Abstract
Voltage-gated K+ (KV) channels are implicated in detrusor smooth muscle (DSM) function. However, little is known about the functional role of the heterotetrameric KV channels in DSM. In this report, we provide molecular, electrophysiological, and functional evidence for the presence of KV2.1 and electrically silent KV channel subunits in guinea pig DSM. Stromatoxin-1 (ScTx1), a selective inhibitor of the homotetrameric KV2.1, KV2.2, and KV4.2 as well as the heterotetrameric KV2.1/6.3 and KV2.1/9.3 channels, was used to examine the role of these KV channels in DSM function. RT-PCR indicated mRNA expression of KV2.1, KV6.2–6.3, KV8.2, and KV9.1–9.3 subunits in isolated DSM cells. KV2.1 protein expression was confirmed by Western blot and immunocytochemistry. Perforated whole cell patch-clamp experiments revealed that ScTx1 (100 nM) inhibited the amplitude of the KV current in freshly isolated DSM cells. ScTx1 (100 nM) did not significantly change the steady-state activation and inactivation curves for KV current. However, ScTx1 (100 nM) decreased the activation time-constant of the KV current at positive voltages. Although our patch-clamp data could not exclude the presence of the homotetrameric KV2.1 channels, the biophysical characteristics of the ScTx1-sensitive current were consistent with the presence of heterotetrameric KV2.1/silent KV channels. Current-clamp recordings showed that ScTx1 (100 nM) did not change the DSM cell resting membrane potential. ScTx1 (100 nM) increased the spontaneous phasic contraction amplitude, muscle force, and muscle tone as well as the amplitude of the electrical field stimulation-induced contractions of isolated DSM strips. Collectively, our data revealed that KV2.1-containing channels are important physiological regulators of guinea pig DSM excitability and contractility.
Keywords: urinary bladder, patch clamp, reverse transcriptase-polymerase chain reaction, Western blot, immunocytochemistry, stromatoxin-1
detrusor smooth muscle (DSM), which makes up the bladder wall, relaxes during bladder filling and contracts phasically during voiding (2). The underlying cause of the spontaneous phasic DSM contractions is the spontaneous action potential and corresponding Ca2+ transients (19–22, 25–27, 47). Initiation of the action potential in guinea pig DSM arises from the opening of L-type voltage-gated Ca2+ channels followed by an increase in the intracellular Ca2+ concentration (25). The repolarization phase of the DSM action potential is initiated by activation of large-conductance Ca2+-activated K+ (BK) channels, voltage-gated K+ (KV) channels (56), and negative feedback Ca2+-mediated inhibition of the L-type voltage-gated Ca2+ channels (7, 11, 19, 26, 35, 46, 48), whereas small-conductance Ca2+-activated K+ (SK) channels and KV channels are responsible for the slow afterhyperpolarization (16, 20, 56). The resting membrane potential and action potential frequency are considered to be regulated by KATP, BK, and KV channels (7, 16, 19, 46, 47, 56). However, there remains a substantial gap in our knowledge regarding the cellular and functional roles, expression, and regulation of different subtypes of K+ channels in DSM.
Of all ion channels that control DSM excitability, the KV channels are the most diverse superfamily (45, 60). These channels have been identified in many different types of smooth muscle (5, 50, 53), including DSM (9, 13, 56). The expression of KV2.1 and KV2.2 channels has been demonstrated in rat DSM (9, 17, 41). Expression of KV1 channels has been shown in human DSM (13) and of KV2.1, KV5.1, KV6.1, KV6.2, and KV6.3 subunits in mouse DSM (14, 56).
The structural diversity of the KV channels contributes to the variety of their biophysical and pharmacological properties and makes them potentially attractive targets for pharmacological treatment of diseases such as overactive bladder (OAB) and urinary incontinence (UI) (3, 9, 45, 60). Further investigation in this area may lead to development of selective pharmacological or genetic therapies for OAB and UI.
In the human genome, the members of the KV superfamily are encoded by 40 genes and comprise 12 KV channel families (45, 60). Only KV1–KV4, KV7, KV11 (also known as hERG), and KV12 (also known as elk) channel families form pore-conducting channels, homo- or heterotetramers, whereas the other members of the KV families, KV5.1, KV6.1–6.4, KV8.1–8.2, and KV9.1-KV9.3, are electrically silent and do not form functional channels when expressed alone (8, 36, 42, 44, 45, 54, 55). Instead, they coassemble with KV2 family, thus increasing KV channel diversity (45, 52, 60). Those channels form heterotetrameric channels with KV2 subunits, and their activation, deactivation, inactivation, and recovery from inactivation properties differ from those reported for homotetrameric KV2 channels (8, 36, 42, 44, 51, 52). For example, the KV9.3 subunit causes an important alteration of the channel biophysical properties and increases the single-channel conductance of the heterotetrameric KV2.1 channel (44). This modulatory KV subunit combines with KV2.1 to form a functional heterotetrameric KV2.1/9.3 channel consisting of three KV2.1 subunits and one KV9.3 subunit (33). Expression of the electrically silent KV6.3 and KV9.3 subunit has been reported in vascular smooth muscle cells (4, 12, 23, 38–40, 43, 44, 49, 58, 59, 61). It is suggested that the heterotetrameric KV2.1/9.3 channel contributes to the excitability in oxygen-sensitive pulmonary myocytes, and it is involved in hypoxic pulmonary vasoconstriction (44). Structural, biophysical, and pharmacological properties of the heterotetrameric KV2.1/silent KV channels have been extensively studied (14, 32–34, 40, 44), but their physiological roles in smooth muscle cells, including DSM cells, remain unclear.
In the past, the study of KV channels' function has been hampered by the lack of selective inhibitors. Stromatoxin-1 (ScTx1), a spider venom peptide isolated from the African tarantula (Stromatopelma calceata), was recently identified as a highly selective inhibitor of the KV2.1, KV2.2, and KV4.2 homotetramers as well as the KV2.1/6.3 and KV2.1/9.3 heterotetramers (14, 15, 40). Studies in neurons, cerebral arteries, urethra, and DSM have confirmed that ScTx1 is a useful pharmacological tool to study the functional role of these KV channels (1, 6, 9, 37, 40, 61).
The aim of this study was to examine the expression and function of KV2.1 and electrically silent KV subunits in guinea pig DSM excitability and contractility. We applied a multidisciplinary approach to this investigation, using molecular biological techniques, patch-clamp electrophysiology, isometric tension recordings of isolated guinea pig DSM strips, and pharmacological protocols using ScTx1.
MATERIALS AND METHODS
Animal care and tissue collection.
All animal studies were reviewed and approved by the University of South Carolina Institutional Animal Care and Use Committee and carried out in accordance with the Animal Use Protocol 1747. Eighty-five adult Harley albino guinea pigs (Charles River Laboratories) were used in this study (54 males and 31 females, average weight 372.8 ± 2.1 g). Guinea pigs were euthanized with CO2, followed by thoracotomy. The urinary bladder was quickly removed and placed in an ice-cold, Ca2+-free dissection solution (see Solutions and drugs) or in RNAlater solution (Qiagen, Hilden, Germany) for the RT-PCR experiments. The urinary bladder was then cut open, the entire mucosal and urothelium layers were carefully removed, and only the smooth muscle tissue from the detrusor was used. For RT-PCR and Western blot experiments, the guinea pig brains were also removed and placed in RNAlater solution.
DSM single-cell isolation.
DSM single cells for RT-PCR, immunocytochemistry, and electrophysiology were freshly isolated and collected as previously described (7, 9, 10, 31). Briefly, isolated DSM was cut in strips (5–8 mm long, 2–4 mm wide), and 1–2 strips were placed in dissection solution (2 ml) supplemented with 1 mg/ml bovine serum albumin (BSA), 1 mg/ml papain (Worthington Biochemical, Lakewood, NJ), and 1 mg/ml dl-dithiothreitol and incubated for 25–30 min at 37°C. The DSM strips were transferred to dissection solution (2 ml) containing 1 mg/ml BSA, 1 mg/ml collagenase (type II from Sigma-Aldrich, St. Louis, MO), and 100 μM CaCl2 and incubated for 5–6 min at 37°C. DSM strips were then washed with fresh BSA-containing dissection solution. The individual DSM cells were dispersed when the enzyme-treated tissue was passed through the tip of a Pasteur pipette and then used for electrophysiology, RT-PCR, and immunocytochemistry studies. DSM cells for patch-clamp experiments were stored at 4°C for further use.
RNA extraction, RT-PCR, and sequencing.
For RT-PCR experiments, whole DSM tissue and freshly isolated single DSM cells were immediately used for RNA extraction. RT-PCR experiments were conducted as previously described, and the single-cell RT-PCR experiments were carefully controlled for contaminations from non-DSM cells (9, 10). Specific primers for KV2.1, KV2.2, KV4.2, KV5.1, KV6.1–6.3, KV8.1–8.2, and KV9.1–9.3 were designed based on the sequences of multiple species present in GenBank and aligned using Primer Premier 5 software (PREMIER Biosoft International, Palo Alto, CA). Specific primer pair sequences are listed in Table 1. The cDNA production was amplified using GoTaq green master mix (Promega) and specific primers for KV channel subunits. The PCR annealing temperature for each primer pair was optimized using a Mastercycler gradient thermocycler (Eppendorf, Hamburg, Germany). Negative control experiments for PCR were performed in the absence of the reverse transcriptase (−RT). Guinea pig brain tissue, which is known to express the studied KV channels, was used as a positive control. PCR products were purified using the GenElute PCR clean-up kit (Sigma-Aldrich) and sequenced at the University of South Carolina Environmental Genomics Facility for sequence confirmation.
