Abstract
Incremental advances in medical technology, such as the development of sensitive hormonal assays for routine clinical care, are the drivers of medical progress. This principle is exemplified by the creation of the concept of multiple endocrine neoplasia type 2, encompassing medullary thyroid cancer, pheochromocytoma, and primary hyperparathyroidism, which did not emerge before the early 1960s.
This review sets out to highlight key achievements, such as joint biochemical and DNA-based screening of individuals at risk of developing multiple endocrine neoplasia type 2, before casting a spotlight on current challenges which include: (i) ill-defined upper limits of calcitonin assays for infants and young children, rendering it difficult to implement the biochemical part of the integrated DNA-based/biochemical concept; (ii) our increasingly mobile society in which different service providers are caring for one individual at various stages in the disease process. With familial relationships disintegrating as a result of geographic dispersion, information about the history of the origin family may become sketchy or just unavailable. This is when DNA-based gene tests come into play, confirming or excluding an individual's genetic predisposition to multiple endocrine neoplasia type 2 even before there is any biochemical or clinical evidence of the disease.
However, the unrivaled molecular genetic progress in multiple endocrine neoplasia type 2 does not come without a price. Screening may uncover unknown gene sequence variants representing either harmless polymorphisms or pathogenic mutations. In this setting, functional characterization of mutant cells in vitro may generate helpful ancillary evidence with regard to the pathogenicity of gene variants in comparison with established mutations.
Keywords: Medullary thyroid carcinoma, Pheochromocytoma, Primary hyperparathyroidism, RET proto-oncogene, Age-related progression, Calcitonin screening, DNA-based screening
INTRODUCTION
Incremental advances in medical technology, such as the development of sensitive hormonal assays for routine clinical care, are the drivers of medical progress. This principle is exemplified by the creation of the concept of multiple endocrine neoplasia type 2 (MEN2), which did not emerge before the early 1960s (1). This hereditable syndrome combines three distinct disease entities into one clinical syndrome: medullary thyroid cancer (MTC); pheochromocytoma; and primary hyperparathyroidism (2),(3). MEN2B patients who do not have primary hyperparathyroidism also develop conjunctival, corneal, buccal, lip and tongue neuromas, intestinal ganglioneuromas (dubbed “pseudo-Hirschsprung's disease”), and skeletal anomalies subsumed under the designation of “Marfanoid habitus” as they grow older (2)–(4).
Before this landmark event, individuals operated on for MTC used to be diagnosed with papillary, follicular, or undifferentiated thyroid cancer. After completing their fourth decade of life, some of these patients succumbed to metastatic MTC or to acute hypertensive crisis owing to adrenergic hormonal excess from a previously undetected pheochromocytoma. In the absence of a postmortem examination, these deaths were frequently ascribed to acute cardiovascular or cerebrovascular events. Without clinical–pathologic characterization of the disease, targeted interventions are difficult, if not impossible, to carry out at an early stage in the disease process.
BIOCHEMICAL SCREENING
The widespread availability of commercial calcitonin and catecholamine assays in the 1960s completely changed the scene, enabling biochemical screening of asymptomatic individuals from MEN2 families who were suspected to harbor C-cell disease and adrenal medullary disease (5). At the very beginning, calcitonin screening, augmented through stimulation with intravenous bolus injections of pentagastrin or calcium gluconate short infusions, was largely cross-sectional, including kindreds of various age groups at different stages of tumor development (5). With the increasing inclusion of asymptomatic infants and young children at risk of developing MEN2 tumors, the focus of the screening programs shifted toward longitudinal observation of family members (6). This prompted biochemical screening of individuals at risk of developing MEN2 over many years who were slated for preemptive thyroidectomy as soon as basal serum calcitonin levels exceeded the upper normal limit of the assay. With the exception of a few false-positives from physiologic C-cell disease of the thyroid or calcitonin-secreting small cell lung cancer or pancreatic neuroendocrine cancer (7), this approach worked fairly well, reducing the number of patients newly diagnosed with advanced MTC (5),(6). As a result, family members from the younger generations were found to have more localized disease (“anticipation phenomenon”), as opposed to the metastatic disease that used to haunt earlier generations.
