Abstract
The niacin receptor GPR109A is a Gi-protein coupled receptor which mediates the effects of niacin on inhibiting intracellular triglyceride lipolysis in adipocytes. However, the role of GPR109A in mediating the effects of niacin on high density lipoprotein (HDL) metabolism is unclear. We found niacin has no effect on HDL-C in GPR109A knockout mice. Furthermore, niacin lowered intracellular cAMP in primary hepatocytes mediated by GPR109A. We used an adeno-associated viral (AAV) serotype 8 vector encoding GPR109A under the control of the hepatic-specific thyroxine-binding globulin promoter to specifically overexpress GPR109A in mouse liver. Plasma HDL-C, hepatic ABCA1 and the HDL cholesterol production rate were significantly reduced in mice overexpressing GPR109A. Overexpression of GPR109A reduced primary hepatocyte free cholesterol efflux to apoA-I; conversely, GPR109A deficient hepatocytes had increased ABCA1-mediated cholesterol efflux. These data support the concept that the HDL-C lowering effect of niacin in wild-type mice is mediated through stimulation of GPR109A in hepatocytes; such an effect then leads to reduced hepatocyte ABCA1 expression and activity, decreased cholesterol efflux to nascent apoA-I, and reduced HDL-C levels. These results indicate that niacin-mediated activation of GP109A in liver lowers ABCA1 expression leading to reduced hepatic cholesterol efflux to HDL.
Supplementary key words: niacin, GPR109A, HDL, ABCA1, AAV
Plasma levels of high density lipoprotein (HDL) cholesterol (C) and its major apolipoprotein, apoA-I, show a strong inverse association with the risk of atherosclerotic cardiovascular disease (1,2). HDL-C and apoA-I levels are reduced in acute and chronic inflammatory states (3,4). Niacin (nicotinic acid) is the most effective pharmacologic approach currently available for raising HDL-C levels, but many questions remain regarding the molecular mechanisms responsible for such an effect (5–7). Studies in cultured hepatocytes have suggested that niacin reduces the uptake of HDL (8) while kinetic studies in humans have mixed results, with reports that niacin reduces HDL clearance from plasma (9) or increases production (10) of the major HDL protein apoA-I.
GPR109A (also referred to as HM74A in humans and PUMA-G in mice) and GPR109B (also known as HM74 in human) are highly homologous G-protein coupled receptors (GPCRs) and were identified as receptors for niacin (11–13) in 2003. Both GPR109A and GPR109B are highly expressed in adipocytes and activated immune cells (11–14). GPR109B has only been found in humans, not in rodents (14–16). β-hydroxybutyrate, produced during fasting and starvation, was found to act as endogenous ligand of GPR109A (17). Activation of GPR109A in adipose tissue leads to inhibition of adenylate cyclase, reduced cyclic adenosine monophosphate (cAMP), reduced activity of protein kinase A, reduced adipocyte triglyceride lipolysis, and reduced release of free fatty acids. Studies in GPR109A knockout mice confirmed that GPR109A was responsible for the antilipolytic (and cutaneous flushing) effects of niacin (18). Reduced flux of free fatty acids from adipose to liver is believed to potentially underlie the triglyceride and LDL lowering effects of niacin.
Whether the HDL-raising effects of niacin are related to activation of GPR109A is unknown. Interestingly, niacin treatment of wild-type mice lacking cholesteryl ester transfer protein (CETP) consistently results in reduction of HDL-C, whereas in mice expressing CETP niacin has a modest HDL-raising effect (19–21). The molecular mechanism(s) by which niacin reduces HDL-C in wild-type mice lacking CETP and whether this involves activation of GPR109A in the liver are unknown. The aim of the present study is to establish whether GPR109A activation in liver influences HDL metabolism and the HDL response to niacin. By using GPR109A knockout mice, we demonstrate that GPR109A mediates niacin’s effect of reducing HDL-C in the mouse. We established that GPR109A is expressed in primary murine hepatocytes and human-derived hepatocytes at low basal levels, and that expression is markedly inducible after exposure to inflammatory stimuli. Hepatic-specific overexpression of GPR109A in mice reduced plasma HDL-C levels accompanied by reduction in hepatic cAMP, ABCA1 protein, cholesterol efflux to apoA-I, and a reduced HDL cholesterol production rate. These data show that the reduction of HDL-C seen in mice in response to niacin is mediated by hepatic GPR109A.
Methods
Reagents and plasmid constructs
Forskolin, 3-isobutyl-1-methylxanthine (IBMX), niacin and puromycin were purchased from Sigma-Aldrich (St. Louis, MO, USA). Ketamine was supplied by Fort Dodge Animal Health (Fort Dodge, Iowa, USA), xylazine was supplied by Vedco Inc. (Saint joseph, MO, USA). The cAMP assay was purchased from R&D systems (Minneapolis, MN, USA). pCMV.SPORT6.1-hGPR109A plasmids were purchased from Invitrogen (Carlsbad, CA, USA). Polyclonal antibodies to HM74 and ABCA1 were from Novus Biologicals (Littleton, CO, USA); monoclonal antibodies to β-actin were from Santa Cruz Biotechnology (Santa Cruz, CA, USA); HRP-conjugated anti-mouse, anti-rabbit, anti-goat IgG and percoll were from GE Healthcare (Piscataway, NJ, USA); pQCXIP retroviral vectors were from BD-Clontech (Palo Alto, CA, USA). hGPR109A sequence from pCMV.SPORT6.1-hGPR109A was subcloned to pQCXIP vector. β–hydroxybutyrate liquicolor assay kit was purchased from Stanbio Laboratory (Los Angeles, CA, USA). 3H-cholesteryl ether was supplied by Perkin Elmer Life Sciences Inc. (Boston, MA, USA).
Cell culture
HepG2 cells obtained from ATCC (Manasas, VA, USA) were grown in DMEM supplemented with 10% fetal bovine serum (FBS). For retroviral infections, viruses were produced from Phoenix ecotropic cells (Orbigen Inc., San Diego, CA, USA) by transient transfection of pQCXIP-human GPR109A, GPR109B or pQCXIP vector containing no insert (mock). HepG2 cells were subjected to one round of infection and selected 48 hours after infection using 3 μg/ml puromycin. HepG2-mock (stably expressing pQCXIP vector), HepG2-GPR109A (stably expressing human GPR109A) and HepG2-GPR109B (stably expressing human GPR109B) were grown in DMEM supplemented with 10 % FBS and 1 μg/ml puromycin.
