1990;26(4):209–223. [PubMed] [Google Scholar]
- Ottesen A, Aksglaede L, Garn I, Tartaglia N, Tassone F, Gravholt C, Juul A. Increased number of sex chromosomes affects height in a non-linear fashion. American Journal of Medical Genetics: Part A. 2010 doi: 10.1002/ajmg.a.33334. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratcliffe SG, Butler GE, Jones M. Edinburgh study of growth and development of children with sex chromosome abnormalities. IV. Birth Defects Original Article Series. 1990;26(4):1–44. [PubMed] [Google Scholar]
- Ross J, Roeltgen D, Kushner H, Zinn A, Reiss A, McCauley E, Tartaglia N. Contrasting behavior and social phenotypes in boys with 47,XYY syndrome and 47,XXY Klinefelter syndrome. Pediatrics. doi: 10.1542/peds.2011-0719. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross JL, Roeltgen DP, Stefanatos G, Benecke R, Zeger MP, Kushner H, Zinn AR. Cognitive and motor development during childhood in boys with Klinefelter syndrome. American Journal of Medical Genetics A. 2008;146A(6):708–719. doi: 10.1002/ajmg.a.32232. [DOI] [PubMed] [Google Scholar]
- Ross JL, Samango-Sprouse C, Lahlou N, Kowal K, Elder FF, Zinn A. Early androgen deficiency in infants and young boys with 47,XXY Klinefelter syndrome. Hormone Research. 2005;64(1):39–45. doi: 10.1159/000087313. [DOI] [PubMed] [Google Scholar]
- Sorensen K, Nielsen J, Jacobsen P, Rolle T. The 48,XXYY syndrome. Journal of Mental Deficiency Research. 1978;22(3):197–205. [PubMed] [Google Scholar]
- SPSS. SPSS Statistics 17.0 Algorithms manual. Chicago, IL: 2007a. [Google Scholar]
- SPSS. SPSS Statistics 17.0 Command Syntax Reference manual. Chicago, IL: 2007b. [Google Scholar]
- Tartaglia N, Ayari N, Howell S, D’Epagnier C, Zeitler P. 48,XXYY, 48,XXXY and 49,XXXXY syndromes: not just variants of Klinefelter syndrome. Acta Paediatrica. 2011;100(6):851–860. doi: 10.1111/j.1651-2227.2011.02235.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tartaglia N, Cordeiro L, Howell S, Wilson R, Janusz J. The spectrum of the behavioral phenotype in boys and adolescents 47,XXY (Klinefelter syndrome) Pediatric Endocrinology Reviews. 2010;8(Suppl 1):151–159. [PMC free article] [PubMed] [Google Scholar]
- Tartaglia N, Davis S, Hench A, Nimishakavi S, Beauregard R, Reynolds A, Hagerman R. A new look at XXYY syndrome: medical and psychological features. Americal Journal of Medical Genetics A. 2008;146A(12):1509–1522. doi: 10.1002/ajmg.a.32366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Rijn S, Aleman A, De Sonneville L, Swaab H. Cognitive mechanisms underlying disorganization of thought in a genetic syndrome (47,XXY) Schizophrenia Research. 2009;112(1–3):91–98. doi: 10.1016/j.schres.2009.04.017. [DOI] [PubMed] [Google Scholar]
- van Rijn S, Aleman A, Swaab H, Krijn T, Vingerhoets G, Kahn R. What it is said versus how it is said: comprehension of affective prosody in men with Klinefelter (47,XXY) syndrome. Journal of the International Neuropsychological Society. 2007;13(6):1065–1070. doi: 10.1017/S1355617707071044. [DOI] [PubMed] [Google Scholar]
- van Rijn S, Swaab H, Aleman A, Kahn R. X Chromosomal effects on social cognitive processing and emotion regulation: A study with Klinefelter men (47,XXY) Schizophrenia Research. 2006;84(2–3):194–203. doi: 10.1016/j.schres.2006.02.020. [DOI] [PubMed] [Google Scholar]
- van Rijn S, Swaab H, Aleman A, Kahn RS. Social behavior and autism traits in a sex chromosomal disorder: Klinefelter (47XXY) syndrome. Journal of Autism and Developmental Disorders. 2008;38(9):1634–1641. doi: 10.1007/s10803-008-0542-1. [DOI] [PubMed] [Google Scholar]
- Walzer S, Bashir A, Silbert A. Cognitive and behavioral factors in the learning disabilities of XXY and XYY boys. Birth Defects Original Article Series. 1990;26(4):45–58. [PubMed] [Google Scholar]
- Yan J, Oliveira G, Coutinho A, Yang C, Feng J, Katz C, Sommer SS. Analysis of the neuroligin 3 and 4 genes in autism and other neuropsychiatric patients. Molecular Psychiatry. 2005;10(4):329–332. doi: 10.1038/sj.mp.4001629. [DOI] [PubMed] [Google Scholar]


