Abstract
Platelet-derived growth factor (PDGF) and its receptor are known to be substantially elevated in lung tissues and pulmonary arterial smooth muscle cells (PASMC) isolated from patients and animals with pulmonary arterial hypertension. PDGF has been shown to phosphorylate and activate Akt and mammalian target of rapamycin (mTOR) in PASMC. In this study, we investigated the role of PDGF-mediated activation of Akt signaling in the regulation of cytosolic Ca2+ concentration and cell proliferation. PDGF activated the Akt/mTOR pathway and, subsequently, enhanced store-operated Ca2+ entry (SOCE) and cell proliferation in human PASMC. Inhibition of Akt attenuated the increase in cytosolic Ca2+ concentration due to both SOCE and PASMC proliferation. This effect correlated with a significant downregulation of stromal interacting molecule (STIM) and Orai, proposed molecular correlates for SOCE in many cell types. The data from this study present a novel pathway for the regulation of Ca2+ signaling and PASMC proliferation involving activation of Akt in response to upregulated expression of PDGF. Targeting this pathway may lead to the development of a novel therapeutic option for the treatment of pulmonary arterial hypertension.
Keywords: pulmonary vascular remodeling, platelet-derived growth factor, Akt/mammalian target of rapamycin pathway, store-operated Ca2+ entry
increased levels of platelet-derived growth factor (PDGF) in the blood and lung tissues, and upregulated PDGF receptors (PDGFR) in the pulmonary vasculature are strongly implicated in the development of pulmonary hypertension (2, 17, 25, 39). It was also recently reported that high deposition of PDGF is observed in distal arteries of patients with chronic thromboembolic pulmonary hypertension (CTEPH), and cells isolated from endarterectomized tissues of patients with CTEPH have high expression of PDGFR (24). Such data indicate that, regardless of the type of pulmonary hypertension, PDGF is a potent mitogen that contributes to the development and progression of pulmonary vascular remodeling. The same study also demonstrated a role for the Akt-mammalian target of rapamycin (mTOR)-p70 ribosomal S6 kinase (p70S6K) pathway in the regulation of cell growth and proliferation. It has been previously reported that rapamycin, an inhibitor of mTOR, may be a potential therapeutic option for pulmonary hypertension due to its inhibition of cell proliferation (18, 24, 33, 36), which has been demonstrated in coronary arterial smooth muscle cells and cancer cells (23, 32).
Regulation of cytosolic Ca2+ concentration ([Ca2+]cyt) in pulmonary arterial smooth muscle cells (PASMC) is critical for many cellular processes, such as contraction, migration, and proliferation (8). A rise in [Ca2+]cyt in PASMC is a major trigger for pulmonary vasoconstriction. Additionally, an increase in [Ca2+]cyt, by activating Ca2+-dependent signaling cascades [e.g., calmodulin, calmodulin kinase, nuclear factor of activated T cells (NFAT)], is an important stimulus for PASMC proliferation and migration, two important causes for pulmonary vascular remodeling (4). One mechanism involved in raising [Ca2+]cyt is store-operated Ca2+ entry (SOCE), which is induced by depletion of Ca2+ from the intracellular stores, mainly the sarcoplasmic (SR) or endoplasmic (ER) reticulum.
Although the exact molecular components of SOCE are still debated, the coupling of stromal interacting molecule (STIM), a Ca2+ sensor in the SR/ER membrane, and Orai, a pore-forming channel protein in the plasma membrane, has been shown to contribute to SOCE (5, 30, 31). STIM1 senses decreased [Ca2+] in the SR/ER when inositol-trisphosphate (IP3)-mediated activation of IP3 receptor induces Ca2+ release from the SR/ER to the cytosol. STIM1 then clusters in discrete puncta in the SR/ER membrane and translocates to the plasma membrane. There it activates Orai1, allowing Ca2+ influx into the cell through tetrameric Orai1 channels formed in the surface membrane. This form of Ca2+ signaling is more commonly referred to as Ca2+ release-activated Ca2+ currents (9, 19, 21). It has previously been reported that PDGF significantly increases SOCE and subsequently promotes cell proliferation of PASMC, contributing to pulmonary vascular remodeling (39). Recently, STIM1 and Orai1 were implicated as important components in PDGF-regulated Ca2+ entry in vascular smooth muscle cell migration, and a role for STIM1 and Orai1 has been postulated in neointimal formation in pulmonary hypertension (3).
Both the Akt/mTOR pathway and SOCE are known to be involved in cell proliferation and pulmonary vascular remodeling induced by PDGF (24); however, whether a relationship between these two pathways exists is unclear. In the present study, we show that Akt signaling plays an important role in Ca2+ handling and PASMC proliferation in response to PDGF. The PDGF/Akt-mediated regulation of [Ca2+]cyt and proliferation in human PASMC is partially dependent on the expression and function of STIM and Orai.
MATERIALS AND METHODS
Cell preparation and culture.
Human PASMC and pulmonary arterial endothelial cells (PAEC) from normal subjects were purchased from Lonza (Walkersville, MD). PASMC and PAEC were cultured in smooth muscle growth media (Lonza) and endothelial growth medium (Lonza), respectively. Pulmonary arterial fibroblasts (PAF) and fibroblast medium were purchased from ScienCell Research Laboratories (Carlsbad, CA). When serum starvation was needed, 0.1% fetal bovine serum was added to basal medium without adding other growth factors. All of the cells were incubated in a humidified 5% CO2 atmosphere at 37°C. After reaching confluence, the cells were subcultured by trypsinization with 0.05% trypsin-EDTA (Lonza).
