Summary
Background
Medication errors are common in primary care and are associated with considerable risk of patient harm. We tested whether a pharmacist-led, information technology-based intervention was more effective than simple feedback in reducing the number of patients at risk of measures related to hazardous prescribing and inadequate blood-test monitoring of medicines 6 months after the intervention.
Methods
In this pragmatic, cluster randomised trial general practices in the UK were stratified by research site and list size, and randomly assigned by a web-based randomisation service in block sizes of two or four to one of two groups. The practices were allocated to either computer-generated simple feedback for at-risk patients (control) or a pharmacist-led information technology intervention (PINCER), composed of feedback, educational outreach, and dedicated support. The allocation was masked to general practices, patients, pharmacists, researchers, and statisticians. Primary outcomes were the proportions of patients at 6 months after the intervention who had had any of three clinically important errors: non-selective non-steroidal anti-inflammatory drugs (NSAIDs) prescribed to those with a history of peptic ulcer without co-prescription of a proton-pump inhibitor; β blockers prescribed to those with a history of asthma; long-term prescription of angiotensin converting enzyme (ACE) inhibitor or loop diuretics to those 75 years or older without assessment of urea and electrolytes in the preceding 15 months. The cost per error avoided was estimated by incremental cost-effectiveness analysis. This study is registered with Controlled-Trials.com, number ISRCTN21785299.
Findings
72 general practices with a combined list size of 480 942 patients were randomised. At 6 months' follow-up, patients in the PINCER group were significantly less likely to have been prescribed a non-selective NSAID if they had a history of peptic ulcer without gastroprotection (OR 0·58, 95% CI 0·38–0·89); a β blocker if they had asthma (0·73, 0·58–0·91); or an ACE inhibitor or loop diuretic without appropriate monitoring (0·51, 0·34–0·78). PINCER has a 95% probability of being cost effective if the decision-maker's ceiling willingness to pay reaches £75 per error avoided at 6 months.
Interpretation
The PINCER intervention is an effective method for reducing a range of medication errors in general practices with computerised clinical records.
Funding
Patient Safety Research Portfolio, Department of Health, England.
Introduction
Medication errors are an important cause of potentially avoidable morbidity and mortality in primary1,2 and secondary care3 and reports from the USA, the UK, and elsewhere have shown the urgent need to reduce the risk of occurrence of these errors.4–6 Although important progress has been made in the implementation of interventions for use in specialist care settings,7 particularly in relation to computerised entry of physician orders7,8 and computerised decision support,9 the evidence for primary care—in which most patients are now managed worldwide—is still very weak.8,10
On the basis of systematic reviews of published work10,11 and our own research,12,13 we identified the drugs most commonly associated with medication errors in primary care.11–13 In view of the few known effective interventions, we focused on the identification of the most promising components of any future intervention.10 The evidence was strongest for educational outreach14 and pharmacist-led interventions.10 Furthermore, most preventable adverse drug events in primary care are attributable to errors in prescription and medication monitoring,2,12 and changes in practice enabled by information technology have substantial potential to reduce the frequency of these errors.8 However, translation of this potential into proven benefits is far from straightforward, which relates to the difficulties in making the organisational changes needed to embed information technology into routine models of care.15 The need for a new multifaceted intervention has been further underscored by two trials that have raised serious doubts about the effectiveness of simple pharmacist-centred interventions.16,17
Informed by the Medical Research Council's framework for complex interventions,18 we aimed to test whether an information technology intervention for pharmacists could improve prescription safety and medication monitoring in general practices. We also undertook an indicative analysis of the cost-effectiveness of the intervention.
Methods
Study design and participants
We did a two-group pragmatic cluster randomised trial. Further details of the methods are available in the trial protocol.19 We chose a cluster design because the intervention was applied by the general practice. General practices were eligible to participate if they were computerised with electronic prescribing. Practices were excluded if they did not routinely record morbidities such as asthma or peptic ulcer in patients' computerised records; did not routinely use computers to record prescriptions issued; intended to change their computer systems during the study to that of a different supplier that was not compatible with Quest Browser; were in primary care trusts that were undertaking interventions that might overlap with our intervention; took part in the pilot study of the trial; or expected large changes in list-size (numbers of registered patients) during the study.
The study was approved by the Nottingham 2 research ethics committee. We obtained written consent from all general practices after a face-to-face meeting at which the study was explained in more detail. For the economic analysis, the general practices recruited to the study were asked to write to all patients identified through baseline data collection who appeared in the numerator of one of our outcome measures (ie, they had a potential medication error). Patients were given a leaflet about the study and were asked to give written consent for the research team to access their medical records.
Randomisation and masking
We stratified eligible practices by centre (Manchester and Nottingham) and list size (<2500, 2500–6000, and >6000 patients) and randomly allocated them within strata (1:1 ratio) to receive the intervention or control by block randomisation with block sizes of two or four. The allocation sequence was centrally generated, independent of the investigators, by the clinical trials unit at the University of Nottingham (UK). Practices were enrolled by the research team and allocated by the independent web-based randomisation service provided by the unit. Access to the allocation sequence was restricted to the clinical trials unit data manager and the sequence was concealed until all analyses were completed.
General practices, patients, pharmacists, and researchers visiting practices to extract computerised data were not masked to the allocation. All outcome data for the trial were extracted from patient records by prespecified electronic searches and so these data could not be altered before leaving the practice. Data were then sent electronically by secure file transfer protocol and automatically entered into a database on the trial manager's computer. Researchers who cleaned the data and the trial statisticians were masked to allocation.
Procedures
Because patients were proactively identified as being at high risk of potentially serious errors, we judged that a control group of no intervention was unethical. The control group practices therefore used simple feedback; after collection of data at baseline, control practices received computerised feedback for patients identified as at risk from potentially hazardous prescripting and inadequate blood-test monitoring of medicines plus brief written educational materials explaining the importance of each type of error. Practices were asked to introduce changes they considered necessary within 12 weeks after the collection of data at baseline.
