Abstract
Multiple endocrine neoplasias are autosomal dominant disorders characterized by the occurrence of tumors in at least two endocrine glands. Two MEN syndromes have long been known and are well characterized: the MEN type 1 (MEN1) and type 2 (MEN2). These syndromes are caused by germline mutations in the MEN1 and RET genes, respectively, and have a different tumor spectrum. Recently, a variant of the MEN syndromes arose spontaneously in a rat colony and was named MENX. Affected animals consistently develop multiple endocrine tumors, with a spectrum that shares features with both MEN1 and MEN2 human syndromes.
Genetic studies identified a germline mutation in the Cdkn1b gene, encoding the p27 cell cycle inhibitor, as the causative mutation for MENX. Capitalizing on these findings, heterozygous germline mutations in the human homologue, CDKN1B, were searched for and identified in patients with multiple endocrine tumors. As a consequence of this discovery, a novel human MEN syndrome, named MEN4, was recognized, which is caused by mutations in p27. Altogether, these studies identified Cdkn1b/CDKN1B as a novel tumor susceptibility gene for multiple endocrine tumors in both rats and humans. Here we review the characteristics of the MENX and MEN4 syndromes and we briefly address the main function of p27 and how they are affected by MENX/4-associated mutations.
Keywords: Multiple Endocrine Neoplasias, MENX, MEN4, p27, Cdkn1b Gene
The MENX syndrome in the rat
The multiple endocrine neoplasia X (MENX) multi-tumor syndrome was discovered by accident when we observed that some rats belonging to a Sprague–Dawley colony started to spontaneously develop multiple endocrine tumors. Specifically, these rats presented with multifocal anterior pituitary adenoma and bilateral adrenal pheochromocytoma, as well as extra-adrenal pheochromocytoma (paraganglioma), thyroid C-cell hyperplasia, parathyroid hyperplasia, and pancreatic islet cells hyperplasia (Figure 1) (1). This multi-tumor syndrome was named MENX, as it shares phenotypic features with both MEN1 and MEN2 human tumor syndromes (1).
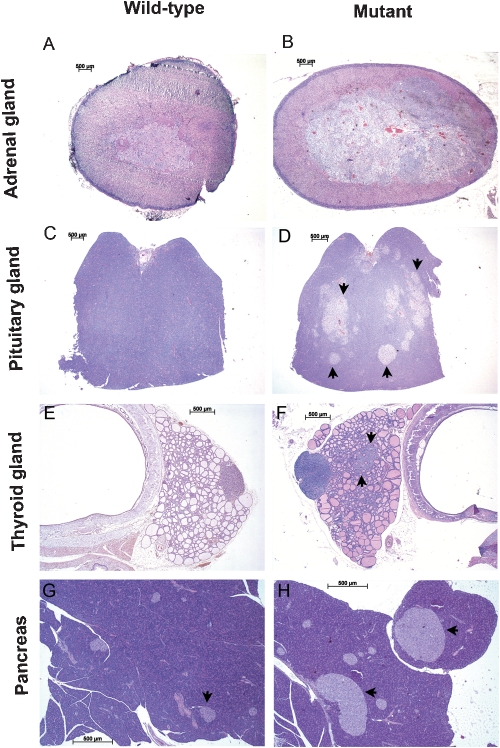
Figure 1.
Histology of rat tissues affected by the MENX syndrome. Adrenal (A, B), pituitary (C, D) and thyroid (E, F) glands, and pancreas (G, H) of wild-type and age-matched MENX-affected (mutant) rats. Multifocal hyperplastic lesions in the anterior pituitary are indicated by arrows (D). A nodule of thyroid C cell hyperplasia is also indicated (F). Pancreatic islet cells are indicated by arrows in panels G and H: as the pictures were taken with the same magnifications the increase in size of the islets in mutant rats can be readily appreciated. Hematoxylin and eosin stain (H&E) original magnification: 20X (A–D); 25X (E, F); 40X (G, H). (Reproduced with permission from: Molatore & Pellegata, 2010 Copyright © 2010 by Elsevier).