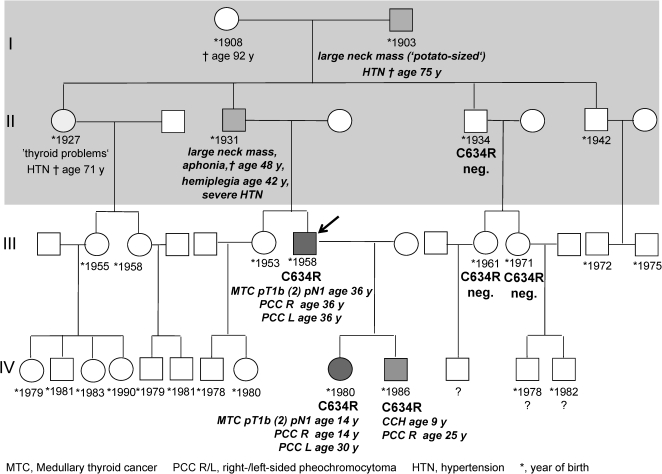
This breakthrough in the management of MEN2 patients is beautifully illustrated in Figure 1 with the pedigree of an MEN2A family of Transylvanian Saxons (ethnic Germans whose ancestors colonized Sibiu (Hermannstadt) county in present-day Romania during the 13th century). The first two affected individuals, I-2 and II-3, developed huge neck lumps (presumably MTC) and severe hypertension (presumably due to pheochromocytoma), causing aphonia (presumably as a result of recurrent laryngeal nerve invasion) and hemiplegia at the age of 42 years (presumably following cathecholamine excess) in individual II-3. Both MTC and pheochromocytoma went unrecognized as such by the rural Communist healthcare system, so that the diagnosis of MEN2A was not made at the time (Figure 1), shaded area). After the fall of the Eastern bloc in 1989, the whole family migrated to Germany where individuals III-7 and IV-9 were found to harbor node-positive MTC and pheochromocytoma at the age of 36 and 14 years in 1994 and 1995, respectively (Figure 1). Unfortunately, several family members declined screening.
Figure 1.
Multigenerational Ethnic German C634R Family from Romania.
DNA-BASED SCREENING
The 1993 and 1994 discoveries of RET (REarranged during Transfection) as the susceptibility gene for MEN2 ushered in the molecular era (8)–(10). Owing to the need for genetic association studies for unequivocal cases of MEN type 2, higher risk mutations in codon 918 (the classic MEN2B mutation; American Thyroid Association/ATA D), codon 634 (the classic MEN2A mutation; ATA C) and codons 609, 611, 618, 620, and 630 (ATA B) were overrepresented in the International MEN2 Consortium (2). The subsequent scanning of the RET proto-oncogene for additional mutations identified more RET mutations in codons 768, 790, 791, 804, and 891 between 1995 and 1998 (11)–(14). These milder mutations were often associated with familial MTC only, an abortive form of MEN2.
GENOTYPE–PHENOTYPE CORRELATIONS
Transformation from hyperplasia to neoplasia
The RET proto-oncogene encodes for a transmembrane tyrosine kinase receptor. The constitutive (i.e., genetically encoded) activation of the mutated RET receptor protein is believed to cause, in decreasing frequency, hyperplasia of the parafollicular C-cells, adrenal medullary cells, and parathyroid chief cells (3). A second mutation in one of these neuroendocrine cells (“second hit”) is thought to cause MTC (C-cell cancer), pheochromocytoma, and primary hyperparathyroidism. Because the acquisition of somatic mutations by these cells reflects the play of chance, the development of the various MEN2 components can vary tremendously even among members of the same family, compromising predictions regarding the age by which the various MEN2 components will have developed. As a rule, the weaker the transforming activity of the inherited RET mutation, the more variable will be the clinical presentation (phenotype) of the various MEN2 components (3),(15),(16), with steep gradients from highest risk ATA-D to very high-risk ATA-C, high-risk ATA-B, and lowest risk ATA-A mutations (Table 1). Carriers of the last two groups of mutations may develop familial MTC only.
Table 1.
