Abstract
Lymphangiogenic cytokines such as vascular endothelial growth factor-C (VEGF-C) are critically required for lymphatic regeneration; however, in some circumstances, lymphatic function is impaired despite normal or elevated levels of these cytokines. The recent identification of anti-lymphangiogenic molecules such as interferon-γ (IFN-γ), transforming growth factor-β1, and endostatin has led us to hypothesize that impaired lymphatic function may represent a dysregulated balance in the expression of pro/anti-lymphangiogenic stimuli. We observed that nude mice have significantly improved lymphatic function compared with wild-type mice in a tail model of lymphedema. We show that gradients of lymphatic fluid stasis regulate the expression of lymphangiogenic cytokines (VEGF-A, VEGF-C, and hepatocyte growth factor) and that paradoxically the expression of these molecules is increased in wild-type mice. More importantly, we show that as a consequence of T-cell-mediated inflammation, these same gradients also regulate expression patterns of anti-lymphangiogenic molecules corresponding temporally and spatially with impaired lymphatic function in wild-type mice. We show that neutralization of IFN-γ significantly increases inflammatory lymph node lymphangiogenesis independently of changes in VEGF-A or VEGF-C expression, suggesting that alterations in the balance of pro- and anti-lymphangiogenic cytokine expression can regulate lymphatic vessel formation. In conclusion, we show that gradients of lymphatic fluid stasis regulate not only the expression of pro-lymphangiogenic cytokines but also potent suppressors of lymphangiogenesis as a consequence of T-cell inflammation and that modulation of the balance between these stimuli can regulate lymphatic function.
Keywords: lymphangiogenesis, vascular endothelial growth factor-C, lymphedema, transforming growth factor-β1, interferon-γ
lymphedema is a dreaded complication of cancer treatment and occurs commonly after lymph node resection. It is estimated that 30–40% of breast cancer survivors who undergo axillary lymph node dissection go on to develop lymphedema (29, 30). Although the clinical risk factors for lymphedema have been identified, the exact pathophysiology of this disease remains unknown. Since resection of lymph nodes results in disruption of lymphatic vessels, one potential mechanism regulating development of lymphedema may be failure of lymphatic regeneration. This hypothesis is supported by recent studies demonstrating that exogenous delivery of lymphangiogenic cytokines such as hepatocyte growth factor (HGF) (34) or vascular endothelial growth factor-C (VEGF-C) promote lymphatic regeneration in animal models of lymphedema and decrease lymphatic fluid stasis (5, 9, 25, 33, 36, 39).
The regulation of lymphangiogenesis appears to be more complicated, however, than simply the expression of lymphangiogenic cytokines. For instance, Rutkowski and colleagues (32) have recently shown that expression of VEGF-C alone is insufficient to promote lymphatic regeneration in the setting of lymphatic fluid stasis. Similarly, Goldman et al. (18) have shown that even exogenous delivery or overexpression of VEGF-C using cell-based gene therapeutic approaches fails to promote lymphatic regeneration in some settings, suggesting that these circumstances activate mechanisms that actively or passively inhibit lymphatic regeneration. This hypothesis is supported by the finding that some cytokines expressed normally during wound repair directly inhibit lymphatic regeneration. For example, our laboratory has previously shown that inhibition of transforming growth factor-β1 (TGF-β1) markedly accelerates lymphatic regeneration in a mouse tail model and that TGF-β1 directly inhibits cellular proliferation and differentiation potential of isolated lymphatic endothelial cells (2, 3, 10). Similar findings were noted by Oka et al. (26) during inflammatory and tumor-associated lymphangiogenesis. Thus lymphangiogenesis, similar to angiogenesis, may represent a balance between pro- and anti-lymphangiogenic molecular signals during wound repair. Failure of lymphatic regeneration after wound healing may occur as a consequence of dysregulation of this balance resulting in a relative decreased expression of lymphangiogenic or increased expression of anti-lymphangiogenic cytokines.
The purpose of this study was to determine the expression patterns of pro- and anti-lymphangiogenic cytokines in a mouse tail model of lymphatic regeneration. In addition, we sought to determine differences in the expression of these molecules in circumstances where regeneration occurs rapidly compared with those in which lymphatic regeneration occurs in a delayed fashion. We demonstrate that the expression of lymphangiogenic and anti-lymphangiogenic cytokines occurs simultaneously during wound repair. Additionally, we demonstrate that lymphatic function is improved in nude mice compared with wild-type mice and that this response is associated with an altered balance in pro- and anti-lymphangiogenic molecular signals, primarily as a result of decreased expression of anti-lymphangiogenic cytokines including TGF-β1 and IFN-γ.
METHODS
Animal models.
To study the role of lymphangiogenic and anti-lymphangiogenic cytokines during wound repair, we used the previously described mouse tail model of lymphatic fluid stasis (10, 32). We have previously shown that wild-type animals have delayed lymphatic regeneration in this model with resultant tail edema lasting as long as 6 wk after surgery (3, 10). Briefly, adult (10–12 wk old) female C57Bl/6 or nude mice (nu/nu based on C57Bl/6 background; both from Jackson Labs, Bar Harbor, ME) were anesthetized, and a 2-mm wide circumferential full-thickness skin excision was performed 20 mm from the mouse tail to remove the superficial lymphatics. In addition, deep lymphatics were identified using a surgical microscope (Leica, StereoZoom SZ-4, Wetzlar, Germany) and carefully ligated taking care to avoid injury to the underlying lateral tail veins. The wound was then dressed with a solution of 1% rat tail collagen (BD Biosciences, San Jose, CA) and an occlusive dressing. Wound dressings were removed on postoperative day 5, and animals were euthanized 2 or 4 wk after surgery for analysis.
An inflammatory lymph node lymphangiogenesis model was used to study the role of IFN-γ in the regulation of lymphangiogenesis (40). Briefly, adult female C57Bl/6 mice were injected with 20 μl of a 1:1 mixture of complete Freund's adjuvant (CFA) and ovalbumin (OVA; 2 mg/ml; both from Sigma) in the hindlimb footpad. To inhibit IFN-γ function, animals in the experimental group (n = 5–7/group) were treated with IFN-γ monoclonal antibodies (500 μg ip every 5 days, clone R4–6A2, Bio-X-Cell, West Lebanon, NH) beginning either immediately after treatment with CFA/OVA (immediate IFN-γ blockade) or 2 days after CFA/OVA injection (delayed IFN-γ blockade). Control animals (n = 5–7/group) were treated with similar doses of a nonspecific isotype control monoclonal antibody. Popliteal lymph nodes were harvested 5 days after initiation of IFN-γ blockade (i.e., 5 or 7 days after injection with CFA/OVA), and frozen sections were prepared. Lymphatic vessel endothelial receptor-1 (LYVE-1)-stained lymph nodes were imaged using MetaMorph Widefield Imaging System (Carl Zeiss), and image analysis was performed using Metamorph Offline software (Molecular Devices, Sunnyvale, CA) (40). Lymphatic vessel density was determined by photographic analysis of fluorescence staining using Metamorph Offline software (Molecular Devices). Vessel density was expressed as a ratio of the area of positive pixels relative to total node area (vessels/mm2, n = 6–8/group) (40). All animals were maintained in temperature- and light-controlled environment and fed ad libitum, and all animal procedures were performed ethically following approval by the Resource Animal Research Center IACUC at Memorial Sloan-Kettering Cancer Center, New York, NY.
Analysis of lymphatic function.