Malignancies in MENX-affected rats show a clear progression with time: these animals develop with complete penetrance adrenal medullary hyperplasia at 3–4 months of age, which progresses to pheochromocytoma by 6–8 months of age. They also present with multifocal anterior pituitary neoplasms at about 4 months of age, which progress to larger adenomas by 8–12 months of age (frequency 100%). MENX mutant rats also develop pancreatic islet cell hyperplasia (frequency 80%) and from these hyperplastic islets, if the animal lives long enough, insulinomas may arise (Figure 1). Due to the development of multiple malignancies, affected rats have an average life span of 10±2 months, whereas their wild-type littermates live approximately 24–30 months (2). Based on the symptomatology of affected rats, high blood pressure seems to be the leading cause of morbidity, although formal proof is still lacking.
Additional phenotypic characteristics of the MENX-affected rats include: macroscopically visible bilateral juvenile cataracts (1), increased body size, and organomegaly, particularly of spleen and thymus, in mutants versus wild-type littermates (2).
The predisposition of MENX-affected rats to develop multiple endocrine tumors is inherited as a recessive trait, indicating that they are homozygous for the underlying genetic mutation (1). Linkage analysis followed by a positional cloning approach allowed us to identify the gene responsible for the MENX syndrome: the Cdkn1b gene encoding the cell cycle inhibitor p27. Affected rats carry a tandem duplication of eight nucleotides (c.520-528dupTTCAGAC, RefSeq: NM_031762 from base 77 to 670) in exon 2 of Cdkn1b, which causes a frameshift (2). At the protein level, the mutated allele encodes a protein predicted to have a novel C-terminal sequence starting at codon 177, referred to as p27fs177. The wild-type p27 (p27wt) protein is 198 amino acids long, whereas the mutated protein, as a result of the frameshift, is predicted to be 221 amino acids long. This Cdkn1b mutation was identified in homozygosity in all MENX-affected rats tested (n>200), whereas it was never observed in unaffected littermates or in control wild-type rats of commercially available inbred strains (2).
Following the identification of the causative genetic mutation, the expression of the Cdkn1b gene in MENX rats was explored in more detail. The analysis of various tissues of 2-month-old affected rats (before they develop malignancies) and wild-type age-matched littermates (controls) showed that the level of Cdkn1b mRNA is highly similar in both animal groups. Furthermore, correct splicing of the mutant mRNA was demonstrated in mutant rat tissues (2). When the expression of the encoded p27 protein was assessed by western blotting, a faint band corresponding in size to the predicted p27 mutant protein (p27fs177) could be observed in only some tissues (i.e. thymus and thyroid). Immunohistochemical staining, performed with an anti-p27-specific antibody on a broader variety of tissues, showed extremely low (thyroid, pituitary, thymus, parathyroid, and brain) or absent (adrenals, lung, kidney, liver, and testis) p27 immunoreactivity in tissues of affected rats before they eventually developed neoplasias, whereas the same tissues obtained from wild-type rats showed strong nuclear positivity for p27 (2). This suggests that the MENX mutation does not affect the transcription or processing of Cdkn1b mRNA, but affects the amount of the encoded protein through post-transcriptional or post-translational mechanisms.
The discovery of CDKN1B mutations in human patients: the MEN4 syndrome
Approximately 30% of patients who have a MEN1–like phenotype do not carry detectable MEN1 gene mutations (3). Although this could be because of the presence of mutations in noncoding or regulatory sequences of the gene that are not commonly analyzed, it also suggests that these patients could potentially bear mutations in additional, not yet identified, susceptibility genes. Following the identification of the genetic mutation causing MENX syndrome in rats, we decided to investigate whether mutations in the human homologue CDKN1B could explain some of the MEN1-like cases without mutations in MEN1. We screened for the presence of CDKN1B germline mutations several patients fulfilling these criteria and we identified a germline heterozygous TGG>TAG (c.227G>A; RefSeq: AY890407) nonsense mutation at codon 76 (p.Trp76stop, W76X) in a female proband with growth hormone (GH)-secreting pituitary adenoma (causing acromegaly; age 30 years) and primary hyperparathyroidism (age 46 years) (2). Analysis of the proband's relatives confirmed that in her family, the p27W76X mutation segregates with the predisposition to tumors belonging to the MEN1 tumor spectrum. Indeed, one of the proband's sisters, also a carrier of the germline c.227G>A mutation, developed renal angiomyolipoma (age 55 years), a tumor type that has been previously associated with the MEN1 syndrome.
