Abstract
Hirschsprung disease is a congenital form of aganglionic megacolon that results from cristopathy. Hirschsprung disease usually occurs as a sporadic disease, although it may be associated with several inherited conditions, such as multiple endocrine neoplasia type 2. The rearranged during transfection (RET) proto-oncogene is the major susceptibility gene for Hirschsprung disease, and germline mutations in RET have been reported in up to 50% of the inherited forms of Hirschsprung disease and in 15–20% of sporadic cases of Hirschsprung disease. The prevalence of Hirschsprung disease in multiple endocrine neoplasia type 2 cases was recently determined to be 7.5% and the co-occurrence of Hirschsprung disease and multiple endocrine neoplasia type 2 has been reported in at least 22 families so far. It was initially thought that Hirschsprung disease could be due to disturbances in apoptosis or due to a tendency of the mutated RET receptor to be retained in the Golgi apparatus. Presently, there is strong evidence favoring the hypothesis that specific inactivating haplotypes play a key role in the fetal development of congenital megacolon/Hirschsprung disease. In the present study, we report the genetic findings in a novel family with multiple endocrine neoplasia type 2: a specific RET haplotype was documented in patients with Hirschsprung disease associated with medullary thyroid carcinoma, but it was absent in patients with only medullary thyroid carcinoma. Despite the limited number of cases, the present data favor the hypothesis that specific haplotypes not linked to RET germline mutations are the genetic causes of Hirschsprung disease.
Keywords: RET, MEN2, HSCR, Hirschsprung, Haplotypes
INTRODUCTION
We will briefly review Hirschsprung disease (HSCR), multiple endocrine neoplasia type 2 (MEN2), and the co-segregation of these conditions in the few families that have been studied to date. In addition, we will discuss the existing hypotheses to explain the finding of HSCR associated with MEN2.
HIRSCHSPRUNG DISEASE
HSCR (MIM #142623) is a congenital intestinal malformation characterized by aganglionic megacolon in a variable length of the intestine; both short- and long-segment forms of the disease exist (1). The incidence of this congenital megacolon is 1 per 5,000 newborns, although it varies from 1.5 in Caucasians to 2.8 in Asians for each 10,000 newborns. HSCR is a neurocristopathy most frequently found in its sporadic form (S-HSCR, ∼70% of cases) which is a non-Mendelian disease with low, sex-dependent penetrance and variable clinical expression (1)–(3). Familial forms of HSCR (F-HSCR) usually occur in association with several inherited syndromes, such as MEN2 (4)–(13). Recently, a Hirschsprung disease consortium verified that germline mutations in the RET proto-oncogene have been reported in up to 50% of patients with F-HSCR and in 15-20% of patients with S-HSCR (2). RET is the major susceptibility gene for HSCR, although up to 10 genes and five different loci have been associated with HSCR development (1),(2). The short-segment form of the condition is more frequent (80%) than the long-segment form [20%], and recently developed surgical approaches for both forms have a high chance of success, markedly decreasing the mortality and morbidity rates of this condition (2),.
Diagnosis of HSCR is usually confirmed by rectal biopsy in newborns with intestinal obstructions with the following findings: failure of meconium delivery in the first 2 days of life, abdominal distention that is alleviated with rectal stimulus and enema, vomiting, and neonatal enterocolitis. The shorter the aganglionic segment, the later the signals and symptoms develop and consequently the more delayed the diagnosis of HSCR (2),(3),(16).
MEN2
MEN2 (MIM #171400) is an inherited syndrome caused by germline mutations in the RET proto-oncogene, which encodes the RET receptor tyrosine kinase. Three neuroendocrine neoplasias are usually present: medullary thyroid carcinoma (MTC), pheochromocytoma (PHEO), and primary hyperparathyroidism. Two other subtypes of MEN2 are familial MTC (FMTC; MTC only) and MEN2B (MTC; PHEO, and ganglioneuromas) (7),(13),(18),(19).
HIRSCHSPRUNG DISEASE CO-OCCURRING WITH MEN2
In MEN2A, MTC and PHEO are the most prevalent causes of morbidity and mortality, although HSCR is also an infrequent but potentially life-threatening MEN2 phenotype (2),(18),(19). In contrast, in sporadic MTC there are no other associated neuroendocrine tumors and HSCR has not been reported to date (20),(21).