Table 1.
RT-PCR primers for subunits of the KV channels sensitive to ScTx1 and for the silent KV channel subunits
| Sense | Antisense | Product Size, bp | |
|---|---|---|---|
| KV2.1 | CTCCACCATTGCCCTGTC | TCCGCTTGATTGCTTTCTC | 695 |
| KV2.2 | TCCCAGGAACAGATGAGC | GAGTGGTGAGCGGAAAGT | 324 |
| KV4.2 | CTTCACTATCCCCGCCAT | GTTTCCACCACATTCGCG | 308 |
| Kv5.1 | GCGAAGACATTGAGATCGTG | CGTCCAAGATGAGCTGCAC | 393 |
| Kv6.1 | CTGGACAGCGAGGATCAAG | TACCATGTCTCCGTAGCCT | 731 |
| Kv6.2 | CGAACGTGTACTGTCATCA | GCTCGTGCACCTGGCTG | 309 |
| Kv6.3 | ATGACGACGGTGGGCTAT | CTGCTCCTGCTCCCTCTTG | 256 |
| Kv8.1 | ACCTCCTTGCCATCTTGC | TTGCCTGTCGTGGTGTCT | 385 |
| Kv8.2 | GAGGAGCAGCGGGACAGCAA | AGCGGCGGAGAAGGCAAAG | 181 |
| Kv9.1 | CCCCATCACCATCATCTTCAA | CGTCAACGCTACTCAGCAAGTC | 119 |
| Kv9.2 | TGTGCGTCTTCTCCTTCA | CTGGTCACTCTGCTCGTC | 137 |
| KV9.3 | ACAGTGGGCTATGGAGAC | GATGCCAATGGAAGACAG | 273 |
ScTx1, stromatoxin-1.
Western blot analysis.
Protein extraction and Western blot analysis were performed as previously described (9, 10). The blots were incubated with the affinity-purified rabbit polyclonal primary antibodies anti-KV2.1 (DRK1, 1:400), anti-KV2.2 (CDRK, 1:2,000), and anti-KV4.2 (Kcnd2, 1:200; Alomone Labs, Jerusalem, Israel) overnight at 4°C. The membrane was then incubated with goat anti-rabbit IgG conjugated with horseradish peroxidase diluted to 1:3,000 in the blocking buffer for 1 h at room temperature. Bound antibodies were detected using an ECL substrate kit (Amersham, Piscataway, NJ) according to the manufacturer's instructions. Staining specificity was verified by preincubation of the antibodies with a competing peptide.
Immunocytochemistry.
Immunocytochemistry was performed as previously described (9, 10). DSM cells were incubated with the rabbit polyclonal primary antibodies anti-KV2.1, anti-KV2.2, and anti-KV4.2 (DRK1, CDRK, and Kcnd2, 1:100; Alomone Labs) at 37°C for 1 h. Next, cells were labeled with secondary antibodies (Cy3-conjugated anti-rabbit IgG, 1:200 in PBS/3% normal donkey serum/0.01% Triton X-100; Jackson ImmunoResearch, West Grove, PA) for 1 h in a dark room. After labeling, DSM cells were washed with PBS and incubated with phalloidin for 2 h in the dark. Cells were then washed two more times, incubated with 4′,6-diamidino-2-phenylindole for 15 min and washed again, and then mounted onto slides with DABCO (1,4-diazabicyclo-2–2-2-octane). Control treatments were carried out as follows: 1) omission of the primary antibody for confirming the specificity of the secondary antibody and 2) absorption of the primary antibody by a competing peptide for confirming the specificity of the primary antibody. Images at ×63 magnification were acquired with an LSM 510 META confocal microscope (Carl Zeiss, Göttingen, Germany). The slides for each group were imaged with the same laser power, gain settings, and pinhole for the controls and antibody treatment.
Electrophysiological patch-clamp recordings.
To preserve the physiological environment of the DSM cells, the whole cell perforated patch-clamp technique was applied in all electrophysiological experiments (18, 29). Freshly isolated DSM cells were used for patch-clamp experiments within 12 h after isolation. Isolated DSM cells were placed in an experimental chamber for at least 20 min to allow them to adhere to the glass bottom and were then washed with extracellular solution (see Solutions and drugs). Only DSM cells with a spindle shape, bright appearance, and well-defined cell edges were used. The ionic currents were recorded using an Axopatch 200B amplifier, Digidata 1440A, and pCLAMP 10.2 software (Molecular Devices, Sunnyvale, CA) and then filtered using an eight-pole Bessel filter 900CT/9L8L (Molecular Devices). The glass pipettes used for the patch-clamp experiments were made from borosilicate glass (Sutter Instruments, Novato, CA) and pulled using a Narishige PP-830 vertical puller (Narishige Group, Tokyo, Japan). The pipettes were polished with a Micro Forge MF-830 fire polisher (Narishige Group) and coated with dental wax to reduce capacitance.
In all voltage-clamp experiments, the BK channel was blocked with 100 nM paxilline, a BK channel-selective inhibitor. To study the KV currents, we applied various voltage-step protocols. The leak current was not subtracted during the experiments. The average current value during the last 50 ms of the 400-ms depolarization step was taken for the KV current. For the controls, current traces of at least 5 pulses within 10 min were recorded and averaged. Only cells with stable control currents in response to depolarization steps within at least 10 min were used to study ScTx1 effects. All patch-clamp experiments were carried out at room temperature.
Isometric DSM tension recordings.
Isometric tension recordings of guinea pig DSM isolated strips were performed as previously described (7, 9, 31). Briefly, small DSM muscle strips (5–8 mm long, 2–3 mm wide) were placed between a stationary mount and a force-displacement transducer in thermostatically controlled (at 37°C) 10-ml tissue baths filled with Ca2+-containing physiological salt solution (see Solutions and drugs). Strips were stretched to 1 g of tension and were then washed every 15 min during an equilibration period of 45–60 min. Electrical field stimulation (EFS) was generated using a PHM-152I stimulator (Med Associates, Georgia, VT). DSM contractile responses were recorded using MyoMed software (Med Associates).
Solutions and drugs.
The Ca2+-free dissection solution had the following composition (in mM): 80 monosodium glutamate, 55 NaCl, 6 KCl, 10 glucose, 10 HEPES, and 2 MgCl2, pH 7.3, adjusted with NaOH. The Ca2+-containing physiological salt solution was prepared daily and contained (in mM) 119 NaCl, 4.7 KCl, 24 NaHCO3, 1.2 KH2PO4, 2.5 CaCl2, 1.2 MgSO4, and 11 glucose and was aerated with 95% O2-5% CO2 to obtain pH 7.4. The extracellular solution used in the electrophysiological experiments contained (in mM) 134 NaCl, 6 KCl, 1 MgCl2, 2 CaCl2, 10 glucose, and 10 HEPES; pH was adjusted to 7.4 with NaOH. The pipette solution contained (in mM) 110 potassium aspartate, 30 KCl, 10 NaCl, 1 MgCl2, 10 HEPES, and 0.05 EGTA, pH adjusted to 7.2 with NaOH, and was supplemented with freshly dissolved 200 μg/ml amphotericin-B. ScTx1 was purchased from Alomone Labs. dl-Dithiothreitol, paxilline, tetrodotoxin (TTX), carbachol, and collagenase type II were obtained from Sigma-Aldrich. BSA and amphotericin-B were obtained from Fisher Scientific. Papain was obtained from Worthington Biochemical.
Data analysis and statistics.
Clampfit version 10.2 software (Molecular Devices) was used for electrophysiological data analyses, MiniAnalysis software (Synaptosoft, Decatur, GA) was used for data analyses of DSM contraction, and GraphPad Prism 4.03 software (GraphPad Software, La Jolla, CA) was used for statistical analyses. For data presentation, CorelDraw Graphic Suite X3 software (Corel, Ottawa, ON, Canada) was used. Results are summarized as means ± SE for the number of DSM strips or cells (n) and the number of guinea pigs (N). Data were compared using ANOVA or paired Student's t-test. P values <0.05 were considered statistically significant.
RESULTS
RT-PCR.