Western blot analysis.
The cells were washed with ice-cold PBS, suspended into lysis buffer (1% Nonidet P-40, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate, 100 μg/ml phenylmethylsulfonyl fluoride, phosphatase inhibitors, and protease inhibitors), and incubated for 30 min on ice. The cell lysates were then sonicated and centrifuged at 12,000 rpm for 10 min, and the supernatant was collected. Protein concentrations were determined by DC protein assay (Bio-Rad Laboratories, Hercules, CA) using BSA as a standard. Samples were applied on SDS-PAGE (4–20%), and proteins were transferred onto nitrocellulose membranes by electroblot. Membranes were blocked in 5% nonfat milk and incubated overnight at 4°C with primary antibodies and then with secondary antibody. Blots were developed using the SuperSignal West Pico Chemiluminescent Substrate (Pierce Biotechnology, Rockford, IL).
Measurement of [Ca2+]cyt.
Cells were plated on 25-mm coverslips and placed in a recording chamber on the stage of an inverted Nikon Eclipse/TE 200 microscope with the TE-FM epifluorescence attachment. [Ca2+]cyt was measured in each cell using the membrane-permeable Ca2+-sensitive fluorescent indicator, fura-2 AM (Invitrogen, Carlsbad, CA). The cells were incubated at room temperature for 30 min in modified Krebs solution containing 4 μM fura-2 AM. The loaded cells were then washed with modified Krebs solution for 30 min to remove excess extracellular dye and allow intracellular esterases to cleave cytosolic fura-2 AM into active fura-2. Fura-2 fluorescence was observed as 510-nm wavelength light emission with excitation wavelengths of 340 and 380 nm by use of the digital fluorescence imaging system from Intracellular Imaging (Cincinnati, OH). In all experiments, multiple cells were imaged in a single field, and one arbitrarily chosen peripheral cytosolic area from each cell was spatially averaged. [Ca2+]cyt was expressed as fura-2 fluorescence emission ratio excited at 340 and 380 nm (R340/380); the change in [Ca2+]cyt in response to different agonists was normalized by the baseline ratio (F0, the R340/380 measured before application of agonists) and expressed as F/F0 (where F is the R340/380 measured during or after application of agonists).
Proliferation assay.
[3H]thymidine incorporation assay was performed to assess DNA synthesis. PASMC were seeded in 12-well plates and treated for each experiment. Twenty-four hours before treatment was finished, 1 μCi [3H]thymidine was added to each conditioned media. One day later, cells were washed with cold PBS once and washed twice with cold 7.5% trichloracetic acid, and then lysed with 0.5 M NaOH. The radioactivity was measured in a liquid scintillation counter. The data were obtained as counts per minute.
Chemicals and antibodies.
PDGF-BB, hydrogen peroxide, EGTA, cyclopiazonic acid (CPA), carbonyl cyanide-p-trifluoromethoxyphenylhydrazone (FCCP) were purchased from Sigma-Aldrich (St. Louis, MO). Ionomycin calcium salt was from Ascent Scientific (Princton, NJ). Rapamycin and Akt inhibitor (VIII) were purchased from Calbiochem (Gibbstown, NJ). Antibodies to mTOR, phospho-mTOR (Ser2448), p70S6K, phospho-p70S6K (Thr389), Akt, phospho-Akt (Ser473), eukaryotic initiation factor 4E binding protein 1 (4EBP1), phospho-4EBP1 (Ser65), eukaryotic initiation factor 4E (eIF4E), and phospho-eIF4E (Ser209) were purchased from Cell Signaling Technology (Beverly, MA). Antibodies to STIM1 and Orai1 were purchased from ProSci (Poway, CA). Antibody to GAPDH and horseradish peroxidase-conjugated donkey anti-mouse IgG antibody were purchased from Millipore (Billerica, MA). Horseradish peroxidase-conjugated anti-rabbit IgG antibody was from Santa Cruz Biotechnology (Santa Cruz, CA).
Statistical analysis.
The data are expressed as means ± SE. Differences between groups were examined for statistical significance using Student's t-test. Differences were considered to be statistically significant when P < 0.05.
RESULTS
PDGF induces phosphorylation of Akt and mTOR in PASMC.
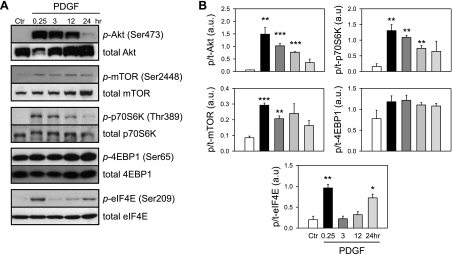
To investigate whether PDGF affects the Akt/mTOR signaling pathway in the pulmonary vasculature, we examined the effects of PDGF on Akt and mTOR in PASMC. PDGF-induced phosphorylation of Akt and downstream signaling proteins in PASMC were investigated over a time course of 0.25, 3, 12, and 24 h subsequent to 48-h serum starvation in 0.1% fetal bovine serum-containing media (Fig. 1). Western blots indicate that PDGF treatment transiently increased phosphorylation of Akt; the phosphorylated Akt (p-Akt) subsequently caused significant increase in phosphorylated mTOR (p-mTOR), p70S6K (p-p70S6K), and eIF4E (p-eIF4E). Treatment of PASMC with PDGF for 0.25 h increased p-Akt by 24.8-fold (P < 0.01), p-mTOR by 3.6-fold (P < 0.001), p-p70S6K by 8.1-fold (P < 0.01) and p-eIF4E by 5.1-fold (P < 0.01) (Fig. 1). PDGF treatment, however, did not significantly increase phosphorylation of 4EBP1, another downstream signaling protein that has been reported to be phosphorylated by Akt/mTOR. 4EBP1 acts as a transcriptional repressor by binding to and inhibiting eIF4E. Phosphorylation of 4EBP1 by mTOR results in the dissociation of 4EBP1 from eIF4E, thereby relieving the inhibitory effect of 4EBP1 on eIF4E-dependent transcription (15). It has been noted that the basal level of phosphorylated 4EBP1 (p-4EBP1) is high in control human PASMCs. PDGF treatment appeared to slightly increase p-4EBP1 in PASMC, but the effect was not statistically significant (Fig. 1). These results suggest that PDGF induces a rapid and transient phosphorylation of the Akt/mTOR pathway and that 4EBP1 phosphorylation at Ser65 may be regulated by factors other than p-Akt/mTOR after PDGF stimulation. These results demonstrate that treatment with PDGF results in activation of the Akt/mTOR pathway. Our laboratory has previously reported a similar observation in cells isolated from endarterectomized tissues of patients with CTEPH (24).