As a consequence of this discovery, other groups identified germline mutations in patients with features suggestive of MEN1. First, Georgitsi et al. (4) identified a heterozygous 19-bp duplication (c.59_77dup19) in exon 1 of the CDKN1B gene in one out of 36 European patients with a MEN1-like phenotype and no MEN1 gene mutations. The mutation carrier was a woman who had developed a small-cell neuroendocrine cervical carcinoma (aged 45 years), adrenocorticotropic hormone (ACTH)-secreting pituitary adenoma (Cushing's disease) (aged 46 years), and hyperparathyroidism (aged 47 years). The c.59_77dup19 duplication causes a frameshift, and the variant mRNA is predicted to encode a p27 protein 69 amino acids shorter than p27wt and with a different amino acid sequence after codon 25 (p.Lys25fs, K25fs).
Subsequently, in a study of American patients with a family history of endocrine tumors and showing primary hyperparathyroidism (1° HPT) as a common feature, three new germline CDKN1B changes were identified: ATG-7(g>c); c.283C>T (Pro95Ser, P95S); and c.592G>G (stop>Gln, stop>Q) (5). The patient who had the ATG-7(g>c) nucleotide change presented with primary hyperparathyroidism (aged 61 years) and a non-functioning bilateral adrenal mass (aged 63 years). The patient with the missense variant at codon 95 presented with 1° HPT, Zollinger-Ellison syndrome (ZES) and masses in the duodenum and pancreas (aged 50 years). The individual with the stop>Q mutation showed primary hyperparathyroidism (aged 50 years).
By screening 27 Italian patients with a tumor phenotype suggestive of MEN1, we identified a heterozygous germline change (c.206C>T) which, at the protein level, translates to a Pro>Leu amino acid substitution at codon 69 (p.Pro69Leu, P69L) (6). The mutation-positive patient developed bilateral multiple metastases of bronchial carcinoid and pituitary non-functioning microadenoma (aged 79 years), 1° HPT caused by a parathyroid adenoma (aged 67 years), and also a papillary thyroid carcinoma (aged 64 years). The patient's relatives refused to undergo genetic screening, therefore the inheritance of this mutation could not be assessed.
Recently, two novel germline CDKN1B mutations were found by analyzing 90 common sporadic parathyroid adenomas with non-familial presentation (7). Specifically, one patient carried a heterozygous germline single nucleotide change c.25G>A at base 25 in CDKN1B exon 1, which would result in a Gly9Arg (G9R) substitution in the translated p27 protein product. This patient, a 68-year old man, had a typical, single-gland, mildly symptomatic presentation. Another tumor contained a heterozygous single nucleotide substitution c.397C>A, directing a Pro133Thr (P133T) substitution in the translated p27 protein. This alteration was also present in the germline DNA of another patient, a 53-year-old woman presenting with fatigue, slightly elevated serum calcium and parathyroid hormone levels, and a single parathyroid adenoma (7).
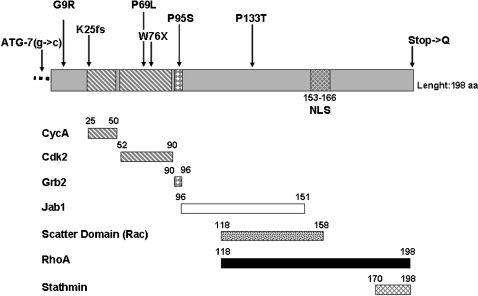
The CDKN1B mutations above described and the phenotype of the mutation carriers are summarized in Table 1, while the location of these changes within the p27 protein is schematically outlined in Figure 2. It is important to point out that the CDKN1B changes described here were not found in 192–650 alleles (depending on the study) from healthy control individuals with no history of endocrine tumors, nor were they identified in >2000 CDKN1B alleles sequenced in the world literature.
Table 1.
Germline mutations in the CDKN1B gene, phenotype of the mutation carriers and molecular phenotype of the p27 variant proteins. Mutations are numbered referenced to the cDNA sequence AY890407 (GenBank).