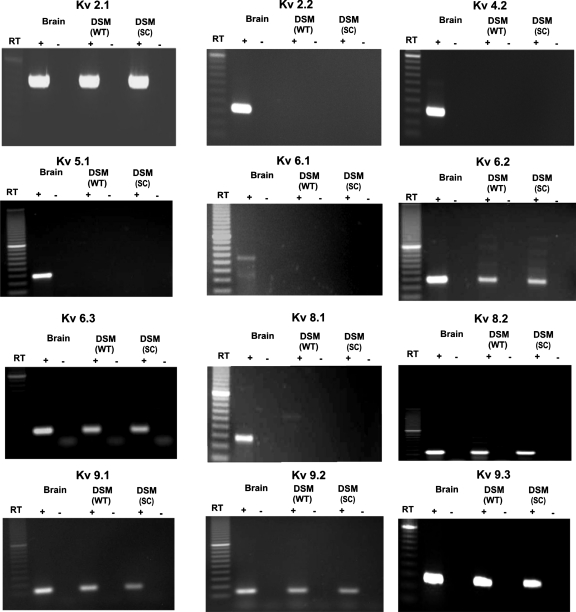
RT-PCR experiments were conducted in both whole DSM tissues and freshly isolated single DSM cells to determine the expression of mRNA messages for the subunits of the known ScTx1-sensitive KV channels (KV2.1, KV2.2, and KV4.2) as well as all KV silent subunits (KV5.1, KV6.1–6.3, KV8.1–8.2, and KV9.1–9.3) that associate with the KV2 family. The expression of mRNA message for all these KV channel subunits was detected in guinea pig brain, which was used as a positive control (Fig. 1). However, the whole DSM tissue expressed detectible mRNA messages for KV2.1, KV6.2, KV6.3, KV8.2, and KV9.1–9.3 but not for KV2.2, KV4.2, KV5.1, KV6.1, and KV8.1 channel subunits (Fig. 1). The presence of other cell types within the DSM layer, such as neurons, fibroblasts, and vascular myocytes, may lead to the detection of KV channel subunits expressed in cell types other than DSM cells. To eliminate the possible contamination from other cell types, we applied single-cell RT-PCR experiments to freshly isolated DSM cells. Again, the single-cell RT-PCR confirmed the expression of mRNA messages for KV2.1, KV6.2, KV6.3, KV8.2, and KV9.1–9.3 but not for KV2.2, KV4.2, KV5.1, KV6.1, and KV8.1 subunits (Fig. 1). A lack of genomic DNA contamination in mRNA isolated from single DSM cells was also confirmed by using the negative control reactions lacking the reverse transcriptase. All purified PCR products were sequenced to confirm their identity. Results were verified in 18 different preparations, obtained from 16 guinea pigs. We next applied Western blot analysis and immunocytochemistry to detect whether proteins for the three main channel subunits that can form their own homotetramers (KV2.1, KV2.2, and KV4.2) are expressed in DSM.
Fig. 1.
Detection of mRNA message for KV channel subunits in detrusor smooth muscle (DSM) whole tissue (WT) and freshly isolated DSM single cells (SC). mRNA message was detected for KV2.1, KV6.2, KV6.3, KV8.2, and KV9.1–9.3 but not for KV2.2, KV4.2, KV5.1, KV6.1, and KV8.1 channel subunits. The base pair sizes for each product are included in Table 1. No products were observed in the negative controls in which reverse transcriptase (RT) was left out of the reaction. Guinea pig brain tissue was used as a positive control.
Western blot analysis.
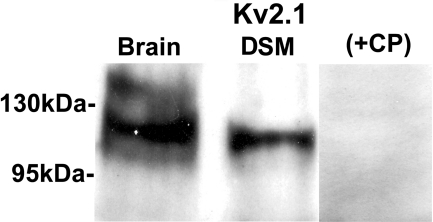
Using commercial KV channel subunit-specific antibodies, we confirmed protein expression of KV2.1 subunit in whole DSM tissue with Western blot experiments (Fig. 2). Preabsorption of the primary antibody with its antigenic competing peptide indicated the specificity of the antibodies for their intended epitope. Consistent with our RT-PCR data, no KV2.2 and KV4.2 proteins were detected in whole DSM tissue (data not shown). The protein expression for each separate KV channel subunit was verified in three separate Western blot reactions using proteins isolated from three guinea pigs.
Fig. 2.
Western blot detection of KV2.1 channel protein expression in whole DSM tissues. The immunoreactive band in DSM tissue was eliminated by a competing peptide (+CP). Guinea pig brain tissue was used as a positive control.
Immunocytochemistry.
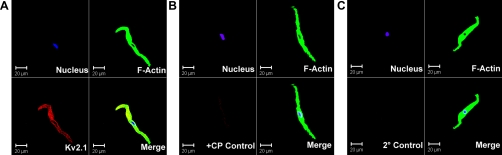
To further verify the presence of KV2.1 channel subunits in guinea pig DSM, we immunocytochemically labeled freshly isolated DSM cells. Freshly isolated DSM cells had bright, distinct edges under phase-contrast optics. Anti-KV2.1 antibody specifically labeled the freshly isolated cells (Fig. 3). However, immunofluorescence staining was not detected for KV2.2 and KV4.2 channel subunits in isolated DSM cells (data not shown), which is consistent with our RT-PCR and Western blot experiments. The immunocytochemical experiments were carefully controlled for specificity by the omission of the primary antibody or absorption of the primary antibody by a competing peptide (Fig. 3). Results were verified in eight cells, obtained from three guinea pigs.
Fig. 3.
Immunocytochemical detection of KV2.1 channel in freshly isolated single DSM cells using channel-specific antibody. Red staining (bottom left panels) indicates detection of KV2.1 subunit (A); no staining was visible after absorption of the primary antibody with a competing peptide (B; +CP control) or after the primary antibody was omitted and cells were incubated with the secondary antibody only (C; 2° control). Cells nuclei are shown in blue (top left panels); F-actin is shown in green (top right panels). The merged images are also shown (bottom right panels). Images at ×63 magnification were obtained via confocal microscopy.
Voltage dependence and ScTx1 sensitivity of guinea pig DSM whole cell KV current.
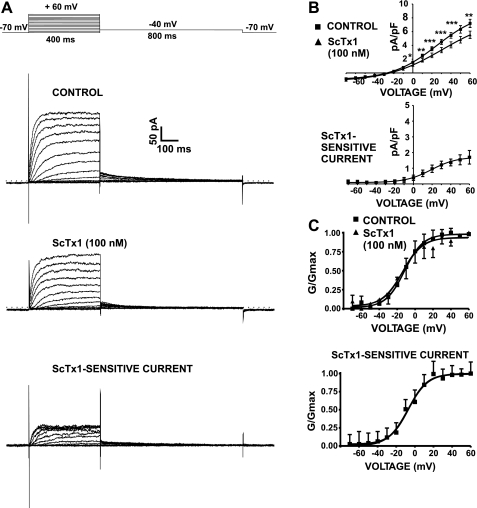
All voltage-clamp experiments were conducted in the presence of 100 nM paxilline to eliminate the contribution of the BK channels to the total whole cell current and to study the remaining KV current. The average DSM cell capacitance was 28.2 ± 1.2 pF (n = 155 cells; N = 61 guinea pigs). In the first experimental series, we applied a 400-ms voltage-step protocol, illustrated in Fig. 4A. A series of potentials between −70 and +60 mV were applied from a holding potential (Vh) of −70 mV in 10-mV increments, and then cells were repolarized to −40 mV for another 800 ms. The KV current amplitude increased steeply with increasing the membrane potential, and small deactivating tail currents were recorded on repolarization to −40 mV (Fig. 4A). Application of ScTx1 (100 nM) caused a significant inhibition of the whole cell KV current amplitude (n = 12; N = 9; P < 0.05; Fig. 4). The remaining ScTx1-insensitive current is possibly determined by other types of KV channels. The inhibitory effect of ScTx1 (100 nM) on the current-voltage relationship in guinea pig DSM cells is shown in Fig. 4B (n = 12; N = 9; P < 0.05).
Fig. 4.
Voltage-dependent activation of stromatoxin-1 (ScTx1)-sensitive KV currents in guinea pig DSM cells. A: representative recording of the effect of ScTx1 on the whole cell KV current elicited by a short (400 ms) voltage-step protocol (top). ScTx1 (100 nM) significantly decreased the voltage step-induced whole cell KV current. ScTx1-sensitive current was obtained after subtraction of the remaining whole cell current from the control current. The scale bar applies to all recordings. B: the current-voltage relations show that ScTx1 (100 nM) decreased voltage-dependent whole cell KV current in guinea pig DSM cells (n = 12; N = 9). *P < 0.05; **P < 0.005; ***P < 0.001. The current-voltage relation of the subtracted ScTx1-sensitive current is also shown. C: steady-state activation curves of the KV current in the absence (control) or presence of 100 nM ScTx1 (ScTx1-insensitive current). Data were obtained from the current recordings shown in A. The tail currents recorded after stepping back to −40 mV for 800 ms were used to build the normalized conductance (G/Gmax) curve. The curves were fitted using a single-component Boltzmann equation. The steady-state activation curve of the ScTx1-sensitive current is also shown. Values are means ± SE. All experiments were performed in the presence of the selective BK channel inhibitor paxilline (100 nM).
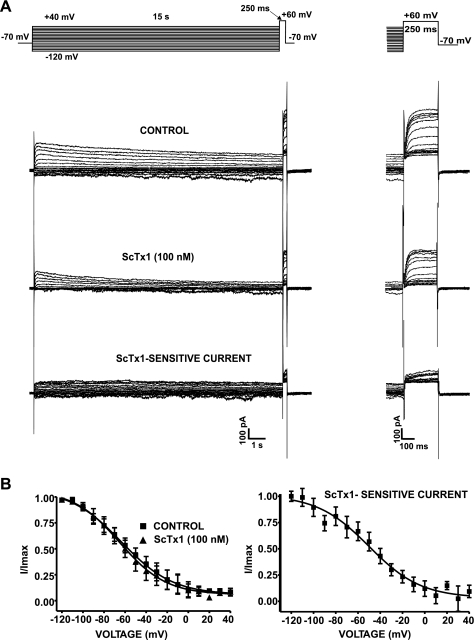
In the second experimental series, a long-lasting double-pulse protocol was applied (Fig. 5A). The cell membrane potential was stepped from a Vh of −70 mV to potentials between −120 and +40 mV for 15 s, before stepping to +60 mV for 250 ms to determine the current availability at the end of each presiding step. As is evident from Fig. 5A, the KV currents at various voltages showed a slow pattern of inactivation. Typical current traces of KV current elicited by this protocol before and after application of 100 nM ScTx1 are shown in Fig. 5A (n = 7; N = 5).
Fig. 5.