Murine primary hepatocytes were isolated from adult female C57BL/6 mice. Using aseptic techniques, mice were anesthetized with ketamine/xylazine (70/7 mg/kg, respectively, IP) and subject to a mid-line laparotomy. Following a nonrecirculating in situ perfusion with Liver Perfusion Medium (Invitrogen, Carlsbad, CA, USA) through the portal vein, liver was digested with Liver Digestion Medium (Invitrogen) and minced. After washing with hepatocyte wash medium (Invitrogen), viable cells were purified by Percoll gradient centrifugation, and plated in collagen I-coated plates at 2 × 105 cells per well in a 12-well plate in Williams E medium supplemented with 10% FBS and antibiotics (22,23).
Animal studies
All animal procedures were approved by the University of Pennsylvania Institutional Animal Care and Use Committee. Breeder GPR109A knockout (KO) mice were kindly provided by Dr. Klaus Pfeffer (University of Düsseldorf, Düsseldorf, Germany) and then bred in our facility. GPR109A knockout mice have been backcrossed eight times to C57BL/6 to ensure a 98.3% similarity in genetic backgrounds with wild-type C57BL/6 mice. C57BL/6N mice were purchased from Taconic Company as wild-type (WT) control. Female C57BL/6J mice (six- to eight-week-old) were obtained from the Jackson Laboratory and maintained on a chow diet unless otherwise indicated. Diets were supplied by Research Diets, Inc. (New Brunswick, NJ). A simple purified ingredient diet, AIN-76A rodent diet, was used as the control diet and a 1% niacin diet consisted of the AIN-76A diet containing 10 g niacin per kilogram.
To overexpress hepatic GPR109A or GPR109B, C57BL/6 mice were injected with an AAV consisting of serotype 2/8 encoding human GPR109A (AAV2/8-TBG-hGPR109A) or GPR109B (AAV2/8-TBG-hGPR109B) under the control of the liver-specific, thyroxine-binding globulin (TBG) promoter, or with control null AAV2/8 (AAV2/8-TBG-Null) into the peritoneum (1×1012 particles/mouse) (24). The AAV2/8-TBG-hGPR109A and AAV2/8-TBG-hGPR109B cis plasmids were constructed by inserting human GPR109A or GPR109B cDNA followed by SV-40 poly A tail in front of liver-specific human TBG promoter flanked by AAV2-inverted terminal repeat sequence. The chimeric vector AAV2/8 with human GPR109A, GPR109B or null was then formed by fusing the capsid gene of serotype 8 with AAV2 rep gene by triple transfection into 293 cells with adenovirus helper plasmid, cis-plasmid, and a chimeric packaging construct.
Plasma lipid and lipoprotein analyses
Plasma total cholesterol and HDL cholesterol were measured on a Cobas Fara (Roche Diagnostics, Indianapolis, IN, USA) with the use of Sigma Diagnostic reagents. Fast protein liquid chromatography (FPLC) (Amersham Pharmacia Biotech, Uppsala, Sweden) analysis was performed on pooled samples. The cholesterol concentrations in the FPLC fractions were determined via an enzymatic assay (Wako Pure Chemical Industries Ltd, Richmond, VA, USA).
Kinetic study
The in vivo kinetic study was performed by co-injection of human HDL3 labeled with 125I-tyramine cellobiose, which labels primarily apoA-I on HDL3, and human HDL3 exchange labeled with 3H-cholesteryl ether, a tracer of the cholesteryl ester core of HDL, as previously described (25). Tyramine cellobiose was prepared and provided by William Cain, University of Delaware. Both tracers are non-hydrolyzable allowing tissue uptake to be accurately assessed. 125I-Tyramine cellobiose-HDL3 and 3H-cholesteryl ether-HDL3 were co-injected by the tail vein in each mouse, and blood samples were obtained by retro-orbital bleeding at 2 minutes and at 1, 3, 6, 9, 24 and 48 hours. Forty-eight hours after dose injection, fasted animals were sacrificed, the vascular system was flushed with 15 ml ice-cold PBS, the liver and kidneys were harvested and radioactivity counted. The fractional catabolic rate (FCR) of apoA-I and HDL cholesteryl ester was determined by fitting the corresponding tracer data to a single pool model containing an extravascular exchange using data normalized to the 2-min time point. Selective uptake was calculated as the difference between the HDL cholesteryl ester and apoA-I FCRs. The production rate for HDL cholesteryl ester was calculated by multiplying the FCR by the estimated HDL cholesteryl ester pool size.
Statistics
Statistics were conducted using GraphPad Prism Software version 5.0. Results are presented as mean ± SD or ± SEM as indicated. Differences were determined using 2-tailed Student’s t test or a one-way ANOVA using a Newman-Keuls test to determine post-hoc differences. Differences were considered significant at a two-tailed P value of < 0.05
Results
Niacin reduces plasma HDL-C in WT mice but not in GPR109A KO mice
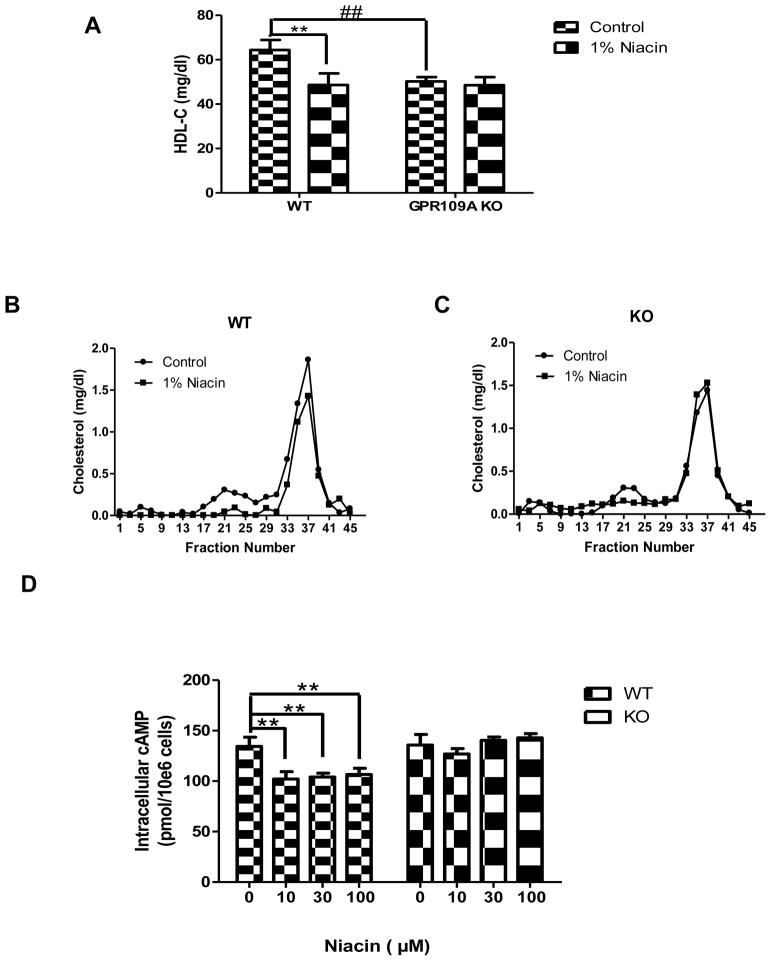
It is well-established that in wild-type mice, niacin treatment reduces plasma HDL-C levels (19–21). To test whether this HDL-lowering effect of niacin is GPR109A dependent, GPR109A knockout and wild-type control mice were treated with 1% niacin diet for 3 weeks. There were no differences in food consumption or weight gain between mice consuming the 1% niacin diet and control diet (data not shown). We found that niacin reduced HDL-C levels in wild-type mice as expected (Fig 1A); interestingly, in GPR109A KO mice niacin had no effect on HDL-C levels (Fig 1A). These results were confirmed by FPLC, showing that niacin reduced HDL in wild-type mice (Figure 1B) but not in GPR109A knockout mice (Figure 1C). This suggests that the HDL-lowering effect of niacin in mice is GPR109A-dependent.