Intervention practices received simple feedback plus a pharmacist-led information technology complex intervention (PINCER) lasting 12 weeks. The pharmacist arranged to meet with members of the practice team to discuss the computer-generated feedback for patients with medication errors. All doctors were encouraged to attend this meeting with at least one member of the nursing staff, the practice manager, and at least one member of the reception staff. Before the meeting, whenever possible, all relevant members of staff were provided with a brief summary of the objectives of the PINCER intervention and a summary of the findings from the computer search. At the meeting the pharmacists were asked to use the principles of educational outreach while taking account of human error theory in their discussions. After this initial meeting, pharmacists were expected to use various techniques to help correct medication errors that had been identified and prevent future medication errors. Interventions included review of patients' medical records; discussions with family doctors to decide on actions to be taken; invitation of patients to be reviewed or to have blood tests; and working with members of the practice team to improve local safety systems. Details are provided in the trial protocol.19 All general practices had access to some computerised decision support, such as drug interactions, for prescription.
Data were extracted at baseline and at 6 months and 12 months after the intervention with Quest Browser software (version 2.3.39).
We chose our primary and secondary outcomes on the basis of medicines management difficulties that are important in overall burden and severity of iatrogenic harm in primary care,11–13 and those that are detectable from general practice computer systems. The three primary outcomes assessed after 6 months were (1) prescription of non-selective non-steroidal anti-inflammatory drugs (NSAIDs) to patients with a history of peptic ulcer without co-prescription of a proton-pump inhibitor (PPI); (2) prescription of β blockers to patients with a history of asthma; and (3) long-term prescription of angiotensin converting enzyme (ACE) inhibitors or loop diuretics to patients 75 years or older without assessment of urea and electrolytes in the preceding 15 months. Panel 1 shows the primary, secondary, and composite outcomes (see also the trial protocol19).
Panel 1. Outcomes.
Primary
-
1
Patients with a history of peptic ulcer who have been prescribed a non-selective non-steroidal anti-inflammatory drug without co-prescription of a proton-pump inhibitor
-
2
Patients with asthma who have been prescribed a β blocker
-
3
Patients aged 75 years and older who have been prescribed an angiotensin converting enzyme inhibitor or a loop diuretic long-term who have not had a computer-recorded check of their renal function and electrolytes in the previous 15 months
Secondary
-
2a
Patients with asthma (and no history of coronary heart disease) who had been prescribed a β blocker
-
4
Proportions of women with a past medical history of venous or arterial thrombosis who had been prescribed the combined oral contraceptive pill
-
5
Patients receiving methotrexate for at least 3 months who had not had a full blood count recorded (5a), or liver function test (5b), in the previous 3 months
-
6
Patients receiving warfarin for at least 3 months who had not had a recorded check of their international normalised ratio in the previous 12 weeks
-
7
Patients receiving lithium for at least 3 months who had not had a recorded check of their lithium concentrations in the previous 3 months
-
8
Patients receiving amiodarone for at least 6 months who had not had a thyroid function test in the previous 6 months
-
9
Patients receiving prescriptions of methotrexate without instructions that the drug should be taken every week
-
10
Patients receiving prescriptions of amiodarone for at least 1 month who are receiving a dose of more than 200 mg per day
Composite secondary outcome measures
-
11
Patients with at least one prescription problem (a combination of outcome measures 1, 2, or 4)
-
12
Patients with at least one monitoring problem (a combination of outcome measures 3, 5, 6, 7, and 8)
In the trial protocol19 composite outcome measures for patients with two or more prescription or monitoring problems were suggested. However, the number of patients with two or more problems was very small and so these findings are not reported in this Article.
Statistical analysis
Characteristics of practices and participants were recorded at baseline and compared informally between treatment groups.19 The prevalences of primary and secondary outcomes were measured at each timepoint by allocation group, at the level of the patient, using the numerator, denominator and percentage.
Analysis was by intention to treat.20,21 All outcome measures were binary and were compared between groups by random effects logistic regression, with patient at level one and practice at level two, to estimate odds ratios and 95% CIs. The primary analysis adjusted for stratum as a fixed effect (practice level), errors related to medication at baseline (patient level), deprivation,22 and training status (practice level). We estimated intraclass correlation coefficients from these models.
Subgroup analyses for primary outcome measures assessed whether the intervention effect varied by practice size or practice deprivation by incorporation of a term for the interaction between treatment group and the (continuous) covariate of interest into regression models.23 We categorised the covariate at the median value when there was evidence of non-linearity.
Significance was assessed with likelihood ratio tests with a p value of less than 0·05. All analyses were done with Stata (version 10.1).24 We obtained outcome data for all participating practices at both the 6 months (primary) and 12 months assessment points, no data were missing. No adjustments were made for multiple endpoints. We checked models by examining plots of standardised empirical Bayes estimates for the random effects and did sensitivity analyses by excluding practices with estimates above or below two standard deviations.
We calculated sample sizes separately for all primary outcomes. To calculate sample sizes unadjusted for clustering we used nQuery Advisor (version 6.0),25 and to inflate to adjust for clustering26 we used intraclass correlation coefficients and average cluster sizes estimated from 43 general practices that contributed anonymous clinical data to the QResearch database (webappendix).19 66 practices were needed to detect a difference between an 11% reduction in error rate in the simple feedback group and a 50% reduction in the PINCER group for the primary outcome measures with 80% power and a two-tailed α of 0·05.
In a prospective economic evaluation (webappendix), we compared PINCER with simple feedback for reduction of proportions of patients at risk from prescription errors in general practice, from the perspective of the UK NHS. Costs and outcomes associated with sequelae of errors were not included.
The outcome for the economic analysis was the number of errors detected in the PINCER and simple feedback groups at 6 months and 12 months after the intervention. We used outcome measures 1, 2, 3, 5a, 5b, 7, and 8 (table 1). We excluded outcome measure 4 because the number of patients with errors was very small and outcome measures 6, 9, and 10 because obtaining full data in all practices was difficult.19
Table 1.