| Location number | Codon | Mutation | Predicted effect | Clinical Phenotype of proband | Molecular Phenotype | Reference | ||||
| Protein expression in vitro | Protein localization in vitro | Protein Interaction | Protein expression in patient's tumor | Protein localization in patient's tumor | ||||||
| 5′ UTR | ||||||||||
| -7 | - | ATG-7(g>c) | 1°HPT (1 PT tumor), bilateral adrenal mass NF, uterine fibroids | Reduced | N.D. | N.D. | N.D. | N.D. | Agarwal et al., 2009 | |
| Exon 1 | ||||||||||
| 25 | 9 | GGG>AGG | ms, G9R | 1°HPT (1 PT tumor) | Reduced | Nuclear | N.D. | Reduced | Nuclear | Costa-Guda et al., 2011 |
| 59-77 | 25 | nt59_77dup19 | fs, K25fs | 1°HPT, ACTH- pituitary adenoma, carcinoid tumor of the uterine cervix | N.D. | N.D. | N.D. | Absent | - | Georgitsi et al., 2007 |
| 206 | 69 | CCC>CTC | ms, P69L | 1°HPT, NF pituitary adenoma, bronchial carcinoids, PTC | Reduced | Nuclear cytoplasm | GRB2/Reduced | Absent/reduced*) | Nuclear | Molatore et al., 2010 |
| 227 | 76 | TGG>TAG | ns, W76X | 1°HPT, GH-pituitary adenoma | Wt | Cytoplasm | N.D. | Absent | - | Pellegata et al., 2006 |
| 283 | 95 | CCC>TCC | ms, P95S | 1°HPT (2 PT tumors), ZES, mass in duodenum and tail of pancreas | Wt | N.D. | GRB2/Reduced | N.D. | N.D. | Agarwal et al., 2009 |
| 397 | 133 | CCC>ACA | ms, P133T | 1°HPT (1 PT tumor) | Wt | Nuclear | N.D. | N.D. | N.D. | Costa-Guda et al., 2011 |
| Exon 2 | ||||||||||
| 592 | 198 | TAG>CAG | if, stop>Q | 1°HPT (3 PT tumors) | Reduced | N.D. | CDK2/wt | N.D. | N.D. | Agarwal et al., 2009 |
From this patient 2 tumor tissues were available (PT and pituitary adenoma).
UTR, untranslated region; 1°HPT, primary hyperparathyroidism; PT, parathyoir tumor; NF, non-functioning; ms, missense; fs, frameshift; PTC, papillary thyroid carcinoma; ns, nonsense, if, in frame; ZES, Zollinger-Ellison syndrome.
Figure 2.
Schematic structure of the p27 protein and position of the germline mutations identified. The regions mediating the binding of p27 to its major interacting partners, which are reported below the protein, are indicated. The germline mutations so far identified in MEN4 patients are indicated above the protein sequence. NLS = nuclear localization signal.
As a result of the identification of patients bearing germline CDKN1B mutations and showing multiple endocrine tumors, a novel MEN syndrome was recognized and submitted to the Online Mendelian Inheritance in Man (OMIM) database in 2007 under the name of MEN4. Owing to the limited number of patients with MEN4 so far reported, the precise tumor spectrum associated with this syndrome is still not well defined (Table 1.
Function of p27 protein
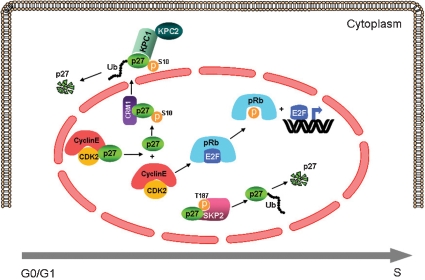
p27 belongs to the kinase inhibitory protein/cyclin-dependent kinase (CDK) inhibitor interacting protein (KIP/CIP) family of cell cycle inhibitors, and its main function is to control the progression from G1 to S phase (8). p27 binds to, and inhibits, cyclinE/CDK2 and cyclinA/CDK2 complexes in response to either mitogenic or anti-mitogenic stimuli. The targets of the CDK2 kinase are members of the retinoblastoma (pRb) family, which upon phosphorylation, release E2F transcription factors, thereby allowing the transcription of genes responsible for the progression into the S phase (8). Thus, binding of p27 to cyclinE,A/CDK2 prevents pRb phosphorylation and stops the cells in the G1 phase (Figure 3). p27 is also required for the cytoplasmic assembly and nuclear import of cyclinD/CDK complexes, molecules that promote cell cycle progression (8). Because p27 is a critical regulator of cell cycle progression, its activity is tightly regulated at different levels: transcriptional, translational, and post-translational. Transcriptional regulation of the Cdkn1b promoter (9),(10) and control of mRNA translation (11) have been described, but the best known mechanism that regulates p27 function is post-translational, and it modulates the abundance of the protein through ubiquitin-mediated proteasomal degradation (12). In growth-arrested cells, p27 accumulates in the nucleus, where it binds the cyclin/CDK complexes to block cell cycle progression (13). Upon mitogenic stimulation, the cells need to re-enter the cell cycle, so they rapidly eliminate p27 through proteasome-mediated degradation following two molecular pathways. The first involves the ubiquitylation-promoting complex KPC1/KPC2 and occurs in the cytoplasm (14), while the second pathway takes place in the nucleus and is mediated by the ubiquitin ligase SKP2 (15). Degradation through the KPC1 pathway occurs following phosphorylation of p27 at the Ser10 residue and its export to the cytoplasm (16). SKP2-mediated degradation requires the phosphorylation of p27 at the Thr187 residue by cyclinE,A/CDK2 complexes, an event that takes place in late G1. The phosphorylation at Thr187 creates a recognition site for the SKP2 ubiquitin ligase which induces p27 poly-ubiquitylation and subsequent degradation by the proteasome (Figure 3) (15). The complex regulation of the intracellular amount of p27 suggests that its dose is critical for tissue homeostasis. This hypothesis is supported by the observation that mice heterozygous for a null Cdkn1b allele are predisposed to tumor formation, thereby indicating that p27 is a dose-dependent (haplo-insufficient) tumor suppressor in mice (17).
