Abstract
Human Na+-d-glucose cotransporter (hSGLT) inhibitors constitute the newest class of diabetes drugs, blocking up to 50% of renal glucose reabsorption in vivo. These drugs have potential for widespread use in the diabetes epidemic, but how they work at a molecular level is poorly understood. Here, we use electrophysiological methods to assess how they block Na+-d-glucose cotransporter SGLT1 and SGLT2 expressed in human embryonic kidney 293T (HEK-293T) cells and compared them to the classic SGLT inhibitor phlorizin. Dapagliflozin [(1S)-1,5,5-anhydro-1-C-{4-chloro-3-[(4-ethoxyphenyl)methyl]phenyl}-d-glucitol], two structural analogs, and the aglycones of phlorizin and dapagliflozin were investigated in detail. Dapagliflozin and fluoro-dapagliflozin [(1S)-1,5-anhydro-1-C-{4-chloro-3-[(4-ethoxyphenyl)methyl]phenyl}-4-F-4-deoxy-d-glucitol] blocked glucose transport and glucose-coupled currents with ≈100-fold specificity for hSGLT2 (Ki = 6 nM) over hSGLT1 (Ki = 400 nM). As galactose is a poor substrate for SGLT2, it was surprising that galacto-dapagliflozin [(1S)-1,5-anhydro-1-C-{4-chloro-3-[(4-ethoxyphenyl)methyl]phenyl}-d-galactitol] was a selective inhibitor of hSGLT2, but was less potent than dapagliflozin for both transporters (hSGLT2 Ki = 25 nM, hSGLT1 Ki = 25,000 nM). Phlorizin and galacto-dapagliflozin rapidly dissociated from SGLT2 [half-time off rate (t1/2,Off) ≈ 20–30 s], while dapagliflozin and fluoro-dapagliflozin dissociated from hSGLT2 at a rate 10-fold slower (t1/2,Off ≥ 180 s). Phlorizin was unable to exchange with dapagliflozin bound to hSGLT2. In contrast, dapagliflozin, fluoro-dapagliflozin, and galacto-dapagliflozin dissociated quickly from hSGLT1 (t1/2,Off = 1–2 s), and phlorizin readily exchanged with dapagliflozin bound to hSGLT1. The aglycones of phlorizin and dapagliflozin were poor inhibitors of both hSGLT2 and hSGLT1 with Ki values > 100 μM. These results show that inhibitor binding to SGLTs is composed of two synergistic forces: sugar binding to the glucose site, which is not rigid, and so different sugars will change the orientation of the aglycone in the access vestibule; and the binding of the aglycone affects the binding affinity of the entire inhibitor. Therefore, the pharmacophore must include variations in both the structure of the sugar and the aglycone.
Keywords: renal glucose reabsorption, diabetes mellitus, sodium-glucose cotransporter inhibitors
the pharmaceutical industry has targeted human renal Na+-d-glucose cotransporters (hSGLTs) to control blood glucose concentrations in patients with type 2 diabetes. A number of specific hSGLT inhibitors are in phase III clinical trials (4). Companies seek inhibitors with high specificity for hSGLT2 over hSGLT1 for three major reasons: first, hSGLT2 is believed to be responsible for the majority (>80%) of glucose reabsorption in the kidney under normal and hyperglycemic conditions; second, mutations in hSGLT2 cause familial renal glycosuria, a benign condition characterized by increased urinary glucose excretion (36, 37); and third, hSGLT1 inhibitors may cause diarrhea, mimicking the phenotype of individuals deficient in functional hSGLT1 protein (40).
Drug development has focused on modifying the chemical structure of the natural Na+-d-glucose cotransporter (SGLT) inhibitor phlorizin {1-[2-(β-d-glucopyranosyloxy)-4,6-dihydroxyphenyl]-3-(4-hydroxyphenyl)-1-propanone} to identify compounds with high potency and selectivity for hSGLT2 over hSGLT1. Early candidate drugs were O-glucosides like phlorizin, e.g., remogliflozin (10) and sergliflozin (15, 16, 21), but development was discontinued because they were rapidly hydrolyzed by glucosidases in vivo (17). This problem was overcome by removing the glycosidic linkage, e.g., C-aryl glycosides such as dapagliflozin (43). A number of studies have reported on structure-activity relationships in the development of C-aryl glycoside inhibitors with extensive modifications to both the sugar and chalcone moieties (11, 28, 35, 43).
This approach has led to promising candidate drugs, including dapagliflozin (28) and canagliflozin (30). Dapagliflozin increased urinary glucose excretion in control subjects and improved glucose homeostasis and insulin sensitivity in diabetic rats (12, 22). In diabetic human subjects, they improved glycemic control by lowering fasting and postprandial plasma glucose, all without major adverse events (2, 9, 23, 44). Despite promising clinical data, little is known about how these drugs work at a molecular level.
In our laboratory's recent study of SGLTs expressed in human embryonic kidney 293T (HEK-293T) cells (14), we showed that differences in inhibitor dissociation rates accounted for differences in phlorizin inhibitory constant (Ki) for hSGLT2 and hSGLT1. Here we have extended these studies to dapagliflozin and several of its structural analogs. Our results provide insights into both dapagliflozin's mechanism of action and its effects on renal glucose reabsorption.
MATERIALS AND METHODS
Cell Culture and Transfection
HEK-293T and COS-1 cells were maintained, passaged, and transfected with hSGLT2 or hSGLT1 cDNA, as described previously (14). HEK-293T is a highly transfectable derivative of the 293 cell line into which the temperature-sensitive gene for SV40 T-antigen was inserted.
Radioactive Tracer Determinations
[14C]α-methyl-d-glucopyranoside (α-MDG) uptakes into COS-1 cells expressing hSGLT2 or hSGLT1 were measured as described (14). Cells were incubated in sodium buffer with 50 μM [14C]α-MDG at 37°C for 1–40 min in the presence or absence of inhibitors: 100 μM phlorizin and 10 nM or 1 μM of each hSGLT2 inhibitor (dapagliflozin, fluoro-dapagliflozin, or galacto-dapagliflozin). Uptakes were expressed as picomoles per minute per microgram total protein, mean ± SE (Fig. 1). [14C]α-MDG uptakes were linear for 15 min for hSGLT2 and 2 min for hSGLT1, and so the effects of inhibitors on initial rates of uptake were determined at 10 min for hSGLT2 and 1 min for hSGLT1.
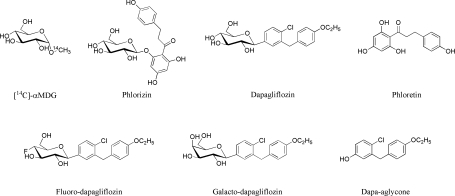
Fig. 1.
Chemical structures of the Na+-d-glucose cotransporter (SGLT) substrate and inhibitors used in this study. α-MDG, [14C]α-methyl-d-glucopyranoside.
Electrophysiological Experiments
Whole cell patch-clamp recordings were performed on HEK-293T cells 2 days posttransfection (14). The extracellular solution contained (in mM) 150 NaCl, 1 CaCl2, 1 MgCl2, 10 HEPES, pH 7.4 (“Na+ buffer”), or 150 choline chloride, 1 CaCl2, 1 MgCl2, pH 7.4 (“choline+” buffer). For experiments at 37°C, mannitol (100 mM) was added to the external solution to reduce noise and increase the stability of the whole cell recordings. The pipette (internal) solution contained (in mM) 145 CsCl, 5 NaCl, 11 EGTA, and 10 HEPES. Membrane potential was held at −60 mV. For hSGLT2, all experiments were performed at 37°C, and for hSGLT1, at 37 or 22°C.