RET genotype–phenotype relationships and ATA recommendations for thyroidectomy.
| ATAgroup | RETgenotype | Relative frequency of genotype among RET families (%) | RET phenotype:youngest age at tissue diagnosis (years) (2,15–28) | ATA recommendations for thyroidectomy (31) | |||||
| Exon | Codon | Germany(n = 140) (19) | Italy(n = 235) (22) | MTC | PCC | pHPT | DNAbased | b+stCT based | |
| D | 16 | 918 | 15 | 8 | 0.2 | 12 | – | ASAP (≤1 year) | if ↑ |
| 15*) | 883*) | 0 | 0.4 | 39*) | 39*) | N/A | |||
| C | 11 | 634 | 41 | 37 | 0.8 | 12 | 5 | <5 years | if ↑ |
| B | 11†) | 630†) | 0.7 | 2 | 1†) | – | 32 | <5 years (may delay if b+stCT WNL) | if ↑ |
| 10 | 620 | 7 | 4 | 5 | 19 | N/A | |||
| 10 | 618 | 5 | 6 | 5 | 19 | 41 | |||
| 10 | 611 | 1 | 0.4 | 7 | 30 | 40 | |||
| 10 | 609 | 0.7 | 3 | 4 | 19 | 34 | |||
| A | 8 | 533 | 0‡) | 0‡) | 21 | 35 | N/A | May delay >5 years if b+stCT WNL | if ↑ |
| 13 | 768 | 1 | 4 | 9 | 59 | N/A | |||
| 13 | 790 | 12 | 3 | 10 | 28 | N/A | |||
| 13 | 791 | 7 | <1 | 15 | 38 | 38 | |||
| 14 | 804 | 6 | 22 | 6, 12 | 28–33 | 54 | |||
| 15 | 891 | 2 | 10 | 39 | 39 | N/A | |||
ASAP = as soon as possible; ATA = American Thyroid Association; b+stCT = basal and stimulated serum calcitonin levels; MTC = medullary thyroid cancer; PCC = pheochromocytoma; pHPT = primary hyperparathyroidism; RET = REarranged during Transfection.
May need regrouping if confirmed in subsequent studies (ATA group A?).
May need regrouping if confirmed in subsequent studies (ATA group C?).
May be more common in Brazilians of Catalan/Spanish ancestry (17) and the Greek population (20).
Youngest age of tumor development
A systematic review of the literature (2),(15)–(28), focused on the youngest carrier age at which a given MEN2-related tumor was ever reported, yielded the following chart of earliest tumor development (Table 1): medullary thyroid cancer as early as age 2 months (carriers of mutations in codon 918), 10 months (carriers of mutations in codon 634), 12 months (carriers of mutations in codon 630), 4–7 years (carriers of mutations in codons 609, 611, 618 and 620) and 9–21 years (carriers of mutations in codons 533, 768, 790, 791, 804 and 891); pheochromocytoma as early as age 12 years (carriers of mutations in codon 918 and 634), 19–30 years (carriers of mutations in codons 609, 611, 618, and 620), and 28–59 years (carriers of mutations in codons 768, 790, 791, 804, and 891); primary hyperparathyroidism as early as 5 years (carriers of mutations in codon 634), 34–41 years (carriers of mutations in codons 609, 611, 618, and 620), and 38–54 years (carriers of mutations in codons 533, 768, 790, 791, 804, and 891), but for unknown reasons, never in MEN2B patients (carriers of mutations in codon 918).
With more data set to be forthcoming, the current ATA assignments of the rare RET mutations in codon 630 (currently ATA-B) and 883 (currently ATA D) may need to be revised.
Youngest age of node-positive MTC
Another literature review of the youngest age of carriers harboring lymph node metastases from MTC revealed a comparable pattern (16). Barring rare exceptions, lymph node metastases have not yet developed before age 2 years (carriers of mutations in codon 918), not before age 5 years (carriers of mutations in codon 634) and 15 years (carriers of mutations in codons 630) and not before age 20 years in the remaining RET carriers.