Recently, an international consortium on RET exon 10 studied 340 affected subjects from 103 MEN2 families and assessed the risk profiles and penetrance estimations of MEN2 patients who were diagnosed with germline RET mutations in exon 10. In this study, 267 MEN2 patients were investigated for HSCR disease and an overall penetrance of 7.5% was observed (22). Of note, carriers of germline RET mutations in codon 620 were more prone to HSCR (13%), especially those with C620R or C620G substitutions (14–33%), although RET 618 and 609 codon mutation carriers may also present with HSCR (22).
Present hypotheses
The co-occurrence of the HSCR and MEN2 (HSCR/MEN2) phenotypes raises an apparent paradox because MTC is caused by activation of cellular growth signaling of the RET tyrosine kinase receptor, while HSCR is largely associated with disruption of this pathway because of inactivating RET mutations (1). Initially it was thought that disturbed mechanisms of apoptosis might lead to HSCR (23). Several investigations have examined this topic (24)–(33).
At present, there are two main hypotheses to explain the occurrence of HSCR/MTC within MEN2 families. The first states that MEN2-associated RET mutations, mostly in exon 10, may play a dual activating and inactivating role in the RET tyrosine kinase molecular pathway, leading to constitutive activation of the RET receptor. The mutated RET receptor would present a tissue-specific tendency to be retained in the Golgi apparatus, leading to a reduced number of RET membrane receptors in the intestine and, therefore, decreased intracellular tyrosine signaling (24).
The second hypothesis is that the RET-activating mutations play either no role, or only a minor role, in HSCR development (2). In this scenario, low-penetrant and dose-dependent, specific predisposition to RET haplotypes may lead to a reduction in RET tyrosine receptor activation during embryonic life, leading to neuronal and intestinal cell disturbances and, finally, HSCR. Of note, both protective and predisposing haplotypes to HSCR have been described (2). There is strong evidence favoring the hypothesis that specific haplotypes play a key role in the fetal development of congenital megacolon (2),(32). Recent data suggest that mutations in RET ligand genes may be responsible for a small proportion of HSCR cases (33).
As previously mentioned, the prevalence of HSCR in MEN2 cases has been recently determined to be 7.5% (22). Borrego et al. (10) reported that 13 HSCR/MEN2 families had been described up to 1998. At present, up to 22 HSCR/MEN2 families have been reported, although the number of families that have been genetically investigated is relatively limited (2),(4),(12). S-HSCR or F-HSCR cases have been extensively studied clinically; however, further genetic haplotype analyses may contribute to a better understanding of the molecular causes of familial HSCR associated with MEN2.
LOCAL EXPERIENCE
Although a limited number of affected familial cases from this new FMTC/MEN2 genealogy could be studied, data from patients with either MTC alone or HSCR associated with MTC/MEN2 were available to be analyzed.
A novel HSCR/FMTC family
Herein we report the results of the genotyping analysis of a novel HSCR/FMTC family that carries the germline c.1858T>C (p.C620R) RET activating mutation in exon 10. The patients come from a rural area of northeast Brazil and have been diagnosed, treated and followed for several years at the University of São Paulo School of Medicine, Academic Hospital (Hospital das Clínicas, southeast Brazil) (Figure 1). Early recognition of HSCR in the studied cases was achieved with routine investigations for HSCR (15),(16). The recommended surgical approach for HSCR was used, allowing the affected patients in the study to survive longer (14),(17). The two patients with HSCR/MEN2 who were operated on in our hospital had the long-segment form of HSCR. No renal/urinary disturbances were observed in our cases, consistent with the findings from the HSCR mouse model (34). Several RET gene mutation carriers from this family underwent preventive or curative total thyroidectomy. We have recently reported partial phenotypic data obtained from this large FMTC/MEN2 family (22).
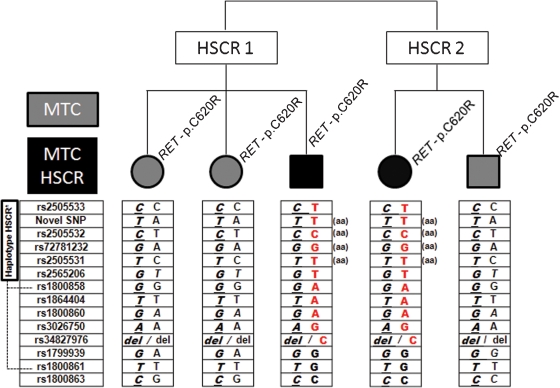
Figure 1.