Inhibitor Kinetics
Our general approach to the interaction of inhibitors with the SGLTs followed that used previously by Oulianova et al. (32) to investigate phlorizin binding to SGLTs in rabbit renal brush-border membranes, but here we measure binding to specific human SGLTs using electrophysiological assays. Inhibition of steady-state, glucose-induced hSGLT current was measured as a function of external inhibitor concentration to determine the inhibition constant Ki. As described previously, when the glucose concentration is equal to the sugar half-maximal inhibition constant (K0.5), the concentration of the inhibitor producing 50% inhibition (IC50) is twice the Ki (14, 38). The hSGLT2 d-glucose K0.5 was 5 mM at 37°C (14) and for hSGLT1 it was 2 mM at 37°C and 0.5 mM at 22°C.
The sequence of each experiment was as follows. Current was induced by the K0.5 concentration of d-glucose. The solution was changed to d-glucose plus inhibitor. Once a new steady-state current was achieved, the cell was washed with d-glucose and Na+-free (choline+) buffer for ≥3 min to remove the inhibitor. This protocol was repeated at multiple inhibitor concentrations to estimate the Ki.
Some of the hSGLT1 galacto-dapagliflozin Ki determinations were performed using the two-electrode voltage clamp on Xenopus laevis oocytes expressing hSGLT1 to minimize the quantity of the inhibitor needed to complete the experiments. Oocyte isolation, preparation, injections, and electrophysiological methods were performed as described previously (24, 25).
Inhibitor off rates (kOff) were determined by recording the time course of recovery of the d-glucose-coupled current after removal of an inhibitor [such as phlorizin (Pz)], assuming pseudo-first-order binding and dissociation kinetics (14):
The d-glucose-coupled current recovery followed an exponential time course with time constant 1/kOff, and half-time for recovery was related to the off rate (t1/2,Off): t1/2,Off = ln(2)/kOff. The on rate (kOn) was calculated from the empirically measured Ki values and kOff values (Ki = kOff/kOn). The kOff for hSGLT1 at 37°C was within the half-time of the solution change (5 s), and so the temperature was lowered to 22°C, and the SF-77B Fast-step solution changer (Warner Instruments, Hamden, CT) was utilized to wash out inhibitors (solution change half-time of ≤20 ms).
Synthesis of Inhibitors
Dapagliflozin [(1S)-1,5-anhydro-1-C-{4-chloro-3-[(4-ethoxyphenyl)methyl]phenyl}-d-glucitol] was synthesized by a reported procedure (28). Galacto-dapagliflozin was synthesized starting from dapagliflozin by adequate sugar protection and selective configuration conversion at C4′, and this was followed by fluorination to give fluoro-dapagliflozin. The phenol-aglycone of dapagliflozin [4-chloro-3-(4-ethoxybenzyl)phenol] was obtained by hydrolysis of the corresponding bromo-aglycone, prepared from commercial 4-bromo-2-chlorobenzoic acid (28). The synthesized products were characterized by Electrospray ionization mass spectrometry, 1H-NMR, 19F-NMR, and 13C-NMR, and their anomeric configuration at C1′ and the epimeric configuration at C4′ were confirmed by advanced two-dimensional NMR techniques and X-ray crystallography data. [The syntheses and spectroscopic characterization will be published elsewhere.] Figure 1 shows the structures of the substrate and inhibitors used in this study.
Energy-Minimized Inhibitor Structures
Representative energy-minimized in silico chemical structures of phlorizin, dapagliflozin, fluoro-dapagliflozin, and galacto-dapagliflozin were generated using HyperChem version 6.0 (Hypercube, Gainesville, FL) (see also Ref. 13).
Statistical Analysis
Data fitting and t-tests for significance were performed using either Sigma Plot 10.0 (Systat Software, San Jose, CA) or Excel (Microsoft, Redmond, WA).
Reagents
All reagents were purchased from Fisher Scientific or Sigma and were of the highest purity available. [14C]α-MDG was purchased from Moravek Biochemicals (Brea, CA).
RESULTS
Biochemical
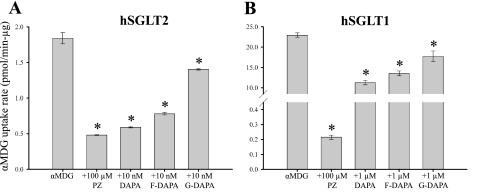
To provide a direct comparison with previous work on SGLT inhibitors, we first measured the effects on quasi-steady-state (40 min) [14C]α-MDG uptakes into COS-1 cells expressing hSGLT1 and hSGLT2. Dapagliflozin at 10 nM blocked 90 ± 1% of phlorizin-sensitive sugar transport by hSGLT2, whereas fluoro-dapagliflozin and galacto-dapagliflozin blocked 80 ± 1 and 30 ± 3%, respectively (Fig. 2A). In parallel experiments with hSGLT1, we found that 1 μM dapagliflozin blocked 50 ± 3% of the phlorizin-sensitive α-MDG uptake, whereas fluoro-dapagliflozin and galacto-dapagliflozin blocked 40 ± 3 and 20 ± 6%, respectively (Fig. 2B). The results with dapagliflozin and phlorizin are comparable with the published data (12). Next we measured the effect of the inhibitors on the initial rates of α-MDG transport, and IC50 values for dapagliflozin and phlorizin were 6 ± 1 and 65 ± 10 nM for hSGLT2 and 400–800 and 400 ± 100 nM for hSGLT1, respectively. For the initial rate measurements, the potency of dapagliflozin for hSGLT2 was ∼100-fold greater than for hSGLT1, as opposed to 1,200-fold difference reported for steady-state uptakes (12, 28).
Fig. 2.
Radioactive tracer flux experiments in human SGLT 2 (hSGLT2) and hSGLT1. α-MDG (50 μM) uptake was measured in hSGLT2- (A) and hSGLT1-transfected (B) COS-1 cells at 37°C. Uptake is expressed as picomoles per minute per microgram of total protein. Values are means ± SE; N = 4 per condition. As shown previously (14), the SGLT inhibitor phlorizin (Pz, 100 μM) abolished uptake to background levels (“+Pz”, 0.2–0.5 pmol·min−1·μg−1). A: dapagliflozin (DAPA) derivatives (10 nM) all significantly (*P < 0.05) reduced the hSGLT2-specific (i.e., PZ-sensitive) accumulation of α-MDG: DAPA, 90%; fluoro-DAPA (F-DAPA), 80%; and galacto-DAPA (G-DAPA), 30%. B: for hSGLT1, 1 μM concentrations of each inhibitor reduced α-MDG uptake (*P < 0.05), by 50, 40, and 20% for DAPA, F-DAPA, and G-DAPA.
Role of sugar moiety.