Window of opportunity for cure
The lead time provided by early screening of infants and young children from RET families defines a “window of opportunity”, setting the time frame within which total thyroidectomy alone will eliminate all C-cell disease (3). If this time frame has been exceeded, systematic dissection of the central and lateral lymph nodes on either side of the neck is unavoidable. In this situation, the patient's prospects of cure fall from 95% with no lymph node metastases to 57–31% with 1–10 lymph node metastases and 0–4% with >10 lymph node metastases (29),(30).
INTEGRATED DNA-BASED/BIOCHEMICAL CONCEPT OF EARLY THYROIDECTOMY
The impossibility of anticipating the development of the various MEN2 components has prompted both overtreatment (based on the above worst-case scenarios) and undertreatment (practicing the law of averages) of gene carriers. Although the respective RET mutation sets the stage for tumor development, defining a time corridor, this corridor often is too wide to be useful for individual decisions with regard to the timing of prophylactic thyroidectomy.
This imperfection of the DNA-based approach prompted researchers to build on the strengths of the DNA-based and biochemical screening programs, giving rise to the integrated DNA-based/biochemical concept of early thyroidectomy (16). Herein, biochemical information (basal serum calcitonin levels) is utilized to spot imminent or early malignant transformation from C-cell hyperplasia to MTC while it is still confined to the thyroid gland. There is good evidence to suggest that MTC has not developed as long as basal calcitonin levels remain within normal limits. No more than one-third of pediatric RET gene carriers with increased basal calcitonin levels are believed to harbor occult MTC (16). It may be interesting to note that lymph node metastases have not yet been reported with basal serum calcitonin levels ≤40 pg/ml (upper normal limit <10 pg/ml) (16),(26),(31).
What precisely constitutes a normal or elevated serum calcitonin level for infants and young children remains to be determined. As a rule, calcitonin levels tend to be higher in infants younger than 6 months but are thought to approximate to adult levels by the age of 3 years (32). In male and female children older than 6 months (i.e., aged 1–9 years), normal calcitonin values as high as 17 pg/ml and 8.5 pg/ml, respectively, have been noted, exceeding the manufacturer's upper normal limits of <11.5 pg/ml for male adults and <4.6 pg/ml for female adults (33). The application of adult reference data to young children for whom upper normal limits are rarely, if ever, available from the manufacturer of the calcitonin assay may yield incorrect information for children whose seemingly abnormal test results may actually be within the normal limits for that age group. This renders it challenging to implement the calcitonin-based part of the ATA guidelines (Table 1); right panel) for young children unless calcitonin serum levels are quite high.
CURRENT CHALLENGES
In our increasingly mobile society, clinical care for MEN2 has become fragmented, with different service providers caring for one individual at various stages in the disease process. Geographical dispersion of family members, some of whom may be living abroad, can add to a screening program's difficulty in reaching out to all relatives who may have inherited the trait running in the family. These issues frustrated screening efforts directed at all 86 members of a large French L790F RET family. Of these potential gene carriers, no more than 22 family members (26%) ended up being tested despite the authors' vigorous attempts at complete ascertainment (34). Moreover, not every newly identified gene carrier is prepared to share personal genetic information with his/her next of kin.