Characteristics of patients at baseline by allocation group according to outcome measure
| Patient characteristics | Simple feedback | PINCER | |
|---|---|---|---|
| Primary outcomes | |||
| 1 | History of peptic ulcer prescribed an NSAID without a PPI/history of peptic ulcer without a PPI | 93/1970 (5%) | 87/1828 (5%) |
| 2 | Asthma prescribed a β blocker/asthma | 628/20 634 (3%) | 537/18 906 (3%) |
| 3 | Aged ≥75 years receiving long term ACE inhibitors or loop diuretics without urea and electrolyte monitoring in the previous 15 months/aged ≥75 years receiving long-term ACE inhibitors or diuretics | 483/4722 (10%) | 549/4349 (13%) |
| Secondary outcomes | |||
| 2a | Asthma and not CHD prescribed a β blocker/asthma and not CHD | 375/19 528 (2%) | 337/17 968 (2%) |
| 4 | History of venous or arterial thrombosis prescribed combined oral contraceptives/history of venous or arterial thrombosis (women) | 16/2588 (1%) | 5/2284 (<1%) |
| 5a | Methotrexate for ≥3 months without full blood count in past 3 months/methotrexate for ≥3 months | 202/483 (42%) | 170/480 (35%) |
| 5b | Methotrexate for ≥3 months without a liver function test in past 3 months/methotrexate for ≥3 months | 184/483 (38%) | 172/480 (36%) |
| 6 | Warfarin for ≥3 months without an INR in past 3 months/warfarin for ≥3 months | 99/1496 (7%) | 92/1591 (6%) |
| 7 | Lithium for ≥3 months without a lithium concentration measurement in past 3 months/lithium for ≥3 months | 101/224 (45%) | 97/194 (50%) |
| 8 | Amiodarone for ≥6 months without a thyroid function test in the past 6 months/amiodarone for ≥6 months | 130/253 (51%) | 111/240 (46%) |
| 9 | Methotrexate without instructions to take weekly/patients prescribed methotrexate | 12/345 (3%) | 7/305 (2%) |
| 10 | Amiodarone for ≥1 month at a dose of >200 mg per day/amiodarone for ≥1 month | 1/223 (<1%) | 1/222 (<1%) |
| 11 | At least one prescription problem/at risk of at least one prescription problem | 736/24 550 (3%) | 629/22 473 (3%) |
| 12 | At least one monitoring problem/at risk of at least one monitoring problem | 1015/6756 (15%) | 1018/6371 (16%) |
Data are numerator/denominator (%). NSAID=non-steroidal anti-inflammatory drug. PINCER=pharmacist-led information technology intervention. ACE=angiotensin converting enzyme. PPI=proton-pump inhibitor. CHD=coronary heart disease. INR=international normalised ratio.
Costs were the direct costs of provision of an intervention to reduce prescription errors in general practice. The study was not powered to detect differences in costs because no previous study on which to base a power calculation exists. The time horizon was 6 months in the base case. Because all costs were incurred by the practices, correction for clustering was not needed.
The only cost for the simple feedback group was from the researchers returning to the practices at set times to generate error reports from general practice systems. This cost was retained in the model to account for the equivalent resource that would be used to generate these error reports in the real world. The PINCER intervention consisted of these report-generation costs, plus pharmacist training sessions, facilitated meetings, monthly meetings, practice feedback meetings, and time spent in each practice outside meetings following up errors.
Before incremental cost-effectiveness analysis, we adjusted costs and outcomes for a range of practice characteristics. We used the negative binomial model27 for regression analysis of errors because variance was greater than the mean for errors per practice in both groups (overdispersion), and the variation differed between groups. Poisson regression would underestimate the standard errors of the coefficients. We estimated adjusted costs by generalised linear modelling. A simple probabilistic decision-analytic model was populated to generate incremental cost-effectiveness ratios, which were calculated for differences in error rates between the PINCER and simple feedback groups:
To identify the magnitude of uncertainty of the incremental cost-effectiveness ratios, we generated a bootstrap estimate of the incremental cost-effectiveness ratio sampling distribution. We constructed a cost-effectiveness acceptability curve to express the probability that the cost per extra unit of outcome (error avoided in this study) gained from the trial was cost effective as a function of the decision-maker's ceiling cost-effectiveness ratio (λ). We did a sensitivity analysis to establish incremental cost-effectiveness ratios when the time horizon was 12 months. A detailed description of the analysis is provided in the trial protocol.19 This study is registered, number ISRCTN21785299.
Role of the funding source
The sponsor of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the manuscript. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
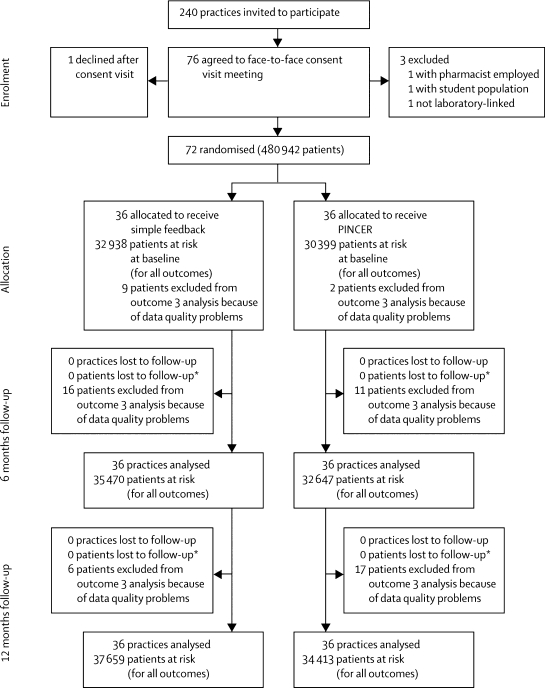
240 practices in Nottinghamshire, Staffordshire, and central and eastern Cheshire, England, were invited to participate, of which 72 (30%) were recruited between July 11, 2006, and Aug 8, 2007 (figure 1). Participating and non-participating practices had much the same numbers of family doctors and socioeconomic profiles, however, participating practices were larger, more likely to be training practices, and had slightly higher quality and outcomes framework scores, than did non-participating practices (webappendix). Table 2 shows the number of patients per practice with each primary outcome measure. The main reason practices gave for not taking part was that they were too busy. In the simple feedback group, three practices had a small list size, 14 a medium list size, and 19 a large list size. In the PINCER group, four practices had a small list size, 13 a medium list size, and 19 a large list size. Table 1 shows participant charactersistics and the webappendix shows practice characteristics at baseline for the two treatment groups.