Voltage-dependent inactivation of ScTx1-sensitive KV currents in guinea pig DSM cells. A: representative recording of the effect of ScTx1 on the whole cell KV current elicited by a 15-s long-lasting double-pulse voltage-step protocol (top). The scale bar applies to all recordings. The expanded scale at right shows the currents during the 250-ms test pulse to +60 mV. B: steady-state KV current inactivation curves show voltage dependence of inactivation in the absence (control) or presence of 100 nM ScTx1 (n = 7; N = 5). The inactivation curves were obtained by plotting the normalized peak currents (I/Imax) recorded during the 250-ms test pulse to +60 mV as a function of the voltage of the 15-s prepulse. Data were fitted using a single-component Boltzmann equation. The steady-state inactivation curve of the subtracted ScTx1-sensitive current is also shown. Values are means ± SE. All experiments were performed in the presence of the selective BK channel inhibitor paxilline (100 nM).
Effects of ScTx1 on the steady-state activation and inactivation of the KV current.
We examined whether ScTx1 (100 nM) can shift the steady-state activation and inactivation curves of the KV current. Voltage-dependent activation and inactivation of ScTx1-sensitive current was studied with the voltage protocols shown in Figs. 4A and 5A, respectively. Activation curves of the KV current in the presence or absence of 100 nM ScTx1 (Fig. 4C) were obtained from the tail currents recorded after stepping back to −40 mV (Fig. 4A). The activation curves are plotted as normalized conductance (G/Gmax) against voltage and are fitted using a single-component Boltzmann equation (Fig. 4C). ScTx1 (100 nM) did not significantly affect the half-maximal activation potential (V0.5) of the steady-state activation. The V0.5 was −11.7 ± 0.8 mV in the absence of ScTx1 and −14.5 ± 2.2 mV in the presence of 100 nM ScTx1 (Fig. 4B; Table 2). The slope factor (k) of the steady-state activation curve remained unchanged by 100 nM ScTx1, and it was 10.1 ± 0.8 mV under control conditions and 11.5 ± 2.0 mV in the presence of 100 nM ScTx1 (Fig. 4B).
Table 2.
Comparison of steady-state properties of total whole cell KV, ScTx1-sensitive, ScTx1-insensitive currents and published data on the homotetrameric KV2.1 and heterotetrameric KV2.1/silent KV channel currents
| Steady-State Activation |
Steady-State Inactivation |
|||
|---|---|---|---|---|
| V0.5, mV | Ref. | V0.5, mV | Ref. | |
| Control current in guinea pig DSM | −11.7 ± 0.8 | −63.6 ± 1.7 | ||
| ScTx1-insensitive current in guinea pig DSM | −14.5 ± 2.2 | −65.3 ± 1.7 | ||
| ScTx1-sensitive current in guinea pig DSM | −6.9 ± 1.4 | −50.1 ± 3.7 | ||
| KV current in mouse DSM | 1.1 ± 1.3 | 56 | −61.4 ± 1.2 | 56 |
| ScTx1-sensitive current in rabbit urethra | −7.0 ± 5.0 | 37 | −55.0 ± 3.0 | 37 |
| KV2.1 current | −1.7 | 36 | −30.1 | 36 |
| +12.2 ± 1.4 | 42 | −15.9 ± 2 | 42 | |
| +1.0 ± 1.0 | 34 | −18.4 ± 2.8 | 6 | |
| +7.8 | 12a | −28.3 | 12a | |
| +20.4 | 44 | −34.7 ± 0.6 | 34 | |
| −20.5 ± 0.9 | 52 | |||
| KV2.1/6.2 current | −10 | 62 | NA | |
| KV2.1/6.3 current | −13 ± 1.8 | 52a | −55.6 ± 1.1 | 42 |
| KV2.1/8.2 current | −2.8 | 12a | −30.6 | 12a |
| KV2.1/9.1 current | NA | −33.3 ± 0.2 | 52 | |
| KV2.1/9.2 current | NA | −35.7 ± 3.2 | 52 | |
| KV2.1/9.3 current | −9.5 ± 2 | 34 | −54.8 ± 1.8 | 34 |
| +7.2 | 14 | −47.2 ± 0.7 | 6 | |
| +3.2 | 44 | −44.9 | 44 | |
Values are means ± SE of half-maximal potentials (V0.5) of steady-state activation and inactivation from our experiments in guinea pig detrusor smooth muscle (DSM), as well as data from the literature for currents in other species and for homotetrameric KV2.1 and heterotetrameric KV2.1/silent KV channel currents. NA, not available.
We next evaluated the effect of ScTx1 (100 nM) on the steady-state inactivation curve. Using the protocol shown in Fig. 5A, steady-state inactivation curves were obtained from the maximum value of the normalized end-pulse current amplitudes (I/Imax) recorded at +60 mV and plotted against test potentials. KV current steady-state inactivation curves in the presence and absence of 100 nM ScTx1, fitted by a single-component Boltzmann equation, are shown in Fig. 5B. There was no significant difference in the V0.5, which was −63.6 ± 1.7 mV in the absence of ScTx1 and −65.3 ± 1.7 mV in the presence of 100 nM ScTx1 (n = 7; N = 5; P > 0.05; Fig. 5B). This V0.5 value is consistent with data from mouse DSM cells (56). The slope factor k of the steady-state inactivation curves also remained unchanged, and it was −24.5 ± 1.7 mV under control conditions and −22.1 ± 1.8 mV in the presence of 100 nM ScTx1 (n = 7; N = 5; P > 0.05; Fig. 5B). We also found that the total KV current of guinea pig DSM cells did not completely inactivate (Fig. 5B).
The ScTx1-sensitive current was evaluated after subtraction from the total KV current and the remaining current after application of 100 nM ScTx1 (Figs. 4 and 5). All parameters of the ScTx1-sensitive current were elucidated and are shown in Tables 2 and 3. The half-maximal activation of the ScTx1 current was V0.5 = −6.9 ± 1.4 mV, and the slope factor k was 10.2 ± 1.2 mV (Fig. 4C). The half-maximal steady-state inactivation was V0.5 = −50.1 ± 3.7 mV, and the slope factor k was −23.4 ± 3.9 mV (n = 7; N = 5; P > 0.05; Fig. 5B). Comparisons of the steady-state activation and inactivation parameters of the ScTx1-sensitive current are shown in Table 2. Table 2 also presents published data on homotetrameric KV2.1 and heterotetrameric KV2.1/silent KV channels in heterologous expression systems and native tissues such as neurons, arterial and urethral smooth muscle, and mouse DSM. Most importantly, as shown in Table 2, the steady-state properties of the ScTx1-sensitive KV current correspond to a channel population of homotetrameric KV2.1 and heterotetrameric KV2.1/silent KV channels.
Table 3.
Comparison of time constants of total whole cell KV, ScTx1-sensitive, and ScTx1-insensitive currents and published data on the homotetrameric KV2.1 and heterotetrameric KV2.1/silent KV channel currents
| Time Constant of Activation |
Time Constant of Inactivation |
|||
|---|---|---|---|---|
| τ, ms | Ref. | τ, s | Ref. | |
| Control current in guinea pig DSM | 25.5 ± 2.1 (+40 mV) | 5.4 ± 0.8 (+40 mV) | ||
| ScTx1-insensitive current in guinea pig DSM | 23.7 ± 1.2 (+40 mV) | 5.4 ± 1.3 (+40 mV) | ||
| ScTx1-sensitive current in guinea pig DSM | 35.3 ± 3.6 (+40 mV) | 5.9 ± 1.6 (+40 mV) | ||
| KV2.1 current | 15.6 ± 0.9 (+30 mV) | 52 | 7.0 (+40 mV) | 34 |
| 21 ± 1 (+20 mV) | 34 | |||
| 7 (+10 mV) | 44 | |||
| 74 ± 3 (−10 mV) | 34 | |||
| 68.4 ± 6.8 (+30 mV) | 42 | |||
| KV current in mouse DSM | 29.9 ± 2.0 (+30 mV) | 56 | 3.4 ± 0.2 (+20 mV) | 56 |
| KV2.1/KV6.3 | 22.6 ± 3.2 (+30 mV) | 42 | NA | |
| KV2.1/9.1 current | 14.3 ± 0.8 (+30 mV) | 52 | NA | |
| KV2.1/9.2 current | 20.3 ± 1.3 (+30 mV) | 52 | NA | |
| KV2.1/9.3 current | 20.1 ± 1 (+20 mV) | 34 | 16.2 ± 1.8 (+40 mV) | 34 |
| 79 ± 2 (−10 mV) | 34 | |||
| 4.9 (+10 mV) | 44 | |||
| 7.3 ± 0.6 (+60 mV) | 31a | |||
Values are means ± SE of the time constants (τ) of activation and inactivation (with action potential given in parentheses) from our experiments in guinea pig DSM, as well as data from the literature for homotetrameric KV2.1 and heterotetrameric KV2.1/silent KV channel currents.
ScTx1 affects the kinetics of KV current activation.