Figure 1.
Treatment of mice with a diet containing 1% niacin decreases plasma HDL-C in wild-type mice but not in GPR109A knockout mice. GPR109A knockout and wild-type control mice (n=8) were fed a 1% niacin diet for three weeks. (A) Plasma HDL-C levels. Data are expressed as mean ± SEM, **P<0.01, compared with control diet; ##P<0.01 compared with wild type. Total cholesterol FPLC profile in GPR109A knockout (C) and wild-type mice(B). (D) Intracellular cAMP levels in the primary hepatocyte. Primary hepatocytes from GPR109A deficient or wild-type C57BL/6 mice were isolated and plated in 12-well plates. After pretreatment with or without 0, 10, 30 or 100 μM niacin for 1 hour, hepatocytes were treated with 1 mM IBMX and 30 μM forskolin for 15 min at 37° C. The amount of cAMP present was calculated from a standard curve prepared using nonradioactive cAMP and was expressed as picomoles per 1 × 106 cells. Data are mean ± SEM, n = 3, **P<0.01, compared with no niacin treatment.
GPR109A is a Gi protein coupled receptor that when activated in adipocytes and GPR109A stably expressing cells reduces cAMP levels (11,12,14,26–29). We hypothesized that niacin acts via GPR109A to modulate cAMP levels in hepatocytes. Primary hepatocytes from wild-type and GPR109A knockout mice were isolated and cAMP levels in response to niacin stimulation were measured. Niacin inhibited intracellular cAMP release in hepatocytes from wild-type mice whereas there was no effect of niacin on cAMP release from hepatocytes isolated from GPR109A knockout mice (Figure 1D). These results suggest that primary hepatocytes respond to niacin via GPR109A despite low basal levels of receptor expression.
LPS induces expression of GPR109A in murine hepatocytes in vivo and ex vivo
GPR109A is expressed in murine liver but the basal expression of GPR109A is low. However, after injection of very low dose endotoxin (LPS), expression of GPR109A was markedly upregulated in liver (Supplemental Figure A). The maximal upregulation of GPR109A mRNA by LPS treatment occurred at 2 hours (>80-fold) and returned to baseline by 24 hours. The peak expression of GPR109A protein at 2 hours was confirmed in the liver lysates by Western blotting (Supplemental Figure B).
Macrophages are known to express GPR109A and therefore the increased GPR109A expression in liver after LPS stimulation could be due primarily to Kupffer cell expression. To determine whether LPS upregulates GPR109A expression specifically in parenchymal cells, C57BL/6 mice were injected intraperitoneally with LPS and hepatocytes were isolated from liver at baseline and 2 hours after LPS injection. RNA was isolated and GPR109A mRNA determined by QRT-PCR. Hepatocyte GPR109A was markedly upregulated (>500-fold) by LPS injection (Supplemental Figure C), establishing that parenchymal cells respond to inflammation in vivo with pronounced upregulation of the receptor. Finally, mouse primary hepatocytes were isolated from C57BL/6 mice and were treated ex vivo with 100 ng/ml LPS for various time periods. GPR109A mRNA was increased by about 4-fold at 6 hours compared with the baseline (Supplemental Figure D). These studies establish that parenchymal hepatocytes express GPR109A at low levels under basal conditions and markedly upregulate its expression in response to inflammation.
Hepatocyte-specific overexpression of GPR109A in WT mice reduces HDL-C levels
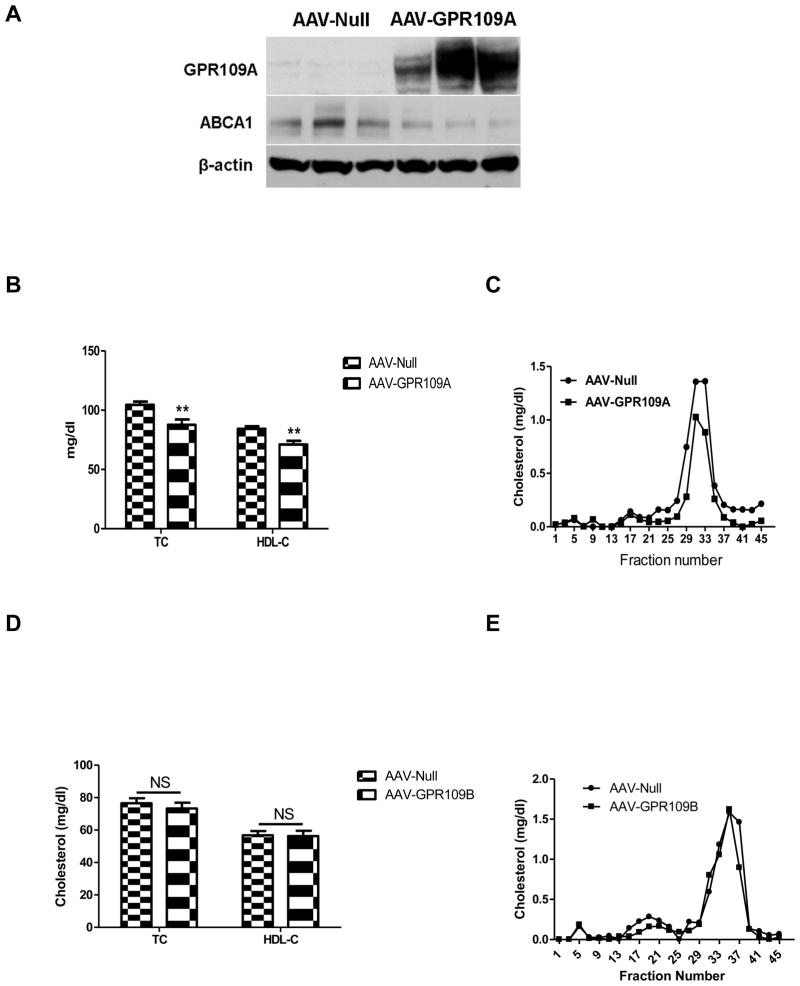
Having established that GPR109A is expressed in mouse hepatocytes and is upregulated by inflammation, we wished to probe the effects of specific hepatocyte GPR109A overexpression on HDL metabolism. To test this, we utilized an AAV2/8-based vector encoding human GPR109A under the control of the liver-specific TBG promoter. GPR109B shows a high degree of sequence similarity to GPR109A; thus to test whether specific hepatic GPR109B overexpression has a similar effect as GPR109A on HDL metabolism, the AAV2/8-TBG-GPR109B virus was made as a control. Injection of the AAV-GPR109A virus into wild-type mice resulted in detectable increase in the amount of GPR109A protein in liver (Figure 2A). Eight weeks after injection, plasma total and HDL cholesterol (Figure 2B) levels were significantly lower in GPR109A overexpressing mice compared with mice injected with control vector. The decrease in HDL-C in mice overexpressing GPR109A in liver was confirmed by measurement of cholesterol in FPLC fractions (Figure 2C). Furthermore, GPR109B overexpression had no effect on either total or HDL cholesterol (Figure 2D and E).