Identification of different haplotypes, including the rs2505533 and rs34827976 SNPs, in relatives with MTC/HSCR or MTC alone (non-HSCR) who carry the C620R RET mutation. The observed haplotype in the HSCR/MTC patients (red) contains the region previously recognized as related to the sporadic and familial forms of HSCR (1),(2). Four SNPs within this region are only homozygous (aa) in patients with HSCR.
Visit to the area
During a recent visit to the area where the family originally lived and where most patients still live (2,500 km from São Paulo), written informed consent was obtained and blood samples for DNA analysis were collected from several at-risk family members. The researchers were informed that the family had experienced three neonatal deaths of babies who were operated on for congenital megacolon at a local hospital, but not in time to save their lives. The institutional ethics committee has approved this research project.
Haplotype investigation
To verify if an additional and inactivating RET mutation was present in the C620R-mutated HSCR patients in this family, the 21 exons of the RET proto-oncogene were analyzed by polymerase chain reaction amplification and bidirectional sequencing, as previously reported (35). No germline RET mutation other than C620R was observed. We also investigated whether RET single nucleotide polymorphisms (SNPs) or specific haplotypes were involved. Haplotypes were estimated using the EHPlus and Hapi software programs (http://linkage.rockefeller.edu). Genotyping data on 16 RET intragenic SNPs were as follows: rs2505533, novel SNP-1, rs2505532, rs72781232, rs2505531, rs2565206, rs1800858, rs1864404, novel SNP-2, rs1800860, rs3026750, rs34827976, rs1799939, rs1800861, rs1800862, and rs1800863 (Table 1).
Table 1.
Sixteen RET intragenic SNPs that were genotyped in patients with MTC alone or HSCR/MTC from a newly reported FMTC/MEN2 family.
| SNP*) | Location | DNA | Protein |
| rs2505533 | Intron 1 | c.74-1454T>C | / |
| Novel SNP | c.74-1379T>A | / | |
| rs2505532 | c.74-1362C>T | / | |
| rs72781232 | c.74-1329G>A | / | |
| rs2505531 | c.74-1290T>C | / | |
| rs2565206 | c.74-126G>T | / | |
| rs1800858 | Exon 2 | c.135G>A | p.A45A |
| rs1864404 | Intron 5 | c.1064-89A>T | / |
| Novel SNP | Intron 7 | c.1522+58C>A | / |
| rs1800860 | Exon 7 | c.1296A>G | p.A432A |
| rs3026750 | Intron 8 | c.1648+84G>A | / |
| rs34827976 | c.1648+88delC | / | |
| rs1799939 | Exon 11 | c.2071G>A | p.G691S |
| rs1800861 | Exon 13 | c.2307T>A | p.L769L |
| rs1800862 | Exon 14 | c.2508C>T | p.S836S |
| rs1800863 | Exon 15 | c.2712C>G | p.S904S |
Please see http://www.ncbi.nlm.nih.gov/snp.
Initially, the haplotype CTCGTGGTGAdel (rs2505533, IVS1-126, rs2505532, rs72781232, rs2505531, rs2565206, rs1800858, rs1864404, rs1800860, rs3026750 and rs34827976) was documented in all of the studied C620R-mutated relatives in the present family, indicating that this haplotype is linked to the RET mutation.
Because not all of family members with the RET mutation had developed HSCR, the emerging hypothesis was that HSCR disease maybe associated with genetic variants in the remaining chromosome region. In accordance with this assumption, the MEN2-affected relatives who did not have HSCR were believed to have inherited the CATACTGTAAdel haplotype, while the HSCR/MEN2 patients had inherited a different haplotype, TTCGTTAAAGC (Figure 1).
The RET gene region that includes the observed haplotypes in this new MEN2 family, especially the combination of the SNPs from rs2505533 to rs2565206 in linkage disequilibrium with SNPs rs1800858 and rs1800861, has been previously implicated in the development of both sporadic and familial forms of HSCR (1),(2). Furthermore, analysis of four additional members of this family with the C620R mutation (three cases without and one with HSCR) strengthened the proposal that this RET gene region is involved in the development of HSCR (n = 9; p = 0.012; Fisher exact test). It is worth noting that only HSCR patients were homozygous for the four SNPs located in the implicated region: rs2565206, rs2505532, rs72781232, and rs2505531 (Figure 1). Concordantly, RET exon 2 A45A SNP has been found in the homozygous state in cases with HSCR only, whereas MEN2 patients and healthy controls are frequently heterozygous for this SNP (2),(31).