Figure 3.
Graphic representation of the nuclear and cytoplasmic interactions of p27. Upon mitogenic stimulation, p27 is released from cyclinE/CDK2 complexes and this dissociation from p27 activates CDK2, which, in turn, phosphorylates pRb. Phosphorylated pRb releases the transcription factor E2F, which induces the expression of genes required for the G1 to S progression. After the dissociation of p27 from the cyclinE/CDK2 complex in early G1 a portion of p27 is phosphorylated on Ser10 and exported into the cytoplasm through the interaction with CRM1 (exportin). Once in the cytoplasm, p27 is ubiquitylated by the KPC1/KPC2 complex and degraded by the proteasome. Upon mitogenic stimulation p27 becomes the substrate of the cyclinE/CDK2 complex, which phosphorylates the protein at the Thr187 residue, thereby creating a recognition site for the SKP2 ligase, which promotes ubiquitylation-mediated degradation of p27 by the proteasome in S phase.
It is important to mention that the amount of p27 is reduced/lost in many cancers, including those of the colon, breast, prostate, and stomach, and this down-regulation is associated with poor prognosis (18). It was demonstrated that in aggressive colorectal carcinoma samples, the loss/reduction of p27 expression is caused by accelerated proteasome-dependent degradation of the protein (19), probably caused by increased expression of the ubiquitin ligase SKP2 (20). This molecular mechanism to reduce the amount of p27 has been subsequently identified in other human tumors (18). The observation that low p27 protein levels are often associated with no change in CDKN1B mRNA expression in many human tumors has been considered indirect evidence that enhanced proteolysis is responsible for p27 down-regulation in such tumors, even when formal evidence has not been presented.
In addition to the amount of protein, the intracellular localization of p27 can also profoundly affect the protein's function. Indeed, p27wt is mainly localized in the nucleus and in this compartment it can bind to, and inhibit, cyclin/CDK complexes, thereby acting as a cell cycle inhibitor. However, p27 can be mislocalized in the cytoplasm through phosphorylation or protein binding (21), and, once confined in this compartment, p27 may acquire additional functions that are not completely understood. Studies on engineered mouse strains with mutations in p27 that abolish cyclin/CDK binding have suggested that cytoplasmic p27 may play a pro-oncogenic role (22), possibly by interacting with Rac and RhoA, molecules, which are involved in cell migration (23),(24). Mislocalization of p27 in the cytoplasm through phosphorylation of Thr157 by protein kinase b (AKT) has frequently been observed in carcinoma of the breast, and is associated with poorer prognosis (25).
Germline mutations in p27
MENX-associated mutation
Functional in vitro studies have been performed to understand the effect of the Cdkn1b mutation causing MENX in rats. These analyses have demonstrated that the p27fs177 mutant protein retains some properties of the p27wt protein: it can localize to the nucleus and interact with cyclin-dependent kinases (Cdk2 and Cdk4) and, to a lesser extent, with cyclins (26). However, differently from p27wt, p27fs177 is highly unstable and rapidly degraded, so that its steady-state level in vitro is always very low. The p27-unrelated C-terminal domain of p27fs177 is responsible for the rapid degradation of the protein, probably through protein misfolding. Studies of primary rat newborn fibroblasts established from wild-type unaffected and mutant rats confirmed the rapid degradation of p27fs177, which can be rescued by proteasome inhibition (i.e. MG132, epoxomycin, or bortezomib treatment) and thus is mediated by the proteasome. Specifically, degradation of p27fs177 is, at least in part, mediated by Skp2-dependent proteasomal proteolysis (26).