Fig. 1.
Platelet-derived growth factor (PDGF)-induced phosphorylation of the Akt/mammalian target of rapamycin (mTOR) pathway in normal human pulmonary arterial smooth muscle cells (PASMC). A: representative Western blots showing time-dependent changes in the phosphorylated [p-Akt, p-mTOR, p-p70S6K (p70 ribosomal S6 kinase), p-4EBP1 (eukaryotic initiation factor 4E binding protein 1), and p-eIF4E (eukaryotic initiation factor 4E)] components and total proteins (t-Akt, t-mTOR, t-p70S6K, t-4EBP1, and t-eIF4E) of the Akt/mTOR signaling pathway induced by PDGF stimulation (10 ng/ml). Protein expression was assessed at 0.25, 3, 12, and 24 h after PDGF treatment. B: summary data (means ± SE, n = 3) were quantitated and compared at each time point, with the control (Ctr) being without PDGF treatment. au, Arbitrary units. *P < 0.05, **P < 0.01, and ***P < 0.001 vs. Ctr.
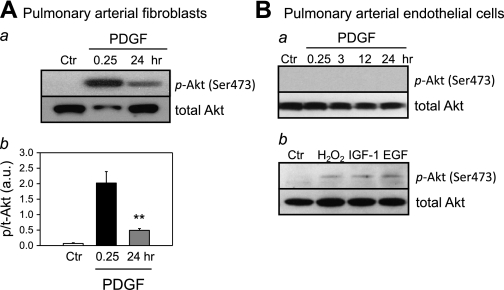
The pulmonary artery, similar to other systemic arteries, is structurally composed of the intima, media, and adventitia. The three major cell types that constitute pulmonary artery are PAF, which are the predominant cells found in the adventitia, PASMC which form the media, and PAEC, which form the endothelium of the intima. As in PASMC, treatment of PAF with PDGF also resulted in significant and rapid phosphorylation of Akt; 0.25-h treatment of PAF with PDGF caused a 10-fold increase in p-Akt (Fig. 2A). The level of p-Akt in PDGF-treated PAF was still significantly higher at 24 h of the treatment than in control PAF, but much less than in PDGF-treated PAF at 0.25 h (Fig. 2Aa). In human PAEC, however, PDGF failed to induce phosphorylation of Akt (Fig. 2Ba), whereas hydrogen peroxide (H2O2), insulin-like growth factor-I, and epidermal growth factor all induced Akt phosphorylation (Fig. 2Bb). These data indicate that PDGF selectively phosphorylates Akt in human pulmonary vascular smooth muscle cells and fibroblasts, but not in pulmonary vascular endothelial cells.
Fig. 2.
Akt phosphorylation in pulmonary arterial fibroblasts (PAF) and pulmonary arterial endothelial cells (PAEC). A, a: representative Western blots showing time-dependent changes in the p-Akt and t-Akt induced by PDGF stimulation (10 ng/ml). Protein expression was assessed at 0.25 and 24 h after PDGF treatment. b: Summarized data (means ± SE, n = 3) were quantitated and compared at each time point. **P < 0.01 vs. Ctr. B, a: representative Western blots showing no phosphorylation of Akt induced by PDGF stimulation (10 ng/ml). b: Treatment with hydrogen peroxide (500 μM), insulin-like growth factor-I (IGF-I; 50 ng/ml), and EGF (50 ng/ml) for 0.25 h induced phosphorylation of Akt. Blots were representative of 3 different sets of experiments.
The Akt/mTOR pathway mediates PDGF-enhanced SOCE in PASMC.