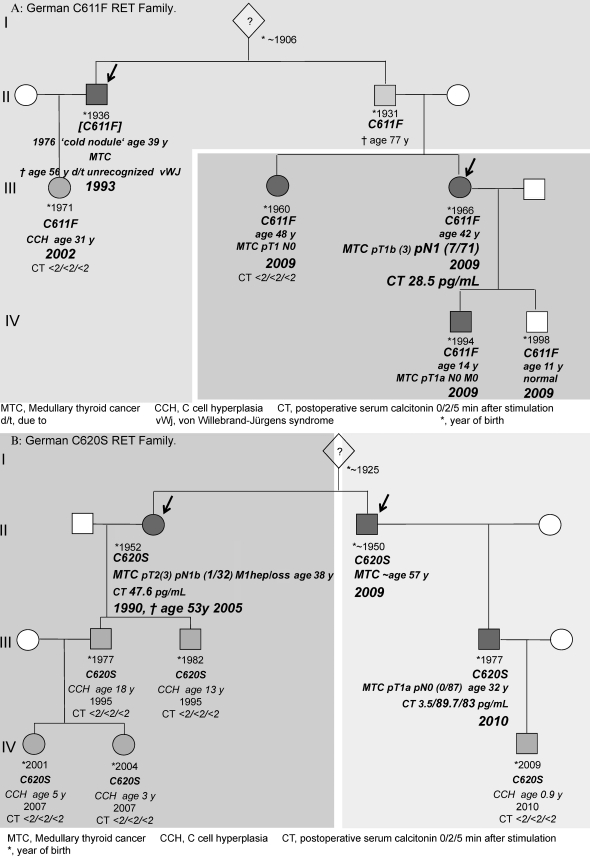
This attitude explains why there can be more than one index patient within an extended RET family whose members, living in silos as it were, underwent RET screening after developing MTC independently of one another. Figure 2 depicts the pedigrees of two unrelated RET families in whom a longstanding breakdown of communication had occurred. This disruption delayed the diagnosis of hereditary MTC in the second index patient by 7 years in the C611F family (Figure 2a) and 19 years in the C620S family (Figure 2b). This significant delay may have contributed to our inability to biochemically cure the second index patient, giving the C-cell disease 7 and 19 additional years to spread beyond curability. There is a paucity of research into the reasons behind some gene carriers' unwillingness to share genetic risk with individuals at risk within their families. Hypothetical reasons include repression, feelings of guilt and resentment, emotional distress, and poor familial interaction.
Figure 2.
German RET Families with 2 Index Patients.
This is when DNA-based screening, requiring only a venous blood draw, comes into play. Barring sample mix-up, the RET gene test confirms or rules out an individual's genetic predisposition to MEN2 even before there is any biochemical or clinical evidence of disease. This is most relevant in the event of adopted children or broken families in whom information about the history of the origin family is sketchy or just unavailable.
However, this unrivaled molecular genetic progress does not come without a price. DNA-based screening may occasionally uncover previously unknown RET sequence variants dubbed “variants of unknown significance” (VUS), which may represent harmless polymorphisms or pathogenic mutations. This distinction is difficult, if not impossible, to make when one sole family member is clinically affected so that there is no demonstrable segregation of the trait with the disease. This scenario is common in small and younger families whose members conceivably may not have been given sufficient time to develop the disease. In this setting, functional characterization of RET mutant cells in vitro has been harnessed to generate ancillary evidence regarding the pathogenicity of unknown sequence variants in comparison with established RET mutations (35)–(38). This experimental approach, albeit promising, will need further validation before being considered for routine clinical use.
Footnotes
No potential conflict of interest was reported.
REFERENCES
- 1.Sipple JH. The association of pheochromocytoma with carcinoma of the thyroid gland. Am J Med. 1961;31:163–6. [Google Scholar]
- 2.Eng C, Clayton D, Schuffenecker I, Lenoir G, Cote G, Gagel RF, et al. The relationship between specific RET proto-oncogene mutations and disease phenotype in multiple endocrine neoplasia type 2. J Am Med Assoc. 1996;276:1575–9. [PubMed] [Google Scholar]
- 3.Machens A, Lorenz K, Dralle H. Constitutive RET tyrosine kinase activation in hereditary medullary thyroid cancer: clinical opportunities. J Intern Med. 2009;266((1)):114–25. doi: 10.1111/j.1365-2796.2009.02113.x. [DOI] [PubMed] [Google Scholar]
- 4.Brauckhoff M, Machens A, Hess S, Lorenz K, Gimm O, Brauckhoff K, et al. Premonitory symptoms preceding metastatic medullary thyroid cancer in MEN 2B: an exploratory analysis. Surgery. 2008;144((6)):1044–50; discussion 1050–3. doi: 10.1016/j.surg.2008.08.028. [DOI] [PubMed] [Google Scholar]