Changes in tail edema were analyzed using tail volume measurements performed by blinded reviewers using the truncated cone formula as previously reported (3). Briefly, the tail circumference of each animal (4–5/group/time point) was measured at 10-mm intervals starting at the distal margin of the wound using a digital caliper, and volumes were calculated. Microlymphangiography was performed in 3–4 animals/group 4 wk after surgery to evaluate the gross structure of the dermal capillary lymphatics. This was performed by injecting 15 μl of a 10 mg/ml solution of fluorescein isothiocyanate-conjugated dextran (2,000 kDa, Invitrogen, Carlsbad, CA) at the tip of the mouse tail under constant physiological pressure (38). The dextran molecule used in this study is too large to enter the blood stream and is transported by the lymphatics enabling visualization of dermal lymphatic vessels using a fluorescent microscope (Leica MZFL3 Stereoscope, Wetzlar, Germany). Images obtained by fluorescence microscopy were overlaid on brightfield images and exposed using consistent exposure, gain, and magnification with Volocity software (PerkinElmer, Waltham, MA).
Lymphoscintigraphy was performed 4 wk after tail skin and lymphatic excision to quantify lymphatic transport using our previously published methods (10). Briefly, 50 μl of technetium-99m/sulfur colloid (100-nm particle size) were injected intradermally 20 mm from the tip of the tail (10). Dynamic planar gamma camera images were then acquired for up to 2.5 h after injection using an X-SPECT camera (Gamma Medica, Northridge, CA), and region-of-interest analysis was performed to derive decay-adjusted activity using ASIPro software (CTI Molecular Imaging, Knoxville, TN).
Finally, we identified the number of podoplanin-positive lymphatic vessels in tail sections using our methods (3). Briefly, lymphatic vessels were identified using podoplanin immunohistochemistry, and the number of vessels located 1 cm distal to the wound center were counted by two blinded reviewers in a minimum of 4 animals per group/time [3–4 high-powered fields (×20) per section].
Specimen preparation, histology, and protein expression.
One-centimeter longitudinal tail sections centered on the wound as well as cross sections located 1 cm proximal/distal to the wound were harvested and fixed overnight in 4% paraformaldehyde. Tissues were decalcified in immunocal (Decal Chemical, Tallman, NY), embedded in paraffin, and sectioned using a microtome. Sections were stained using hematoxylin and eosin and visualized using the Mirax Slide Scanner (Carl Zeiss Microimaging, Munich, Germany). Subcutaneous tissue thickness was determined by measuring the thickness of the skin extending from the dermis to the tail muscle below (i.e., encompassing the dermis and subcutaneous fat) in tissue sections localized 1 cm distal to the wound using Image J Software (software available at http://rsweb.nih.gov/ij/). Two blinded reviewers performed measurements in identically processed tissue sections at 5× magnification in 5–6 animals per group/time point.
Immunohistochemical staining was performed using our previously published methods to identify podoplanin or LYVE–positive lymphatic vessels (primary antibodies from Abcam, Cambridge, MA) (3). IFN-γ staining was performed using monoclonal mouse antibodies directed against mouse IFN-γ (Abcam, Cambridge, MA). Immunohistochemical staining was visualized with secondary antibodies from the VECTASTAIN ABC Kit (Vector, Burlingame, CA) and developed using diaminobenzamine (Vector). Negative control sections were incubated with secondary antibody only. Brightfield images were visualized using a Mirax slide scanner (Carl Zeiss), and a minimum of four animals per group/time point were evaluated.
We identified proliferating lymphatic endothelial cells by costaining with antibodies directed against LYVE-1 and proliferating cell nuclear antigen (PCNA, Santa Cruz Biotechnology, Santa Cruz, CA) using our previously published methods (2, 10). Briefly, LYVE-1 and PCNA were colocalized using Alexa Fluor immunofluorescent secondary antibodies (Invitrogen Molecular Probes, Carlsbad, CA), and the number of single-positive (LYVE-1 only) and double-positive (LYVE-1+/PCNA+) cells were counted in 4–5 high-powered fields (×20) located 0.5 and 1.5 centimeters distal to the wound. Two blinded reviewers evaluated a minimum of four animals per group/time point (n = 3–4 animals evaluated per time point).
Western blot analysis was performed using our previously published methods (3, 40). Briefly, total cellular protein was harvested from the skin and subcutaneous tissues of mouse tails 5 mm distal and proximal to the wound from both nude and wild-type animals. Protein was isolated using the Qiagen DNA/RNA/Protein mini kit according to the manufacturer's directions (Qiagen, Valencia, CA) and quantified using the Bradford method. Protein from 3 to 4 animals per group was pooled and Western blotting performed as previously described for VEGF-A, VEGF-C, HGF, LYVE-1, prospero homeobox protein 1 (Prox1), CD45, IFN-γ, TGF-β1, and endostatin. All antibodies were from Abcam except for TGF-β1, which was from Santa Cruz Biotechnology. Equal loading was confirmed with actin or tubulin stains (Abcam). Immunoreactivity was determined using the ECL chemiluminescence detection system (Amersham, Arlington Heights, IL). Each Western blot was repeated in triplicate, and fold change in protein expression was normalized to actin or tubulin controls and quantified using Image J software.
CD3 depletion and flow cytometry.
We used our previously published methods to deplete CD3+ cells (all T-cells) in wild-type C57Bl/6 mice to determine the role of T-cells in the expression of lymphangiogenic and anti-lymphangiogenic cytokines during wound healing (3). We chose to deplete all T-cells rather than a subset since this phenotype would most closely approximate nude mice. Briefly, experimental animals (n = 6–8) were treated with a monoclonal antibody against mouse CD3e (20 mg/kg diluted in 150 μl of PBS, IP), clone 145–2C11 (Bio-x-cell, West Lebanon, New Hampshire) beginning 5 days before surgery and continuing every 5 days for 4 wk following tail excision as described above. This protocol has been previously shown to result in effective T-cell depletion with minimal toxicity (1). Control animals (n = 8) were treated with a similar dose of isotype control monoclonal antibody. We confirmed efficiency of CD3 depletion using flow cytometry of single cell splenic suspensions as previously described (3). In addition, we performed flow cytometry on single cell splenic suspensions from nude and wild-type animals to ensure that nude mice are lacking mature T-cell populations.
Statistical analysis.
Student's t-test was used for analyzing differences between two groups. A minimum of five animals were used in each experimental group. Data are presented as means ± SD unless otherwise noted with P < 0.05 considered significant.
RESULTS
Nude animals have decreased tail edema and lymphatic stasis.
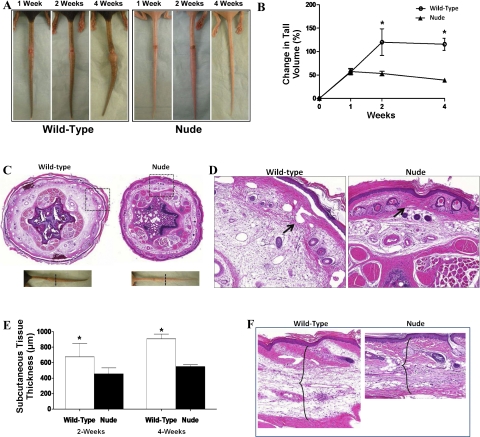
Gross examination of wound healing demonstrated similar rates of wound healing in nude and wild-type mice (Fig. 1A). However, consistent with previous publications, we found that nude mice exhibited less scarring at the wound site than wild-type mice (6, 16). In addition, gross analysis of mouse tails demonstrated that nude animals had markedly less tail edema than wild-type animals 4 wk after surgery (Fig. 1B). In addition, tail skin localized distal to the site of lymphatic injury appeared more hyperkeratotic in wild-type animals. Differences in tail edema were corroborated by tail volume measurements demonstrating a more than twofold increase in tail volume in wild-type animals compared with nude mice (P < 0.01; Fig. 1B). Histological analysis demonstrated markedly decreased subcutaneous tissue thickness in nude animals compared with wild-type controls indicating decreased tail edema (Fig. 1, C–F). This difference did not appear to be a result of simply increased interstitial edema but rather reflected increased deposition of fat in the subcutaneous compartment and is consistent with previous studies demonstrating lymphedema in the mouse tail model (32). In addition, as we have previously shown (2, 3, 10), the tails of wild-type animals had dilated, ectatic dermal lymphatics; in contrast, these lymphatics appeared collapsed in nude animals, indicative of decreased lymphatic fluid stasis.