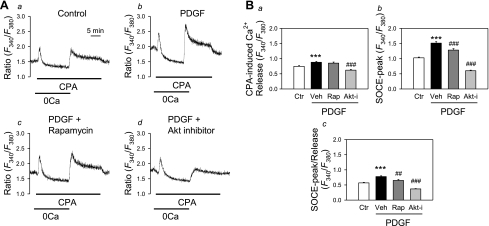
Our laboratory previously reported that PDGF upregulated transient receptor potential canonical (TRPC) 1 channels and enhanced receptor-operated Ca2+ entry and SOCE in normal human and rat PASMC (39). Additionally, inhibition of Akt pathway with rapamycin significantly decreased SOCE in pulmonary vascular cells isolated from patients with CTEPH (24, 39). We, therefore, investigated the effect of inhibition of the Akt/mTOR pathway on PDGF-enhanced SOCE in PASMC. In normal human PASMC, PDGF treatment significantly enhanced SOCE induced by passive depletion of the intracellular Ca2+ stores with CPA, an inhibitor of the Ca2+-Mg2+ ATPase on the SR or ER [sarco(endo)plasmic reticulum Ca2+-ATPase], compared with control PASMC (P < 0.001, Fig. 3, Aa and Ab). In the presence of PDGF, treatment of PASMC with either rapamycin or a specific Akt inhibitor significantly attenuated the amplitude of SOCE (P < 0.001, Fig. 3, Ac and Ad). These results indicate that PDGF enhances SOCE in normal PASMC via, at least in part, activation of the Akt/mTOR signaling pathway.
Fig. 3.
Inhibition of Akt/mTOR attenuates PDGF-induced increase in store-operated Ca2+ entry (SOCE) in PASMC. A: representative traces showing changes in cytosolic Ca2+ concentration ([Ca2+]cyt) [expressed as the ratio of the 340 and 380 fluorescence (F340/F380)] in response to cyclopiazonic acid (CPA)-induced store depletion in the absence of extracellular Ca2+ and subsequent SOCE upon replenishment of extracellular Ca2+. Cells were treated with PDGF and other inhibitors [a: Ctr; b: PDGF (10 ng/ml), c: PDGF and rapamycin (Rap; 10 nM), d: PDGF and Akt inhibitor (Akt-I; 1 μM)] for 24 h before Ca2+ measurement. B: quantitative comparison of the CPA-induced Ca2+ release (a), the peak of SOCE (SOCE-peak; b), and the ratio of SOCE-peak to CPA-induced Ca2+ release (SOCE-peak/release; c) in Ctr (n = 65), PDGF + vehicle (Veh, n = 64), PDGF + Rap (n = 50), and PDGF + Akt-i (n = 58) PASMC. Rap and Akt-i both significantly attenuated PDGF-induced augmentation of SOCE. Values are means ± SE. ***P < 0.001 vs. Ctrl. ##P < 0.01, ###P < 0.001 vs. PDGF + Veh-treated cells.
PDGF-induced expression of STIM1 and Orai1 is dependent on the Akt/mTOR pathway.
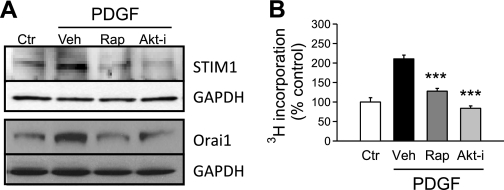
To further understand the role of the Akt/mTOR pathway in PDGF-enhanced SOCE, we examined the expression of STIM1 and Orai1 in PASMC, after treatment with PDGF. STIM1 has recently been described to function as a SR/ER membrane-bound sensor of [Ca2+] in the SR/ER, while Orai1 is a membrane-spanning Ca2+ channel that forms tetrameric store-operated Ca2+ channels (SOC) in the plasma membrane (5). When the intracellular store (i.e., SR/ER) is depleted, or the [Ca2+] in the SR/ER is reduced, STIM1 proteins oligomerize at first and then translocate to the puncta to recruit Orai1 subunits to form SOC and induce SOCE (5, 30). STIM1 has also been shown to interact with and regulate transient receptor potential channels, which are also known to function as SOC and contribute to SOCE (7, 16). The role of STIM1, Orai1, and transient receptor potential channels in PASMC is still not fully understood. We examined the involvement of PDGF and the Akt/mTOR pathway in the regulation of STIM1 and Orai1 proteins in human PASMC. As shown in Fig. 4A, treatment with PDGF for 24 h significantly upregulated protein expression of STIM1 and Orai1, whereas rapamycin or the Akt inhibitor both significantly inhibited the PDGF-mediated upregulation of STIM1 and Orai1. The inhibitory effect of rapamycin and the Akt inhibitor on PDGF-induced expression of STIM1 and Orai1 in PASMC indicates that PDGF-mediated enhancement of SOCE is due, at least partially, to upregulation of STIM1 and Orai1 via activation of the Akt/mTOR signaling pathway.
Fig. 4.
Inhibition of Akt/mTOR blocks PDGF-induced stromal interacting molecule 1 (STIM1)/Orai1 expression and PASMC proliferation. A: representative blots showing PDGF treatment (24 h)-induced upregulation of STIM1 and Orai1 expression in normal PASMC. After treatment with Rap (10 nM) or Akt-i (1 μM), PDGF-induced expression of both molecules was decreased. Experiments were repeated 3 times. B: bar charts comparing the effect of Rap (10 nM) and Akt-i (1 μM) on the PDGF-stimulated proliferation of normal PASMC using 3H incorporation (in counts per minute). ***P < 0.001 vs. PDGF + Veh-treated cells.
Inhibition of the Akt/mTOR pathway significantly suppresses proliferation of PASMC.
Our laboratory has previously reported that CTEPH cells show an enhanced proliferative response to PDGF (24). We, therefore, investigated the involvement of the Akt/mTOR pathway in PDGF-induced proliferation in normal PASMC. Inhibition of Akt/mTOR pathway with rapamycin or the Akt inhibitor suppressed PDGF-induced proliferation of PASMC (Fig. 4B). These results provide evidence that PDGF-induced proliferation of PASMC is dependent on the Akt/mTOR pathway, indicating that the Akt/mTOR pathway may be a potential therapeutic target to reduce pulmonary vascular remodeling due to excessive PASMC proliferation.