- 5.Graze K, Spiler IJ, Tashjian AH, Jr, Melvin KE, Cervi-Skinner S, Gagel RF, et al. Natural history of familial medullary thyroid carcinoma: effect of a program for early diagnosis. N Engl J Med. 1978;299((18)):980–5. doi: 10.1056/NEJM197811022991804. [DOI] [PubMed] [Google Scholar]
- 6.Gagel RF, Tashjian AH, Jr, Cummings T, Papathanasopoulos N, Kaplan MM, DeLellis RA, et al. The clinical outcome of prospective screening for multiple endocrine neoplasia type 2a. An 18-year experience. N Engl J Med. 1988;318((8)):478–84. doi: 10.1056/NEJM198802253180804. [DOI] [PubMed] [Google Scholar]
- 7.Machens A, Haedecke J, Holzhausen HJ, Thomusch O, Schneyer U, Dralle H. Differential diagnosis of calcitonin-secreting neuroendocrine carcinoma of the foregut by pentagastrin stimulation. Langenbecks Arch Surg. 2000;385((8)):398–401. doi: 10.1007/s004230000169. [DOI] [PubMed] [Google Scholar]
- 8.Donis-Keller H, Dou S, Chi D, Carlson KM, Toshima K, Lairmore TC, et al. Mutations in the RET proto-oncogene are associated with MEN 2A and FMTC. Hum Mol Genet. 1993;2((7)):851–6. doi: 10.1093/hmg/2.7.851. [DOI] [PubMed] [Google Scholar]
- 9.Mulligan LM, Kwok JB, Healey CS, Elsdon MJ, Eng C, Gardner E, et al. Germ-line mutations of the RET proto-oncogene in multiple endocrine neoplasia type 2A. Nature. 1993;363((6428)):458–60. doi: 10.1038/363458a0. [DOI] [PubMed] [Google Scholar]
- 10.Hofstra RM, Landsvater RM, Ceccherini I, Stulp RP, Stelwagen T, Luo Y, et al. A mutation in the RET proto-oncogene associated with multiple endocrine neoplasia type 2B and sporadic medullary thyroid carcinoma. Nature. 1994;367((6461)):375–6. doi: 10.1038/367375a0. [DOI] [PubMed] [Google Scholar]
- 11.Bolino A, Schuffenecker I, Luo Y, Seri M, Silengo M, Tocco T, et al. RET mutations in exons 13 and 14 of FMTC patients. Oncogene. 1995;10((12)):2415–19. [PubMed] [Google Scholar]
- 12.Eng C, Smith DP, Mulligan LM, Healey CS, Zvelebil MJ, Stonehouse TJ, et al. A novel point mutation in the tyrosine kinase domain of the RET proto-oncogene in sporadic medullary thyroid carcinoma and in a family with FMTC. Oncogene. 1995;10((3)):509–13. [PubMed] [Google Scholar]
- 13.Hofstra RM, Fattoruso O, Quadro L, Wu Y, Libroia A, Verga U, et al. A novel point mutation in the intracellular domain of the ret protooncogene in a family with medullary thyroid carcinoma. J Clin Endocrinol Metab. 1997;82((12)):4176–8. doi: 10.1210/jcem.82.12.4439. [DOI] [PubMed] [Google Scholar]
- 14.Berndt I, Reuter M, Saller B, Frank-Raue K, Groth P, Grussendorf M, et al. A new hot spot for mutations in the ret protooncogene causing familial medullary thyroid carcinoma and multiple endocrine neoplasia type 2A. J Clin Endocrinol Metab. 1998;83((3)):770–4. doi: 10.1210/jcem.83.3.4619. [DOI] [PubMed] [Google Scholar]
- 15.Machens A, Niccoli-Sire P, Hoegel J, Frank-Raue K, van Vroonhoven TJ, Roeher HD, et al. European Multiple Endocrine Neoplasia (EUROMEN) Study Group. Early malignant progression of hereditary medullary thyroid cancer. N Engl J Med. 2003;349((16)):1517–25. doi: 10.1056/NEJMoa012915. [DOI] [PubMed] [Google Scholar]