Figure 1.
Trial profile
*Repeated cross-sectional design accounts for no loss to follow-up of patients. PINCER=pharmacist-led information technology-based intervention.
Table 2.
Mean number of patients per practice for each primary outcome measure
|
At baseline |
At 6 months |
At 12 months |
||||
|---|---|---|---|---|---|---|
| Simple feedback | PINCER | Simple feedback | PINCER | Simple feedback | PINCER | |
| Outcome 1 | 55 (7–129) | 51 (9–124) | 56 (10–130) | 51 (11–120) | 56 (12–136) | 51 (12–115) |
| Outcome 2 | 573 (93–1215) | 525 (94–1356) | 617 (106–1307) | 564 (104–1438) | 653 (111–1381) | 593 (118–1499) |
| Outcome 3 | 131 (22–379) | 121 (18–287) | 148 (26–462) | 135 (21–331) | 161 (30–492) | 146 (22–365) |
Data are mean (range). PINCER=pharmacist-led information technology intervention.
Table 3 shows results for the primary and secondary outcome measures at 6 months. For the primary outcomes, participants in the PINCER group were significantly less likely to have been prescribed a NSAID without a PPI if they had a history of peptic ulcer, a β blocker if they had asthma, or an ACE inhibitor or diuretic without having had urea and electrolytes measured in the preceding 15 months (table 3). Treatment group and practice size or deprivation did not interact significantly (data not shown). The intraclass correlation coefficients estimated from the models for the NSAID and β-blocker outcomes were smaller than those used in the sample size calculations, whereas those for the ACE inhibitor or diuretic outcome were larger.
Table 3.
Prevalence of prescription and monitoring problems at 6 months' follow-up by allocation group
| Patient characteristics | Simple feedback | PINCER | Adjusted odds ratio (95% CI)* | ICC | |
|---|---|---|---|---|---|
| Primary outcomes | |||||
| 1 | History of peptic ulcer prescribed an NSAID without a PPI/history of peptic ulcer without a PPI | 86/2014 (4%) | 51/1852 (3%) | 0·58 (0·38–0·89) | 4·68×10−7 |
| 2 | Asthma prescribed a β blocker/asthma | 658/22 224 (3%) | 499/20 312 (2%) | 0·73 (0·58–0·91) | 3·50×10−7 |
| 3 | Aged ≥75 years receiving long-term ACE inhibitors or loop diuretics without urea and electrolyte monitoring in the previous 15 months/aged ≥75 years receiving long-term ACE inhibitors or diuretics | 436/5329 (8%) | 255/4851 (5%) | 0·51 (0·34–0·78) | 0·14 |
| Secondary outcomes | |||||
| 2a | Asthma and not CHD prescribed a β blocker/asthma and not CHD | 387/21 048 (2%) | 299/19 286 (2%) | 0·81 (0·63–1·04) | 4·94×10−6 |
| 4 | History of venous or arterial thrombosis prescribed combined oral contraceptives/history of venous or arterial thrombosis (women) | 8/2783 (<1%) | 3/2490 (<1%) | 0·39 (0·07–2·15) | 0·05 |
| 5a | Methotrexate for ≥3 months without full blood count in past 3 months/methotrexate for ≥3 months | 162/518 (31%) | 122/494 (25%) | 0·80 (0·45–1·43) | 0·15 |
| 5b | Methotrexate for ≥3 months without a liver function test in past 3 months/methotrexate for ≥3 months | 154/518 (30%) | 121/494 (24%) | 0·79 (0·43–1·45) | 0·17 |
| 6 | Warfarin for ≥3 months without an INR in past 3 months/warfarin for ≥3 months | 78/1618 (5%) | 52/1720 (3%) | 0·53 (0·29–0·95) | 1·11×10−6 |
| 7 | Lithium for ≥3 months without a lithium concentration measurement in past 3 months/lithium for ≥3 months | 84/211 (40%) | 67/190 (35%) | 0·53 (0·24–1·19) | 0·24 |
| 8 | Amiodarone for ≥6 months without a thyroid function test in the past 6 months/amiodarone for ≥6 months | 106/235 (45%) | 81/242 (33%) | 0·57 (0·36–0·92) | 4·86×10−7 |
| 9 | Methotrexate without instructions to take weekly/patients prescribed methotrexate | 16/310 (5%) | 2/268 (1%) | 0·72 (0·06–9·25) | 5·20×10−7 |
| 10 | Amiodarone for ≥1 month at a dose of >200 mg per day/amiodarone for ≥1 month | 1/228 (<1%) | 1/228 (<1) | 0·96 (0·06–15·55)† | 2·1×105 |
| 11 | At least one prescription problem/at risk of at least one prescription problem | 752/26 329 (3%) | 553/24 073 (2%) | 0·71 (0·59–0·86) | 9·16×10−7 |
| 12 | At least one monitoring problem/at risk of at least one monitoring problem | 868/7409 (12%) | 584/6963 (8%) | 0·56 (0·44–0·70) | 0·04 |
Data are numerator/denominator (%), unless otherwise stated. Numbers of patients does not equal the sum of the denominators in each group because only those with baseline and follow-up data are included. PINCER=pharmacist-led information technology intervention. NSAID=non-steroidal anti-inflammatory drug. ACE=angiotensin converting enzyme. PPI=proton-pump inhibitor. CHD=coronary heart disease. INR=international normalised ratio. ICC=intraclass correlation coefficients.
Adjusted for randomisation stratum, baseline prevalence of errors, deprivation, and training status unless otherwise stated.
Adjustment for other variables not calculable.
We identified issues with three of our secondary outcome measures.19 As a result, we excluded seven practices from the analysis of outcome 6, and 11 practices from outcomes 9 and 10. Participants in the PINCER group were significantly less likely to have a prescription error or monitoring problem (table 3). Participants were also significantly less likely to have been prescribed warfarin without monitoring in the previous 3 months or prescribed amiodarone without a thyroid function test in the past 6 months (table 3). Treatment groups did not differ for other secondary outcome measures. We identified no significant interactions between treatment group and practice size or deprivation (data not shown).