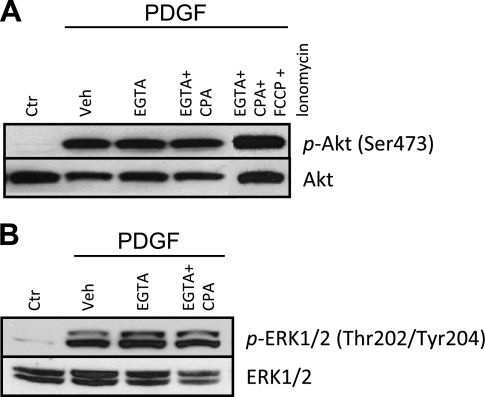
PDGF-induced Akt phosphorylation is independent of extracellular and intracellular Ca2+.
When PDGF binds to and activates its receptors (i.e., PDGFR-α and PDGFR-β) on the plasma membrane, the downstream signaling cascades include, not only Akt/mTOR pathway, but also IP3-diacylglycerol-Ca2+ pathway (1). To investigate whether PDGF-mediated activation of Akt/mTOR pathway is caused by a rise in [Ca2+]cyt due to Ca2+ influx in normal human PASMC, we examined if PDGF-mediated Akt phosphorylation is dependent on extracellular and intracellular Ca2+. After chelation of extracellular Ca2+ by incubation of cells in EGTA containing media, PDGF-induced phosphorylation of Akt was still observed (Fig. 5A). Depletion of Ca2+ in the SR by pretreatment of the cells with CPA in addition to EGTA also did not prevent PDGF-induced phosphorylation of Akt (Fig. 5A). Moreover, complete depletion of intracellular Ca2+ by treatment with FCCP and ionomycin concomitant with CPA and EGTA was unable to prevent the phosphorylation of Akt (Fig. 5A). These results show that phosphorylation of Akt induced by PDGF is independent of both extracellular and intracellular Ca2+. To determine whether extracellular and intracellular Ca2+ was important for PDGF-induced activation of other targets, we also examined the effect of chelation of Ca2+ in media on ERK phosphorylation. There was no effect on PDGF-induced ERK phosphorylation (Fig. 5B). These data imply that PDGF activates the Akt/mTOR signaling pathway by a Ca2+-independent mechanism.
Fig. 5.
Phosphorylation of Akt induced by PDGF is independent of extracellular and intracellular Ca2+. PASMC were treated for 0.25 h with PDGF (10 ng/ml) and/or Veh, EGTA (2 mM), CPA (10 μM), carbonyl cyanide-p-trifluoromethoxyphenylhydrazone (FCCP; 5 μM), or ionomycin (10 μM) to chelate Ca2+. A: representative Western blots showing the expression level of p- and t-Akt. Blots were representative of 3 different sets of experiments. B: representative Western blots showing the expression level of p- and t-ERK1/2. Blots were representative of 3 different sets of experiments.
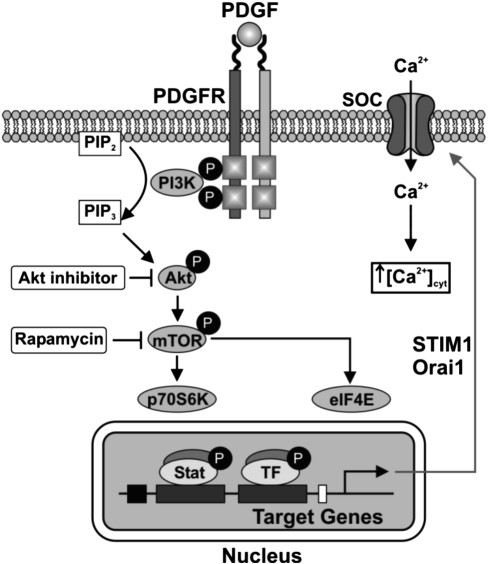
The data presented here define a novel pathway for the regulation of Ca2+ signaling in PASMC. The proposed pathway is shown in Fig. 6. PDGF binds to its receptor and activates the Akt/mTOR signaling pathway, leading to increased STIM1/Orai1 expression, enhanced SOCE, and increased cell proliferation in PASMC. These data provide a target for the development of new therapeutic options for the treatment of pulmonary arterial hypertension.
Fig. 6.
Schematic of proposed pathway. Binding of PDGF to its receptor (PDGFR) activates Akt/mTOR signaling, leading to increased expression of STIM1/Orai1, increased SOCE, and increased cell proliferation. SOC, store-operated Ca2+ channels; PIP2, phosphatidylinositol 4,5-bisphosphate; PIP3, phosphatidylinositol (3,4,5)-trisphosphate; TF, transcription factor.
DISCUSSION
PDGF is a well-known mitogen involved in pulmonary vascular remodeling in pulmonary arterial hypertension. Increased expression of PDGF and its receptors (PDGFR) in pulmonary vasculature are shown in previous studies (14, 27, 29). After PDGF binds to PDGFR, autophosphorylation of PDGFR occurs. It increases kinase activity and causes proliferation and migration of PASMC and pulmonary vascular remodeling. Recently, the inhibition of PDGFR activation by imatinib mesylate was shown to be effective in the reversal of pulmonary vascular remodeling (11, 12).