To assess the contribution of the sugar moiety to inhibitor potency, we measured the effect of phloretin (the aglycone of phlorizin) and dapagliflozin-aglycone [4-chloro-3-(4-ethoxybenzyl)phenol] on the 40-min 50 μM α-MDG uptakes: 250 μM phloretin inhibited hSGLT1 and hSGLT2 transport by 70 ± 5 and 90 ± 8%, respectively, which is consistent with reported IC50 values [140 and 25 μM (33)], and 300 μM dapagliflozin-aglycone inhibited hSGLT1 and hSGLT2 transport by 25 and 60%, respectively (Table 1). Additional experiments showed dapagliflozina-aglycone IC50 values of ≈1,000 μM for hSGLT1 and 200 μM for hSGLT2 (Lu C, Hummel CS, and Wright EM, unpublished observations). These results demonstrate that removing glucose from the phlorizin and dapagliflozin molecules reduces their inhibitory potency by more than three orders of magnitude against both hSGLT isoforms.
Table 1.
Inhibition of [14C]α-methyl-d-glucopyranoside uptake by aglycones
| Compound | Concentration, μM | hSGLT1, %inhibition | hSGLT2, %inhibition |
|---|---|---|---|
| Phloretin | 250 | 70 ± 5 | 90 ± 8 |
| Dapagliflozin aglycone | 300 | 25 ± 7 | 60 ± 3 |
Values are means ± SE for n ≥ 3 wells. hSGLT1 and hSGLT2, human Na+-d-glucose cotransporters 1 and 2, respectively. The “% inhibition” is determined by the ratio of inhibition of the test compound (phloretin, dapagliflozin-aglycone) to that of 100 μM phlorizin (100% inhibition). The phloretin inhibition values were consistent with IC50 values reported in the literature [140 μM for hSGLT1 and 25 μM for hSGLT2 (33)].
Biophysical
Ki values.
To investigate further the kinetics and specificity of drug interactions with hSGLT2 and hSGLT1, we turned to electrophysiological methods and examined the inhibition of the sugar-coupled Na+ currents, as previously developed for phlorizin (14). We first determined the Ki values from blockade of steady-state currents generated by glucose at its half-maximal concentration (K0.5). For hSGLT2, dapagliflozin and fluoro-dapagliflozin had the same Ki values, ≈6 nM, whereas for galacto-dapagliflozin it was 25 nM. These values are comparable to the Ki of 11 nM for phlorizin reported previously (Table 2). For hSGLT1, the dapagliflozin Ki was 360 ± 20 nM compared with 140 nM for phlorizin. These electrophysiological results confirm the higher selectivity of dapagliflozin as an inhibitor for hSGLT2, Ki 6 vs. 360 nM, and this 60-fold selectivity is consistent with our biochemical studies (see above). Again, this selectivity is 10-fold lower than the 1,000-fold ratio reported previously for the inhibition of the steady-state (2 h) accumulation of α-MDG in transfected cells (12, 28).
Table 2.
Binding properties of phlorizin and dapagliflozin derivatives with hSGLT2 (37°C)
| Compound | n | Ki, nM | t1/2,Off, s | kOff, s−1 | kOn, M/s (calculated) |
|---|---|---|---|---|---|
| Phlorizin | 11 ± 0.9 | 24 ± 3 | 0.030 ± 0.003 | 2.7 × 106 | |
| Dapagliflozin | 5 | 6.0 ± 0.6 | >>300 | <<0.002 | <<3.3 × 106 |
| Fluoro-dapagliflozin | 10 | 5.3 ± 0.8 | 170 ± 20 | 0.0048 ± 0.0006 | 0.91 × 106 |
| Galacto-dapagliflozin | 3 | 24 ± 6 | 21 ± 3 | 0.030 ± 0.007 | 1.3 × 106 |
Values are means ± SE; n, no. of cells. Ki, inhibitory constant; t1/2,Off, half-time off rate; kOff, inhibitor off rate; kOn, inhibitor on rate. Phlorizin parameters are from Ref. 14.
We also measured hSGLT1 Ki values at 22°C (Table 3). For dapagliflozin and phlorizin, the Ki values were quite similar at 22°C to those at 37°C, 390 and 80 nM vs. 360 and 140 nM (Table 3), indicating a relatively low temperature dependence of inhibitor binding. This is consistent with the minimal temperature dependence of the hSGLT1 apparent affinities (K0.5) for d-glucose transport over the same 15°C temperature range, 0.5 (22°C) to 1.8 mM (37°C) (14). On the other hand, the temperature dependence of the maximal rate of transport is high, ≈25 kcal/mol (14).
Table 3.
Binding properties of phlorizin and dapagliflozin derivatives with hSGLT1 (22°C)
| Compound | n | Ki, nM | t1/2,Off, s | kOff, s−1 | kOn, M/s (calculated) |
|---|---|---|---|---|---|
| Phlorizin (37°C) | >3 | 140 ± 15 | 4 ± 0.5 | 0.19 ± 0.02 | 1.4 × 106 |
| Phlorizin | 4 | 80 ± 10 | 9.4 ± 0.6 | 0.07 ± 0.01 | 0.88 × 106 |
| Dapagliflozin | 6 | 390 ± 25 | 1.8 ± 0.1 | 0.40 ± 0.05 | 1.0 × 106 |
| Fluoro-dapagliflozin | 7 | 330 ± 30 | 1.2 ± 0.1 | 0.61 ± 0.03 | 1.8 × 106 |
| Galacto-dapagliflozin | 4 | 25,000 ± 6,000 | 0.68 ± 0.08 | 1.1 ± 0.1 | 0.01 × 106 |
Values are means ± SE.; n, no. of cells (galacto-dapagliflozin: n = 3, determined in X. laevis oocytes expressing hSGLT1). Phlorizin parameters at 37°C are from Ref. 14.
The affinity of hSGLT1 for fluoro-dapagliflozin (Ki = 330 nM) at 22°C was similar to the parent compound, but it was 70-fold lower for galacto-dapagliflozin (Ki = 25,000 nM) (Table 3). Inhibitor constants were higher for hSGLT1 relative to hSGLT2 (Tables 2 and 3): 7.2-fold for phlorizin, and 62- to 65-fold for dapagliflozin and fluoro-dapagliflozin, (390 and 330 vs. 6 nM), and 1,000-fold for galacto-dapagliflozin (25,000 vs. 24 nM).
Rates of inhibitor association and dissociation.