At 12-months' follow-up participants in the PINCER group were still significantly less likely to have been prescribed a β blocker if they had a history of asthma, or prescribed an ACE inhibitor or diuretic without assessment of urea and electrolytes in the past 15 months (table 4). However, prescription of a non-selective NSAID without a PPI for patients with a history of peptic ulcer was no longer significant (table 4). The intraclass correlation coefficients estimated from the models for the NSAID outcome were smaller than those used in the sample size calculations, and those for the β blocker and ACE inhibitors or diuretic outcomes were larger.
Table 4.
Prevalence of prescription and monitoring problems at 12 months' follow-up by allocation group
| Patient characteristics | Simple feedback | PINCER | Adjusted odds ratio*(95% CI) | ICC | |
|---|---|---|---|---|---|
| Primary outcomes | |||||
| 1 | History of peptic ulcer prescribed an NSAID without a PPI/history of peptic ulcer without a PPI | 78/2035 (4%) | 61/1852 (3%) | 0·91 (0·59–1·39) | 6·54×10−7 |
| 2 | Asthma prescribed a β blocker/asthma | 692/23 520 (3%) | 545/21 359 (3%) | 0·78 (0·63–0·97) | 0·008 |
| 3 | Aged ≥75 years receiving long-term ACE inhibitors or loop diuretics without urea and electrolyte monitoring in the previous 15 months/aged ≥75 years receiving long-term ACE inhibitors or diuretics | 452/5813 (8%) | 306/5242 (6%) | 0·63 (0·41–0·95) | 0·13 |
| Secondary outcome measures | |||||
| 2a | Asthma and not CHD prescribed a β blocker/asthma and not CHD | 414/22 294 (2%) | 326/20 283 (2%) | 0·79 (0·62–1·02) | 0·009 |
| 4 | History of venous or arterial thrombosis prescribed combined oral contraceptives/history of venous or arterial thrombosis (women) | 15/2987 (1%) | 4/2640 (<1%) | 0·57 (0·05–6·17) | 0·24 |
| 5a | Methotrexate for ≥3 months without full blood count in past 3 months/methotrexate for ≥3 months | 194/552 (35%) | 130/531 (24%) | 0·51 (0·27–0·99) | 0·22 |
| 5b | Methotrexate for ≥3 months without a liver function test in past 3 months/methotrexate for ≥3 months | 186/552 (34%) | 134/531 (25%) | 0·50 (0·28–0·91)† | 0·16 |
| 6 | Warfarin for ≥3 months without an INR in past 3 months/warfarin for ≥3 months | 69/1752 (4%) | 76/1877 (4%) | 0·98 (0·52–1·85) | 0·10 |
| 7 | Lithium for ≥3 months without a lithium concentration measurement in past 3 months/lithium for ≥3 months | 88/213 (41%) | 56/176 (32%) | 0·50 (0·29–0·85) | 0·02 |
| 8 | Amiodarone for ≥6 months without a thyroid function test in the past 6 months/amiodarone for ≥6 months | 92/247 (37%) | 80/233 (34%) | 0·77 (0·41–1·43) | 0·11 |
| 9 | Methotrexate without instructions to take weekly/patients prescribed methotrexate | 13/309 (4%) | 0/271 (0%) | Not calculable | .. |
| 10 | Amiodarone for ≥1 month at a dose of >200 mg per day/amiodarone for ≥1 month | 1/231 (<1%) | 1/232 (<1%) | 0·95 (0·06–15·45)‡ | 1·07×10−5 |
| 11 | At least one prescription problem/at risk of at least one prescription problem | 785/27 808 (3%) | 610/25 246 (2%) | 0·78 (0·64–0·94) | 0·01 |
| 12 | At least one monitoring problem/at risk of at least one monitoring problem | 901/8011 (11%) | 652/7449 (9%) | 0·64 (0·51–0·82)† | 0·05 |
Data are numerator/denominator (%), unless otherwise stated. Number of patients does not equal the sum of the denominators in each group, because only those with baseline and follow-up data are included. PINCER=pharmacist-led information technology intervention. NSAID=non-steroidal anti-inflammatory drug. ACE=angiotensin converting enzyme. PPI=proton-pump inhibitor. CHD=coronary heart disease. INR=international normalised ratio. ICC=intraclass correlation coefficients.
Adjusted for randomisation stratum, baseline prevalence of errors, deprivation, and training status unless otherwise stated.
Includes interaction between treatment group and covariate dichotomised at the median value (≤median vs >median).
Adjustment for other variables not calculable.
Participants in the PINCER group were overall still significantly less likely to have a prescription or monitoring problem at 12 months (table 4), and were significantly less likely to have been prescribed methotrexate without a full blood count or liver function test in the past 3 months (table 4). Patients were also significantly less likely to have been prescribed lithium without measurement of lithium concentration in the past 3 months (table 4). Treatment groups did not differ for other secondary outcome measures (table 4). We identified no significant interactions between treatment group and practice or practice deprivation (data not shown). The models for primary outcomes, and all except three models for secondary outcomes, were robust to the exclusion of practices with standardised empirical Bayes estimates of random effects above or below two standard deviations (data not shown). No adverse events were reported.
Three reports were run in each practice (at baseline, 6 months, and 12 months), at a cost of £92·84 (£1=US$1·56) per practice at 6 months follow-up, and £139·26 per practice at 12 months follow-up. In total, generation of reports cost £3342·24 for 36 simple feedback and 36 PINCER intervention practices at 6 months and £5013·36 for the same number of practices at 12 months. This was the only cost in the simple feedback group. The PINCER group also generated training costs of £9933·26, preparation costs of £102·89, facilitated meeting costs of £6976·81, monthly meeting costs of £1996·30, practice meeting costs of £794·52, and error management costs of £14 641·20. The cost components were added to give the total mean cost per practice in each group of the trial (webappendix).
Adjusted costs were estimated by generalised linear modelling with the assumption of a γ distribution (webappendix). Only baseline list size was significant (webappendix).