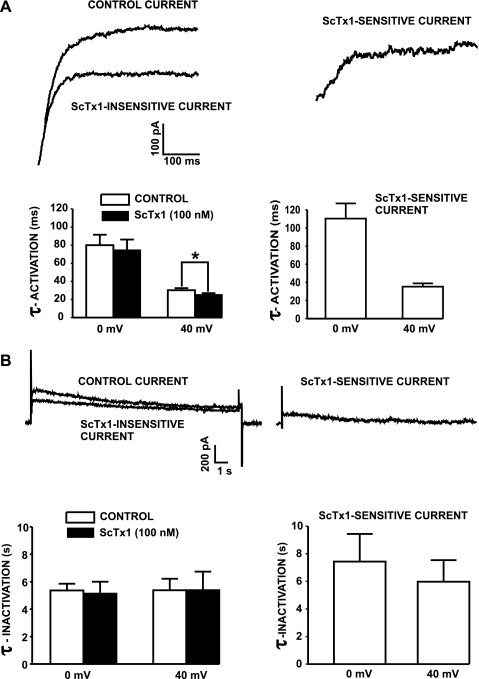
We investigated the effect of 100 nM ScTx1 on the time constants of activation (τ-activation) and inactivation (τ-inactivation) of the KV current. Figure 6A shows representative KV current traces evoked by depolarization steps to +40 mV (400 ms), which is close to the peak of the action potential in DSM. The data analysis showed that ScTx1 (100 nM) decreased the time constant of total outward current activation at +40 mV from 25.5 ± 2.1 to 23.7 ± 1.2 ms (n = 12; N = 9; P < 0.05; Fig. 6A). The time constant of activation of the ScTx1-sensitive current was 35.3 ± 3.6 ms at +40 mV (Fig. 6B).
Fig. 6.
Effects of ScTx1 on time constants (τ) of activation and inactivation of the KV current. A: representative whole cell KV current traces evoked by depolarization steps to +40 mV from a holding potential (Vh) of −70 mV in the presence or absence of 100 nM ScTx1. The subtracted ScTx1-sensitive current is also shown. Bar graphs show the time constants of activation of the control current, ScTx1-insensitive current, and ScTx1-sensitive current at 0 and +40 mV. ScTx1 decreased significantly the time constant of activation at +40 mV (n = 12; N = 9). *P < 0.05. B: ScTx1 (100 nM) did not affect time constant kinetics of inactivation of KV current elicited by a voltage-step depolarization to +40 mV (protocol as shown in Fig. 5A; n = 7; N = 5; P > 0.05). Values are means ± SE. All experiments were performed in the presence of the selective BK channel inhibitor paxilline (100 nM).
The time constant of inactivation was determined using the stimulation protocol shown in Fig. 5A. ScTx1 did not affect the kinetics of inactivation of KV current. The time constant of inactivation was 5.4 ± 0.8 s in the absence of ScTx1 and 5.4 ± 1.3 s in the presence of 100 nM ScTx1 at +40 mV (n = 7; N = 5; P > 0.05; Fig. 6B). The time constant of inactivation of the subtracted ScTx1-sensitive current was 5.9 ± 1.6 s at +40 mV (Fig. 6B).
Collectively, our findings indicate that at positive voltages, ScTx1 has a statistically significant effect on the kinetics of the KV current activation. The time constants of activation and inactivation of the ScTx1-sensitive current obtained experimentally in our study compared with published data on homotetrameric KV2.1 and heterotetrameric KV2.1/silent KV channels are summarized in Table 3.
ScTx1 does not affect the resting membrane potential in guinea pig DSM cells.
We used the amphotericin-perforated, current-clamp mode of the patch-clamp technique to test the effect of ScTx1 on the resting membrane potential in guinea pig DSM cells in the absence of paxilline. In current-clamp mode, we recorded at least 7–10 min of stable control before applying 100 nM ScTx1 for another 10 min. ScTx1 (100 nM) did not affect the resting membrane potential. The values of the resting membrane potential were −32.2 ± 2.3 mV under control conditions and −32.9 ± 3.2 mV in the presence of 100 nM ScTx1 (n = 10; N = 6; P > 0.05).
ScTx1 increases spontaneous and nerve-evoked contractions of isolated DSM strips.
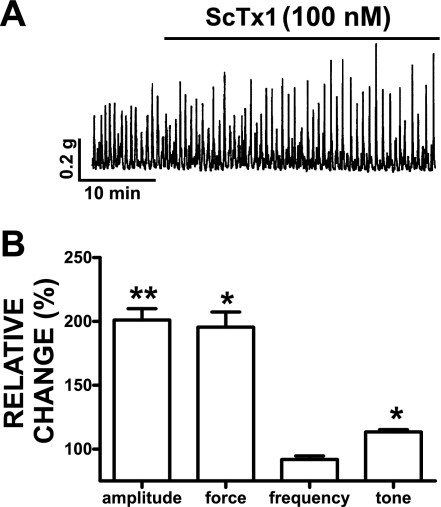
We studied the effect of ScTx1 on physiologically relevant spontaneous (myogenic) phasic and tonic contractions of guinea pig DSM isolated strips. To minimize any possible effects caused by neurotransmitter release, we performed all experiments with the exception of the EFS-induced contractions in the presence of 1 μM TTX, a neuronal Na+ channel blocker. DSM strips were preincubated with 1 μM TTX before the experiments. Isometric DSM tension recordings of isolated guinea pig DSM strips showed that ScTx1 (100 nM) increased spontaneous phasic contraction amplitude, muscle force integral (area under the curve), and muscle tone without any effect on the phasic contraction frequency (n = 13; N = 7; P < 0.05; Fig. 7). Both ScTx1-induced and spontaneous phasic contractions were completely inhibited by 1 μM nifedipine, a selective blocker of the L-type voltage-gated Ca2+ channels (n = 4, N = 4).
Fig. 7.
ScTx1 increases spontaneous phasic contraction amplitude, muscle force integral, and muscle tone in guinea pig DSM strips. A: representative original trace of spontaneous phasic DSM contractions and the effect of 100 nM ScTx1. B: summary data showing a statistically significant increase in DSM spontaneous phasic contraction amplitude, muscle force integral, and muscle tone. For comparison of the phasic contraction parameters of the DSM strips, data were normalized to the spontaneous contractions, which were taken to be 100%, and then expressed as percentages. DSM force is presented as muscle force integral and was determined by integrating the area under the curve of the phasic contractions component. The tone of the DSM strips was determined by measuring the phasic contraction baseline curve. To evaluate the effect of the ScTx1 on the phasic contractions, we analyzed a 5-min period before ScTx1 application for the controls and another 5-min period 30 min after ScTx1 application (100 nM). Values are means ± SE (n = 13; N = 7). *P < 0.05; **P < 0.01. Tetrodotoxin (1 μM) was present throughout the experiments.
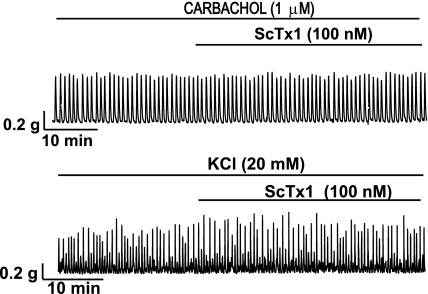
In the next experimental series, we studied the effect of ScTx1 on DSM contractions under conditions of sustained membrane depolarization, induced by either 20 mM KCl or 1 μM carbachol. ScTx1 (100 nM) slightly increased only the carbachol-induced phasic contraction amplitude (n = 9; N = 9; P < 0.05; Fig. 8; Table 4) but had no effect on any other parameters of the DSM contractions induced by 1 μM carbachol (n = 9; N = 9; P > 0.05; Fig. 8; Table 4) or 20 mM KCl (n = 12; N = 10; P > 0.05; Fig. 8; Table 4). After application of ScTx1 (100 nM), nifedipine (1 μM) completely inhibited the DSM contractility of strips precontracted with 1 μM carbachol (n = 8; N = 7) or 20 mM KCl (n = 4; N = 4).
Fig. 8.
Representative original traces showing the effect of ScTx1 on the phasic contractions induced by 1 μM carbachol (top trace) and 20 mM KCl (bottom trace) in DSM isolated strips. ScTx1 (100 nM) did not substantially affect the parameters of the 1 μM carbachol- or 20 mM KCl-induced phasic contractions in DSM isolated strips (see also Table 4).
Table 4.
Effects of ScTx1 on DSM contraction parameters under conditions of KCl- or carbachol-induced membrane depolarization
| Amplitude, %change | Force, %change | Frequency, %change | Tone, %change | |
|---|---|---|---|---|
| Carbachol (1 μM)-induced contractions | 136.8 ± 14.70* | 106.0 ± 9.8 | 100.1 ± 3.3 | 97.8 ± 1.8 |
| K+ (20 mM)-induced contractions | 108.3 ± 10.6 | 105.9 ± 11.7 | 92.7 ± 6.7 | 99.9 ± 2.4 |
Values are means ± SE of %change in DSM contraction parameters under conditions of membrane depolarization induced by 1 μM carbachol or 20 mM KCl (K+) in the presence of 100 nM ScTx1. Carbachol results are from 9 DSM strips obtained from 9 guinea pigs; KCl results are from 12 DSM strips obtained from 10 guinea pigs.
P < 0.05, ScTx1 slightly increased phasic contraction amplitude only under conditions of depolarization with carbachol. ScTx1 had no other statistically significant effect on DSM contraction parameters.