Figure 2.
Effects of liver specific expression of GPR109A or GPR109B on plasma lipids in vivo. C57BL/6 mice (n=7) were injected intraperitoneally with 1x1012 genome copies (GC) AAV2/8 GPR109A or control virus. (A) Western blot of GPR109A and ABCA1 in liver tissue from GPR109A overexpression and control mice. C57BL/6 mice were injected with 1x1012 GC AAV2/8 GPR109A or control virus IP, and 8 weeks later livers were harvested for total protein extraction. Experiments were repeated 3 times. (B) Plasma levels of total cholesterol and HDL-C after 8 weeks of transduction. Data are expressed as mean ± SEM. n=7, **P <0.01. (C) Total cholesterol FPLC Profile. C57BL/6 mice (n=8) were injected intraperitoneally with 1x1012 GC AAV2/8 GPR109B or control virus. (D) Plasma levels of total cholesterol and HDL-C after 8 weeks of transduction. Data are expressed as mean ± SEM. (E) Total cholesterol FPLC Profile.
Ligand activation of GPR109A in hepatocytes reduces HDL-C levels in vivo
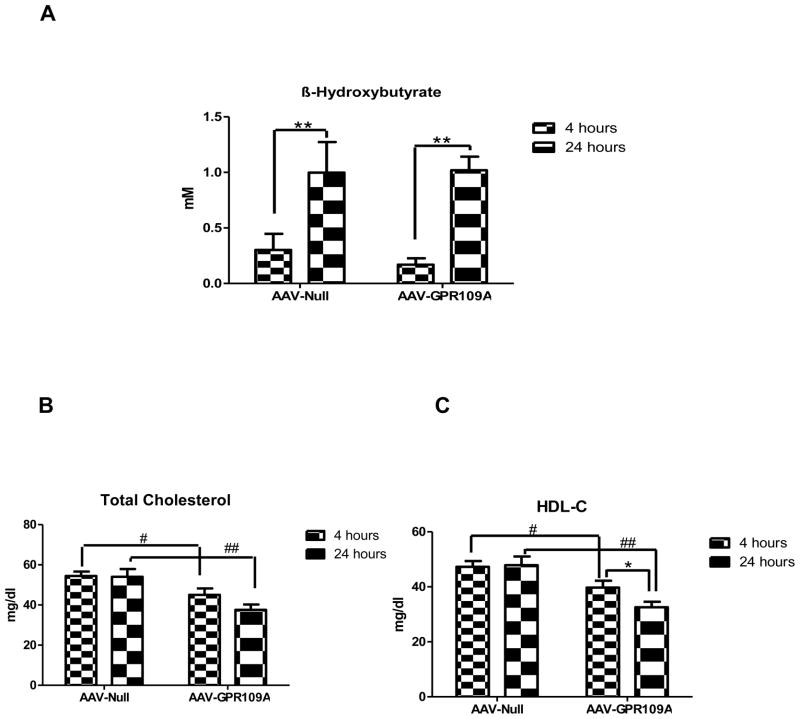
β-hydroxybutyrate is an endogenous ligand for GPR109A (17). In order to test the role of hepatic GPR109A in HDL metabolism under endogenous ligand stimulated conditions in vivo, mice were subjected to 24 hours fasting to stimulate production of β-hydroxybutyrate. Twenty four hour fasting significantly increased plasma β-hydroxybutyrate as expected (Figure 3A). While the increase in β-hydroxybutyrate had no effect on total and HDL-C in control mice, fasting significantly reduced HDL-C in mice overexpressing GPR109A (Figure 3B, C).
Figure 3.
Effects of 24 hour fasting on both control and liver-specific expression of GPR109A. C57BL/6 mice were injected intraperitoneally with 1x1012 genome copies AAV2/8 GPR109A or control AAV2/8 Null. After 11 weeks of virus injection, chow-fed mice were subjected to 24 hour fasting; plasma levels of β– hydroxybutyrate (A), total cholesterol (B) and HDL-C (C) were measured. Data are expressed as mean ± SEM, n=7. *P<0.05 compared with 4 hours fasting, #P <0.05, ##P<0.01 compared with AAV-Null.