Based on these findings, we postulate that reduced p27 levels, not newly acquired molecular phenotypes, trigger tumor formation in MENX-affected rats. This establishes a parallel with p27-deficient mice, which are also predisposed to tumor formation (27).
MEN4-associated mutations
Most of the germline mutations identified in patients have been studied in vitro to understand their link to tumor predisposition. The ATG-7(g>c) change affects the Kozac consensus sequence, which plays a major role in the initiation of mRNA translation. The ATG-7(g>c) nucleotide substitution reduces the translation efficiency of the variant allele, which, in turn, causes a reduced amount of the encoded p27 protein compared with the wild-type CDKN1B allele (5). In vitro studies showed that the p27G9R variant is less stable than p27wt, and that this associates with strongly reduced expression of p27 in the tumor tissue of the mutation-carrier individual. Moreover, p27G9R mimics a p27 protein phosphorylated at the Ser10 residue, the major phosphorylation site of the protein, which regulates its localization and stability (7). The P69L change substitutes one of the proline residues mediating the interaction between p27wt and the CDK2 protein. Functional studies on the p27P69L mutant protein have demonstrated that, indeed, this protein does not efficiently bind CDK2 (6). Moreover, p27P69L is quite unstable in vitro, and is expressed at a reduced level both in transfected cells and in the tissues of mutation-positive patients. p27P69L is also less efficient than p27wt in blocking cell growth (6). The p27W76X peptide has lost the nuclear localization signal (Figure 2), and therefore it is mislocalized to the cytoplasm in vitro and in vivo (in the tissues of a mutation-positive patient) (2),(6). Therefore, p27W76X can no longer bind and inhibit cyclin–CDK complexes and, as a consequence, this peptide has lost the ability to inhibit cell growth when over-expressed in p27-negative GH3 pituitary adenoma cells (6). The p27P95S variant changes one of the residues mediating the binding of p27 to the Grb2 adaptor protein. As a consequence of this change, the p27P95S mutant protein does not efficiently bind to Grb2, and this may ultimately impair the activation of the Ras signal transduction pathway (5). By contrast, no molecular phenotype could be assigned to the p27P133T variant using various in vitro assays (7). The p27 stop>Q variant encodes a protein containing 60 amino acids more than p27wt. The amount of the p27stop>Q mutant protein was lower than that of p27wt in vitro, and it could be rescued by proteasome inhibitor treatment. Therefore, the stop>Q variation encodes a quite unstable p27 protein (5).
Based on how severely they affect the molecular characteristics of p27, the various genetic changes illustrated here have been classified as pathogenic mutations (K25fs, P69L, W76X), potentially pathogenic mutations [ATG-7(g>c), G9R, P95S, stop>Q] or variants of unknown pathogenic significance (P133T). The molecular phenotype of the various p27 mutations is summarized in Table 1.
The recognition of both the MENX (rat) and the MEN4 (human) syndromes has demonstrated that Cdkn1b/CDKN1B is a new tumor susceptibility gene for multiple neuroendocrine tumors in both species. The observations summarized here, together with studies of engineered mouse models with defective or mutant p27, confirm a critical role for p27 in regulating cell proliferation in neuroendocrine cells. Our hypothesis is that in these cells, aberrant/absent p27 activity cannot be compensated for by other cyclin–CDK inhibitors, as it occurs in tissues less affected by lack of functional p27. This would explain the tissue-specific susceptibility to tumor formation associated with p27 germline mutations. As novel CDKN1B mutations are discovered, our understanding of the relationship between p27 and neuroendocrine tumor predisposition will increase. The characterization of the molecular properties of mutant p27 proteins associated with MEN4 may facilitate the development of more effective targeted therapeutic strategies for the patients carrying those mutations.
ACKNOWLEDGMENTS
I thank all members of the laboratory for stimulating discussions. This work was supported by SFB 824 (DFG Sonderforschungsbereich 824) from the Deutsche Forschungsgemeinschaft, Bonn, Germany, and by grant #109223 from the Deutsche Krebshilfe, Bonn, Germany.