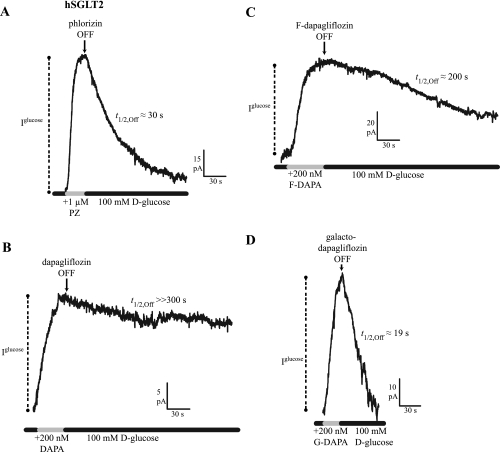
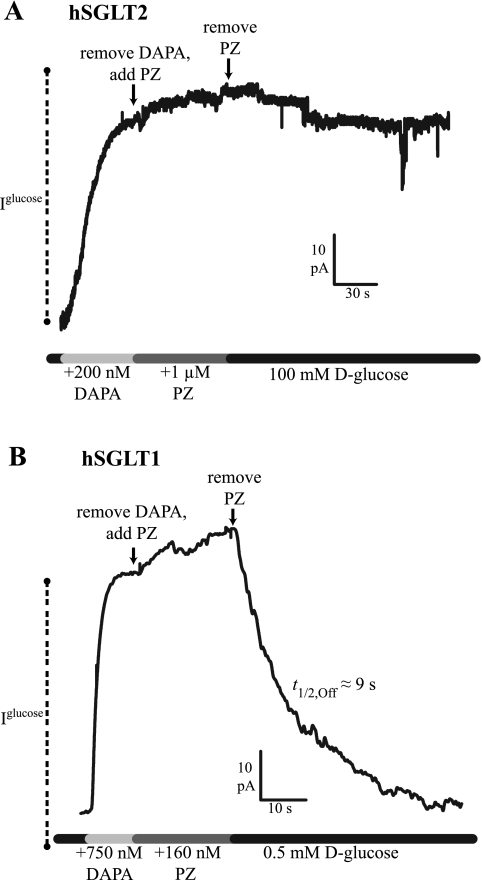
To interpret differences in Ki values among the different inhibitors and between the two hSGLT isoforms, we investigated the rates of association and dissociation. The dissociation rates of the inhibitors were determined by measuring the time course of the recovery of Na+/glucose current following rapid washout of inhibitor, and the association rates were estimated from the Ki and the kOff (see below). In the hSGLT2 experiment shown in Fig. 3A, the half-time of phlorizin (1,000 nM) inhibition of the 100 mM glucose Na+/sugar current was 9 s, and recovery from inhibition (t1/2,Off) was 30 s (see also Ref. 14). The on time course (t1/2,On) of dapagliflozin (200 nM) was 25 s, and t1/2,Off was in excess of 300 s (Fig. 3B). For fluoro-dapagliflozin (200 nM), inhibition occurred with a t1/2,On of 18 s, and t1/2,Off was 200 s (Fig. 3C). Complete reversal of dapagliflozin inhibition required a washout in Na+-free (choline+) buffer of ≈3 min (data not shown). Galacto-dapagliflozin (1 μM) inhibition occurred with t1/2,On = 10 s, and it dissociated with t1/2,Off = 19 s (Fig. 3D). As expected, the time course of the inhibitor dissociation was independent of the glucose concentration used to generate the Na+/sugar currents.
Fig. 3.
Time course of inhibitor dissociation in hSGLT2 (−60 mV, 37°C). Continuous records of sugar current (100 mM d-glucose) show reduction in steady-state transport [represented by glucose-coupled current (Iglucose)] upon application of inhibitor [half-time on rate (t1/2,On), not shown]. Upon removal of inhibitor, the time course of d-glucose current recovery was monitored [half-time off rate (t1/2,Off)]. A: PZ inhibited current with a t1/2,On = 9 s, and Iglucose recovery occurred with t1/2,Off = 30 s. B: DAPA had a t1/2,On = 25 s, but it showed no recovery of current, even after 5 min, and thus t1/2,Off >> 300 s. C: F-DAPA's t1/2,On was 18 s, and it showed a slow recovery of Iglucose, t1/2,Off = 200 s. D: G-DAPA association occurred with t1/2,On = 10 s, and its dissociation was rapid, t1/2,Off = 19 s.
The kOn for inhibitor binding was calculated from kOff and Ki values using the relationship kOn = kOff/Ki (Table 2). The koff for phlorizin and galacto-dapagliflozin were not significantly different (0.03 s−1), while the rates for dapagliflozin and fluoro-dapagliflozin were about an order of magnitude slower (<0.005 s−1). This implies that differences in the kOn also factor into the differences in the inhibitor constants (Table 2).
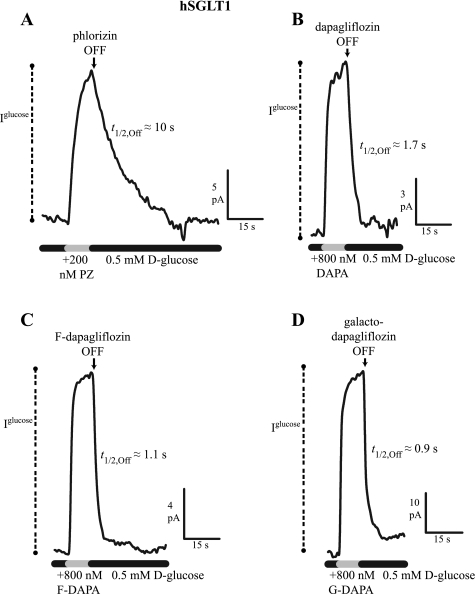
Previous experiments revealed that the lower phlorizin affinity of hSGLT1 relative to hSGLT2 was due to higher dissociation rates, i.e., t1/2,Off = 4 vs. 25 s (14). Initial hSGLT1 experiments at 37°C revealed off time courses faster than the solution change, and thus we lowered the temperature to 22°C and utilized a fast perfusion system (Fig. 4 and Table 3). In hSGLT1, 10 μM phlorizin blocked 0.5 mM d-glucose-induced current, with a t1/2,On = 2 s and t1/2,Off = 10 s (Fig. 4A), whereas the values for 800 nM dapagliflozin, fluoro-dapagliflozin, and galacto-dapagliflozin were t1/2,On = 0.5–0.8 s and t1/2,Off = 0.9–1.7 s. The t1/2,Off, kOff, kOn, and Ki values for each inhibitor are summarized in Table 3. The kOn were comparable for hSGLT1 and hSGL2, ∼1 × 106 M/s, except that for galacto-dapagliflozin that was 0.01 × 106 M/s. The kOff of 2- to 100-fold were faster for hSGLT1 than hSGLT2.
Fig. 4.
Time course of inhibitor dissociation in hSGLT1 (−60 mV, 22°C). Continuous records of sugar current (0.5 mM d-glucose) inhibition, with magnitude = Iglucose, as in Fig. 3. A: PZ inhibited current with t1/2,On = 2 s (not shown) and dissociation t1/2,Off = 12 s. B: DAPA inhibition occurred with t1/2,On = 0.8 s, and it dissociated with t1/2,Off = 1.7 s. Similarly, F-DAPA (C) and G-DAPA (D) showed fast on- and off-time courses: t1/2,On = 0.8 and 0.5 s, t1/2,Off = 1.1 and 0.9 s, respectively.
Phlorizin/dapagliflozin exchange.
We evaluated whether dapagliflozin bound to hSGLT1 or hSGLT2 can be exchanged with phlorizin (Fig. 5). As shown for hSGLT2 in Fig. 5A, the 100 mM d-glucose current was completely blocked by 200 nM dapagliflozin. When dapagliflozin in the buffer was replaced with 1,000 nM phlorizin, the inhibition of Na+/glucose current was maintained, but, on washing out phlorizin, the transporter remained blocked for >5 min. As the phlorizin block of hSGLT2 is normally rapidly reversed (t1/2,Off = 25 s), these data suggest that phlorizin did not exchange with dapagliflozin bound to hSGLT2. In contrast, phlorizin readily exchanged with dapagliflozin bound to hSGLT1 (Fig. 5B). After inhibition of hSGLT1 with 750 nM dapagliflozin, replacement with 160 nM phlorizin maintained the inhibition. With phlorizin washout, the Na+/glucose current returned with a t1/2,Off of 9 s, identical to the time course for phlorizin alone (Table 3).
Fig. 5.