Hepatocyte-specific overexpression of GPR109A in WT mice reduces HDL-C production
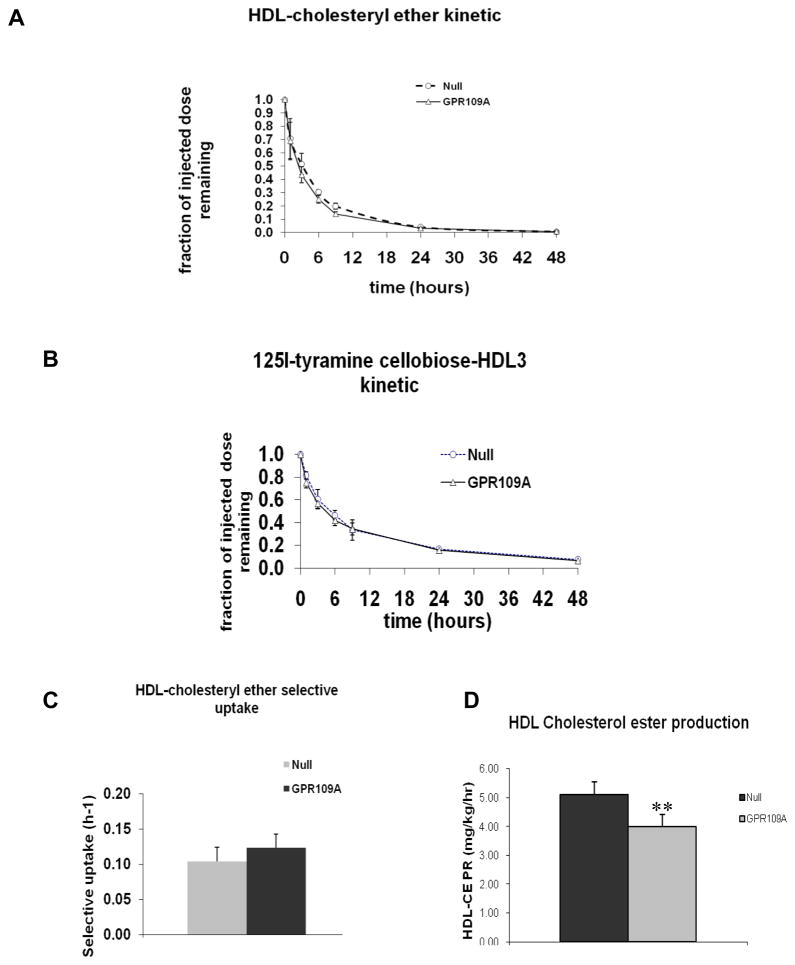
To investigate the effect of hepatic overexpression of GPR109A on plasma HDL metabolism, 125I-tyramine cellobiose and 3H-cholesteryl ether labeled HDL was IV injected into mice treated with AAV2/8-TBG-GPR109A or control vector (Figure 4). There was no difference in the rate of HDL cholesteryl ester (HDL-CE) (Figure 4A) or protein clearance (Figure 4B) between hepatic overexpressing GPR109A mice and control mice and there was no change in selective uptake of HDL-CE (Figure 4C). However, the HDL cholesterol production rate was significantly reduced in mice overexpressing GPR109A (Figure 4D). These data indicate that the lowering of HDL-C in hepatic overexpressing GPR109A mice is not due to increased HDL cholesterol catabolism but due to reduced HDL cholesterol production.
Figure 4.
Plasma kinetics of 3H-cholesteryl ether and 125I-labeled tyramine-cellobiose labeled human HDL3. Blood samples were drawn at the time points indicated and analyzed for radioactivity. (A) 3H-HDL-cholesteryl ether clearance from plasma. (B) 125I-Tyramine cellobiose HDL clearance from plasma. (C) Plasma HDL-cholesteryl ether selective uptake. Data are expressed as mean ± SEM, n=6. (D) Plasma HDL-cholesteryl ester transport rate. Data are expressed as mean ± SEM, n = 6, **P<0.01.
Hepatocyte-specific overexpression of GPR109A in hepatocytes reduces cAMP, ABCA1 protein, and cholesterol efflux to apoA-I
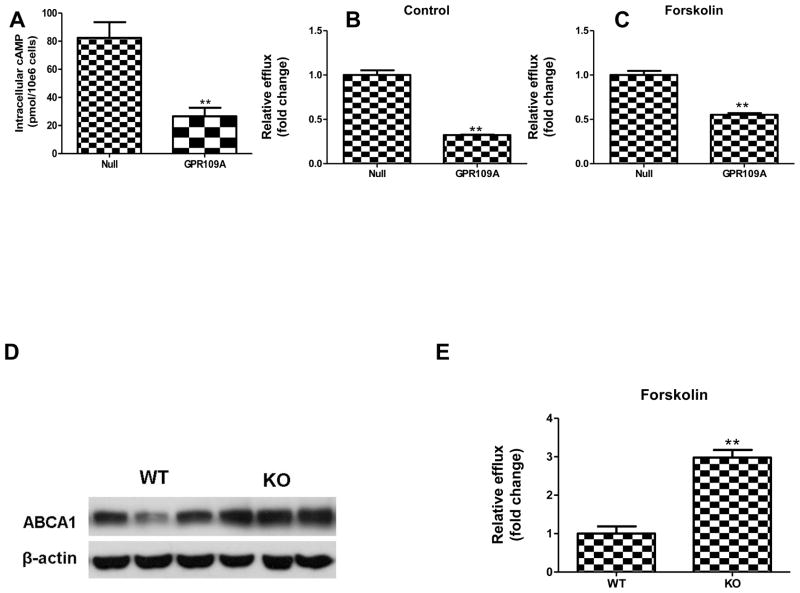
In order to understand the mechanism of the reduced HDL cholesteryl ester production, primary hepatocytes were isolated from GPR109A-expressing and control mice. Expression of GPR109A in hepatocytes dramatically reduced the forskolin-induced intracellular level of cAMP (Figure 5A), indicating that overexpression of the receptor led to its activation, as has been described for other GPCRs (30,31). Hepatic ABCA1 expression is a key determinant of HDL-C levels in mice through promoting cholesterol efflux from hepatocytes to apoA-I (32,33), and in some cells, such as macrophages, ABCA1 is upregulated at the protein level by cAMP (34,35). We hypothesized that in hepatocytes the reduction of cAMP due to GPR109A activation would result in decreased ABCA1 protein abundance and function. We confirmed hepatic ABCA1 protein levels were substantially reduced in overexpressing GPR109A mice (Figure 2A), consistent with the reduction in cAMP (Figure 5A). GPR109A-overexpressing primary hepatocytes had reduced efflux of free cholesterol to apoA-I (a measure of ABCA1 activity) compared with control cells under basal (Figure 5B) and forskolin-treated (Figure 5C) conditions. In contrast, the GPR109A deficient liver had a higher ABCA1 protein expression compared with wild-type control mice liver (Figure 5D). Cholesterol efflux data show that forkolin treatment evokes a higher free cholesterol efflux to apoA-I in GPR109A knockout hepatocytes (Figure 5E).
Figure 5.