In summary, we have presented new evidence supporting the role of a RET haplotype, which is not linked to the C620R mutation, in the development of HSCR in a newly reported FMTC/MEN2 family. Our findings support the hypothesis that the occurrence of HSCR in MEN2 families is not caused by any additional RET-inactivating mutation. Rather, it is most likely due to the combination of low-penetrance RET SNPs in the previously recognized HSCR-risk region between SNPs 2505533 and 2565206. The TTCGTTAAAGC RET haplotype may be helpful as a genetic marker for HSCR, which would ultimately translate into improved medical care for these patients.
Footnotes
No potential conflict of interest was reported.
Funding
RAT is the recipient of a postdoctoral fellowship from Fundação de Amparo à Pesquisa do Estado de São Paulo (2009/15386-6), TS is the recipient of a doctoral FAPESP fellowship (11942/2009), FLC is the recipient of a Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) fellowship and SPAT is supported by a CNPq senior research productivity fellowship. This work was supported by a CNPq grant (401990/2010-9).
REFERENCES
- 1.Amiel J, Lyonnet S. Hirschsprung disease, associated syndromes, and genetics: a review. J Med Genet. 2001;38((11)):729–39. doi: 10.1136/jmg.38.11.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amiel J, Sproat-Emison E, Garcia-Barcelo M, Lantieri F, Burzynski G, Borrego S, et al. Hirschsprung disease, associated syndromes and genetics: a review. J Med Genet. 2008;45((1)):1–14. doi: 10.1136/jmg.2007.053959. [DOI] [PubMed] [Google Scholar]
- 3.Moore SW, Zaahl M. The Hirschsprungs-multiple endocrine neoplasia (MEN) connection. Clinics. 2012;67(S1):63–7. doi: 10.6061/clinics/2012(Sup01)12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Verdy M, Weber AM, Roy CC, Morin CL, Cadotte M, Brochu P. Hirschsprung's disease in a family with multiple endocrine neoplasia type 2. J Pediatr Gastroenterol Nutr. 1982;1((4)):603–7. doi: 10.1097/00005176-198212000-00027. [DOI] [PubMed] [Google Scholar]
- 5.Mulligan LM, Eng C, Attie T, Lyonnet S, Marsh DJ, Hyland VJ, et al. Diverse phenotypes associated with exon 10 mutations of the RET proto-oncogene. Hum Mol Genet. 1994;3((12)):2163–7. doi: 10.1093/hmg/3.12.2163. [DOI] [PubMed] [Google Scholar]
- 6.Borst MJ, VanCamp JM, Peacock ML, Decker RA. Mutational analysis of multiple endocrine neoplasia type 2A associated with Hirschsprung's disease. Surgery. 1995;117((4)):386–91. doi: 10.1016/s0039-6060(05)80057-1. [DOI] [PubMed] [Google Scholar]
- 7.Eng C, Clayton D, Schuffenecker I, Lenoir G, Cote G, Gagel RF, et al. The relationship between specific RET proto-oncogene mutations and disease phenotype in multiple endocrine neoplasia type 2: International RET Mutation Consortium analysis. JAMA. 1996;276((19)):1575–9. [PubMed] [Google Scholar]
- 8.Caron P, Attié T, David D, Amiel J, Brousset F, Roger P, et al. C618R mutation of exon 10 of the RET proto-oncogene in a kindred with multiple endocrine neoplasia type 2A and Hirschsprung's disease. J Clin Endocrinol Metab. 1996;81((7)):2731–3. doi: 10.1210/jcem.81.7.8675603. [DOI] [PubMed] [Google Scholar]
- 9.Peretz H, Luboshitsky R, Baron E, Biton A, Gershoni R, Usher S, et al. Cys618Arg mutation in the RET proto-oncogene associated with familial medullary thyroid carcinoma and maternally transmitted Hirschsprung's disease suggesting a role for imprinting. Hum Mutat. 1997;10((2)):155–9. doi: 10.1002/(SICI)1098-1004(1997)10:2<155::AID-HUMU7>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 10.Borrego S, Eng C, Sanchez B, Saez ME, Navarro E, Antinolo G. Molecular analysis of the ret and GDNF genes in a family with multiple endocrine neoplasia type 2A and Hirschsprung disease. J Clin Endocrinol Metab. 1998;89((9)):3361–4. doi: 10.1210/jcem.83.9.5093. [DOI] [PubMed] [Google Scholar]