Footnotes
No potential conflict of interest was reported.
REFERENCES
- 1.Fritz A, Walch A, Piotrowska K, Rosemann M, Schäffer E, Weber K, et al. Recessive transmission of a multiple endocrine neoplasia syndrome in the rat. Cancer Res. 2002;62((11)):3048–51. [PubMed] [Google Scholar]
- 2.Pellegata NS, Quintanilla-Martinez L, Siggelkow H, Samson E, Bink K, Hofler H, et al. Germ-line mutations in p27kip1 cause a multiple endocrine neoplasia syndrome in rats and humans. Proc Natl Acad Sci USA. 2006;103((42)):15558–63. doi: 10.1073/pnas.0603877103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ozawa A, Agarwal SK, Mateo CM, Burns AL, Rice TS, Kennedy PA, et al. The parathyroid/pituitary variant of multiple endocrine neoplasia type 1 usually has causes other than p27Kip1 mutations. J Clin Endocrinol Metab. 2007;92((5)):1948–51. doi: 10.1210/jc.2006-2563. [DOI] [PubMed] [Google Scholar]
- 4.Georgitsi M, Raitila A, Karhu A, van der Luijt RB, Aalfs CM, Sane T, et al. Germline cdkn1b/p27kip1 mutation in multiple endocrine neoplasia. J Clin Endocrinol Metab. 2007;92((8)):3321–5. doi: 10.1210/jc.2006-2843. [DOI] [PubMed] [Google Scholar]
- 5.Agarwal SK, Mateo CM, Marx SJ. Rare germline mutations in cyclin-dependent kinase inhibitor genes in multiple endocrine neoplasia type 1 and related states. J Clin Endocrinol Metab. 2009;94((5)):1826–34. doi: 10.1210/jc.2008-2083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Molatore S, Marinoni I, Lee M, Pulz E, Ambrosio MR, Uberti EC, et al. A novel germline cdkn1b mutation causing multiple endocrine tumors: Clinical, genetic and functional characterization. Hum Mutat. 2010;31((11)):E1825–35. doi: 10.1002/humu.21354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Costa-Guda J, Marinoni I, Molatore S, Pellegata NS, Arnold A. Germline and Somatic Mutations of CDKN1B in Sporadic Parathyroid Adenomas. J Clin Endocrinol Metab. 2011;96((4)):E701–6. doi: 10.1210/jc.2010-1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wander SA, Zhao D, Slingerland JM. p27: a barometer of signaling deregulation and potential predictor of response to targeted therapies. Clin Cancer Res. 2011;17((1)):12–8. doi: 10.1158/1078-0432.CCR-10-0752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dijkers PF, Medema RH, Pals C, Banerji L, Thomas NS, Lam EW, et al. Forkhead transcription factor fkhr-l1 modulates cytokine-dependent transcriptional regulation of p27(kip1) Mol Cell Biol. 2000;20((24)):9138–48. doi: 10.1128/mcb.20.24.9138-9148.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Servant MJ, Coulombe P, Turgeon B, Meloche S. Differential regulation of p27(kip1) expression by mitogenic and hypertrophic factors: Involvement of transcriptional and posttranscriptional mechanisms. J Cell Biol. 2000;148((11)):543–56. doi: 10.1083/jcb.148.3.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Millard SS, Yan JS, Nguyen H, Pagano M, Kiyokawa H, Koff A. Enhanced ribosomal association of p27(kip1) mRNA is a mechanism contributing to accumulation during growth arrest. J Biol Chem. 1997;272((11)):7093–8. doi: 10.1074/jbc.272.11.7093. [DOI] [PubMed] [Google Scholar]
- 12.Pagano M, Tam SW, Theodoras AM, Beer-Romero P, Del Sal G, Chau V, et al. Role of the ubiquitin-proteasome pathway in regulating abundance of the cyclin-dependent kinase inhibitor p27. Science. 1995;269((5224)):682–5. doi: 10.1126/science.7624798. [DOI] [PubMed] [Google Scholar]
- 13.Sherr CJ, Roberts JM. Cdk inhibitors: Positive and negative regulators of g1-phase progression. Genes Dev. 1999;13:1501–12. doi: 10.1101/gad.13.12.1501. [DOI] [PubMed] [Google Scholar]