PZ exchange with DAPA bound to SGLTs. Iglucose (100 mM for hSGLT2 in A; 0.5 mM for hSGLT1 in B) was blocked by DAPA. The extracellular (bath) solution was subsequently replaced with PZ: 1 μM in hSGLT2 (A) and 160 nM in hSGLT1 (B). Next, solution was replaced with the original d-glucose buffer. A: for hSGLT2, there was no measurable dissociation time course, suggesting PZ did not exchange with DAPA binding to hSGLT2. B: the hSGLT1 time course of current recovery (t1/2,Off = 9 s) was identical to that observed for PZ alone (Fig. 3A), suggesting that PZ can be exchanged for DAPA.
DISCUSSION
Clinical interest in SGLTs has intensified with the recent development of new oral inhibitors for treatment of type 2 diabetes. These drugs (several of them in late phase III clinical trials) are designed to lower blood glucose concentration by preventing renal glucose reabsorption by specifically targeting hSGLT2 (4). Despite this success, relatively little is known about the mechanism of action of these compounds or the basis of selectivity for hSGLT2 over hSGLT1. We have set out to probe the mechanism of one such inhibitor, dapagliflozin (28), which provides a reference point for comparison with other structurally related hSGLT2 inhibitors currently under development by examining the kinetics of interaction with hSGLT1 and hSGLT2 (14, 25, 45). Specifically, we compared and contrasted dapagliflozin, galacto-dapagliflozin, fluoro-dapagliflozin, and phlorizin (Fig. 6) inhibition kinetics of hSGLT2 and hSGLT1, via determinations of Ki, kOn, and kOff (Figs. 3 and 4, Tables 2 and 3).
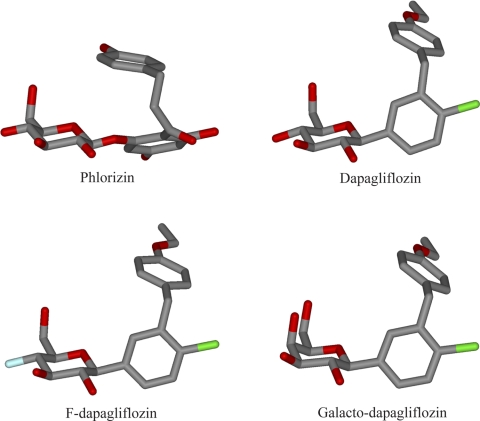
Fig. 6.
Energy-minimized structures of PZ, DAPA, F-DAPA, and G-DAPA. These representative structures show that the aglycone of all varieties of DAPA occupies a similar space and volume as the aglycone of PZ.
We chose to measure inhibition kinetics under initial rate conditions rather than under steady-state conditions used by others (i.e., we measured the kinetics of α-MDG uptake in the absence of intracellular sugar). We have confirmed that dapagliflozin is a selective hSGLT2 inhibitor, and that this selectivity over hSGLT1 is largely due to differences in drug-SGLT dissociation rates: the kOff for hSGLT2 is more than 100-fold slower than that for hSGLT1. Under the conditions in our experiments, the dapagliflozin Ki was 6 nM for hSGLT2 and 360 nM for hSGLT1. By comparison, the phlorizin Ki was 10 nM for hSGLT2 and 140 nM for hSGLT1. Based on 2-h α-MDG uptakes in transfected Chinese hamster ovary cells, IC50 values for dapagliflozin and phlorizin were 1 and 36 nM for hSGLT2 and 1,390 and 330 nM for hSGLT1 (12, 28). The greatest discrepancy between the previous and our present work is the selectivity of dapagliflozin for hSGLT2 over hSGLT1, 1,400-fold, vs. the 60-fold ratio reported here. Such a pronounced difference is likely due to contrasting experimental approaches between the measurement based on initial rates in our biophysical assays compared with the earlier reported steady-state assays (12, 28). In our hands, the dapagliflozin IC50 estimated from initial rates of [14C]α-MDG uptake was 5 nM, whereas, under steady-state conditions, this value was determined to be 1 nM.
Multiple lines of evidence support the conclusion that the inhibitors interact with both hSGLT1 and hSGLT2 at the glucose binding site, as first suggested by Alvarado (1). First, phlorizin binding is competitive with glucose (33). Second, phlorizin and glucose bind to SGLTs only in the presence of Na+ (14, 45). Third, changes in the sugar moiety changes the potency of the inhibitors: the galacto-dapagliflozin Ki was higher than dapagliflozin for both hSGLT2 and hSGLT1 (Tables 2 and 3), and the binding of the aglycones was three orders of magnitude lower than those of the parent drugs (e.g., for hSGLT2, phloretin and phlorizin Ki values were 25 μM and 11 nM; dapagliflozin-aglycone and dapagliflozin Ki values were 200 μM and 5 nM, Tables 1–3). Fourth, modification of the glucose ring produced predictable changes in the inhibitor Ki: the 2-deoxy- and 3-deoxy-sugar derivatives were very poor inhibitors of hSGLT2 (IC50 > 2,000 nM) (35), also in agreement with the observation that these deoxy sugars are poor substrates for hSGLT2 transport (Lu C et al., unpublished observations). In contrast, substitution of -F for -OH at C4 in both glucose and dapagliflozin (fluoro-dapaglifozin, Fig. 6) did not alter the kinetics of transport or inhibition of hSGLT1 or hSGLT2 [Tables 2 and 3 (42); Lu et al., unpublished observations]. Parenthetically, these results also suggest that [18F]fluoro-dapagliflozin (t1/2 of the positron-emitter fluorine-18; t1/2 = 110 min) may also be a useful molecular imaging probe to map and quantify the distribution of SGLT2 in both animal and human subjects using positron emission tomography (3).
The sugar moiety of inhibitors appears to interact with the glucose binding site of hSGLT1 and hSGLT2, but selectivity of inhibitor binding is synergistic and depends on the chemistry of both the sugar moiety and the aglycone, indicating the dual-specific binding elements. This is clearly evident for the studies of galacto-dapagliflozin, where the results were unanticipated when considering selectivity of hexose transport alone: for hSGLT1, both glucose and galactose are transported with the same kinetics (K0.5 and Vmax), whereas galactose is a poor substrate for hSGLT2 transport (K0.5 > 100 mM) (14). In contrast, the Ki for galacto-dapagliflozin compared with dapagliflozin was 25 vs. 6 nM for hSGLT2 and 25,000 vs. 350 nM for hSGLT1.
What accounts for the inhibitor selectivity of hSGLT2 over hSGLT1-phlorizin = 10:1, dapagliflozin = 60:1? Our data offer a kinetic explanation: basically, dissociation rates for inhibitors were orders of magnitude slower for hSGLT2 than for hSGLT1, e.g., the koff for fluoro-dapagliflozion at 37°C was 0.005 s−1 for hSGLT2 vs. >0.6 s−1 for hSGLT1. The reciprocal of the off rate (1/kOff) is the mean occupancy time in the inhibitor-bound conformation. Thus dapagliflozin is bound to hSGLT2 for periods >500 s compared with 30 s for phlorizin. In contrast, dapagliflozin is bound to hSGLT1 for 2.5 s compared with 14 s for phlorizin. The tighter binding of dapagliflozin to hSGLT2 over hSGLT1 is consistent with the inability of phlorizin to exchange with dapagliflozin bound to hSGLT2, unlike the rapid exchange with hSGLT1 (see Fig. 5). The calculated kOn were comparable for hSGLT1 and SGLT2, 1–100 × 104 M/s (Tables 2 and 3), except that for the galacto-dapagliflozin kOn for hSGLT1 is only 1% of that for the other inhibitors.