Fig. 1.
Nude animals have decreased tail edema and lymphatic stasis. A: gross photograph of representative wild-type (left) and nude (right) mouse tails 1, 2, and 4 wk following surgery. Note marked decrease swelling and decreased scarring in nude mice at 4 wk. B: change in tail volume from preoperative to postoperative measures in nude and wild-type mice. Note marked decrease in tail volume changes in nude mice beginning at 2 wk and maintained until 4 wk time point (*P < 0.001). C: representative low-power (×2.5) cross-section of mouse tails from wild-type and nude mice 4-wk after surgery. Sections were harvested 1.5 cm distal to the zone of lymphatic obstruction. Gross photographs (bottom) are shown for orientation. D: dotted box region of C. High-power (×10) view of mouse tail cross sections from wild-type and nude mice 4 wk after surgery. Note increased inflammation and deposition of subcutaneous fat in wild-type compared with nude mice. Also note dilated lymphatic vessels in wild-type (arrow) and contrast to collapsed capillary lymphatics in nude mice (arrow). E: subcutaneous tissue measurements of wild-type and nude mice 2 or 4 wk after surgery. Note significant increase in subcutaneous tissue thickness in wild-type mice (*P < 0.01). F: representative high-power (×20) view of longitudinal section of mouse tails harvested 1 cm distal to the wound demonstrating difference in subcutaneous tissue thickness of wild-type and nude mice (brackets).
Nude animals have improved lymphatic function.
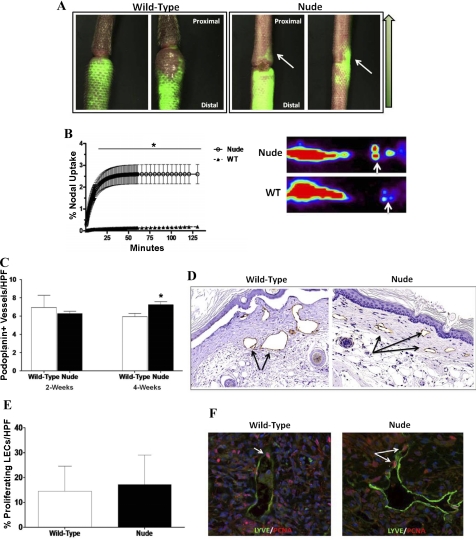
To further analyze lymphatic regeneration and lymphatic function in nude and wild-type animals, we performed microlymphangiography to grossly visualize lymphatic vessels and lymphoscintigraphy to measure lymphatic transport in vivo. Four weeks after surgery, nude mice consistently demonstrate flow of interstitial fluid across the tail wound, whereas wild-type animals demonstrate distal pooling of fluorescent dye (Fig. 2A). Although we were able to observe proximal lymphatic fluid flow in nude mice, we failed to visualize distinctly regenerated capillary lymphatics in the wound bed, indicating that transport of fluorescent dextran dye was occurring primarily as a result of interstitial flow. Furthermore, quantification of lymphatic fluid flow by lymphoscintigraphy demonstrated a marked increase in lymphatic transport in nude mice 4 wk after surgery compared with wild-type controls (P < 0.001, Fig. 2B).
Fig. 2.
Nude animals have more rapid lymphatic regeneration and improved lymphatic function. A: representative microlymphangiography of wild-type (WT, left) and nude (right) mice 4 wk after surgery. Note accumulation of fluorescent dye in the distal tail region in WT mice in contrast to proximal traveling of fluorescent dye across the tail wound (white arrow) in nude mice. Green arrow to the right in A denotes direction of interstitial flow from distal to proximal. B: lymphoscintigraphy comparing lymph node uptake in wild-type and nude mice 4 wk after surgery. Note increased uptake in nude mice compared with WT mice (*P < 0.01). Representative heat map images are shown to the right. Tc99 is injected distally at the tip of the tail and uptake is measured at the lymph nodes at the base of the tail (white arrow). C: number of podoplanin-positive lymphatic vessels/high-powered field (HPF) in WT and nude mice 2 and 4 wk after surgery. Vessels were counted in 3 HPF (×20) located 1 cm away from the distal wound edge in 4–5 animals per group/time point (*P < 0.05). D: representative figures of immunohistochemical localization (×20) of podoplanin-positive capillary lymphatics in WT and nude mice (arrows). Note dilated lymphatics in WT but not nude mice. E: percentage of proliferating lymphatic endothelial cells/HPF in WT and nude mice 4 wk after surgery. The number of lymphatic vessel endothelial receptor-1 (LYVE-1)-positive cells and LYVE-1/proliferating cell-nuclear antigen (PCNA) double-positive cells were calculated in HPF (×20) located just distal to the zone of lymphatic obstruction. Percentage of double-positive cells is presented as a function of total LYVE-1-positive cells. Representative high-powered (×40) view of LYVE-1 (green)- and PCNA (red)-stained lymphatic vessels in WT and nude mice. White arrow shows double-positive cells.
Identification of lymphatic vessels in the distal segments of nude and wild-type animals by immunohistochemical staining for podoplanin confirmed our histological findings of dilated lymphatics in wild-type mice (Fig. 2D). While no difference in the number of lymphatic vessels distal to the wound was present when comparing nude and wild-type animals 2 wk after surgery (Fig. 2, C and D), a subtle increase was present in the number of lymphatic vessels in nude mice just distal to the wound at 4wk postoperatively.
We have previously shown that lymphatic endothelial cell (LEC) proliferation contributes to lymphatic regeneration and function in the mouse tail model (10). Therefore, to determine whether the increased number of lymphatic vessels in nude mice at the 4-wk time point was related to changes in LEC proliferation, we colocalized LECs with PCNA, a marker of cellular proliferation. No differences were present in LEC proliferation comparing wild-type and nude animals 4 wk after surgery in regions of the tail immediately distal to the wound (Fig. 2, E and F). Interestingly, very few proliferating LECs were present upon evaluation of regions located further away from the zone of lymphatic obstruction, indicating gradients of LEC proliferation relative to the wound (not shown).
Taken together, these findings indicate that nude animals have decreased tail edema and chronic lymphatic fluid stasis after injury and that this phenotype is associated with increased interstitial fluid flow and overall minor changes in lymphangiogenesis.
Lymphatic fluid stasis regulates expression of lymphangiogenic and anti-lymphangiogenic cytokines.
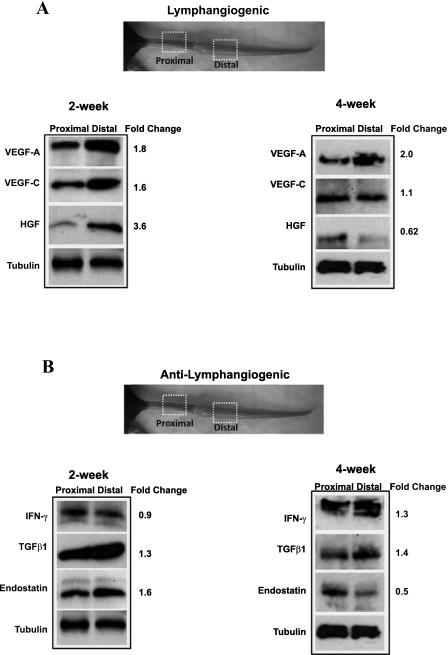
Previous immunohistochemical staining studies have shown that lymphatic fluid flow regulates the expression of VEGF-C (17, 31, 32). Similarly, our group has previously shown that the expression of TGF-β1, a potent anti-lymphangiogenic cytokine, is increased in tissues exposed to lymphatic fluid stasis (3, 10). Therefore, to determine whether gradients of lymphatic fluid stasis regulate the expression of a broad pattern of lymphangiogenic and anti-lymphangiogenic cytokines, we compared protein expression within tissue harvested from regions of the mouse tail proximal to the zone of lymphatic injury (i.e., not exposed to lymphatic stasis) to tissue harvested from regions distal to the wound (i.e., exposed to lymphatic fluid stasis). By this analysis, regions with severe lymphatic fluid stasis demonstrated increased expression of VEGF-A (1.8-fold), VEGF-C (1.6-fold), and HGF (3.6-fold; Fig. 3A) by postoperative week 2. These changes were less obvious 4 wk after surgery at which time only VEGF-A expression remained elevated (twofold higher distally compared with proximal).