In PASMC, phosphorylation of Akt is induced by PDGF. Since there are three layers of different cell types in pulmonary vasculature, all of which are implicated in vascular remodeling, the effects of PDGF on Akt were studied in all of these cell types. Interestingly, PDGF activated Akt in PASMC and PAF, but not in PAEC (Figs. 1 and 2). Since the Akt/mTOR pathway has been well documented to activate/phosphorylate endothelial nitric oxide synthase and upregulate nitric oxide synthesis in PAEC (13, 22), the inability of PDGF to activate the Akt/mTOR pathway in PAEC may further indicate that PDGF is a potent stimulus for the development of vascular remodeling and fibrotic formation in CTEPH patients. The specific effect of PDGF on Akt/mTOR phosphorylation in PASMC and PAF is likely to contribute to the enhanced proliferative nature of these cell types in pulmonary vascular diseases.
Our laboratory has previously reported that PDGF causes phosphorylation of Akt in cells isolated from endarterectomized tissues of patients with CTEPH and augments SOCE (24). In normal PASMC, we observed that PDGF mediated an increase in p-Akt, augmentation of SOCE, and increased cell proliferation (Figs. 1, 3, and 4). Inhibition of the Akt/mTOR pathway by rapamycin or the Akt inhibitor blocked PDGF-enhanced SOCE and proliferation (Fig. 3 and 4). Indeed, Akt inhibitor is more potent than rapamycin in inhibiting both SOCE and cell proliferation.
Our data demonstrate that phosphorylation of Akt/mTOR can regulate expression of STIM and Orai, which have been shown to be molecular correlates for SOCE in several cell types, including PASMC (20, 35). Treatment of PASMC with PDGF increased expression of STIM1 and Orai1, which was inhibited by treatment with either rapamycin or the Akt inhibitor, demonstrating that PDGF-induced activation of Akt/mTOR leads to increased expression of STIM1 and Orai1 (Fig. 4A). We show our proposed pathway in Fig. 6. Upon binding of PDGF to its receptors, the Akt/mTOR pathway is activated, which leads to increased expression of STIM1/Orai1, increased SOCE, and increased cell proliferation (Fig. 6). STIM1 acts as sensor for calcium in the SR/ER, and Orai forms a channel in the cell membrane, which, on interaction with STIM1, opens to allow Ca2+ entry into the cell (SOCE). The mechanism involving STIM and Orai is still somewhat controversial, with some reports suggesting that there is no correlation with TRPC channels (7, 28). Recent reports do suggest that STIM1 may function with TRPC channels in SOCE; however, it remains unknown how STIM, Orai, and TRPC channels interact to regulate SOCE (40).
The data presented here support a potential for the use of Akt inhibitors in the treatment of a variety of forms of pulmonary hypertension. Akt is a member of the serine/threonine-specific kinase family known for potentiating cell survival via inhibition of apoptotic pathways. The phosphatidylinositol 3-kinase-Akt pathway is fundamental in the communication of mitogenic signals to mTOR. Protein synthesis is decreased when mTOR is inhibited by inactivation of p70S6K or activation of 4EBP1 (6, 15, 34). Unfortunately, there is no Akt inhibitor currently available for use in the clinic; however, there are several candidate drugs that are now in clinical trials as a result of their therapeutic effects in cancers (26).
Interestingly, although PDGF regulates SOCE via phosphorylation of Akt, the PDGF-induced phosphorylation of Akt is not dependent on either the intracellular or extracellular [Ca2+] (Fig. 5A). This is consistent with adenosine-activated phosphorylation of Akt, which also is not influenced by removal of extracellular and intracellular calcium (10). This effect is most likely a PDGF-specific effect, as the phosphorylation of Akt is prevented by chelation of calcium in different cell types (37, 38). Furthermore, as shown in Fig. 5B, PDGF-activated phosphorylation of ERK was also not affected by chelation of calcium in PASMC.
In conclusion, we highlight the importance of Akt phosphorylation and signaling in the regulation of PDGF-mediated enhancement of PASMC proliferation and calcium handling. The data importantly highlight the potential for Akt inhibitors in the treatment of pulmonary hypertension by their ability to reduce SOCE and decrease proliferation rates of pulmonary cells responsible for pulmonary vascular remodeling. Although the clinical characterizations of CTEPH and IPAH have both been well documented, the cellular and molecular mechanisms are complex and still remain to be fully elucidated. Further studies into the role of Akt signaling are important, and the Akt/mTOR pathway may be a potential target in the therapeutic intervention for patients with several forms of pulmonary hypertension.
GRANTS
This work was supported, in part, by grants from the National Heart, Lung, and Blood Institute of the National Institutes of Health (HL-066012, HL-098053, HL-098050). A. Ogawa was supported by Japan Heart Foundation/Bayer Yakuhin Research Grant Abroad, and A. L. Firth was supported by a Postdoctoral Training Fellowship from the California Institute of Regenerative Medicine.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: A.O., A.L.F., and J.X.-J.Y. conception and design of research; A.O. and M.V.M. performed experiments; A.O. and A.L.F. analyzed data; A.O., A.L.F., and J.X.-J.Y. interpreted results of experiments; A.O. and K.A.S. prepared figures; A.O. drafted manuscript; A.O., A.L.F., and K.A.S. edited and revised manuscript; A.O., A.L.F., K.A.S., and J.X.-J.Y. approved final version of manuscript.