GPR109A overexpression inversely related to free cholesterol efflux to apoA-I from hepatocytes. C57BL/6 mice were injected with 1x1012 GC AAV2/8 GPR109A or control virus IP, and 8 weeks later liver and hepatocytes were isolated. Primary hepatocytes from mice injected with either AAV-GPR109A or AAV-Null were cultured in 12-well plates. (A) GPR109A overexpression decreased intracellular cAMP release in response to forskolin. The amount of cAMP present was calculated from a standard curve prepared using nonradioactive cAMP and was expressed as picomoles per 1 × 106 cells. Data are mean ± SEM, n = 3, **P<0.01, compared with AAV-Null. Primary hepatocytes were labeled with 2 μCi/ml 3H-cholesterol in medium for 24 h; cells were then equilibrated for 18 h at 37°C with DMEM/BSA (B) or containing the indicated reagents 30 μM forskolin plus 1 mM IBMX (C), separately. For cholesterol efflux, medium containing 10 μg/ml free apoA-I was added to cells. 9 hours cholesterol efflux levels were measured. Values represent the average of triplicate determinations normalized to AAV-Null group (mean ± SD), **P<0.01 compared with control vector. (D) Western blot of ABCA1 in liver tissue from GPR109A knockout and wild-type control mice. Experiments were repeated 3 times. (E) Cholesterol efflux to apoA-I from forskolin treated GPR109A knockout and wild-type control primary hepatocytes. Primary hepatocytes from GPR109A deficient or wild type C57BL/6 mice were isolated, labeled, equilibrated with DMEM/BSA containing 30 μM forskolin plus 1 mM IBMX, then performed 9 hours efflux study to human apoA-I. Values represent the average of triplicate determinations normalized to wild type group (mean ± SD), **P<0.01 compared with wild-type.
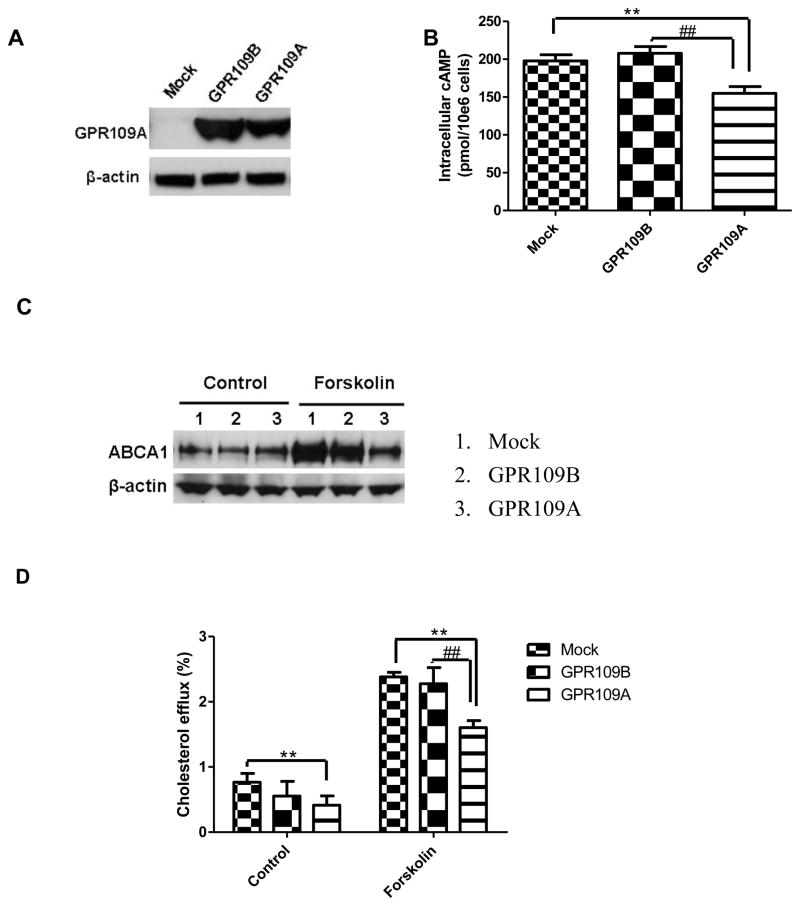
To investigate whether the results observed in mouse hepatocytes were applicable to humans, we conducted experiments using HepG2 cells stably transfected with GPR109A (HepG2-GPR109A) or GPR109B (HepG2-GPR109B). HepG2 cells transduced with a null retroviral vector were used as control cells (HepG2-mock). GPR109B differs from GPR109A in an extended C-terminal tail as well as in 16 amino acids (11,13); despite this difference, the antibody we used recognized both GPR109A and GPR109B. Western blot analysis revealed a low level of GPR109A expression in the control cells and a substantially greater level of GPR109A or GPR109B protein in the stably transfected cells (Figure 6A). As shown in Figure 6B, overexpression of GPR109A reduced cellular cAMP levels in forskolin-treated cells, but not GPR109B. The GPR109A expressing cells had a substantial reduction in ABCA1 protein levels in the presence of forskolin (Figure 6C), accompanied by a 55% decrease in cholesterol efflux to apoA-I (Figure 6D), while GPR109B expressing cells had a similar ABCA1 protein level (Figure 6C) and cholesterol efflux capacity (Figure 6D) with mock cells.
Figure 6.
Overexpression of GPR109A reduces ABCA1 expression and activity in HepG2 cells. (A) Western Blot of GPR109A in HepG2 cells. (B) Intracellular cAMP assay. HepG2 cells transfected with GPR109A or GPR109B or control HepG2 cells were treated with 30 μM forskolin and 1 mM 3-isobutyl-1-methylxanthine for 15 min at 37 °C. Data from 3 independent experiments are expressed as mean ± SD, n =6, **P<0.01, compared with mock group, ##P<0.01, compared with GPR109B group. (C) Western Blot of cell lysate of ABCA1. Experiments were repeated 3 times. (D) Cholesterol efflux to apoA-I from HepG2 cells. Efflux of free cholesterol into the media is expressed as percentage of the total radioactivity in the media and cells (3H-cholesterol in the medium × 100)/(3H-cholesterol in medium + 3H-cholesterol in the cells). Values represent the average of triplicate determinations (mean ± SD), **P<0.01 compared with control vector, ##P<0.01, compared with GPR109B group.
Discussion
The current study is the first to specifically investigate the role of hepatic GPR109A and GPR109B on HDL metabolism and response to niacin therapy. While niacin raises HDL-C levels in humans, it has been reported by others (20,36) and confirmed by us here that niacin treatment reduced serum levels of HDL cholesterol in wild-type mice. This effect of reducing HDL-C was GPR109A dependent. GPR109A is a Gi protein coupled receptor that is known to reduce cAMP and lipase-mediated hydrolysis of triglyceride stores in adipose tissue. We found that niacin treatment also inhibited intracellular cAMP release response to forskolin stimulation in primary hepatocytes from wild-type mice. While hepatocyte expression of GPR109A in the basal state is low, it was markedly upregulated by very low dose endotoxin. GPR109A expression is upregulated in murine macrophages by inflammatory stimuli (37) but this phenomenon has not been reported in other cell types. While the basal expression of GPR109A in liver is low, we show that it is up-regulated after LPS injection; furthermore, we show that this is due to upregulation in hepatocytes and not simply Kupffer cells. These studies establish that hepatocytes express the niacin receptor and upregulate its expression in response to inflammation.