- 11.Decker RA, Peacock ML, Watson P. Hirschsprung disease in MEN 2A: increased spectrum of RET exon 10 genotypes and strong genotype-phenotype correlation. Hum Mol Genet. 1998;7((1)):129–34. doi: 10.1093/hmg/7.1.129. [DOI] [PubMed] [Google Scholar]
- 12.Cohen MS, Phay JE, Albinson C, DeBenedetti MK, Skinner MK, Lairmore TC, et al. Gastrointestinal manifestations of multiple endocrine neoplasia type 2. Ann Surg. 2002;235((5)):648–55. doi: 10.1097/00000658-200205000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Toledo SP, dos Santos MA, Toledo RA, Lourenço DM., Jr Impact of RET proto-oncogene analysis on the clinical management of multiple endocrine neoplasia type 2. Clinics. 2006;61((1)):59–70. [PubMed] [Google Scholar]
- 14.Tannuri U, Tannuri AC. Congenital megacolon in the newborn: which is the best treatment. Rev Assoc Med Bras. 2007;53((2)):100–1. doi: 10.1590/s0104-42302007000200008. [DOI] [PubMed] [Google Scholar]
- 15.Coelho MC, Tannuri U, Benditt I, Santos MM. Studies of RET gene expression and acetylcholinesterase activity in a series of sporadic Hirschsprung''s disease. Pediatr Surg Int. 2008;24((9)):1017–21. doi: 10.1007/s00383-008-2207-8. [DOI] [PubMed] [Google Scholar]
- 16.Santos MM, Tannuri U, Coelho MC. Study of acetylcholinesterase activity in rectal suction biopsy for diagnosis of intestinal dysganglionoses: 17-year experience of a single center. Pediatr Surg Int. 2008;24((6)):715–9. doi: 10.1007/s00383-008-2141-9. [DOI] [PubMed] [Google Scholar]
- 17.Tannuri AC, Tannuri U, Romão RL. Transanal endorectal pull-through in children with Hirschsprung's disease: technical refinements and comparison of results with the Duhamel procedure. J Pediatr Surg. 2009;44((4)):767–72. doi: 10.1016/j.jpedsurg.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 18.Machens A, Lorenz K, Dralle H. Constitutive RET tyrosine kinase activation in hereditary medullary thyroid carcinoma. J Intern Med. 2009;266((1)):114–25. doi: 10.1111/j.1365-2796.2009.02113.x. [DOI] [PubMed] [Google Scholar]
- 19.Machens A, Dralle H. Multiple endocrine neoplasia type 2: achievements and current challenges. Clinics. 2012;67(S2) doi: 10.6061/clinics/2012(Sup01)19. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Correia-Deur JE, Toledo RA, Imazawa AT, Lourenço DM, Jr, Ezabella MC, Tavares MR, et al. Sporadic medullary thyroid carcinoma: clinical data from a university hospital. Clinics (Sao Paulo) 2009;64((5)):379–86. doi: 10.1590/S1807-59322009000500002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Toledo SP, Lourenço DM, Jr, Santos MA, Tavares MR, Toledo RA, Correia-Deur JE. Hypercalcitoninemia is not pathognomonic of medullary thyroid carcinoma. Clinics (Sao Paulo) 2009;64((7)):699–6. doi: 10.1590/S1807-59322009000700015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Frank-Raue K, Rybicki LA, Erlic Z, Schweizer H, Winter A, Milos I, et al. Risk profiles and penetrance estimations in multiple endocrine neoplasia type 2A caused by germline RET mutations located in exon 10. Hum Mut. 2011;32((1)):51–8. doi: 10.1002/humu.21385. [DOI] [PubMed] [Google Scholar]