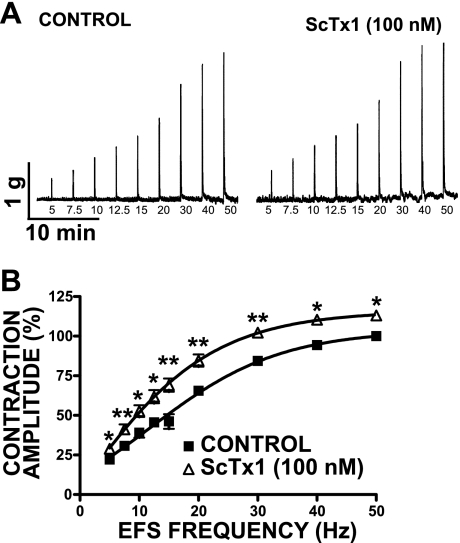
Our next goal was to investigate whether ScTx1-sensitive KV channels modulate the nerve-evoked (EFS-induced) contractions of guinea pig DSM. These experiments were conducted in the absence of TTX. Figure 9 shows original EFS-induced DSM contraction recordings and EFS frequency-response curves created by measuring the amplitude of the EFS-induced contraction at stimulation frequencies of 5, 7.5, 10, 12.5, 15, 20, 30, 40, and 50 Hz. An initial EFS frequency-response curve was generated under control conditions, and then DSM strips were preincubated for 30 min with 100 nM ScTx1. After this procedure, a second EFS frequency-response curve was generated using the same EFS parameters as indicated above. The results showed that EFS frequency-responses of guinea pig DSM strips were significantly increased in the presence of 100 nM ScTx1 at all stimulation frequencies (n = 6; N = 5; P < 0.05; Fig. 9). To confirm the stability of the guinea pig DSM strips, we also performed time controls by generating two consecutive EFS frequency-response curves in a 30-min interval without applying ScTx1. There was no statistically significant difference between the two EFS frequency-response curves in the absence of ScTx1 (n = 4; N = 4; P > 0.05). The EFS experiments suggest that ScTx1-sensitive KV channels also work to oppose the nerve-evoked contractions in guinea pig DSM.
Fig. 9.
ScTx1 increases the amplitude of the electrical field stimulation (EFS)-induced contractions in DSM isolated strips. A: original traces of EFS-induced contractions (5–50 Hz) in the absence and presence of 100 nM ScTx1. B: summary data showing a significant increase in the amplitude of the EFS (5–50 Hz)-induced contractions following 30-min application of 100 nM ScTx1. The maximal EFS contraction amplitude at a stimulation frequency of 50 Hz under control conditions was taken to be 100%, and the contractions were normalized. Values are means ± SE (n = 6; N = 5). *P < 0.05, **P < 0.01.
DISCUSSION
In this study, we revealed that KV2.1-containing channels are physiologically relevant regulators of guinea pig DSM excitability and contractility. On the basis of our data obtained from molecular biology, patch-clamp, and functional studies on DSM contractility, we can draw three important conclusions. First, KV2.1 and several silent KV channel subunits (KV6.2–6.3, KV8.2, and KV9.1–9.3) are expressed in guinea pig DSM. Second, ScTx1-sensitive KV channels contribute to the total KV current in isolated DSM cells. Third, ScTx1-sensitive KV channels regulate DSM contractility by opposing both myogenic and nerve-evoked contractions.
In a recent article (9), we reported that ScTx1-sensitive KV channels are expressed and functional in rat DSM. We have since extended our study and hypothesized that a whole population of ScTx1-sensitive KV2.1-containing channels assembled by various silent KV channel subunits are key regulators of DSM excitability and contractility of guinea pig DSM. To test this hypothesis, we applied a multitechnique molecular biology approach to elucidate the presence of all known ScTx1-sensitive KV channels in guinea pig DSM. The RT-PCR experiments showed mRNA expression of KV2.1, KV6.2–6.3, KV8.2, and KV9.1–9.3 in both whole DSM tissue and single DSM cells and ruled out the expression of two other ScTx1-sensitive channels, the KV2.2 and KV4.2 homotetramers, along with KV5.1, KV6.1, KV8.1 silent subunits (Fig. 1). Using Western blot and immunocytochemistry, we confirmed the expression of the KV2.1 channel protein in guinea pig DSM (Figs. 2 and 3). Therefore, the ScTx1-sensitive KV current in guinea pig DSM cells is determined by KV2.1-containing channels. Consistent with our findings, the KV2.1 subunit has been reported to be the major KV subunit in rat and mouse DSM (9, 17, 41, 56). From the electrically silent subunits that we identified (KV6.2–6.3, KV8.2, and KV9.1–9.3), only KV6.3 and KV9.3 have been reported to contribute to the ScTx1-sensitive current by forming KV2.1/6.3 and KV2.1/9.3 ScTx1-sensitive KV heterotetramers (14, 15, 40). It is also well known that the KV9.3 subunit combines only with the KV2.1 subunit to form a functional heterotetramer and remains electrically silent if expressed alone in artificial systems (14, 32–34, 44, 45). Although ScTx1 sensitivity has not yet been reported for all combinations of KV2.1 and KV silent subunits, the most plausible assumption is that all heterotetrameric KV2.1/KV silent subunits will be ScTx1-sensitive, since the KV2 subunit must be part of the channel.
Using the amphotericin-perforated patch-clamp technique, we investigated the properties of the guinea pig DSM KV current under conditions of paxilline-blocked BK current, which is the main voltage-dependent K+ current in DSM cells (7, 25, 46, 48). The application of 100 nM ScTx1 inhibited ∼22% (at 0 mV) of the remaining KV current amplitude in guinea pig DSM cells (Fig. 4). Most likely, the residual ScTx1-insensitive current is encoded by other types of KV channels. Also, ScTx1 affected the KV current time constant of activation (Fig. 6A).
Our molecular biology studies did not detect the expression of KV2.2 or KV4.2 channel subunits (Fig. 1), and therefore we excluded those channels as possible candidates determining the observed ScTx1-sensitive KV current in guinea pig DSM. Because we identified the mRNA expression of only KV2.1 and electrically silent KV channel subunits, we hypothesized that the ScTx1-sensitive KV current could be attributed to homotetrameric KV2.1 channels and/or heterotetrameric KV2.1/silent KV channels. Our detailed analyses of the biophysical properties of the ScTx1-sensitive KV current in guinea pig DSM demonstrate substantial similarities to those reported for heterotetrameric KV2.1/silent KV channels (Tables 2 and 3). Specifically, our data indicate that the V0.5 of the steady-state inactivation of the ScTx1-senstitive current was about −50 mV, which is more negative than the reported values for homotetrameric KV2.1 channels and consistent with the reported values for heterotetrameric KV2.1/silent KV channels (Table 2). In addition, this V0.5 value of −50 mV is consistent with the recently reported V0.5 for the ScTx1-sensitive current in smooth muscle cells isolated from rabbit urethra (37). Therefore, heterotetrameric assembly of KV2.1 and silent KV channel subunits accounts for the ScTx1-sensitive inhibition of the KV current in guinea pig DSM. Although we do not exclude the possible involvement of the KV2.1 homotetrameric channel, we assume that a population of heterotetrameric KV2.1/silent KV channels most likely underlies the KV current in guinea pig DSM because all these silent subunits cannot form functional channels and associate only with the KV2 family. This assumption is also supported by findings of Thorneloe and Nelson (56) suggesting that KV2.1 does not form its own homotetramer in mouse DSM. They further suggest that in mouse DSM, KV2.1 can potentially coassemble with KV5.1 and KV6 subunits (56). In the present study, however, we did not find KV5.1 or KV6.1 subunit but confirmed the expression of KV6.2–6.3 subunits (Fig. 1). This may be due to species differences. We have also further identified the expression of KV8.2 and KV9.1–9.3 silent subunits in guinea pig DSM cells (Fig. 1).
It has been suggested that KV2.1/9.3 heterotetramer contributes to the excitability in pulmonary and cerebral arteries (1, 44, 61). We observed in current-clamp mode that ScTx1 did not affect the cell resting membrane potential of guinea pig DSM cells. Analysis of steady-state activation and inactivation properties of the KV channels (Figs. 4 and 5) showed that the ScTx1-sensitive channels are likely active at voltages close to the level of the recorded resting membrane potential in guinea pig DSM cells (approximately −32 mV), although their activity at these voltages was relatively low based on the steady-state activation curve. The lack of ScTx1 effect of the resting membrane potential could be explained by the relatively minor contribution of the ScTx1-sensitive current to the total whole cell outward current in DSM cells, as shown in the current-voltage relationship in Fig. 4B. Furthermore, our voltage-clamp experiments were performed in the presence of paxilline to eliminate the BK channel, which is the main contributor to the total whole cell outward current in DSM cells (30, 31, 48), whereas the current-clamp recordings were performed in the absence of paxilline with physiologically active BK channels. On the basis of these results, we conclude that under physiological conditions, ScTx1-sensitive channels are not the main contributors to setting the resting membrane potential in guinea pig DSM cells. However, they regulate excitability at more positive potentials, during the peak of an action potential (Fig. 4). Consistently, Hayase et al. (24) reported no effect of ScTx1 on the resting membrane potential in mouse DSM recorded by intracellular microelectrodes. Our data support the hypothesis that KV2.1-containing channels most likely control the repolarization phase of the action potential and perhaps also the slow afterhyperpolarization in guinea pig DSM.
In the bladder filling phase, spontaneous contractions do not increase substantially intravesical pressure and thus do not initiate voiding (2, 3, 28). During bladder filling, spontaneous contractions occur locally and do not spread throughout the tissue, probably due to a weak electrical coupling between the DSM cells (21, 22). Voiding contractions occur after excitation of the parasympathetic nerves, which causes synchronized contraction of the whole bladder, followed by elevation of the intravesical pressure (2, 3, 26). In guinea pig DSM, a burst of action potentials triggers a single phasic contraction (21). Blocking the KV2.1-containing channels with ScTx1 is expected to increase the action potential frequency within a burst of action potential, and therefore the amplitude of the phasic contraction, without any effect on the phasic contraction frequency (Fig. 7). Our studies on DSM contractility revealed that ScTx1 significantly increased the spontaneous phasic contraction amplitude, muscle force integral, and muscle tone in isolated guinea pig DSM strips (Fig. 7), consistent with previous findings in rat DSM (9). However, ScTx1 had no effect on the DSM contractions induced by 1 μM carbachol or 20 mM KCl with the exception of slightly increased phasic contraction amplitude in the presence of 1 μM carbachol (Table 4; Fig. 8). These results demonstrate that under experimental conditions of membrane depolarization, the KV2.1-containing channels have no further contribution to the membrane excitability and contractility (Table 4; Fig. 8). The KV2.1-containing channels, by decreasing membrane excitability, work to oppose spontaneous contractions under physiological conditions. Our results also showed that ScTx1 significantly increased the amplitude of the EFS-induced contractions over a range of stimulation frequencies, 5–50 Hz (Fig. 9). This indicates that KV2.1-containing channels, by decreasing membrane excitability, also oppose the DSM contractility in response to excitatory neurotransmitters.