- 16.Machens A, Lorenz K, Dralle H. Individualization of lymph node dissection in RET (rearranged during transfection) carriers at risk for medullary thyroid cancer: value of pretherapeutic calcitonin levels. Ann Surg. 2009;250((2)):305–10. doi: 10.1097/SLA.0b013e3181ae333f. [DOI] [PubMed] [Google Scholar]
- 17.Da Silva AM, Maciel RM, Da Silva MR, Toledo SR, De Carvalho MB, Cerutti JM. A novel germ-line point mutation in RET exon 8 (Gly(533)Cys) in a large kindred with familial medullary thyroid carcinoma. J Clin Endocrinol Metab. 2003;88((11)):5438–43. doi: 10.1210/jc.2003-030997. [DOI] [PubMed] [Google Scholar]
- 18.Machens A, Brauckhoff M, Holzhausen HJ, Thanh PN, Lehnert H, Dralle H. Codon-specific development of pheochromocytoma in multiple endocrine neoplasia type 2. J Clin Endocrinol Metab. 2005;90((7)):3999–4003. doi: 10.1210/jc.2005-0064. [DOI] [PubMed] [Google Scholar]
- 19.Machens A, Dralle H. Familial prevalence and age of RET germline mutations: implications for screening. Clin Endocrinol. 2008;69:81–7. doi: 10.1111/j.1365-2265.2007.03153.x. [DOI] [PubMed] [Google Scholar]
- 20.Peppa M, Boutati E, Kamakari S, Pikounis V, Peros G, Panayiotides IG, et al. Multiple endocrine neoplasia type 2A in two families with the familial medullary thyroid carcinoma associated G533C mutation of the RET proto-oncogene. Eur J Endocrinol. 2008;159((7)):767–71. doi: 10.1530/EJE-08-0476. [DOI] [PubMed] [Google Scholar]
- 21.Zenaty D, Aigrain Y, Peuchmaur M, Philippe-Chomette P, Baumann C, Cornelis F, et al. Medullary thyroid carcinoma identified within the first year of life in children with hereditary multiple endocrine neoplasia type 2A (codon 634) and 2B. Eur J Endocrinol. 2009;160((5)):807–13. doi: 10.1530/EJE-08-0854. [DOI] [PubMed] [Google Scholar]
- 22.Romei C, Mariotti S, Fugazzola L, Taccaliti A, Pacini F, Opocher G, et al. Multiple endocrine neoplasia type 2 syndromes (MEN 2): results from the ItaMEN network analysis on the prevalence of different genotypes and phenotypes. Eur J Endocrinol. 2010;163((2)):301–8. doi: 10.1530/EJE-10-0333. [DOI] [PubMed] [Google Scholar]
- 23.Schulte KM, Machens A, Fugazzola L, McGregor A, Diaz-Cano S, Izatt L, et al. The clinical spectrum of multiple endocrine neoplasia type 2a caused by the rare intracellular RET mutation S891A. J Clin Endocrinol Metab. 2010;95((1)):E92–7. doi: 10.1210/jc.2010-0375. [DOI] [PubMed] [Google Scholar]
- 24.Frank-Raue K, Rybicki LA, Erlic Z, Schweizer H, Winter A, Milos I, et al. International RET Exon 10 Consortium. Risk profiles and penetrance estimations in multiple endocrine neoplasia type 2A caused by germline RET mutations located in exon 10. Hum Mutat. 2011;32((1)):51–8. doi: 10.1002/humu.21385. [DOI] [PubMed] [Google Scholar]
- 25.Jasim S, Ying AK, Waguespack SG, Rich TA, Grubbs EG, Jimenez C, et al. Multiple endocrine neoplasia type 2B with a RET proto-oncogene A883F mutation displays a more indolent form of medullary thyroid carcinoma compared with a RET M918T mutation. Thyroid. 2011. 21((2)):189–92. doi: 10.1089/thy.2010.0328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Magalhães PK, Antonini SR, de Paula FJ, de Freitas LC, Maciel LM. Primary hyperparathyroidism as the first clinical manifestation of multiple endocrine neoplasia type 2a in a 5-year-old child. Thyroid. 2011;21((5)):547–50. doi: 10.1089/thy.2010.0336. [DOI] [PubMed] [Google Scholar]