What does dapagliflozin binding reveal about the inhibitor binding sites in hSGLTs? First, as discussed above, it is probable that the sugar moiety of these inhibitors binds in the glucose binding site. The fact that the inhibitor binding constants are 103- to 106-fold lower that the apparent affinity for glucose indicates that the structural and chemical properties of the aglycone are compatible with a specific binding site, probably in the entry vestibule to the glucose binding site. Alvarado (1) was among the first to postulate the synergistic effect of glucose and the aglycone in phlorizin binding. Second, the Ki for phlorizin is 10-fold lower for hSGLT2 than hSGLT1, indicating a difference between hSGLT1 and hSGLT2 in this location and that the interaction between the chalone and the protein is stronger in hSGLT2. Third, changing the sugar moiety of dapagliflozin from glucose to galactose reduced affinity of both hSGLT1 and hSGLT2, but less for hSGLT2, suggesting that the position of the sugar in the binding site is not rigid, and so the position of the aglycone is shifted. In this case, the change affects binding less in hSGLT2 and may be an indication that the hSGLT2 binding site is more constrained than that of hSGLT1, perhaps explaining the low rate of galacto-dapagliflozin binding to hSGLT1 (Table 3).
At this time, it is difficult to pinpoint structural differences between the inhibitor sites in hSGLT1 and hSGLT2 based on the crystal structure of Vibrio parahaemolyticus SGLT1 (vSGLT1) (8), despite the general validity of hSGLT structural models (45). There is 32% amino acid identity (60% similarity) between vSGLT and hSGLT1, and all of the gating and coordinating residues are conserved between vSGLT1, hSGLT1, and hSGLT2. It is possible to dock the inhibitors to the occluded sugar binding site in the bacterial and human SGLTs, but, given the flexibility of the aglycones (Fig. 6), it is not yet possible to draw meaningful conclusions about the differences in inhibitor binding sites between hSGLT1 and hSGLT2 based on existing evidence. The successful determination of the crystal structures of inhibitors bound to the SGLTs would permit a more accurate interpretation of this differential binding.
Clinical Significance
In control human subjects, oral dapagliflozin inhibited up to 50% of the renal glucose reabsorption by the kidney (19, 22). The maximum glucose excretion, ≈60 g/24 h, occurred with 50-mg oral dapagliflozin, and, over this time, the plasma concentration of the drug rose to 4 μM at 1.5 h and decayed to 0.25 μM at 24 h. Ninety percent of dapagliflozin was found to be bound to serum proteins, and only 1% of the injected dose was excreted in the urine (see also Refs. 20, 31). Most of the oral dose appeared in plasma as an inactive glucuronidated metabolite, dapagliflozin-3-O-glucuronide, and this was excreted in the urine. These data, therefore, suggest that the free (unmodified and unbound) drug concentration in plasma and the glomerular filtrate, in the 24 h following a 250-mg dose, ranges from as high as 400 to as low as 25 nM. This is significantly higher than the dapagliflozin Ki for hSGLT2 (5 nM), and so it would expected that glucose excretion due to hSGLT2 inhibition would be close to the filtered glucose load, if hSGLT2 were responsible for 90% of glucose reabsorption.
What accounts for the fact that the selective hSGLT2 inhibitors only produce a 50% block of renal glucose reabsorption, whereas phlorizin produces complete blockage (5)? One possibility is that hSGLT1 accounts for a larger fraction of glucose reabsorption than previously recognized. Three recent studies in transgenic mice support this possibility: homozygous SGLT2 knockout (SGLT2−/−) mice retained up to 40% of renal d-glucose reabsorptive capacity (18, 27, 41). Given the above discussion of the pharmacokinetic data (for a 250-mg maximal dose), we estimate that the mean free dapagliflozin concentration in the glomerular filtrate is well below the hSGLT1 Ki (100 nM).
Another important question is why only traces of dapagliflozin are found in the urine. Since the major metabolite, the 3-O-glucuronide, is excreted, it is likely that free dapagliflozin in plasma is also passed into the glomerular filtrate. If dapagliflozin inhibits by binding to the luminal SGLTs, once those binding sites are saturated, any additional dapagliflozin in the glomerular filtrate should be passed through the tubule and be excreted. Since this does not happen, and only a trace of dapagliflozin is found in urine, it suggests that there is a mechanism for dapagliflozin absorption somewhere in the renal tubule, possibly by one or both of the SGLTs, as transport of β-d-glucosides by SGLT1 is well documented (6, 26). Another explanation for dapagliflozin's low in vivo potency and low urinary excretion is that it may only block hSGLT2 by gaining access to the apical membrane of tubular cells across the basolateral membrane from plasma. This possibility would depart from known phlorizin binding mechanisms to SGLT1; e.g., phlorizin does not inhibit intestinal absorption from the blood side (29) and does not inhibit SGLT1 from the cytosolic side of the plasma membrane (7, 34). These alternative possibilities are currently being investigated.
Aside from dapagliflozin, there currently is a paucity of published data on other hSGLT2 inhibitors. In only one study has the major functional differences between dapagliflozin and canagliflozin been reported: whereas the potency and specificity of canagliflozin in vitro are very similar to dapagliflozin, hSGLT2 Ki = 2 nM, hSGLT1 Ki = 1,000 nM (30), significantly higher oral doses of canagliflozin than dapagliflozin are required to produce equivalent effects on renal glucose excretion (39). Kinetic specificity for hSGLT2 vs. SGLT1 alone cannot explain this discrepancy at this time, and so other pharmacokinetic factors must be in play.
In summary, the high affinity of dapagliflozin for hSGLT2 is the result of its tight binding, reflected by its surprisingly slow dissociation from the transporter, and this is the biophysical basis for the difference in dapagliflozin affinity between hSGLT1 and hSGLT2. Inhibitor affinity is the result of a synergistic relationship between binding sites for sugar and the aglycone, with alterations in the sugar resulting in surprising differences in selectivity. The sugar moiety has an important role in determining inhibitor specificity and is likely essential for positioning of the aglycone for interaction with residues in the sugar pathway, and the aglycone also influences the inhibitor interaction in ways we have yet to determine.
GRANTS
J. R. Barrio gratefully acknowledges the support of the Elizabeth and Thomas Chair Endowment in Gerontology. This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grants DK019567 and DK0077133 (E. M. Wright) and National Research Service Award DK082153 (C. S. Hummel).
DISCLOSURES
E. M. Wright serves on an SGLT2 Inhibitor Advisory Board for Boehringer-Ingleheim, has been a consultant for BMS/AZ, Roche, Merck, and Novartis on SGLT biology, and has been a speaker on SGLT biology at industry-sponsored symposia and workshops.
AUTHOR CONTRIBUTIONS
Author contributions: C.S.H., J.L., C.G., B.A.H., D.D.L., V.K., J.R.B., and E.M.W. conception and design of research; C.S.H., C.L., J.L., and C.G. performed experiments; C.S.H., C.L., J.L., C.G., D.D.L., V.K., J.R.B., and E.M.W. analyzed data; C.S.H., C.L., C.G., B.A.H., D.D.L., V.K., J.R.B., and E.M.W. interpreted results of experiments; C.S.H. and E.M.W. prepared figures; C.S.H. and E.M.W. drafted manuscript; C.S.H., C.L., J.L., B.A.H., D.D.L., J.R.B., and E.M.W. edited and revised manuscript; C.S.H., C.L., J.L., C.G., B.A.H., D.D.L., V.K., J.R.B., and E.M.W. approved final version of manuscript.