Under pathological conditions, such as some forms of OAB, spontaneous myogenic contractions can also cause a rise of intravesical pressure, initiating involuntary voiding (3). These pathological conditions are poorly understood, and the current pharmacological treatment is limited to antimuscarinics that have many adverse effects (3). Our study shows that pharmacological block of KV2.1-containing channels with ScTx1 increases DSM contractility (Figs. 7 and 9). Therefore, genetic mutations causing KV2.1 channel malfunction would be expected to cause DSM dysfunction. Consistently, other studies also suggest the implication of the KV2.1 channels in OAB (17, 41). Thus a better understanding of KV2.1-containing channels is an important step for developing new therapeutic strategies for treatment of OAB and UI. The KV2.1-containing channels may represent new targets for pharmacological or genetic control of OAB.
GRANTS
This study was supported by National Institutes of Health Grants DK084284, DK083687, and UL1RR029882 (to G. V. Petkov).
DISCLAIMER
The contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: K.L.H., M.C., and G.V.P. conception and design of research; K.L.H., M.C., R.P.S., S.P.P., Q.C., and W.F.K. performed experiments; K.L.H., M.C., R.P.S., Q.C., and W.F.K. analyzed data; K.L.H., M.C., R.P.S., S.P.P., and G.V.P. interpreted results of experiments; K.L.H., M.C., R.P.S., Q.C., W.F.K., and G.V.P. prepared figures; K.L.H. and G.V.P. drafted manuscript; K.L.H., M.C., R.P.S., S.P.P., Q.C., W.F.K., and G.V.P. edited and revised manuscript; K.L.H., M.C., R.P.S., S.P.P., Q.C., W.F.K., and G.V.P. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank S. Afeli for help with some of the DSM contraction experiments and Drs. J. Schnellmann, J. Malysz, and W. Xin, as well as A. Smith, for critical evaluation of the manuscript.
REFERENCES
- 1. Amberg GC, Santana LF. Kv2 channels oppose myogenic constriction of rat cerebral arteries. Am J Physiol Cell Physiol 291: C348–C356, 2006 [DOI] [PubMed] [Google Scholar]
- 2. Andersson KE, Arner A. Urinary bladder contraction and relaxation: physiology and pathophysiology. Psychol Rev 84: 935–986, 2004 [DOI] [PubMed] [Google Scholar]
- 3. Andersson KE, Wein AJ. Pharmacology of the lower urinary tract: basis for current and future treatments of urinary incontinence. Pharmacol Rev 56: 581–631, 2004 [DOI] [PubMed] [Google Scholar]
- 4. Archer SL, Wu XC, Thebaud B, Nsair A, Bonnet S, Tyrrell B, McMurtry MS, Hashimoto K, Harry G, Michelakis ED. Preferential expression and function of voltage-gated, O2-sensitive K+ channels in resistance pulmonary arteries explains regional heterogeneity in hypoxic pulmonary vasoconstriction: ionic diversity in smooth muscle cells. Circ Res 95: 308–318, 2004 [DOI] [PubMed] [Google Scholar]
- 5. Beech DJ, Bolton TB. Two components of potassium current activated by depolarization of single smooth muscle cells from the rabbit portal vein. J Physiol 418: 293–309, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bocksteins E, Raes AL, Van de Vijver G, Bruyns T, Van Bogaert PP, Snyders DJ. Kv2.1 and silent Kv subunits underlie the delayed rectifier K+ current in cultured small mouse DRG neurons. Am J Physiol Cell Physiol 296: C1271–C1278, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Brown SM, Bentcheva-Petkova LM, Liu L, Hristov KL, Chen M, Kellett WF, Meredith AL, Aldrich RW, Nelson MT, Petkov GV. β-Adrenergic relaxation of mouse urinary bladder smooth muscle in the absence of large-conductance Ca2+-activated K+ channel. Am J Physiol Renal Physiol 295: F1149–F1157, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Castellano A, Chiara MD, Mellstrom B, Molina A, Monje F, Naranjo JR, Lopez-Barneo J. Identification and functional characterization of a K+ channel alpha-subunit with regulatory properties specific to brain. J Neurosci 17: 4652–4661, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chen M, Kellett WF, Petkov GV. Voltage-gated K+ channels sensitive to stromatoxin-1 regulate myogenic and neurogenic contractions of rat urinary bladder smooth muscle. Am J Physiol Regul Integr Comp Physiol 299: R177–R184, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chen M, Petkov GV. Identification of large conductance calcium activated potassium channel accessory beta4 subunit in rat and mouse bladder smooth muscle. J Urol 182: 374–381, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Collier ML, Ji G, Wang Y, Kotlikoff MI. Calcium-induced calcium release in smooth muscle: loose coupling between the action potential and calcium release. J Gen Physiol 115: 653–662, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Coppock EA, Martens JR, Tamkun MM. Molecular basis of hypoxia-induced pulmonary vasoconstriction: role of voltage-gated K+ channels. Am J Physiol Lung Cell Mol Physiol 281: L1–L12, 2001 [DOI] [PubMed] [Google Scholar]
- 12a. Czirják G, Tóth ZE, Enyedi P. Characterization of the heteromeric potassium channel formed by Kv2.1 and the retinal subunit Kv8.2 in Xenopus oocytes. J Neurophysiol 98: 1213–1222, 2007 [DOI] [PubMed] [Google Scholar]
- 13. Davies AM, Batchelor TJ, Eardley I, Beech DJ. Potassium channel KValpha1 subunit expression and function in human detrusor muscle. J Urol 167: 1881–1886, 2002 [PubMed] [Google Scholar]
- 14. Escoubas P, Diochot S, Celerier ML, Nakajima T, Lazdunski M. Novel tarantula toxins for subtypes of voltage-dependent potassium channels in the Kv2 and Kv4 subfamilies. Mol Pharmacol 62: 48–57, 2002 [DOI] [PubMed] [Google Scholar]
- 15. Escoubas P, Rash L. Tarantulas: eight-legged pharmacists and combinatorial chemists. Toxicon 43: 555–574, 2004 [DOI] [PubMed] [Google Scholar]
- 16. Fujii K, Foster CD, Brading AF, Parekh AB. Potassium channel blockers and the effects of cromakalim on the smooth muscle of the guinea-pig bladder. Br J Pharmacol 99: 779–785, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gan XG, An RH, Bai YF, Zong DB. Expressions of voltage-gated K+ channel 2.1 and 22 in rat bladder with detrusor hyperreflexia. Chin Med J (Engl) 121: 1574–1577, 2008 [PubMed] [Google Scholar]
- 18. Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflügers Arch 391: 85–100, 1981 [DOI] [PubMed] [Google Scholar]
- 19. Hashitani H, Brading AF. Electrical properties of detrusor smooth muscles from the pig and human urinary bladder. Br J Pharmacol 140: 146–158, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hashitani H, Brading AF. Ionic basis for the regulation of spontaneous excitation in detrusor smooth muscle cells of the guinea-pig urinary bladder. Br J Pharmacol 140: 159–169, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hashitani H, Brading AF, Suzuki H. Correlation between spontaneous electrical, calcium and mechanical activity in detrusor smooth muscle of the guinea-pig bladder. Br J Pharmacol 141: 183–193, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hashitani H, Fukuta H, Takano H, Klemm MF, Suzuki H. Origin and propagation of spontaneous excitation in smooth muscle of the guinea-pig urinary bladder. J Physiol 530: 273–286, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hayama E, Imamura S, Wu C, Nakazawa M, Matsuoka R, Nakanishi T. Analysis of voltage-gated potassium channel beta1 subunits in the porcine neonatal ductus arteriosus. Pediatr Res 59: 167–174, 2006 [DOI] [PubMed] [Google Scholar]
- 24. Hayase M, Hashitani H, Kohri K, Suzuki H. Role of K+ channels in regulating spontaneous activity in detrusor smooth muscle in situ in the mouse bladder. J Urol 181: 2355–2365, 2009 [DOI] [PubMed] [Google Scholar]
- 25. Heppner TJ, Bonev AD, Nelson MT. Ca2+-activated K+ channels regulate action potential repolarization in urinary bladder smooth muscle. Am J Physiol Cell Physiol 273: C110–C117, 1997 [DOI] [PubMed] [Google Scholar]
- 26. Heppner TJ, Bonev AD, Nelson MT. Elementary purinergic Ca2+ transients evoked by nerve stimulation in rat urinary bladder smooth muscle. J Physiol 564: 201–212, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Heppner TJ, Herrera GM, Bonev AD, Hill-Eubanks D, Nelson MT. Ca2+ sparks and KCa channels: novel mechanisms to relax urinary bladder smooth muscle. Adv Exp Med Biol 539: 347–357, 2003 [DOI] [PubMed] [Google Scholar]