Fig. 3.
Lymphatic fluid stasis regulates expression of lymphangiogenic and anti-lymphangiogenic cytokines. Representative (of triplicate) Western-blot analysis of lymphangiogenic (A) and anti-lymphangiogenic (B) cytokines 2 or 4 wk after surgery comparing expression in regions of the tail located 1 cm proximal to 1 cm distal to the zone of lymphatic obstruction. Gross photograph and boxed regions of the tail are shown for orientation. Fold changes are calculated comparing expression in the distal region to the proximal region after correction for equal loading with tubulin.
Interestingly, lymphatic fluid stasis was also associated with increased expression of known anti-lymphangiogenic cytokines such that, in the earlier postoperative period (2 wk), TGF-β1 (1.3-fold) and endostatin (1.6-fold) expression were increased in distal regions of the tail (Fig. 3B); however, IFN-γ expression was relatively unchanged. By 4 wk postoperatively, IFN-γ expression was increased in distal tail tissues (1.3-fold), TGF-β1 expression remained elevated (1.4-fold increase), and endostatin expression decreased. Taken together, these results indicate that lymphatic fluid stasis is a potent regulator of cytokine expression and that this stimulus can activate the expression of opposing mechanisms regulating lymphangiogenesis.
Wild-type animals have increased expression of lymphangiogenic cytokines.
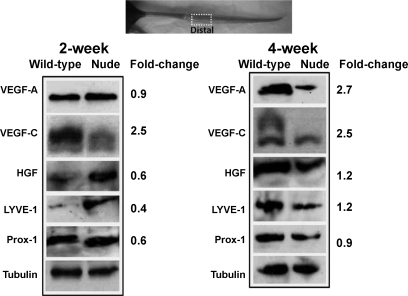
To determine whether differences in lymphatic function between wild-type and nude mice were due to alterations in lymphangiogenic cytokines, we compared the expression of VEGF-A, VEGF-C, and HGF in the tail tissues harvested distal to the zone of lymphatic obstruction in these animals. Interestingly, wild-type animals had markedly increased expression of VEGF-C (2.5-fold) compared with nude animals 2 wk after surgery (Fig. 4A). In contrast, VEGF-A and HGF were relatively unchanged. Distinctions in expression patterns became more obvious at the 4-wk time point at which time there was a 2.7-, 2.5-, and 1.2-fold increase in VEGF-A, VEGF-C, and HGF expression, respectively, in wild-type tissues compared with nude animal tissue. In support of podoplanin vessel counts, relatively minor differences in LYVE-1 and Prox-1 expression were observed at 2- and 4-wk time points. These findings indicate that although wild-type animals have increased expression of lymphangiogenic cytokines, this response does not translate to improved lymphatic function or significantly increased lymphangiogenesis.
Fig. 4.
Wild-type animals have increased expression of lymphangiogenic cytokines. A: representative (of triplicate) Western blot analysis of lymphangiogenic cytokines comparing distal tissues (1 cm distal to the zone of lymphatic obstruction) in wild-type and nude mice 2 or 4 wk after surgery. Gross photograph and boxed regions of the tail are shown for orientation. Fold changes are calculated comparing expression in distal region to proximal region after correction for equal loading with tubulin staining.VEGF, vascular endothelial growth factor; HGF, hepatocyte growth factor; Prox-1, prospero homeobox protein 1.
T-cell deficiency decreases expression of anti-lymphangiogenic cytokines.
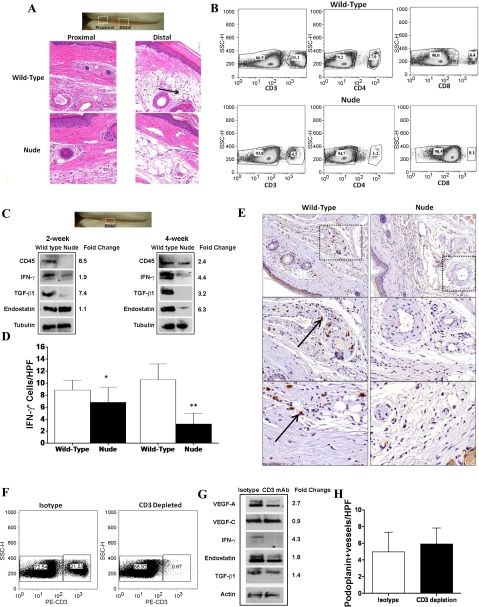
Not surprisingly, wild-type animals had significantly more inflammation in their tail tissues compared with nude animal tissue (Fig. 5A). This pattern of inflammation correlated with gradients of lymphatic fluid stasis since there were no differences in inflammatory cell infiltrate in proximal regions of the tail. Consistent with our previous studies (3, 10), we found that wild-type animals had a marked mononuclear cell inflammatory response in the distal tail tissues. In contrast, nude animals had minimal tissue inflammation in these regions.
Fig. 5.
T cell deficiency decreases expression of anti-lymphangiogenic cytokines. A: representative high-power (×20) hematoxylin and eosin sections of proximal and distal cross-sections of wild-type and nude mice 4 wk after surgery. Note marked mononuclear cell inflammatory reaction in wild-type mice (black arrow) localized to the subcutaneous fat. B: representative flow cytometry analysis of wild-type (top) and nude (bottom) mice for CD3-, CD4-, and CD8-positive cells in single cell suspensions of the spleen. Note marked decreases in total T-cells (CD3+) as well as near complete absence of CD4 and CD8+ cells in nude mice. C: representative (of triplicate) Western blot comparing expression of anti-lymphangiogenic cytokines in wild-type and nude mice 2 or 4 wk after surgery. Gross photograph and boxed region of the tail are shown for orientation. Fold changes are calculated comparing expression in wild-type to nude mice after correction for equal loading with tubulin staining. D: number of IFN-γ-positive cells/HPF in wild-type and nude mice 2 or 4 wk after surgery. Note marked increase in positive cells in wild-type mice (*P < 0.01; **P < 0.001). E: representative high (×10; top 2 panels) and higher (×20; bottom 4 panels) magnification views of IFN-γ staining in wild-type (left) and nude (right) mice. Boxed region is shown in magnified views in bottom panels. Note positive cells surrounding capillary lymphatic and microvascular blood vessels in wild-type mice (arrow). F: representative flow cytometry of single cell suspensions of isotype or CD3-depleted mice 4 wk after tail surgery. Note near complete loss of CD3+ cells in CD3 monoclonal antibody-treated mice. G: representative (of triplicate) Western blot comparing distal tail tissues from isotype and CD3 monoclonal antibody (mAb)-treated animals 4 wk after lymphatic ablation. Fold change is calculated comparing isotype to CD3 mAb-treated animals after correction for equal loading with tubulin staining. H: number of podoplanin-positive lymphatic vessels/HPF in isotype and CD3-depleted animals 4 wk after surgery. Vessels were counted in 3 HPF (×20) located 1 cm away from the distal wound edge in 4–5 animals per group/time point. No significant differences were noted between groups.
Because some animal models of T-cell deficiency demonstrate “leakiness” in their genetic phenotype resulting in a less than complete loss of T-cells, we analyzed white blood cell populations in single cell suspensions of wild-type and nude mice (Fig. 5B). While more than 30% of wild-type mice splenic cells were CD3+, this number was greatly reduced in nude mice (4.5%). The reduction in CD3+ cells in nude mice translated to a near-complete absence of mature T-cell subtypes (<1.1% CD4+ and <0.1% CD8+), indicating that the phenotype of these nude mice is not leaky with regard to T-cell production and maturation.