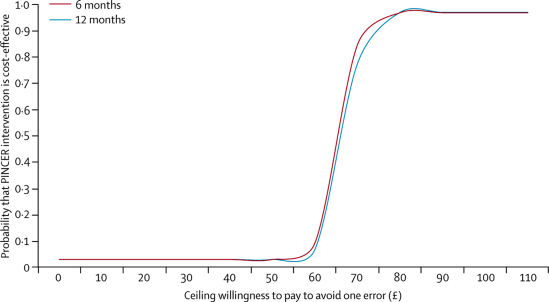
Table 5 summarises the costs and outcomes used in the incremental cost-effectiveness analysis and figure 2 shows the cost-effectiveness acceptability curves at 6 months and 12 months. This analysis suggests that PINCER had a 95% probability of being cost effective if the decision-maker's ceiling willingness to pay reached £75 per error avoided (at 6 months) or £85 per error avoided (at 12 months). Because the error reduction is sustained at 12 months, this analysis suggests that the intervention could be delivered yearly, rather than every 6 months, and still retain equivalent cost-effectiveness.
Table 5.
Costs, outcomes, and incremental economic analyses associated with PINCER intervention and simple feedback
| Simple feedback | PINCER intervention | Group comparison | ||
|---|---|---|---|---|
| Mean cost per practice (median, range) | ||||
| Report generation | ||||
| 6 months | £92·84 (NA) | £92·84 (NA) | .. | |
| 12 months | £139·26 (NA) | £139·26 (NA) | .. | |
| Pharmacist training | £0 | £275·92 (267·76, 79·54 to 591·23) | .. | |
| Quarterly facilitated strategic meetings | £0 | £195·23 (189·45, 56·28 to 418·33) | .. | |
| Monthly operational meetings | £0 | £56·88 (55·20, 16·40 to 121·88) | .. | |
| Practice feedback | £0 | £22·07 (21·42, 6·36 to 47·29) | .. | |
| Management of errors | £0 | £406·70 (320·93, 57·04 to 1318·68) | .. | |
| Total | ||||
| 6 months | £92·84 (NA) | £1049·67 (967·86, 329·22 to 2086·78) | .. | |
| 12 months | £139·26 (NA) | £1096·09 (1014·28, 375·64 to 2133·20) | .. | |
| Mean incremental cost (SD, 95% CI) | ||||
| 6 months | .. | .. | £871·88 (54·04, 765·96 to 977·79) | |
| 12 months | .. | .. | £870·63 (53·60, 858·42 to 1 068·52) | |
| Mean incremental errors (SD, 95% CI) | ||||
| 6 months | .. | .. | −12·90 (0·26, −13·42 to −12·39) | |
| 12 months | .. | .. | −12·71 (0·29, −13·27 to −12·14) | |
| Mean ICER (2·5th–97·5th percentile; cost per error avoided) | ||||
| 6 months | .. | .. | £65·60 (58·2 to 73·0) | |
| 12 months | .. | .. | £66·53 (66·8 to 81·5) | |
£1=US$1·56. NA=not applicable. ICER=incremental cost-effectiveness ratio. PINCER=pharmacist-led information technology-based intervention.
Figure 2.
Cost-effectiveness acceptability curves at 6 and 12 months
PINCER=pharmacist-led information technology-based intervention.
Discussion
This trial shows that the PINCER intervention is more effective than simple feedback for reduction of the numbers of patients at risk from hazardous prescribing and inadequate blood-test monitoring of medicines in general practice. In view of the high risk of serious iatrogenic harm associated with errors, reductions of the magnitude noted in this trial are likely to be clinically important.
The main strengths of this study include the pragmatic design, the large numbers of practices enrolled, and the range of clinically relevant outcomes tested. We avoided bias in allocation of practices by use of an independent centralised web-based randomisation service, and the sequence of treatment allocations was masked until all data analyses had been completed. Also, none of the practices were lost to follow-up. Because the data were extracted electronically from general practice computer systems, and those who cleaned the data were masked to treatment allocation, no risk of bias existed. Analyses were undertaken independently by two statisticians, masked to treatment allocation, both producing similar findings. When minor differences were identified, the reasons for these were explored and discrepancies resolved. Although some of the intraclass correlation coefficients estimated from our models were larger than those used in our sample size calculations, this difference did not result in insufficient power because significant effects were shown for those outcomes.
The simple feedback we used as a control is superior to routine models of care used in the UK, therefore the true effect size of PINCER in comparison to standard care might have been underestimated. We have also sought to assess the sustained effect of PINCER at 12 months after the intervention.
We have undertaken an embedded longitudinal qualitative assessment, which shows that general practice staff are receptive to the PINCER intervention and that the introduction of the intervention was a valuable educational experience for them (Creswell K, unpublished). Our parallel cohort study in 438 practices throughout England confirms that the trial's findings can be generalised widely (Shiekh A, unpublished).
However, our trial does have some limitations. Practices that agreed to participate and those that did not differed slightly (webappendix). The characteristics of the groups were much the same, but the intervention practices were slightly more deprived and likely to be training practices than were control practices (webappendix). We therefore adjusted for these two factors in our analysis. Moreover, results of our parallel national cohort study showed that the frequency of these errors was much the same in a large sample of non-intervention practices, suggesting that the practices enrolled in this trial are likely to be representative of practices nationwide. Although data extraction for our prespecified primary outcome measures worked successfully in all general practices recruited for the study, we encountered some difficulties with three of the secondary outcome measures. Finally, our sample size calculations were made on the basis of assessment of outcomes at the main 6 month assessment point; therefore the study might have been underpowered to assess outcomes at 12 months.
We deliberately focused on potential medication errors rather than adverse events and therefore we cannot be certain that the pharmacist-led intervention will reduce harm to patients. Nevertheless, a strong argument can be made for focus on the measurement of errors rather than on adverse events for assessment of the quality of clinical practice, as the former relate most closely to actions that are within the control of health-care professionals.28
We are developing an economic model incorporating the clinical and economic effects of changes in error rates to allow calculation of costs per quality-adjusted life-years. Little equivalent work about this topic has been done. An exploratory economic modelling study29 estimated that a pharmacy-based intervention to reduce medication errors would have a probability of being cost effective with a quality-adjusted life-year value of £10 000 of more than 60%. This finding suggests that pharmacist-led interventions to reduce error rates have the potential to be cost effective according to currently used cost-effectiveness thresholds. The webappendix contains further discussion of the economic analysis.