- 28. Herrera GM, Pozo MJ, Zvara P, Petkov GV, Bond CT, Adelman JP, Nelson MT. Urinary bladder instability induced by selective suppression of the murine small conductance calcium-activated potassium (SK3) channel. J Physiol 551: 893–903, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Horn R, Marty A. Muscarinic activation of ionic currents measured by a new whole-cell recording method. J Gen Physiol 92: 145–159, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hristov KL, Chen M, Kellett WF, Rovner ES, Petkov GV. Large-conductance voltage- and Ca2+-activated K+ channels regulate human detrusor smooth muscle function. Am J Physiol Cell Physiol 301: C903–C912, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hristov KL, Cui X, Brown SM, Liu L, Kellett WF, Petkov GV. Stimulation of β3-adrenoceptors relaxes rat urinary bladder smooth muscle via activation of the large-conductance Ca2+-activated K+ channels. Am J Physiol Cell Physiol 295: C1344–C1353, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31a. Hulme JT, Coppock EA, Felipe A, Martens JR, Tamkun MM. Oxygen sensitivity of cloned voltage-gated K(+) channels expressed in the pulmonary vasculature. Circ Res 85: 489–497, 1999 [DOI] [PubMed] [Google Scholar]
- 32. Kerschensteiner D, Monje F, Stocker M. Structural determinants of the regulation of the voltage-gated potassium channel Kv2.1 by the modulatory alpha-subunit Kv9.3. J Biol Chem 278: 18154–18161, 2003 [DOI] [PubMed] [Google Scholar]
- 33. Kerschensteiner D, Soto F, Stocker M. Fluorescence measurements reveal stoichiometry of K+ channels formed by modulatory and delayed rectifier alpha-subunits. Proc Natl Acad Sci USA 102: 6160–6165, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kerschensteiner D, Stocker M. Heteromeric assembly of Kv2.1 with Kv9.3: effect on the state dependence of inactivation. Biophys J 77: 248–257, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kotlikoff MI. Calcium-induced calcium release in smooth muscle: the case for loose coupling. Prog Biophys Mol Biol 83: 171–191, 2003 [DOI] [PubMed] [Google Scholar]
- 36. Kramer JW, Post MA, Brown AM, Kirsch GE. Modulation of potassium channel gating by coexpression of Kv2.1 with regulatory Kv5.1 or Kv6.1 α-subunits. Am J Physiol Cell Physiol 274: C1501–C1510, 1998 [DOI] [PubMed] [Google Scholar]
- 37. Kyle B, Bradley E, Ohya S, Sergeant GP, McHale NG, Thornbury KD, Hollywood MA. Contribution of Kv2.1 channels to the delayed rectifier current in freshly dispersed smooth muscle cells from rabbit urethra. Am J Physiol Cell Physiol 301: C1186–C1200, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. McDaniel SS, Platoshyn O, Yu Y, Sweeney M, Miriel VA, Golovina VA, Krick S, Lapp BR, Wang JY, Yuan JX. Anorexic effect of K+ channel blockade in mesenteric arterial smooth muscle and intestinal epithelial cells. J Appl Physiol 91: 2322–2333, 2001 [DOI] [PubMed] [Google Scholar]
- 39. Michelakis ED, Rebeyka I, Wu X, Nsair A, Thebaud B, Hashimoto K, Dyck JR, Haromy A, Harry G, Barr A, Archer SL. O2 sensing in the human ductus arteriosus: regulation of voltage-gated K+ channels in smooth muscle cells by a mitochondrial redox sensor. Circ Res 91: 478–486, 2002 [DOI] [PubMed] [Google Scholar]
- 40. Moreno-Dominguez A, Cidad P, Miguel-Velado E, Lopez-Lopez JR, Perez-Garcia MT. De novo expression of Kv6.3 contributes to changes in vascular smooth muscle cell excitability in a hypertensive mice strain. J Physiol 587: 625–640, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ohya S, Tanaka M, Watanabe M, Maizumi Y. Diverse expression of delayed rectifier K+ channel subtype transcripts in several types of smooth muscles of the rat. J Smooth Muscle Res 36: 101–115, 2000 [DOI] [PubMed] [Google Scholar]
- 42. Ottschytsch N, Raes A, VanHoorick D, Snyders DJ. Obligatory heterotetramerization of three previously uncharacterized Kv channel alpha-subunits identified in the human genome. Proc Natl Acad Sci USA 99: 7986–7991, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Patel AJ, Honore E. Molecular physiology of oxygen-sensitive potassium channels. Eur Respir J 18: 221–227, 2001 [DOI] [PubMed] [Google Scholar]
- 44. Patel AJ, Lazdunski M, Honore E. Kv2.1/Kv9.3, a novel ATP-dependent delayed-rectifier K+ channel in oxygen-sensitive pulmonary artery myocytes. EMBO J 16: 6615–6625, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Petkov GV. Ion channels. In: Pharmacology: Principles and Practice, edited by Hacker M, Messer W, Bachmann K. Boston, MA: Elsevier/Academic, 2009, chapt. 16, p. 385–425 [Google Scholar]
- 46. Petkov GV, Bonev AD, Heppner TJ, Brenner R, Aldrich RW, Nelson MT. Beta1-subunit of the Ca2+-activated K+ channel regulates contractile activity of mouse urinary bladder smooth muscle. J Physiol 537: 443–452, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Petkov GV, Heppner TJ, Bonev AD, Herrera GM, Nelson MT. Low levels of KATP channel activation decrease excitability and contractility of urinary bladder. Am J Physiol Regul Integr Comp Physiol 280: R1427–R1433, 2001 [DOI] [PubMed] [Google Scholar]
- 48. Petkov GV, Nelson MT. Differential regulation of Ca2+-activated K+ channels by β-adrenoceptors in guinea pig urinary bladder smooth muscle. Am J Physiol Cell Physiol 288: C1255–C1263, 2005 [DOI] [PubMed] [Google Scholar]
- 49. Platoshyn O, Yu Y, Golovina VA, McDaniel SS, Krick S, Li L, Wang JY, Rubin LJ, Yuan JX. Chronic hypoxia decreases KV channel expression and function in pulmonary artery myocytes. Am J Physiol Lung Cell Mol Physiol 280: L801–L812, 2001 [DOI] [PubMed] [Google Scholar]
- 50. Robertson BE, Nelson MT. Aminopyridine inhibition and voltage dependence of K+ currents in smooth muscle cells from cerebral arteries. Am J Physiol Cell Physiol 267: C1589–C1597, 1994 [DOI] [PubMed] [Google Scholar]
- 51. Salinas M, de Weille J, Guillemare E, Lazdunski M, Hugnot JP. Modes of regulation of shab K+ channel activity by the Kv8.1 subunit. J Biol Chem 272: 8774–8780, 1997 [DOI] [PubMed] [Google Scholar]
- 52. Salinas M, Duprat F, Heurteaux C, Hugnot JP, Lazdunski M. New modulatory alpha subunits for mammalian Shab K+ channels. J Biol Chem 272: 24371–24379, 1997 [DOI] [PubMed] [Google Scholar]
- 52a. Sano Y, Mochizuki S, Miyake A, Kitada C, Inamura K, Yokoi H, Nozawa K, Matsushime H, Furuichi K. Molecular cloning and characterization of Kv6.3, a novel modulatory subunit for voltage-gated K(+) channel Kv2.1. FEBS Lett 512: 230–234, 2002 [DOI] [PubMed] [Google Scholar]
- 53. Smirnov SV, Beck R, Tammaro P, Ishii T, Aaronson PI. Electrophysiologically distinct smooth muscle cell subtypes in rat conduit and resistance pulmonary arteries. J Physiol 538: 867–878, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Stocker M, Hellwig M, Kerschensteiner D. Subunit assembly and domain analysis of electrically silent K+ channel alpha-subunits of the rat Kv9 subfamily. J Neurochem 72: 1725–1734, 1999 [DOI] [PubMed] [Google Scholar]
- 55. Stocker M, Kerschensteiner D. Cloning and tissue distribution of two new potassium channel alpha-subunits from rat brain. Biochem Biophys Res Commun 248: 927–934, 1998 [DOI] [PubMed] [Google Scholar]
- 56. Thorneloe KS, Nelson MT. Properties and molecular basis of the mouse urinary bladder voltage-gated K+ current. J Physiol 549: 65–74, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Wang J, Weigand L, Wang W, Sylvester JT, Shimoda LA. Chronic hypoxia inhibits Kv channel gene expression in rat distal pulmonary artery. Am J Physiol Lung Cell Mol Physiol 288: L1049–L1058, 2005 [DOI] [PubMed] [Google Scholar]
- 59. Wareing M, Bai X, Seghier F, Turner CM, Greenwood SL, Baker PN, Taggart MJ, Fyfe GK. Expression and function of potassium channels in the human placental vasculature. Am J Physiol Regul Integr Comp Physiol 291: R437–R446, 2006 [DOI] [PubMed] [Google Scholar]
- 60. Wulff H, Castle NA, Pardo LA. Voltage-gated potassium channels as therapeutic targets. Nat Rev Drug Discov 8: 982–1001, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Zhong XZ, Abd-Elrahman KS, Liao CH, El-Yazbi AF, Walsh EJ, Walsh MP, Cole WC. Stromatoxin-sensitive, heteromultimeric Kv2.1/Kv9.3 channels contribute to myogenic control of cerebral arterial diameter. J Physiol 588: 4519–4537, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Zhu XR, Netzer R, Böhlke K, Liu Q, Pongs O. Structural and functional characterization of Kv6.2 a new gamma-subunit of voltage-gated potassium channel. Receptors Channels 6: 337–350, 1999 [PubMed] [Google Scholar]