To determine whether T-cell inflammatory responses regulate the expression of anti-lymphangiogenic cytokines in response to lymphatic fluid stasis, we analyzed the expression of these cytokines in wild-type and nude mice (Fig. 5C). Consistent with our histological analysis, we found that the expression of CD45, a pan-leukocyte antigen, was markedly elevated in wild-type mice (8.5-fold at 2 wk and 2.4-fold at 4 wk). Similarly, the expression of IFN-γ and TGF-β1 was markedly upregulated in wild-type mice at the 2-wk time point (1.9- and 7.4-fold increase, respectively). These differences became even more obvious at the 4-wk time point at which time there was a 4.4-, 3.2-, and 6.3-fold increase in IFN-γ, TGF-β1, and endostatin expression, respectively.
We have previously analyzed the expression patterns of TGF-β1 in tissues exposed to lymphatic fluid stasis, and here we performed immunohistochemistry to localize IFN-γ expression in these regions. Consistent with Western blot analysis, nude animals had significantly fewer IFN-γ-positive cells 2 and 4 wk after surgery (Fig. 5D). In fact, at the 4-wk time point, nude animals demonstrated a more than fourfold decrease in the number of IFN-γ cells (P < 0.001). Furthermore, IFN-γ expression in wild-type animals was localized to mononuclear inflammatory cells as well as macrophages localized to perivascular and perilymphatic areas in the deep dermis and subcutaneous tissues (Fig. 5E). In contrast, nude animals had only scattered IFN-γ-expressing cells in perivascular areas.
It is possible that the changes we noted in the expression of anti-lymphangiogenic cytokines between wild-type and nude mice are due to other changes in these animals related to a chronic T-cell deficiency rather than alterations in response to lymphatic fluid stasis, per se. In an effort to study this response in a controlled setting, we depleted CD3 cells from wild-type mice after lymphatic excision using monoclonal antibodies. Flow cytometry demonstrated that monoclonal antibody treatment was highly efficient at depleting CD3+ cells systemically, resulting in a marked reduction in the number of these cells in single cell splenic suspensions 4 wk after tail skin excision (0.7% in treated animals versus 21.5% in controls; Fig. 5F).
We next compared expression of lymphangiogenic and anti-lymphangiogenic cytokines in distal tail tissues of isotype control and CD3-depleted animals 4 wk after surgery (Fig. 5G). This analysis demonstrated that CD3 depletion, similar to the nude phenotype, resulted in decreased VEGF-A expression (2.7-fold decrease compared with isotype controls) but little change in VEGF-C expression. Similar to the nude phenotype, CD3 depletion also markedly decreased the expression of anti-lymphangiogenic cytokines (4.3-fold decreased IFN-γ, 1.8-fold decreased endostatin, and 1.4-fold decrease in TGF-β1 expression). Finally, there was no change in the number of lymphatic vessels in animals depleted of CD3 cells at 4 wk postoperatively compared with isotype-treated controls (Fig. 5H), suggesting CD3 depletion, similar to our findings with nude mice, does not significantly alter lymphangiogenesis.
Inhibition of IFN-γ augments inflammatory lymphangiogenesis.
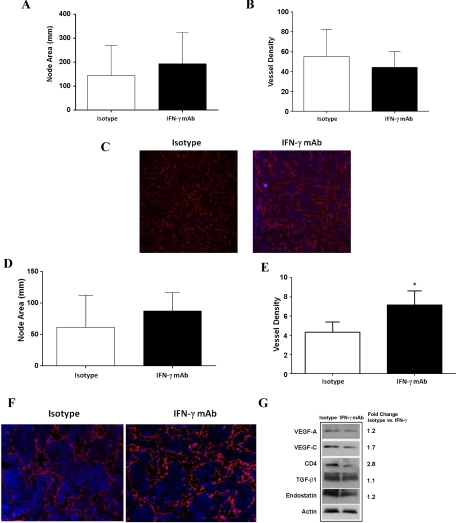
Kataru et al. (22) have recently shown that T-cells negatively regulate lymph node lymphangiogenesis under physiological conditions as well as in response to inflammation or tumor metastasis (22) In addition, using IFN-γ knockout mice, the authors demonstrated that this effect is in part due to IFN-γ expression within the lymph node. However, IFN-γ-deficient mice are known to have alterations in their T cell differentiation profile and responses to inflammatory stimuli (12). Therefore, we chose to study the role of IFN-γ in the regulation of inflammatory lymph node lymphangiogenesis using neutralizing antibodies. This approach is advantageous as it affords the opportunity to change the timing of IFN-γ neutralization. Interestingly, IFN-γ neutralizing antibodies delivered at the time of CFA/OVA inoculation in the hindlimb paw resulted in no significant differences in lymph node area or lymphatic vessel density when popliteal lymph nodes were analyzed 5 days later (Fig. 6, A–C).
Fig. 6.
Inhibition of IFN-γ augments inflammatory lymphangiogenesis. A: popliteal lymph node area in isotype and IFN-γ mAb-treated animals (means ± SD). Animals were treated with IFN-γ mAb immediately after complete Freunds adjunant (CFA)/ovalbumin (OVA) injection into the paw, and lymph nodes were harvested 5 days later. B: lymphatic vessel density in isotype and IFN-γ mAb-treated animals. IFN-γ mAb was administered immediately after CFA/OVA injection. C: representative high-power (×20) photomicrograph of fluorescent LYVE-1 staining (red) in isotype and IFN-γ mAb-treated animals. D: popliteal lymph node area in isotype and IFN-γ mAb-treated animals (means ± SD). Animals were treated with IFN-γ mAb beginning 2 days after CFA/OVA injection into the paw, and lymph nodes were harvested 5 days after initiation of IFN-γ mAb treatment. E: lymphatic vessel density in isotype and IFN-γ mAb-treated animals. IFN-γ mAb was administered beginning 2 days after CFA/OVA injection and lymph nodes were harvested 5 days after initiation of mAb treatment (*P < 0.05). F: representative high-power (×20) photomicrograph of fluorescent LYVE-1 staining (red) in isotype and IFN-γ mAb-treated animals. IFN-γ mAb treatment was started 2 days after CFA/OVA injection. Note increased number and branching of LYVE-1+ vessels in IFN-γ mAb-treated lymph node. G: representative (of triplicate) Western blot comparing expression of lymphangiogenic and anti-lymphangiogenic cytokines in popliteal lymph nodes of mice injected with CFA/OVA and then treated with IFN-γ mAb or isotype control antibodies beginning 2 days after injection. Fold changes are calculated comparing ratio of expression in isotype controls compared with IFN-γ mAb-treated animals corrected for equal loading with actin staining.
It is possible that inhibition of IFN-γ immediately after CFA/OVA injection failed to alter lymphangiogenesis by decreasing the inflammatory stimulus for lymph node lymphangiogenesis. To test this hypothesis, we initiated treatment with IFN-γ-neutralizing antibodies (IFN-γ mAb) 2 days after CFA/OVA injection such that the inflammatory stimulus of CFA/OVA was established and then harvested popliteal lymph nodes 5 days later. This time period has been shown to precede the spontaneous resolution of inflammatory lymphangiogenesis that ordinarily occurs beginning 10–12 days after inflammation (22). Interestingly, this analysis again demonstrated no differences in lymph node area (Fig. 6D). However, analysis of lymph node vessel density demonstrated a significant increase in IFN-γ antibody-treated animals (1.8-fold increase; P < 0.01; Fig. 6, E and F). Interestingly, Western blot analysis of popliteal lymph nodes demonstrated that even though IFN-γ antibody-treated animals had increased lymphatic vessel density, they had decreased expression of lymphangiogenic cytokines (VEGF-A 1.2-fold decreased, VEGF-C 1.7-fold decreased; Fig. 6G). IFN-γ-neutralizing antibody treatment resulted in a 2.8-fold decrease in CD4 expression but only minor changes in the expression of anti-lymphangiogenic cytokines TGF-β1 or endostatin. Taken together, our results suggest that IFN-γ expression in response to inflammation does not alter initiation of lymphangiogenesis but rather inhibits lymphatic vessel formation after this process has started.