In order to study the specific effects of increased hepatocyte expression of GPR109A on lipid, and particularly HDL, metabolism, we made an AAV2/8-based vector encoding murine GPR109A driven by the hepatocyte-specific TBG promoter. Intraperitoneal injection of this vector into wild-type mice resulted in expression of human GPR109A at levels substantially greater than basal and in the range of what we observed after LPS injection. Several G protein-coupled receptors have been shown to exist as homo-and hetero-oligomeric complexes in living cells and can exhibit ligand-independent activation, particularly when overexpressed (30,31). We found that overexpression of GPR109A in hepatocytes resulted in activation as evidenced by marked suppression of forskolin-stimulated cAMP. This activation may be caused by dimerization of the GPR109A, oligomerization with another GPCR in the setting of overexpression, or simply increased receptor activation (38). Although GPR109A and GPR109B are highly similar at the protein level and share a similar expression pattern, they are the products of different genes and clearly differ in their ability to be activated by niacin and β-hydroxybutyrate (16). Our data establish that GPR109B expressed in hepatocytes does not have the same effects on cAMP and ABCA1 activity as does the expression of GPR109A.
Overexpression of GPR109A caused a reproducible and significant decrease in plasma HDL-C levels that we showed was not due to increased HDL catabolism but rather to reduced HDL cholesterol production. Hepatic ABCA1 plays a critical role in the biosynthesis of nascent HDL by promoting the efflux of cholesterol from hepatocytes to lipid-poor apoA-I (32). Because activation of GPR109A is known to reduce cAMP in adipocytes, and ABCA1 protein and activity are known to be increased by cAMP in macrophages, we hypothesized that activation of GPR109A in hepatocytes would reduce cAMP and thus ABCA1 protein and activity. Our data support this model and provide a mechanistic basis for the decreased HDL-C levels with both niacin treatment and GPR109A overexpression in mice. In contrast, GPR109A deficiency was associated with significantly increased cholesterol efflux to apoA-I in primary hepatocytes, which is consistent with a role for GPR109A in modulating HDL-C metabolism in liver.
In summary, while basal expression of GPR109A in hepatocytes is low, it is substantially upregulated by inflammatory stimuli. Overexpression of GPR109A in mouse liver unexpectedly reduced levels of HDL cholesterol in plasma, apparently due to constitutive activation of the receptor with reduced cAMP production, leading to reduced ABCA1 protein and activity, reduced cholesterol efflux to apoA-I and reduced HDL-C levels in wild-type mice. The role of hepatocyte GPR109A expression in human physiology and the response to niacin in humans remains to be fully elucidated.
Supplementary Material
Acknowledgments
We thank Dr. Klaus Pfeffer who kindly provided GPR109A knockout mice for these experiments. We thank Dr. Weijun Jin for his valuable assistance and scientific discussions. We are indebted to Dr Hiroyuki Tanigawa for his valuable assistance and Aisha Wilson and Edwige Edouard for excellent technical assistance.
Sources of Funding
This work was supported by NIH grant P50-HL-083799 from the National Heart Lung and Blood Institute (to D.J.R.).
Abbreviations
- ABCA1
ATP binding cassette transporter A1
- ApoA-I
apolipoprotein A-I
- cAMP
cyclic adenosine monophosphate
- CETP
cholesteryl ester transfer protein
- GPCR
G protein-coupled receptor
- HDL-C
high density lipoprotein cholesterol
- IBMX
3-isobutyl-1-methylxanthine
- LPS
lipopolysaccharide
- TBG
thyroxine-binding globulin
Footnotes
Disclosures
None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gordon DJ, Rifkind BM. N Engl J Med. 1989;321(19):1311–1316. doi: 10.1056/NEJM198911093211907. [DOI] [PubMed] [Google Scholar]
- 2.Goldbourt U, Yaari S, Medalie JH. Arterioscler Thromb Vasc Biol. 1997;17(1):107–113. doi: 10.1161/01.atv.17.1.107. [DOI] [PubMed] [Google Scholar]
- 3.Rossner S. Atherosclerosis. 1978;31(1):93–99. doi: 10.1016/0021-9150(78)90041-2. [DOI] [PubMed] [Google Scholar]
- 4.Bausserman LL, Bernier DN, McAdam KP, Herbert PN. Eur J Clin Invest. 1988;18(6):619–626. doi: 10.1111/j.1365-2362.1988.tb01277.x. [DOI] [PubMed] [Google Scholar]
- 5.Drexel H. Fundam Clin Pharmacol. 2007;21(Suppl 2):5–6. doi: 10.1111/j.1472-8206.2007.00530.x. [DOI] [PubMed] [Google Scholar]
- 6.Bodor ET, Offermanns S. Br J Pharmacol. 2008;153(Suppl 1):S68–75. doi: 10.1038/sj.bjp.0707528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carlson LA. J Intern Med. 2005;258(2):94–114. doi: 10.1111/j.1365-2796.2005.01528.x. [DOI] [PubMed] [Google Scholar]
- 8.Jin FY, Kamanna VS, Kashyap ML. Arterioscler Thromb Vasc Biol. 1997;17 (10):2020–2028. doi: 10.1161/01.atv.17.10.2020. [DOI] [PubMed] [Google Scholar]
- 9.Shepherd J, Packard CJ, Patsch JR, Gotto AM, Jr, Taunton OD. J Clin Invest. 1979;63(5):858–867. doi: 10.1172/JCI109385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lamon-Fava S, Diffenderfer MR, Barrett PH, Buchsbaum A, Nyaku M, Horvath KV, Asztalos BF, Otokozawa S, Ai M, Matthan NR, Lichtenstein AH, Dolnikowski GG, Schaefer EJ. Arterioscler Thromb Vasc Biol. 2008;28(9):1672–1678. doi: 10.1161/ATVBAHA.108.164541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Soga T, Kamohara M, Takasaki J, Matsumoto S, Saito T, Ohishi T, Hiyama H, Matsuo A, Matsushime H, Furuichi K. Biochem Biophys Res Commun. 2003;303(1):364–369. doi: 10.1016/s0006-291x(03)00342-5. [DOI] [PubMed] [Google Scholar]