REFERENCES
- 1. Alvarado F. Hypothesis for the interaction of phlorizin and phloretin with membrane carriers for sugars. Biochim Biophys Acta 135: 483–495, 1967 [DOI] [PubMed] [Google Scholar]
- 2. Bailey CJ, Gross JL, Pieters A, Bastien A, List JF. Effect of dapagliflozin in patients with type 2 diabetes who have inadequate glycaemic control with metformin: a randomised, double-blind, placebo-controlled trial. Lancet 375: 2223–2233, 2010 [DOI] [PubMed] [Google Scholar]
- 3. Barrio JR. The molecular biology of disease. In: PET Molecular Imaging and Its Biological Implications, edited by Phelps ME. New York: Springer, 2004, p. 621 [Google Scholar]
- 4. Chao EC, Henry RR. SGLT2 inhibition–a novel strategy for diabetes treatment. Nat Rev Drug Discov 9: 551–559, 2010 [DOI] [PubMed] [Google Scholar]
- 5. Chasis H, Jolliffe N, Smith HW. The action of phlorizin on the excretion of glucose, xylose, sucrose, creatinine, and urea by man. J Clin Invest 12: 1083–1090, 1933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Diez-Sampedro A, Lostao MP, Wright EM, Hirayama BA. Glycoside binding and translocation in Na(+)-dependent glucose cotransporters: comparison of SGLT1 and SGLT3. J Membr Biol 176: 111–117, 2000 [DOI] [PubMed] [Google Scholar]
- 7. Eskandari S, Wright EM, Loo DD. Kinetics of the reverse mode of the Na+/glucose cotransporter. J Membr Biol 204: 23–32, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Faham S, Watanabe A, Besserer GM, Cascio D, Specht A, Hirayama BA, Wright EM, Abramson J. The crystal structure of a sodium galactose transporter reveals mechanistic insights into Na+/sugar symport. Science 321: 810–814, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ferrannini E, Ramos SJ, Salsali A, Tang W, List JF. Dapagliflozin monotherapy in type 2 diabetic patients with inadequate glycemic control by diet and exercise: a randomized, double-blind, placebo-controlled, phase 3 trial. Diabetes Care 33: 2217–2224, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fujimori Y, Katsuno K, Nakashima I, Ishikawa-Takemura Y, Fujikura H, Isaji M. Remogliflozin etabonate, in a novel category of selective low-affinity sodium glucose cotransporter (SGLT2) inhibitors, exhibits antidiabetic efficacy in rodent models. J Pharmacol Exp Ther 327: 268–276, 2008 [DOI] [PubMed] [Google Scholar]
- 11. Goodwin NC, Mabon R, Harrison BA, Shadoan MK, Almstead ZY, Xie Y, Healy J, Buhring LM, DaCosta CM, Bardenhagen J, Mseeh F, Liu Q, Nouraldeen A, Wilson AG, Kimball SD, Powell DR, Rawlins DB. Novel l-xylose derivatives as selective sodium-dependent glucose cotransporter 2 (SGLT2) inhibitors for the treatment of type 2 diabetes. J Med Chem 52: 6201–6204, 2009 [DOI] [PubMed] [Google Scholar]
- 12. Han S, Hagan DL, Taylor JR, Xin L, Meng W, Biller SA, Wetterau JR, Washburn WN, Whaley JM. Dapagliflozin, a selective SGLT2 inhibitor, improves glucose homeostasis in normal and diabetic rats. Diabetes 57: 1723–1729, 2008 [DOI] [PubMed] [Google Scholar]
- 13. Hirayama BA, Diez-Sampedro A, Wright EM. Common mechanisms of inhibition for the Na+/glucose (hSGLT1) and Na+/Cl−/GABA (hGAT1) cotransporters. Br J Pharmacol 134: 484–495, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hummel CS, Lu C, Loo DD, Hirayama BA, Voss AA, Wright EM. Glucose transport by human renal Na+/d-glucose cotransporters SGLT1 and SGLT2. Am J Physiol Cell Physiol 300: C14–C21, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hussey EK, Clark RV, Amin DM, Kipnes MS, O'Connor-Semmes RL, O'Driscoll EC, Leong J, Murray SC, Dobbins RL, Layko D, Nunez DJ. Single-dose pharmacokinetics and pharmacodynamics of sergliflozin etabonate, a novel inhibitor of glucose reabsorption, in healthy volunteers and patients with type 2 diabetes mellitus. J Clin Pharmacol 50: 623–635, 2010 [DOI] [PubMed] [Google Scholar]
- 16. Hussey EK, Dobbins RL, Stoltz RR, Stockman NL, O'Connor-Semmes RL, Kapur A, Murray SC, Layko D, Nunez DJ. Multiple-dose pharmacokinetics and pharmacodynamics of sergliflozin etabonate, a novel inhibitor of glucose reabsorption, in healthy overweight and obese subjects: a randomized double-blind study. J Clin Pharmacol 50: 636–646, 2010 [DOI] [PubMed] [Google Scholar]
- 17. Isaji M. SGLT2 inhibitors: molecular design and potential differences in effect. Kidney Int 79, Suppl 120: S14–S19, 2011 [DOI] [PubMed] [Google Scholar]
- 18. Jurczak MJ, Lee HY, Birkenfeld AL, Jornayvaz FR, Frederick DW, Pongratz RL, Zhao X, Moeckel GW, Samuel VT, Whaley JM, Shulman GI, Kibbey RG. SGLT2 deletion improves glucose homeostasis and preserves pancreatic beta-cell function. Diabetes 60: 890–898, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kasichayanula S, Chang M, Hasegawa M, Liu X, Yamahira N, LaCreta FP, Imai Y, Boulton DW. Pharmacokinetics and pharmacodynamics of dapagliflozin, a novel selective inhibitor of sodium-glucose co-transporter type 2, in Japanese subjects without and with type 2 diabetes mellitus. Diabetes Obes Metab 13: 357–365, 2011 [DOI] [PubMed] [Google Scholar]
- 20. Kasichayanula S, Chang M, Hasegawa M, Liu X, Yamahira N, LaCreta FP, Imai Y, Boulton DW. Pharmacokinetics and pharmacodynamics of dapagliflozin, a novel selective inhibitor of sodium–glucose co-transporter type 2, in Japanese subjects without and with type 2 diabetes mellitus. Diabetes Obes Metab 13: 357–365, 2011 [DOI] [PubMed] [Google Scholar]
- 21. Katsuno K, Fujimori Y, Ishikawa-Takemura Y, Isaji M. Long-term treatment with sergliflozin etabonate improves disturbed glucose metabolism in KK-A(y) mice. Eur J Pharmacol 618: 98–104, 2009 [DOI] [PubMed] [Google Scholar]
- 22. Komoroski B, Vachharajani N, Boulton D, Kornhauser D, Geraldes M, Li L, Pfister M. Dapagliflozin, a novel SGLT2 inhibitor, induces dose-dependent glucosuria in healthy subjects. Clin Pharmacol Ther 85: 520–526, 2009 [DOI] [PubMed] [Google Scholar]