DISCUSSION
In this study, nude mice had significantly improved lymphatic function after tail lymphatic ablation compared with wild-type mice resulting in more rapid resolution of edema and increased lymphatic transport. Interestingly, these marked improvements were only associated with minor increases in lymphangiogenesis. This apparent paradox led us to explore potential mechanisms by which interstitial fluid transport and lymphatic function can be augmented without marked changes in lymphangiogenesis.
We first sought to determine whether differences in lymphatic function were due to changes in lymphangiogenic cytokine expression. We elected to evaluate the expression of VEGF-A, VEGF-C, and HGF because these cytokines have all been shown to play a critical role in wound healing-associated lymphangiogenesis. Similar to previous studies (17, 32), we found that gradients of lymphatic fluid stasis regulate the expression of VEGF-C, resulting in higher levels of expression in the regions of the tail located distal to the zone of lymphatic injury. Furthermore, we showed that lymphatic fluid stasis also potently upregulates expression of VEGF-A and HGF in the early postoperative period. However, these changes are likely not responsible for improved lymphatic function in nude mice since we found that the expression of these cytokines (particularly VEGF-C) was in fact potently upregulated in wild-type mice compared with nude mice (rather than the converse as would be expected by our observation of improved lymphatic function in nude mice). A number of previous studies support this finding and suggest that other mechanisms may contribute to the resolution of tissue edema. For example, in 2006, Rutowski et al. (32) also showed that lymph stasis in the mouse tail model results in increased VEGF-C expression and lymphatic vessel hyperplasia but that these changes did not augment lymphatic function. Similarly, using the mouse tail model, in 2005, Goldman et al. (18) showed that even when excess VEGF-C was delivered to wounds using transfected cells that overexpressed this cytokine, this treatment failed to improve lymphatic function, increase LEC migration, or significantly increase lymphatic density. More recently, Goldman's group (27) has shown that edema in the mouse tail model can resolve even if lymphangiogenesis was markedly inhibited by VEGF-R2 or VEGF-R3 (ligands for VEGF-C) neutralization. These findings have led the authors to conclude that lymphangiogenesis-independent mechanisms can lead to resolution of experimental lymphedema.
We next hypothesized that differences in the expression of anti-lymphangiogenic cytokine expression may contribute to improved lymphatic function in nude mice. This hypothesis is based on our recent studies demonstrating that inhibition of TGF-β1, a potent anti-lymphangiogenic cytokine, markedly improves lymphatic function in the mouse tail model (3, 11). In addition, this hypothesis is also supported by our previous studies demonstrating that delivery of exogenous TGF-β1 to tail wounds markedly delays lymphatic repair and worsens tail edema (11). We found that similar to lymphangiogenic cytokines, the expression of anti-lymphangiogenic cytokines is also regulated by gradients of lymphatic fluid stasis resulting in increased expression of these molecules in regions of the tail located distal to the zone of lymphatic obstruction. More importantly, we found that the expression of anti-lymphangiogenic cytokines was markedly increased in the distal tail tissues harvested from wild-type animals compared with that of nude animals. These changes were striking and occurred shortly after surgery (2 wk) and persisted as long as 4 wk, corresponding temporally and spatially to decreased lymphatic function in wild-type mice. Interestingly, we found large numbers of IFN-γ-expressing mononuclear inflammatory cells localized in the vicinity of dilated lymphatic vessels in the distal tail, localizing this potent anti-lymphangiogenic cytokine in the direct vicinity of capillary lymphatics. Together, these findings suggest that increased expression of anti-lymphangiogenic cytokines in wild-type mice relative to nude mice contributes to the observed differences in lymphatic function. This hypothesis is supported by previous studies demonstrating potent anti-lymphangiogenic roles for these molecules in inflammatory and tumor-associated lymphangiogenesis. For example, Kataru et al. (22) have recently shown that IFN-γ potently inhibits inflammatory lymph node lymphangiogenesis. This result is due to direct effects on lymphatic endothelial cells since in vitro studies have shown that IFN-γ decreases LEC tubule formation, migration, and proliferation. A number of studies have also shown potent anti-lymphangiogenic effects for endostatin. For example, endostatin treatment has been shown to decrease lung tumor metastasis in mice (13), decrease lymph node metastasis (15), and decrease lymphangiogenesis (8, 28). Future studies will determine whether direct blockade or augmentation of these anti-lymphangiogenic cytokines, similar to our findings with TGF-β1, will alter lymphatic function.
Our current study provides some evidence that improved lymphatic function in T cell-deficient mice is due to improved lymphangiogenesis. For example, we noted a small, though statistically significant, increase in the number of podoplanin-positive vessels in nude mice at the 4-wk time point. However, it is likely that other mechanisms contribute to these changes since the improvements we noted in lymphatic function were not subtle. Based on our previous work demonstrating that fibrosis is a significant regulator of lymphatic function (2–4, 10), as well as our finding and previous reports demonstrating that nude mice display decreased scarring at the wound site (6, 16), we hypothesize that anti-lymphangiogenic cytokines such as IFN-γ, TGF-β1, and endostatin may exert some of their effects on lymphatic function by inducing changes in the extracellular matrix. This hypothesis is supported by previous studies demonstrating changes in lymphatic function as a consequence of interstitial flow (17, 37). In addition, our hypothesis is also consistent with the pathological changes associated with lymphatic injury and lymphedema as evidenced by fibrosis and obliteration of lymphatic vessels in these circumstances (35). Thus it is possible that sustained lymphatic fluid stasis, either clinically or in our mouse tail model, results in the expression of molecules such at IFN-γ, TGF-β1, and endostatin, which in turn regulates lymphatic function not only by direct effects on lymphangiogenesis but also as a consequence of indirect effects on extracellular matrix turnover and remodeling. Future studies will be needed to study this hypothesis.
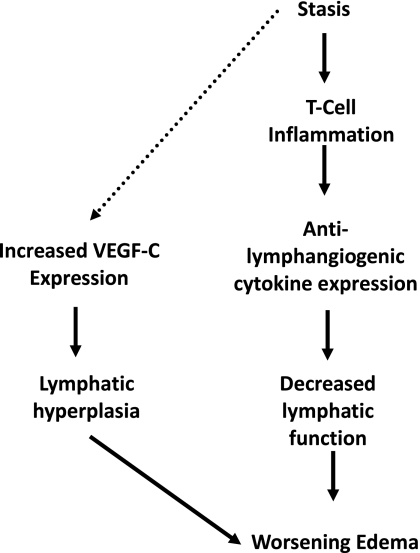
The fact that nude mice lack mature T-cells suggests that T-cell inflammatory reactions are necessary for the production of anti-lymphangiogenic cytokines in response to lymphatic fluid stasis. In fact, we have previously shown that sustained lymphatic fluid stasis in the mouse tail model results in chronic T-cell inflammation and that blockade of TGF-β1 function results not only in improved lymphatic function but also marked reductions in this inflammatory response (3). This hypothesis is also supported by our finding that depletion of CD3+ cells markedly decreases the expression of anti-lymphangiogenic cytokines in the mouse tail model, thereby recapitulating our observations with nude mice. Our findings are also supported by recent studies by Kataru et al. (22) demonstrating that T-cells suppress lymph node lymphangiogenesis under physiological and inflammatory conditions. Our study, together with the findings of Kataru et al. (22),therefore suggest that chronic T-cell inflammation resulting from sustained lymphatic fluid stasis decreases lymphatic function and increases tissue edema. These responses may then increase the expression of lymphangiogenic cytokines including VEGF-C as observed in wild-type mice leading to lymphatic hyperplasia and worsening edema (Fig. 7). Future studies will determine the source of anti-lymphangiogenic cytokines and also whether the T-cell inflammatory response is sufficient for the expression of these factors.
Fig. 7.