- 12.Tunaru S, Kero J, Schaub A, Wufka C, Blaukat A, Pfeffer K, Offermanns S. Nat Med. 2003;9(3):352–355. doi: 10.1038/nm824. [DOI] [PubMed] [Google Scholar]
- 13.Wise A, Foord SM, Fraser NJ, Barnes AA, Elshourbagy N, Eilert M, Ignar DM, Murdock PR, Steplewski K, Green A, Brown AJ, Dowell SJ, Szekeres PG, Hassall DG, Marshall FH, Wilson S, Pike NB. J Biol Chem. 2003;278(11):9869–9874. doi: 10.1074/jbc.M210695200. [DOI] [PubMed] [Google Scholar]
- 14.Knowles HJ, te Poele RH, Workman P, Harris AL. Biochem Pharmacol. 2006;71(5):646–656. doi: 10.1016/j.bcp.2005.11.019. [DOI] [PubMed] [Google Scholar]
- 15.Ahmed K, Tunaru S, Offermanns S. Trends Pharmacol Sci. 2009;30(11):557–562. doi: 10.1016/j.tips.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 16.Irukayama-Tomobe Y, Tanaka H, Yokomizo T, Hashidate-Yoshida T, Yanagisawa M, Sakurai T. Proc Natl Acad Sci U S A. 2009;106(10):3930–3934. doi: 10.1073/pnas.0811844106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Taggart AK, Kero J, Gan X, Cai TQ, Cheng K, Ippolito M, Ren N, Kaplan R, Wu K, Wu TJ, Jin L, Liaw C, Chen R, Richman J, Connolly D, Offermanns S, Wright SD, Waters MG. J Biol Chem. 2005;280(29):26649–26652. doi: 10.1074/jbc.C500213200. [DOI] [PubMed] [Google Scholar]
- 18.Benyo Z, Gille A, Kero J, Csiky M, Suchankova MC, Nusing RM, Moers A, Pfeffer K, Offermanns S. J Clin Invest. 2005;115(12):3634–3640. doi: 10.1172/JCI23626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van der Hoorn JW, de Haan W, Berbee JF, Havekes LM, Jukema JW, Rensen PC, Princen HM. Arterioscler Thromb Vasc Biol. 2008;28(11):2016–2022. doi: 10.1161/ATVBAHA.108.171363. [DOI] [PubMed] [Google Scholar]
- 20.Hernandez M, Wright SD, Cai TQ. Biochem Biophys Res Commun. 2007;355(4):1075–1080. doi: 10.1016/j.bbrc.2007.02.079. [DOI] [PubMed] [Google Scholar]
- 21.Declercq V, Yeganeh B, Moshtaghi-Kashanian GR, Khademi H, Bahadori B, Moghadasian MH. J Cardiovasc Pharmacol. 2005;46(1):18–24. doi: 10.1097/01.fjc.0000162764.12309.25. [DOI] [PubMed] [Google Scholar]
- 22.Millar JS, Stone SJ, Tietge UJ, Tow B, Billheimer JT, Wong JS, Hamilton RL, Farese RV, Jr, Rader DJ. J Lipid Res. 2006;47(10):2297–2305. doi: 10.1194/jlr.M600213-JLR200. [DOI] [PubMed] [Google Scholar]
- 23.Goncalves LA, Vigario AM, Penha-Goncalves C. Malar J. 2007;6:169. doi: 10.1186/1475-2875-6-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lebherz C, Sanmiguel J, Wilson JM, Rader DJ. Cardiovasc Diabetol. 2007;6:15. doi: 10.1186/1475-2840-6-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tietge UJ, Maugeais C, Cain W, Grass D, Glick JM, de Beer FC, Rader DJ. J Biol Chem. 2000;275(14):10077–10084. doi: 10.1074/jbc.275.14.10077. [DOI] [PubMed] [Google Scholar]
- 26.Zhou L, Tang Y, Cryan EV, Demarest KT. Mol Cell Biochem. 2007;294(1–2):243–248. doi: 10.1007/s11010-006-9150-6. [DOI] [PubMed] [Google Scholar]
- 27.Zhang Y, Schmidt RJ, Foxworthy P, Emkey R, Oler JK, Large TH, Wang H, Su EW, Mosior MK, Eacho PI, Cao G. Biochem Biophys Res Commun. 2005;334(2):729–732. doi: 10.1016/j.bbrc.2005.06.141. [DOI] [PubMed] [Google Scholar]
- 28.Walters RW, Shukla AK, Kovacs JJ, Violin JD, DeWire SM, Lam CM, Chen JR, Muehlbauer MJ, Whalen EJ, Lefkowitz RJ. J Clin Invest. 2009;119(5):1312–1321. doi: 10.1172/JCI36806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kostylina G, Simon D, Fey MF, Yousefi S, Simon HU. Cell Death Differ. 2008;15(1):134–142. doi: 10.1038/sj.cdd.4402238. [DOI] [PubMed] [Google Scholar]
- 30.Salim K, Fenton T, Bacha J, Urien-Rodriguez H, Bonnert T, Skynner HA, Watts E, Kerby J, Heald A, Beer M, McAllister G, Guest PC. J Biol Chem. 2002;277(18):15482–15485. doi: 10.1074/jbc.M201539200. [DOI] [PubMed] [Google Scholar]
- 31.Overton MC, Chinault SL, Blumer KJ. Eukaryot Cell. 2005;4(12):1963–1970. doi: 10.1128/EC.4.12.1963-1970.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Timmins JM, Lee JY, Boudyguina E, Kluckman KD, Brunham LR, Mulya A, Gebre AK, Coutinho JM, Colvin PL, Smith TL, Hayden MR, Maeda N, Parks JS. J Clin Invest. 2005;115(5):1333–1342. doi: 10.1172/JCI23915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tsujita M, Wu CA, Abe-Dohmae S, Usui S, Okazaki M, Yokoyama S. J Lipid Res. 2005;46(1):154–162. doi: 10.1194/jlr.M400402-JLR200. [DOI] [PubMed] [Google Scholar]
- 34.Oram JF, Lawn RM, Garvin MR, Wade DP. J Biol Chem. 2000;275(44):34508–34511. doi: 10.1074/jbc.M006738200. [DOI] [PubMed] [Google Scholar]
- 35.Haidar B, Denis M, Krimbou L, Marcil M, Genest J., Jr J Lipid Res. 2002;43 (12):2087–2094. doi: 10.1194/jlr.m200235-jlr200. [DOI] [PubMed] [Google Scholar]
- 36.Krause BR, Princen HM. Atherosclerosis. 1998;140(1):15–24. doi: 10.1016/s0021-9150(98)00141-5. [DOI] [PubMed] [Google Scholar]
- 37.Schaub A, Futterer A, Pfeffer K. Eur J Immunol. 2001;31(12):3714–3725. doi: 10.1002/1521-4141(200112)31:12<3714::aid-immu3714>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 38.Herrlich A, Daub H, Knebel A, Herrlich P, Ullrich A, Schultz G, Gudermann T. Proc Natl Acad Sci U S A. 1998;95(15):8985–8990. doi: 10.1073/pnas.95.15.8985. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.