Since the start of our trial, important studies have questioned the effectiveness of isolated pharmacist-centred interventions in general practice. For example, the HOMER trial17 aimed to assess whether home-based medication review by pharmacists in older people would affect hospital readmission rates. The researchers reported an increase in hospital admissions and no improvement in quality of life or death rate. The RESPECT trial16 showed no benefit of involvement of community pharmacists in the moderation of drug management (pharmaceutical care) in older people in general practice. Findings are awaited from a trial in the USA assessing the effectiveness of a pharmacist intervention for reduction of medication errors after hospital discharge for high-risk patients with cardiovascular disease.30
In view of the conflicting evidence for the effectiveness of pharmacist-led interventions in primary care, the effectiveness of PINCER should be considered (panel 2). First, we used an educational outreach approach, which is a moderately powerful intervention for changing professional behaviour.14 Pharmacists who took part in the trial received training on the use of these techniques and the evidence base for the outcome measures used in the trial. Second, we focused on specific examples of hazardous prescription or inadequacies in medication monitoring, which might have increased our ability to detect change compared with more generalised measures. Third, the pharmacists established working relations with the practices, which granted them access to patient records for contextual information and the mandate to provide practical support to make changes to patients' medications and organise blood tests. Fourth, the intervention was multifaceted, used the potential offered by information technology, and aimed to simultaneously tackle different barriers to change; such interventions are known to be more effective than are simpler interventions.34
Panel 2. Research in context.
Systematic review
Systematic reviews have shown the high incidence of prescription errors in primary care, identified the errors that are most likely to result in patient harm, and identified the processes in which such errors most frequently occur, which suggest potential interventions to reduce the frequency of these errors.31–33 We searched the Cochrane Library, Medline, and Embase with the term “systematic review AND prescribing errors AND primary care” for reports published between Jan 1, 1980, and Aug 31, 2011, in any language. Our searches identified only one systematic review,10 which focuses on primary care, assessing the effectiveness and cost-effectiveness of interventions aiming to reduce the frequency of prescription errors in primary care settings.
Interpretation
This trial shows that a pharmacist-delivered information technology intervention substantially reduced the frequency of a range of clinically important prescription and medication monitoring errors. Related qualitative work shows the acceptability of the PINCER intervention to general practices and a parallel longitudinal observational study of prescription errors in over 400 practices shows the high probable generalisability of these findings across the UK.
Because of the pressing need to reduce errors in health care,4–6 PINCER offers an effective method for reducing a range of medication errors in general practice. An essential prerequisite is the use of electronic health records, which effectively reduces errors.7–9 The intervention that we have developed will be suitable for implementation in the increasing number of countries where clinical records are now computerised and where the roles of pharmacists to monitor proactively for clinically important medication errors can be extended.
Acknowledgments
Acknowledgments
The study was funded by a research grant awarded by the Department of Health's Patient Safety Research Portfolio. We thank the PINCER trial pharmacists, primary care trusts, general practices, and patients who took part in the study; Rachel Illingworth for facilitating the employment and management of the trial pharmacists; Sharon Mills for administrative support and assistance with collation of the costs for the pharmacists' interventions; Clive Morris and colleagues from The Computer Room (www.tcrnottingham.com) and Ed Longridge for their input about data extraction from general practices; Casey Quinn for contributions to the analysis of incremental cost-effectiveness ratios; Tom Turner for provision of advice about written materials that were issued to patients; Richard Lilford and colleagues from the Patient Safety Research Portfolio for their support. Finally, we thank the members of the independent Trial Steering Committee, Philip Hannaford (chair), Martin Buxton, and Marjorie Weiss; and the Data Monitoring and Ethics Committee, Richard Baker (chair), Christine Bond, and Peter Donnan for overseeing the trial.
Contributors
AJA helped design the study, was partly responsible for the overall administration and direction of the study, the analysis and interpretation of data. AS and JAC were partly responsible for the conception, overall design, and administration of the study. SR was the trial manager, helped design the Quest Browser queries, and piloted the data extraction methods. SA, RAE, RH, DK, CJM, and RJP helped design the project. AS, SA, DK, and RJP designed the statistical analyses. SA and DK did the statistical analyses. RE did the economic analysis with assistance from KP, MB, and MF, who contributed to the design, data collection, data processing, data analysis, and interpretation of the findings. GS advised on the study and commented on trial documentation. KC and ME collected data and interpreted the findings; ME processed and cleaned data. AJA, SA, JAC, KC, ME, RAE, RH, DK, RJP, SR, AS, and GS helped design and do the trial, analysed data, and interpreted the findings. AJA, SR, and AS wrote the first draft. All authors read and approved the final manuscript. AJA had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Web Extra Material
References
- 1.Gandhi TK, Weingart SN, Borus J. Adverse drug events in ambulatory care. N Engl J Med. 2003;348:1556–1564. doi: 10.1056/NEJMsa020703. [DOI] [PubMed] [Google Scholar]
- 2.Gurwitz JH, Field TS, Harrold LR. Incidence and preventability of adverse drug events among older persons in the ambulatory setting. JAMA. 2003;289:1107–1116. doi: 10.1001/jama.289.9.1107. [DOI] [PubMed] [Google Scholar]
- 3.Bates DW, Cullen DJ, Laird N. Incidence of adverse drug events and potential adverse drug events: implications for prevention. JAMA. 1995;274:29–34. [PubMed] [Google Scholar]
- 4.Department of Health . An organisation with a memory: report of an expert group on learning from adverse events in the NHS. Department of Health; London: 2000. [Google Scholar]