Conceptual diagram of the role of anti-lymphangiogenic cytokines in the regulation of lymphatic function after lymphatic injury. The molecular mechanisms by which lymphatic stasis is translated to increased VEGF-C expression remain unknown and this link is presented as a dashed line.
In the current study, we found that neutralization of IFN-γ resulted in significantly increased lymphatic vessel density after injection of CFA/OVA into the hindlimb paw. This response only occurred if IFN-γ mAb was delivered in a delayed fashion (i.e., beginning 2 days after CFA/OVA injection). The most likely explanation for this finding was that immediate IFN-γ blockade was associated with decreased inflammation at the injection site. This finding is not surprising given the important role of IFN-γ in regulation of inflammatory responses in general including macrophage activation, migration, and T-cell differentiation (21).
We also found that IFN-γ blockade increased lymph node lymphangiogenesis without increasing the expression of lymphangiogenic cytokines VEGF-A and VEGF-C. In fact, we found that IFN-γ blockade slightly decreased the expression of these cytokines, further supporting the hypothesis that IFN-γ independently contributes to the regulation of inflammatory lymphangiogenesis. Our findings are supported by Kataru et al. (22) who recently showed that IFN-γ decreases LEC tubule formation in vitro and decreases expression of Prox-1 and LYVE-1. In addition, Kataru et al. used IFN-γ-deficient mice to show that this cytokine contributes to baseline and resolution of inflammatory lymph node lymphangiogenesis. Our findings add to those of Kataru et al. since we blocked IFN-γ activity using neutralizing antibodies. This distinction is important because IFN-γ-deficient mice are known to have other immunological phenotypes, including changes in T-cell differentiation, which can, at least in theory, result in observed effects on lymphangiogenesis. Furthermore, our results are clinically relevant since they show that even short-term blockade of IFN-γ can augment lymphangiogenesis independent of effects on lymphangiogenic cytokines. This is important since some clinical scenarios could potentially benefit from such a response. For example, the use of VEGF-C or VEGF-A to augment lymphangiogenesis in patients with breast cancer, the treatment of which is a leading cause for lymphedema of the upper extremity, may result in inadvertent tumor metastasis or growth since these cytokines are also key regulators of tumor growth and lymphatic metastasis (7, 14, 19, 20, 23, 24). In contrast, blockade of anti-lymphangiogenic cytokines may augment lymphangiogenesis without these unwanted side effects.
In conclusion, we have shown that nude mice have significantly improved lymphatic function after tail lymphatic ablation compared with wild-type mice. This response is not associated with significant alterations in the expression of lymphangiogenic cytokines or marked changes in lymphangiogenesis. This finding together with marked increases in the expression of anti-lymphangiogenic cytokines in wild-type mice suggests that the balance of pro- and anti-lymphangiogenic forces regulate lymphatic function and play a critical role in the resolution of edema after lymphatic injury. T-cell inflammatory responses to sustained lymphatic fluid stasis are necessary for the expression of anti-lymphangiogenic cytokines. Finally, even short-term neutralization of IFN-γ can markedly increase lymphangiogenesis without altering the expression of lymphangiogenic cytokines.
GRANTS
Sources of funding for this work are gratefully acknowledged and include an National Institutes of Health T32 grant tor J. Zampell and E. Weitman, Society of Memorial Sloan-Kettering Grant to B. J. Mehrara, Plastic Surgery Education Foundation Research Fellowship Grants to J. Zampell and T. Avraham, and the Sloan-Kettering Institute Department of Surgery.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: J.Z., T.A., N.Y., N.F., and A.Y. performed experiments; J.Z., T.A., N.Y., N.F., A.Y., and E.S.W. analyzed data; J.Z., T.A., and A.Y. interpreted results of experiments; J.Z., N.Y., N.F., E.S.W., and B.J.M. prepared figures; J.Z. and B.J.M. drafted manuscript; J.Z., E.S.W., and B.J.M. edited and revised manuscript; J.Z. and B.J.M. approved final version of manuscript; B.J.M. conception and design of research.
REFERENCES
- 1. Alegre ML, Tso JY, Sattar HA, Smith J, Desalle F, Cole M, Bluestone JA. An anti-murine CD3 monoclonal antibody with a low affinity for Fc gamma receptors suppresses transplantation responses while minimizing acute toxicity and immunogenicity. J Immunol 155: 1544–1555, 1995 [PubMed] [Google Scholar]
- 2. Avraham T, Clavin NW, Daluvoy SV, Fernandez J, Soares MA, Cordeiro AP, Mehrara BJ. Fibrosis is a key inhibitor of lymphatic regeneration. Plast Reconstr Surg 124: 438–450, 2009 [DOI] [PubMed] [Google Scholar]
- 3. Avraham T, Daluvoy S, Zampell J, Yan A, Haviv YS, Rockson SG, Mehrara BJ. Blockade of transforming growth factor-beta1 accelerates lymphatic regeneration during wound repair. Am J Pathol 177: 3202–3214, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Avraham T, Yan A, Zampell J, Daluvoy S, Haimovitz-Friedman A, Cordeiro A, Mehrara B. Radiation therapy causes loss of dermal lymphatic vessels and interferes with lymphatic function by TGF-β1-mediated tissue fibrosis. Am J Physiol Cell Physiol 299: C589–C605, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Baker A, Kim H, Semple JL, Dumont D, Shoichet M, Tobbia D, Johnston M. Experimental assessment of pro-lymphangiogenic growth factors in the treatment of post-surgical lymphedema following lymphadenectomy. Breast Cancer Res 12: R70, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Barbul A, Shawe T, Rotter SM, Efron JE, Wasserkrug HL, Badawy SB. Wound healing in nude mice: a study on the regulatory role of lymphocytes in fibroplasia. Surgery 105: 764–769, 1989 [PubMed] [Google Scholar]
- 7. Boone B, Blokx W, De Bacquer D, Lambert J, Ruiter D, Brochez L. The role of VEGF-C staining in predicting regional metastasis in melanoma. Virchows Arch 453: 257–265, 2008 [DOI] [PubMed] [Google Scholar]
- 8. Brideau G, Makinen MJ, Elamaa H, Tu H, Nilsson G, Alitalo K, Pihlajaniemi T, Heljasvaara R. Endostatin overexpression inhibits lymphangiogenesis and lymph node metastasis in mice. Cancer Res 67: 11528–11535, 2007 [DOI] [PubMed] [Google Scholar]
- 9. Cheung L, Han J, Beilhack A, Joshi S, Wilburn P, Dua A, An A, Rockson SG. An experimental model for the study of lymphedema and its response to therapeutic lymphangiogenesis. BioDrugs 20: 363–370, 2006 [DOI] [PubMed] [Google Scholar]
- 10. Clavin NW, Avraham T, Fernandez J, Daluvoy SV, Soares MA, Chaudhry A, Mehrara BJ. TGF-β1 is a negative regulator of lymphatic regeneration during wound repair. Am J Physiol Heart Circ Physiol 295: H2113–H2127, 2008 [DOI] [PubMed] [Google Scholar]
- 11. Clavin NW, Avraham T, Fernandez JG, Daluvoy SV, Soares M, Chaudhry A, Mehrara BJ. TGF-β1 is a negative regulator of lymphatic regeneration during wound repair. Am J Physiol Heart Circ Physiol 295: H2113–H2127, 2008 [DOI] [PubMed] [Google Scholar]