- 23. Komoroski B, Vachharajani N, Feng Y, Li L, Kornhauser D, Pfister M. Dapagliflozin, a novel, selective SGLT2 inhibitor, improved glycemic control over 2 weeks in patients with type 2 diabetes mellitus. Clin Pharmacol Ther 85: 513–519, 2009 [DOI] [PubMed] [Google Scholar]
- 24. Loo DD, Hazama A, Supplisson S, Turk E, Wright EM. Relaxation kinetics of the Na+/glucose cotransporter. Proc Natl Acad Sci U S A 90: 5767–5771, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Loo DD, Hirayama BA, Sala-Rabanal M, Wright EM. How drugs interact with transporters: SGLT1 as a model. J Membr Biol 223: 87–106, 2008 [DOI] [PubMed] [Google Scholar]
- 26. Lostao MP, Hirayama BA, Loo DD, Wright EM. Phenylglucosides and the Na+/glucose cotransporter (SGLT1): analysis of interactions. J Membr Biol 142: 161–170, 1994 [DOI] [PubMed] [Google Scholar]
- 27. Ly JP, Onay T, Sison K, Sivaskandarajah G, Sabbisetti V, Li L, Bonventre JV, Flenniken A, Paragas N, Barasch JM, Adamson SL, Osborne L, Rossant J, Schnermann J, Quaggin SE. The sweet pee model for Sglt2 mutation. J Am Soc Nephrol 22: 113–123, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Meng W, Ellsworth BA, Nirschl AA, McCann PJ, Patel M, Girotra RN, Wu G, Sher PM, Morrison EP, Biller SA, Zahler R, Deshpande PP, Pullockaran A, Hagan DL, Morgan N, Taylor JR, Obermeier MT, Humphreys WG, Khanna A, Discenza L, Robertson JG, Wang A, Han S, Wetterau JR, Janovitz EB, Flint OP, Whaley JM, Washburn WN. Discovery of dapagliflozin: a potent, selective renal sodium-dependent glucose cotransporter 2 (SGLT2) inhibitor for the treatment of type 2 diabetes. J Med Chem 51: 1145–1149, 2008 [DOI] [PubMed] [Google Scholar]
- 29. Newey H, Parsons BJ, Smyth DH. The site of action of phlorrhizin in inhibiting intestinal absorption of glucose. J Physiol 148: 83–92, 1959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Nomura S, Sakamaki S, Hongu M, Kawanishi E, Koga Y, Sakamoto T, Yamamoto Y, Ueta K, Kimata H, Nakayama K, Tsuda-Tsukimoto M. Discovery of canagliflozin, a novel C-glucoside with thiophene ring, as sodium-dependent glucose cotransporter 2 inhibitor for the treatment of type 2 diabetes mellitus. J Med Chem 53: 6355–6360, 2010 [DOI] [PubMed] [Google Scholar]
- 31. Obermeier M, Yao M, Khanna A, Koplowitz B, Zhu M, Li W, Komoroski B, Kasichayanula S, Discenza L, Washburn W, Meng W, Ellsworth BA, Whaley JM, Humphreys WG. In vitro characterization and pharmacokinetics of dapagliflozin (BMS-512148), a potent sodium-glucose cotransporter type II inhibitor, in animals and humans. Drug Metab Dispos 38: 405–414, 2010 [DOI] [PubMed] [Google Scholar]
- 32. Oulianova N, Falk S, Berteloot A. Two-step mechanism of phlorizin binding to the SGLT1 protein in the kidney. J Membr Biol 179: 223–242, 2001 [DOI] [PubMed] [Google Scholar]
- 33. Pajor AM, Randolph KM, Kerner SA, Smith CD. Inhibitor binding in the human renal low- and high-affinity Na+/glucose cotransporters. J Pharmacol Exp Ther 324: 985–991, 2008 [DOI] [PubMed] [Google Scholar]
- 34. Quick M, Tomasevic J, Wright EM. Functional asymmetry of the human Na+/glucose transporter (hSGLT1) in bacterial membrane vesicles. Biochemistry 42: 9147–9152, 2003 [DOI] [PubMed] [Google Scholar]
- 35. Robinson RP, Mascitti V, Boustany-Kari CM, Carr CL, Foley PM, Kimoto E, Leininger MT, Lowe A, Klenotic MK, Macdonald JI, Maguire RJ, Masterson VM, Maurer TS, Miao Z, Patel JD, Preville C, Reese MR, She L, Steppan CM, Thuma BA, Zhu T. C-aryl glycoside inhibitors of SGLT2: exploration of sugar modifications including C-5 spirocyclization. Bioorg Med Chem Lett 20: 1569–1572, 2010 [DOI] [PubMed] [Google Scholar]
- 36. Santer R, Kinner M, Lassen CL, Schneppenheim R, Eggert P, Bald M, Brodehl J, Daschner M, Ehrich JH, Kemper M, Li Volti S, Neuhaus T, Skovby F, Swift PG, Schaub J, Klaerke D. Molecular analysis of the SGLT2 gene in patients with renal glucosuria. J Am Soc Nephrol 14: 2873–2882, 2003 [DOI] [PubMed] [Google Scholar]
- 37. Scholl-Burgi S, Santer R, Ehrich JH. Long-term outcome of renal glucosuria type 0: the original patient and his natural history. Nephrol Dial Transplant 19: 2394–2396, 2004 [DOI] [PubMed] [Google Scholar]
- 38. Segel IH. Enzyme Kinetics: Behavior and Analysis of Rapid Equilibrium and Steady State Enzyme Systems. New York: Wiley, 1975, p. 957 [Google Scholar]
- 39. Sha S, Devineni D, Ghosh A, Polidori D, Chien S, Wexler D, Shalayda K, Demarest K, Rothenberg P. Canagliflozin, a novel inhibitor of sodium glucose co-transporter 2, dose dependently reduces calculated renal threshold for glucose excretion and increases urinary glucose excretion in healthy subjects. Diabetes Obes Metab 13: 669–672, 2011 [DOI] [PubMed] [Google Scholar]
- 40. Turk E, Zabel B, Mundlos S, Dyer J, Wright EM. Glucose/galactose malabsorption caused by a defect in the Na+/glucose cotransporter. Nature 350: 354–356, 1991 [DOI] [PubMed] [Google Scholar]
- 41. Vallon V, Platt KA, Cunard R, Schroth J, Whaley J, Thomson SC, Koepsell H, Rieg T. SGLT2 mediates glucose reabsorption in the early proximal tubule. J Am Soc Nephrol 22: 104–112, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Voss AA, Diez-Sampedro A, Hirayama BA, Loo DD, Wright EM. Imino sugars are potent agonists of the human glucose sensor SGLT3. Mol Pharmacol 71: 628–634, 2007 [DOI] [PubMed] [Google Scholar]
- 43. Washburn WN. Development of the renal glucose reabsorption inhibitors: a new mechanism for the pharmacotherapy of diabetes mellitus type 2. J Med Chem 52: 1785–1794, 2009 [DOI] [PubMed] [Google Scholar]
- 44. Wilding JP, Norwood P, T'Joen C, Bastien A, List JF, Fiedorek FT. A study of dapagliflozin in patients with type 2 diabetes receiving high doses of insulin plus insulin sensitizers: applicability of a novel insulin-independent treatment. Diabetes Care 32: 1656–1662, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Wright EM, Loo DD, Hirayama BA. Biology of human sodium glucose transporters. Physiol Rev 91: 733–794, 2011 [DOI] [PubMed] [Google Scholar]