- 5.Kohn L, Corrigan J, Donaldson M. To err is human—building a safer health system. Institute of Medicine; Washington, DC: 1999. [Google Scholar]
- 6.Smith J. Building a safer NHS for patients: improving medication safety. Department of Health; London: 2004. [Google Scholar]
- 7.Bates DW, Gawande AA. Improving safety with information technology. N Engl J Med. 2003;348:2526–2534. doi: 10.1056/NEJMsa020847. [DOI] [PubMed] [Google Scholar]
- 8.Schedlbauer A, Prasad V, Mulvaney C. What evidence supports the use of computerized alerts and prompts to improve clinicians' prescribing behavior? J Am Med Inform Assoc. 2009;16:531–538. doi: 10.1197/jamia.M2910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garg AX, Adhikari NK, McDonald H. Effects of computerized clinical decision support systems on practitioner performance and patient outcomes: a systematic review. JAMA. 2005;293:1223–1238. doi: 10.1001/jama.293.10.1223. [DOI] [PubMed] [Google Scholar]
- 10.Royal S, Smeaton L, Avery AJ, Hurwitz B, Sheikh A. Interventions in primary care to reduce medication related adverse events and hospital admissions: systematic review and meta-analysis. Qual Saf Health Care. 2006;15:23–31. doi: 10.1136/qshc.2004.012153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Howard RL, Avery AJ, Slavenburg S. Which drugs cause preventable admissions to hospital? A systematic review. Br J Clin Pharmacol. 2007;63:136–147. doi: 10.1111/j.1365-2125.2006.02698.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Howard RL, Avery AJ, Howard PD, Partridge M. Investigation into the reasons for preventable drug related admissions to a medical admissions unit: observational study. Qual Saf Health Care. 2003;12:280–285. doi: 10.1136/qhc.12.4.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen YF, Avery AJ, Neil KE, Johnson C, Dewey ME, Stockley IH. Incidence and possible causes of prescribing potentially hazardous/contraindicated drug combinations in general practice. Drug Saf. 2005;28:67–80. doi: 10.2165/00002018-200528010-00005. [DOI] [PubMed] [Google Scholar]
- 14.Thomson O'Brien MA, Oxman AD, Davis DA, Haynes RB, Freemantle N, Harvey EL. Educational outreach visits: effects on professional practice and health care outcomes. Cochrane Database Syst Rev. 2000;2:CD000409. doi: 10.1002/14651858.CD000409. [DOI] [PubMed] [Google Scholar]
- 15.Catwell L, Sheikh A. Evaluating eHealth interventions: the need for continuous systemic evaluation. PLoS Med. 2009;6:e1000126. doi: 10.1371/journal.pmed.1000126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Richmond S, Morton V, Cross B, RESPECT Trial Team Effectiveness of shared pharmaceutical care for older patients: RESPECT trial findings. Br J Gen Pract. 2010:e10–e19. doi: 10.3399/bjgp09X473295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Holland R, Lenaghan E, Harvey I. Does home based medication review keep older people out of hospital? The HOMER randomised controlled trial. BMJ. 2005;330:293. doi: 10.1136/bmj.38338.674583.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.MRC . A framework for the development and evaluation of RCT's for complex interventions. Medical Research Council; London: 2000. [Google Scholar]
- 19.Avery AJ, Rodgers S, Cantrill JA. Protocol for the PINCER trial: a cluster randomised trial comparing the effectiveness of a pharmacist-led IT-based intervention with simple feedback in reducing rates of clinically important errors in medicines management in general practices. Trials. 2009;10:28. doi: 10.1186/1745-6215-10-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hollis S, Campbell F. What is meant by intention to treat analysis? Survey of published randomised controlled trials. BMJ. 1999;319:670–674. doi: 10.1136/bmj.319.7211.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shih WJ. Problems in dealing with missing data and informative censoring in clinical trials. Curr Control Trials Cardiovasc Med. 2002;3:4. doi: 10.1186/1468-6708-3-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Department for Communities and Local Government Indices of Deprivation 2004. http://webarchive.nationalarchives.gov.uk/+/http://www.communities.gov.uk/archived/general-content/communities/indicesofdeprivation/216309/ (accessed Jan 20, 2012).
- 23.Assmann SF, Pocock SJ, Enos LE, Kasten LE. Subgroup analysis and other (mis)uses of baseline data in clinical trials. Lancet. 2000;355:1064–1069. doi: 10.1016/S0140-6736(00)02039-0. [DOI] [PubMed] [Google Scholar]
- 24.StataCorp LP. Stata data analysis and statistical software. Special Edition Release. 10.1 ed. Stata; College Station: 2008. [Google Scholar]
- 25.Elashoff JD. nQuery Advisor®. Version 6.0 software. Los Angeles; 2005.
- 26.Kerry SM, Bland JM. Statistics notes: the intracluster correlation coefficient in cluster randomisation. BMJ. 1998;316:1455–1460. doi: 10.1136/bmj.316.7142.1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hilbe JM. Negative binomial regression. Cambridge University Press; New York: 2007. [Google Scholar]
- 28.Lilford RJ, Brown CA, Nicholl J. Use of process measures to monitor the quality of clinical practice. BMJ. 2007;335:648–650. doi: 10.1136/bmj.39317.641296.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Karnon J, Campbell F, Czoski-Murray C. Model-based cost-effectiveness analysis of interventions aimed at preventing medication error at hospital admission (medicines reconciliation) J Eval Clin Pract. 2009;15:299–306. doi: 10.1111/j.1365-2753.2008.01000.x. [DOI] [PubMed] [Google Scholar]
- 30.Schnipper JL, Roumie CL, Cawthon C. Rationale and design of the Pharmacist Intervention for Low Literacy in Cardiovascular Disease (PILL–CVD) study. Circ Cardiovasc Qual Outcomes. 2010;3:212–219. doi: 10.1161/CIRCOUTCOMES.109.921833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Winterstein AG, Sauer BC, Hepler CD, Poole C. The incidence of preventable drug-related hospital admissions. Pharmacoepidemiol Drug Saf. 2000;9:S118–S147. doi: 10.1345/aph.1A225. [DOI] [PubMed] [Google Scholar]
- 32.Garfield S, Barber N, Walley P, Willson A, Eliasson L. Quality of medication use in primary care—mapping the problem, working to a solution: a systematic review of the literature. BMC Med. 2009;7:50. doi: 10.1186/1741-7015-7-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Black AD, Car J, Pagliari C. The impact of eHealth on the quality and safety of health care: a systematic overview. PLoS Med. 2011;8:e1000387. doi: 10.1371/journal.pmed.1000387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Grimshaw JM, Shirran L, Thomas R. Changing provider behavior: an overview of systematic reviews of interventions. Med Care. 2001;39:II2–I45. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.