REFERENCES
- 1. Balciunaite E, Jones S, Toker A, Kazlauskas A. PDGF initiates two distinct phases of protein kinase C activity that make unequal contributions to the G0 to S transition. Curr Biol 10: 261–267, 2000 [DOI] [PubMed] [Google Scholar]
- 2. Barst RJ. PDGF signaling in pulmonary arterial hypertension. J Clin Invest 115: 2691–2694, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bisaillon JM, Motiani RK, Gonzalez-Cobos JC, Potier M, Halligan KE, Alzawahra WF, Barroso M, Singer HA, Jourd'heuil D, Trebak M. Essential role for STIM1/Orai1-mediated calcium influx in PDGF-induced smooth muscle migration. Am J Physiol Cell Physiol 298: C993–C1005, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bonnet S, Paulin R, Sutendra G, Dromparis P, Roy M, Watson KO, Nagendran J, Haromy A, Dyck JRB, Michelakis ED. Dehydroepiandrosterone reverses systemic vascular remodeling through the inhibition of the Akt/GSK3-β/NFAT axis. Circulation 120: 1231–1240, 2009 [DOI] [PubMed] [Google Scholar]
- 5. Cahalan MD. STIMulating store-operated Ca(2+) entry. Nat Cell Biol 11: 669–677, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chiang GG, Abraham RT. Phosphorylation of mammalian target of rapamycin (mTOR) at Ser-2448 is mediated by p70S6 kinase. J Biol Chem 280: 25485–25490, 2005 [DOI] [PubMed] [Google Scholar]
- 7. DeHaven WI, Jones BF, Petranka JG, Smyth JT, Tomita T, Bird GS, Putney JW., Jr TRPC channels function independently of STIM1 and Orai1. J Physiol 587: 2275–2298, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Firth AL, Remillard CV, Platoshyn O, Fantozzi I, Ko EA, Yuan JX. Functional ion channels in human pulmonary artery smooth muscle cells: voltage-dependent cation channels. Pulm Circ 1: 48–71, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Frischauf I, Schindl R, Derler I, Bergsmann J, Fahrner M, Romanin C. The STIM/Orai coupling machinery. Channels (Austin) 2: 261–268, 2008 [DOI] [PubMed] [Google Scholar]
- 10. Germack R, Dickenson JM. Activation of protein kinase B by the A(1)-adenosine receptor in DDT(1)MF-2 cells. Br J Pharmacol 130: 867–874, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ghofrani HA, Morrell NW, Hoeper MM, Olschewski H, Peacock AJ, Barst RJ, Shapiro S, Golpon H, Toshner M, Grimminger F, Pascoe S. Imatinib in pulmonary arterial hypertension patients with inadequate response to established therapy. Am J Respir Crit Care Med 182: 1171–1177, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ghofrani HA, Seeger W, Grimminger F. Imatinib for the treatment of pulmonary arterial hypertension. N Engl J Med 353: 1412–1413, 2005 [DOI] [PubMed] [Google Scholar]
- 13. Hisamoto K, Ohmichi M, Kurachi H, Hayakawa J, Kanda Y, Nishio Y, Adachi K, Tasaka K, Miyoshi E, Fujiwara N, Taniguchi N, Murata Y. Estrogen induces the Akt-dependent activation of endothelial nitric-oxide synthase in vascular endothelial cells. J Biol Chem 276: 3459–3467, 2001 [DOI] [PubMed] [Google Scholar]
- 14. Humbert M, Monti G, Fartoukh M, Magnan A, Brenot F, Rain B, Capron F, Galanaud P, Duroux P, Simonneau G, Emilie D. Platelet-derived growth factor expression in primary pulmonary hypertension: comparison of HIV seropositive and HIV seronegative patients. Eur Respir J 11: 554–559, 1998 [PubMed] [Google Scholar]
- 15. Inoki K, Ouyang H, Li Y, Guan KL. Signaling by target of rapamycin proteins in cell growth control. Microbiol Mol Biol Rev 69: 79–100, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jardin I, Lopez JJ, Salido GM, Rosado JA. Orai1 mediates the interaction between STIM1 and hTRPC1 and regulates the mode of activation of hTRPC1-forming Ca2+ channels. J Biol Chem 283: 25296–25304, 2008 [DOI] [PubMed] [Google Scholar]
- 17. Jones R, Capen D, Jacobson M, Munn L. PDGF and microvessel wall remodeling in adult rat lung: imaging PDGF-AA and PDGF-R alpha molecules in progenitor smooth muscle cells developing in experimental pulmonary hypertension. Cell Tissue Res 326: 759–769, 2006 [DOI] [PubMed] [Google Scholar]
- 18. Krymskaya VP, Snow J, Cesarone G, Khavin I, Goncharov DA, Lim PN, Veasey SC, Ihida-Stansbury K, Jones PL, Goncharova EA. mTOR is required for pulmonary arterial vascular smooth muscle cell proliferation under chronic hypoxia. FASEB J 25: 1922–1933, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Liao Y, Erxleben C, Abramowitz J, Flockerzi V, Zhu MX, Armstrong DL, Birnbaumer L. Functional interactions among Orai1, TRPCs, and STIM1 suggest a STIM-regulated heteromeric Orai/TRPC model for SOCE/Icrac channels. Proc Natl Acad Sci USA 105: 2895–2900, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lioudyno MI, Kozak JA, Penna A, Safrina O, Zhang SL, Sen D, Roos J, Stauderman KA, Cahalan MD. Orai1 and STIM1 move to the immunological synapse and are up-regulated during T cell activation. Proc Natl Acad Sci U S A 105: 2011–2016, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Luik RM, Wang B, Prakriya M, Wu MM, Lewis RS. Oligomerization of STIM1 couples ER calcium depletion to CRAC channel activation. Nature 454: 538–542, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Morales-Ruiz M, Lee MJ, Zollner S, Gratton JP, Scotland R, Shiojima I, Walsh K, Hla T, Sessa WC. Sphingosine 1-phosphate activates Akt, nitric oxide production, and chemotaxis through a Gi protein/phosphoinositide 3-kinase pathway in endothelial cells. J Biol Chem 276: 19672–19677, 2001 [DOI] [PubMed] [Google Scholar]