- 14.Kamura T, Hara T, Matsumoto M, Ishida N, Okumura F, Hatakeyama S, et al. Cytoplasmic ubiquitin ligase kpc regulates proteolysis of p27(kip1) at g1 phase. Nat Cell Biol. 2004;6((12)):1229–35. doi: 10.1038/ncb1194. [DOI] [PubMed] [Google Scholar]
- 15.Carrano AC, Eytan E, Hershko A, Pagano M. Skp2 is required for ubiquitin-mediated degradation of the cdk inhibitor p27. Nat Cell Biol. 1999;1((4)):193–9. doi: 10.1038/12013. [DOI] [PubMed] [Google Scholar]
- 16.Ishida N, Hara T, Kamura T, Yoshida M, Nakayama K, Nakayama KI. Phosphorylation of p27kip1 on serine 10 is required for its binding to crm1 and nuclear export. J Biol Chem. 2002;277((17)):14355–8. doi: 10.1074/jbc.C100762200. [DOI] [PubMed] [Google Scholar]
- 17.Fero ML, Randel E, Gurley KE, Roberts JM, Kemp CJ. The murine gene p27Kip1 is haplo-insufficient for tumour suppression. Nature. 1998;396((6707)):177–80. doi: 10.1038/24179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chu IM, Hengst L, Slingerland JM. The Cdk inhibitor p27 in human cancer, prognostic potential and relevance to anticancer therapy. Nat Rev Cancer. 2008;8((4)):253–67. doi: 10.1038/nrc2347. [DOI] [PubMed] [Google Scholar]
- 19.Loda M, Cukor B, Tam SW, Lavin P, Fiorentino M, Draetta GF, et al. Increased proteasome-dependent degradation of the cyclin-dependent kinase inhibitor p27 in aggressive colorectal carcinomas. Nat Med. 1997;3((2)):231–4. doi: 10.1038/nm0297-231. [DOI] [PubMed] [Google Scholar]
- 20.Hershko D, Bornstein G, Ben-Izhak O, Carrano A, Pagano M, Krausz MM, et al. Inverse relation between levels of p27(Kip1) and of its ubiquitin ligase subunit Skp2 in colorectal carcinomas. Cancer. 2001;91:1745–51. doi: 10.1002/1097-0142(20010501)91:9<1745::aid-cncr1193>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 21.Slingerland J, Pagano M. Regulation of the cdk inhibitor p27 and its deregulation in cancer. J Cell Physiol. 2000;183:10–7. doi: 10.1002/(SICI)1097-4652(200004)183:1<10::AID-JCP2>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 22.Besson A, Hwang HC, Cicero S, Donovan SL, Gurian-West M, Johnson D, et al. Discovery of an oncogenic activity in p27kip1 that causes stem cell expansion and a multiple tumor phenotype. Genes Dev. 2007;21((14)):1731–46. doi: 10.1101/gad.1556607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McAllister SS, Becker-Hapak M, Pintucci G, Pagano M, Dowdy SF. Novel p27(kip1) C-terminal scatter domain mediates Rac-dependent cell migration independent of cell cycle arrest functions. Mol Cell Biol. 2003;23((1)):216–28. doi: 10.1128/MCB.23.1.216-228.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Besson A, Gurian-West M, Schmidt A, Hall A, Roberts JM. P27kip1 modulates cell migration through the regulation of rhoa activation. Genes Dev. 2004;18((8)):862–76. doi: 10.1101/gad.1185504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shin I, Yakes FM, Rojo F, Shin NY, Bakin AV, Baselga J, et al. PKB/Akt mediates cell-cycle progression by phosphorylation of p27(Kip1) at threonine 157 and modulation of its cellular localization. Nat Med. 2002;8((10)):1145–52. doi: 10.1038/nm759. [DOI] [PubMed] [Google Scholar]
- 26.Molatore S, Kiermaier E, Jung CB, Pulz E, Höfler H, Atkinson MJ, et al. Characterization of a naturally-occurring p27 mutation predisposing to multiple endocrine tumors. Mol Cancer. 2010;9:116. doi: 10.1186/1476-4598-9-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fero ML, Rivkin M, Tasch M, Porter P, Carow CE, Firpo E, et al. A syndrome of multiorgan hyperplasia with features of gigantism, tumorigenesis, and female sterility in p27(Kip1)-deficient mice. Cell. 1996;85:733–44. doi: 10.1016/s0092-8674(00)81239-8. [DOI] [PubMed] [Google Scholar]