- 12. Dalton DK, Pitts-Meek S, Keshav S, Figari IS, Bradley A, Stewart TA. Multiple defects of immune cell function in mice with disrupted interferon-gamma genes. Science 259: 1739–1742, 1993 [DOI] [PubMed] [Google Scholar]
- 13. Dong X, Zhao X, Xiao T, Tian H, Yun C. Endostar, a recombined humanized endostatin, inhibits lymphangiogenesis and lymphatic metastasis of Lewis lung carcinoma xenograft in mice. Thorac Cardiovasc Surg 59: 133–136, 2011 [DOI] [PubMed] [Google Scholar]
- 14. Feng Y, Wang W, Hu J, Ma J, Zhang Y, Zhang J. Expression of VEGF-C and VEGF-D as significant markers for assessment of lymphangiogenesis and lymph node metastasis in non-small cell lung cancer. Anat Rec (Hoboken) 293: 802–812, 2010 [DOI] [PubMed] [Google Scholar]
- 15. Fukumoto S, Morifuji M, Katakura Y, Ohishi M, Nakamura S. Endostatin inhibits lymph node metastasis by a down-regulation of the vascular endothelial growth factor C expression in tumor cells. Clin Exp Metastasis 22: 31–38, 2005 [DOI] [PubMed] [Google Scholar]
- 16. Gawronska-Kozak B, Bogacki M, Rim JS, Monroe WT, Manuel JA. Scarless skin repair in immunodeficient mice. Wound Repair Regen 14: 265–276, 2006 [DOI] [PubMed] [Google Scholar]
- 17. Goldman J, Conley KA, Raehl A, Bondy DM, Pytowski B, Swartz MA, Rutkowski JM, Jaroch DB, Ongstad EL. Regulation of lymphatic capillary regeneration by interstitial flow in skin. Am J Physiol Heart Circ Physiol 292: H2176–H2183, 2007 [DOI] [PubMed] [Google Scholar]
- 18. Goldman J, Le TX, Skobe M, Swartz MA. Overexpression of VEGF-C causes transient lymphatic hyperplasia but not increased lymphangiogenesis in regenerating skin. Circ Res 96: 1193–1199, 2005 [DOI] [PubMed] [Google Scholar]
- 19. Gu Y, Qi X, Guo S. Lymphangiogenesis induced by VEGF-C and VEGF-D promotes metastasis and a poor outcome in breast carcinoma: a retrospective study of 61 cases. Clin Exp Metastasis 25: 717–725, 2008 [DOI] [PubMed] [Google Scholar]
- 20. Hirakawa S, Brown LF, Kodama S, Paavonen K, Alitalo K, Detmar M. VEGF-C-induced lymphangiogenesis in sentinel lymph nodes promotes tumor metastasis to distant sites. Blood 109: 1010–1017, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hu X, Ivashkiv LB. Cross-regulation of signaling pathways by interferon-gamma: implications for immune responses and autoimmune diseases. Immunity 31: 539–550, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kataru RP, Kim H, Jang C, Choi DK, Koh BI, Kim M, Gollamudi S, Kim YK, Lee SH, Koh GY. T lymphocytes negatively regulate lymph node lymphatic vessel formation. Immunity 34: 96–107, 2011 [DOI] [PubMed] [Google Scholar]
- 23. Kazama S, Watanabe T, Kanazawa T, Hatano K, Nagawa H. Vascular endothelial growth factor-C (VEGF-C) is a more specific risk factor for lymph node metastasis than VEGF-D in submucosal colorectal cancer. Hepatogastroenterology 54: 71–76, 2007 [PubMed] [Google Scholar]
- 24. Kondo K, Kaneko T, Baba M, Konno H. VEGF-C and VEGF-A synergistically enhance lymph node metastasis of gastric cancer. Biol Pharm Bull 30: 633–637, 2007 [DOI] [PubMed] [Google Scholar]
- 25. Lahteenvuo M, Honkonen K, Tervala T, Tammela T, Suominen E, Lahteenvuo J, Kholova I, Alitalo K, Yla-Herttuala S, Saaristo A. Growth factor therapy and autologous lymph node transfer in lymphedema. Circulation 123: 613–620, 2011 [DOI] [PubMed] [Google Scholar]
- 26. Oka M, Iwata C, Suzuki HI, Kiyono K, Morishita Y, Watabe T, Komuro A, Kano MR, Miyazono K. Inhibition of endogenous TGF-beta signaling enhances lymphangiogenesis. Blood 111: 4571–4579, 2008 [DOI] [PubMed] [Google Scholar]
- 27. Ongstad EL, Bouta EM, Roberts JE, Uzarski JS, Gibbs SE, Sabel MS, Cimmino VM, Roberts MA, Goldman J. Lymphangiogenesis-independent resolution of experimental edema. Am J Physiol Heart Circ Physiol 299: H46–H54, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ou J, Li J, Pan F, Xie G, Zhou Q, Huang H, Liang H. Endostatin suppresses colorectal tumor-induced lymphangiogenesis by inhibiting expression of fibronectin extra domain a and integrin alpha9. J Cell Biochem 112: 2106–2114, 2011 [DOI] [PubMed] [Google Scholar]
- 29. Petrek JA, Heelan MC. Incidence of breast carcinoma-related lymphedema. Cancer 83: 2776–2781, 1998 [DOI] [PubMed] [Google Scholar]
- 30. Petrek JA, Senie RT, Peters M, Rosen PP. Lymphedema in a cohort of breast carcinoma survivors 20 years after diagnosis. Cancer 92: 1368–1377, 2001 [DOI] [PubMed] [Google Scholar]
- 31. Rutkowski JM, Boardman KC, Swartz MA. Characterization of lymphangiogenesis in a model of adult skin regeneration. Am J Physiol Heart Circ Physiol 291: H1402–H1410, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rutkowski JM, Moya M, Johannes J, Goldman J, Swartz MA. Secondary lymphedema in the mouse tail: Lymphatic hyperplasia, VEGF-C upregulation, and the protective role of MMP-9. Microvasc Res 72: 161–171, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Saaristo A, Veikkola T, Tammela T, Enholm B, Karkkainen MJ, Pajusola K, Bueler H, Yla-Herttuala S, Alitalo K. Lymphangiogenic gene therapy with minimal blood vascular side effects. J Exp Med 196: 719–730, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Saito Y, Nakagami H, Morishita R, Takami Y, Kikuchi Y, Hayashi H, Nishikawa T, Tamai K, Azuma N, Sasajima T, Kaneda Y. Transfection of human hepatocyte growth factor gene ameliorates secondary lymphedema via promotion of lymphangiogenesis. Circulation 114: 1177–1184, 2006 [DOI] [PubMed] [Google Scholar]
- 35. Suami H, Pan WR, Taylor GI. Changes in the lymph structure of the upper limb after axillary dissection: radiographic and anatomical study in a human cadaver. Plast Reconstr Surg 120: 982–991, 2007 [DOI] [PubMed] [Google Scholar]
- 36. Szuba A, Skobe M, Karkkainen MJ, Shin WS, Beynet DP, Rockson NB, Dakhil N, Spilman S, Goris ML, Strauss HW, Quertermous T, Alitalo K, Rockson SG. Therapeutic lymphangiogenesis with human recombinant VEGF-C. FASEB J 16: 1985–1987, 2002 [DOI] [PubMed] [Google Scholar]
- 37. Uzarski J, Drelles MB, Gibbs SE, Ongstad EL, Goral JC, McKeown KK, Raehl AM, Roberts MA, Pytowski B, Smith MR, Goldman J. The resolution of lymphedema by interstitial flow in the mouse tail skin. Am J Physiol Heart Circ Physiol 294: H1326–H1334, 2008 [DOI] [PubMed] [Google Scholar]
- 38. Yan A, Avraham T, Zampell J, Aschen S, Mehrara B. Mechanisms of lymphatic regeneration after tissue transfer. PLos One 6: e17201, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Yoon YS, Murayama T, Gravereaux E, Tkebuchava T, Silver M, Curry C, Wecker A, Kirchmair R, Hu CS, Kearney M, Ashare A, Jackson DG, Kubo H, Isner JM, Losordo DW. VEGF-C gene therapy augments postnatal lymphangiogenesis and ameliorates secondary lymphedema. J Clin Invest 111: 717–725, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zampell JC, Yan A, Avraham T, Andrade V, Malliaris S, Aschen SZ, Rockson SG, Mehrara BJ. Temporal and spatial patterns of endogenous danger signal expression after wound healing and in response to lymphedema. Am J Physiol Cell Physiol 300: C1107–C1121, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]