- 23. Moses JW, Leon MB, Popma JJ, Fitzgerald PJ, Holmes DR, O'Shaughnessy C, Caputo RP, Kereiakes DJ, Williams DO, Teirstein PS, Jaeger JL, Kuntz RE. Sirolimus-eluting stents versus standard stents in patients with stenosis in a native coronary artery. N Engl J Med 349: 1315–1323, 2003 [DOI] [PubMed] [Google Scholar]
- 24. Ogawa A, Firth AL, Yao W, Madani MM, Kerr KM, Auger WR, Jamieson SW, Thistlethwaite PA, Yuan JX. Inhibition of mTOR attenuates store-operated Ca2+ entry in cells from endarterectomized tissues of patients with chronic thromboembolic pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 297: L666–L676, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ogawa A, Firth AL, Yao W, Rubin LJ, Yuan JX. Prednisolone inhibits PDGF-induced nuclear translocation of NF-kappaB in human pulmonary artery smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 295: L648–L657, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pal SK, Reckamp K, Yu H, Figlin RA. Akt inhibitors in clinical development for the treatment of cancer. Expert Opin Investig Drugs 19: 1355–1366, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Perros F, Montani D, Dorfmuller P, Durand-Gasselin I, Tcherakian C, Le Pavec J, Mazmanian M, Fadel E, Mussot S, Mercier O, Herve P, Emilie D, Eddahibi S, Simonneau G, Souza R, Humbert M. Platelet-derived growth factor expression and function in idiopathic pulmonary arterial hypertension. Am J Respir Crit Care Med 178: 81–88, 2008 [DOI] [PubMed] [Google Scholar]
- 28. Potier M, Gonzalez JC, Motiani RK, Abdullaev IF, Bisaillon JM, Singer HA, Trebak M. Evidence for STIM1- and Orai1-dependent store-operated calcium influx through ICRAC in vascular smooth muscle cells: role in proliferation and migration. FASEB J 23: 2425–2437, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Schermuly RT, Dony E, Ghofrani HA, Pullamsetti S, Savai R, Roth M, Sydykov A, Lai YJ, Weissmann N, Seeger W, Grimminger F. Reversal of experimental pulmonary hypertension by PDGF inhibition. J Clin Invest 115: 2811–2821, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Soboloff J, Spassova MA, Tang XD, Hewavitharana T, Xu W, Gill DL. Orai1 and STIM reconstitute store-operated calcium channel function. J Biol Chem 281: 20661–20665, 2006 [DOI] [PubMed] [Google Scholar]
- 31. Song MY, Makino A, Yuan JX. STIM2 contributes to enhanced store-operated Ca entry in pulmonary artery smooth muscle cells from patients with idiopathic pulmonary arterial hypertension. Pulm Circ 1: 84–94, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sparks CA, Guertin DA. Targeting mTOR: prospects for mTOR complex 2 inhibitors in cancer therapy. Oncogene 29: 3733–3744, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Stenmark KR, Rabinovitch M. Emerging therapies for the treatment of pulmonary hypertension. Pediatr Crit Care Med 11, Suppl 2: S85–S90, 2010 [DOI] [PubMed] [Google Scholar]
- 34. Sun SY, Rosenberg LM, Wang X, Zhou Z, Yue P, Fu H, Khuri FR. Activation of Akt and eIF4E survival pathways by rapamycin-mediated mammalian target of rapamycin inhibition. Cancer Res 65: 7052–7058, 2005 [DOI] [PubMed] [Google Scholar]
- 35. Wang Y, Deng X, Mancarella S, Hendron E, Eguchi S, Soboloff J, Tang XD, Gill DL. The calcium store sensor, STIM1, reciprocally controls Orai and CaV1.2 channels. Science 330: 105–109, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wessler JD, Steingart RM, Schwartz GK, Harvey BG, Schaffer W. Dramatic improvement in pulmonary hypertension with rapamycin. Chest 138: 991–993, 2010 [DOI] [PubMed] [Google Scholar]
- 37. Whitehead JP, Molero JC, Clark S, Martin S, Meneilly G, James DE. The role of Ca2+ in insulin-stimulated glucose transport in 3T3–L1 cells. J Biol Chem 276: 27816–27824, 2001 [DOI] [PubMed] [Google Scholar]
- 38. Worrall DS, Olefsky JM. The effects of intracellular calcium depletion on insulin signaling in 3T3–L1 adipocytes. Mol Endocrinol 16: 378–389, 2002 [DOI] [PubMed] [Google Scholar]
- 39. Yu Y, Sweeney M, Zhang S, Platoshyn O, Landsberg J, Rothman A, Yuan JX. PDGF stimulates pulmonary vascular smooth muscle cell proliferation by upregulating TRPC6 expression. Am J Physiol Cell Physiol 284: C316–C330, 2003 [DOI] [PubMed] [Google Scholar]
- 40. Zhang S, Dong H, Rubin LJ, Yuan JX. Upregulation of Na+/Ca2+ exchanger contributes to the enhanced Ca2+ entry in pulmonary artery smooth muscle cells from patients with idiopathic pulmonary arterial hypertension. Am J Physiol Cell Physiol 292: C2297–C2305, 2007 [DOI] [PubMed] [Google Scholar]