- 23.Bordeaux MC, Forcet C, Granger L, Corset V, Bidaud C, Billaud M, et al. The RET proto-oncogene induces apoptosis: a novel mechanism for Hirschsprung disease. EMBO J. 19((15)):4056–63. doi: 10.1093/emboj/19.15.4056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nishikawa M, Murakumo Y, Imai T, Kawai K, Nagaya M, Funahashi H, et al. Cys611Ser mutation in RET proto-oncogene in a kindred with medullary thyroid carcinoma and Hirschsprung's disease. Eur J Hum Genet. 2003;11((5)):364–8. doi: 10.1038/sj.ejhg.5200971. [DOI] [PubMed] [Google Scholar]
- 25.Pasini B, Borrello MG, Greco A, Bongarzone I, Luo Y, Mondellini P, et al. Loss of function effect of RET mutations causing Hirschsprung disease. Nat Genet. 1995;10((1)):35–40. doi: 10.1038/ng0595-35. [DOI] [PubMed] [Google Scholar]
- 26.Pelet A, Geneste O, Edery P, Pasini A, Chappuis S, Atti T, et al. Various mechanisms cause RET-mediated signaling defects in Hirschsprung's disease. J Clin Invest. 1998;101((6)):1415–23. doi: 10.1172/JCI375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Carlomagno F, De Vita G, Berlingieri MT, De Franciscis V, Mellilo RM, Colantuoni V, et al. Molecular heterogeneity of RET loss of function in Hirschsprung's disease. EMBO J. 1996;15((11)):2717–25. [PMC free article] [PubMed] [Google Scholar]
- 28.Iwashita T, Murakami H, Asai N, Takahashi M. Mechanism of Ret dysfunction by Hirschsprung mutations affecting its extra cellular domain. Hum Mol Genet. 1996;5((10)):1578–80. doi: 10.1093/hmg/5.10.1577. [DOI] [PubMed] [Google Scholar]
- 29.Romeo G, Ceccherini I, Celli J, Priolo M, Betsos N, Bonardi G, et al. Association of multiple endocrine neoplasia type 2 and Hirschsprung disease. J Intern Med. 1998;243((6)):515–20. doi: 10.1046/j.1365-2796.1998.00332.x. [DOI] [PubMed] [Google Scholar]
- 30.Carrasquillo MM, McCallion AS, Puffenberger EG, Kashuk CS, Nouri N, Chakravarti A. Genome-wide association study in Mennonites identifies multiple genes for oligogenic Hirschsprung disease. Am J Hum Genet. 2002;71((1)):193–7. [Google Scholar]
- 31.Borrego S, Wright FA, Fernández RM, Williams N, Lopez-Alonso M, Davuluri R, et al. A founding locus within the RET proto-oncogene may account for a large proportion of apparently sporadic Hirschsprung disease and a subset of cases of sporadic medullary thyroid carcinoma. Am J Hum Genet. 2003;72((1)):88–100. doi: 10.1086/345466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fernández RM, Antiñolo G, Eng C, Borrego S. The RET C620S mutation causes multiple endocrine neoplasia type 2A (MEN2A) but not Hirschsprung disease (HSCR) in a family cosegregating both phenotypes. Hum Mut. 2003;22((5)):412–5. doi: 10.1002/humu.10273. [DOI] [PubMed] [Google Scholar]
- 33.Ruiz-Ferrer M, Torroglosa A, Luzón-Toro B, Fernandez RM, Antinolo G, Mulligan LM, et al. Novel mutations at RET ligand genes preventing receptor activation are associated to Hirschsprung's disease. J Mol Med (Berl) 2011;89((5)):471–80. doi: 10.1007/s00109-010-0714-2. [DOI] [PubMed] [Google Scholar]
- 34.Carniti C, Belluco S, Riccardi E, Cranston AN, Mondellini P, Ponder BA, et al. The Ret(C620R) mutation affects renal and enteric development in a mouse model of Hirschsprung's disease. Am J Pathol. 2006;168((4)):1262–75. doi: 10.2353/ajpath.2006.050607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Toledo RA, Wagner SM, Coutinho FL, Lourenço DM, Jr, Azevedo JA, Longuini VC, et al. High penetrance of pheochromocytoma associated with the novel C634Y/Y791F double germline mutation in the RET protooncogene. J Clin Endocrinol Metab. 2010;95((3)):1318–27. doi: 10.1210/jc.2009-1355. [DOI] [PubMed] [Google Scholar]