- 27.Mukherjee S, Zakalik D. RET codon 804 mutations in multiple endocrine neoplasia 2: genotype–phenotype correlations and implications in clinical management. Clin Genet. 2011;79((1)):1–16. doi: 10.1111/j.1399-0004.2010.01453.x. [DOI] [PubMed] [Google Scholar]
- 28.Rohmer V, Vidal-Trecan G, Bourdelot A, Niccoli P, Murat A, Wemeau JL, et al. Prognostic factors of disease-free survival after thyroidectomy in 170 young patients with a RET germline mutation: a multicenter study of the Groupe Francais d'Etude des Tumeurs Endocrines. J Clin Endocrinol Metab. 2011;96((3)):E509–18. doi: 10.1210/jc.2010-1234. [DOI] [PubMed] [Google Scholar]
- 29.Machens A, Gimm O, Ukkat J, Hinze R, Schneyer U, Dralle H. Improved prediction of calcitonin normalization in medullary thyroid carcinoma patients by quantitative lymph node analysis. Cancer. 2000;88((8)):1909–15. [PubMed] [Google Scholar]
- 30.Scollo C, Baudin E, Travagli JP, Caillou B, Bellon N, Leboulleux S, et al. Rationale for central and bilateral lymph node dissection in sporadic and hereditary medullary thyroid cancer. J Clin Endocrinol Metab. 2003;88((5)):2070–5. doi: 10.1210/jc.2002-021713. [DOI] [PubMed] [Google Scholar]
- 31.Kloos RT, Eng C, Evans DB, Francis GL, Gagel RF, Gharib H, et al. American Thyroid Association Guidelines Task Force. Medullary thyroid cancer: management guidelines of the American Thyroid Association. Thyroid. 2009;19:565–612. doi: 10.1089/thy.2008.0403. [DOI] [PubMed] [Google Scholar]
- 32.Basuyau JP, Mallet E, Leroy M, Brunelle P. Reference intervals for serum calcitonin in men, women, and children. Clin Chem. 2004;50((10)):1828–30. doi: 10.1373/clinchem.2003.026963. [DOI] [PubMed] [Google Scholar]
- 33.Verga U, Morpurgo PS, Vaghi I, Radetti G, Beck-Peccoz P. Normal range of calcitonin in children measured by a chemiluminescent two-site immunometric assay. Horm Res. 2006;66((1)):17–20. doi: 10.1159/000092848. [DOI] [PubMed] [Google Scholar]
- 34.Bihan H, Baudin E, Meas T, Leboulleux S, Al Ghuzlan A, Hannoteaux V, et al. for the French Group of Endocrine Tumors (GTE). Role of prophylactic thyroidectomy in RET 790 familial medullary thyroid carcinoma. Head Neck. 2011 doi: 10.1002/hed.21763. (e-pub ahead of print 17 Jun 2011; http://dx.doi.org/10.1002/hed.21763). [DOI] [PubMed] [Google Scholar]
- 35.Ercolino T, Lombardi A, Becherini L, Piscitelli E, Cantini G, Gaglianò MS, et al. The Y606C RET mutation causes a receptor gain of function. Clin Endocrinol. 2008;69((2)):253–8. doi: 10.1111/j.1365-2265.2008.03197.x. [DOI] [PubMed] [Google Scholar]
- 36.Fazioli F, Piccinini G, Appolloni G, Bacchiocchi R, Palmonella G, Recchioni R, et al. A new germline point mutation in Ret exon 8 (cys515ser) in a family with medullary thyroid carcinoma. Thyroid. 2008;18((7)):775–82. doi: 10.1089/thy.2007.0365. [DOI] [PubMed] [Google Scholar]
- 37. Borrello MG, Aiello A, Peissel B, Rizzetti MG, Mondellini P, Degl'innocenti D, et al. Functional characterization of the MTC-associated germ line RET-K666E mutation: evidence of oncogenic potential enhanced by the G691S polymorphism Endocr Relat Cancer. 2011;18(4):519-27 [DOI] [PubMed] [Google Scholar]
- 38.Machens A, Spitschak A, Lorenz K, Pützer BM, Dralle H. Germline RET sequence variation I852M and occult medullary thyroid cancer: harmless polymorphism or causative mutation. Clin Endocrinol. 2011;75((6)):801–5. doi: 10.1111/j.1365-2265.2011.04158.x. [DOI] [PubMed] [Google Scholar]