Abstract
Staphylococcus aureus is a common aetiological agent of bacterial brain abscesses. We have previously established that a considerable IL-1 (interleukin-1) response is elicited immediately following S. aureus infection, where the cytokine can exert pleiotropic effects on glial activation and blood–brain barrier permeability. To assess the combined actions of IL-1α and IL-1β during CNS (central nervous system) infection, host defence responses were evaluated in IL-1RI (IL-1 receptor type I) KO (knockout) animals. IL-1RI KO mice were exquisitely sensitive to intracerebral S. aureus infection, as demonstrated by enhanced mortality rates and bacterial burdens within the first 24 h following pathogen exposure compared with WT (wild-type) animals. Loss of IL-1RI signalling also dampened the expression of select cytokines and chemokines, concomitant with significant reductions in neutrophil and macrophage infiltrates into the brain. In addition, the opening of astrocyte hemichannels during acute infection was shown to be dependent on IL-1RI activity. Collectively, these results demonstrate that IL-1RI signalling plays a pivotal role in the genesis of immune responses during the acute stage of brain abscess development through S. aureus containment, inflammatory mediator production, peripheral immune cell recruitment, and regulation of astrocyte hemichannel activity. Taken in the context of previous studies with MyD88 (myeloid differentiation primary response gene 88) and TLR2 (Toll-like receptor 2) KO animals, the current report advances our understanding of MyD88-dependent cascades and implicates IL-1RI signalling as a major antimicrobial effector pathway during acute brain-abscess formation.
Keywords: acute brain slices, brain abscess, hemichannel, interleukin-1 receptor type I (IL-1RI), microglia, myeloid differentiation primary response gene 88 (MyD88)
Abbreviations: Ab, antibody; ACSF, artificial cerebral spinal fluid; cfu, colony forming unit; CNS, central nervous system; CTB, CellTracker Blue; CXCL1, CXC chemokine ligand 1; MIP, macrophage inflammatory protein; CCL2, CC chemokine ligand 2; EtBr, ethidium bromide; IFNγ, interferon γ; IL, interleukin; IL-1RI, IL-1 receptor type I; KO, knockout; MyD88, myeloid differentiation primary response gene 88; PFA, paraformaldehyde; ROI, region of interest; TLR, Toll-like receptor; TNFα, tumour necrosis factor α; WT, wild-type
INTRODUCTION
Brain abscesses ensue following pyogenic parenchymal infections and are typified by widespread inflammation and necrosis (Mathisen and Johnson, 1997; Lu et al., 2002). Despite modern neurosurgical interventions, new antibiotics, and advanced imaging modalities, this suppurative infection often results in significant neurological disability or death. Staphylococcus aureus is a common aetiological agent of brain abscess and the incidence of infections caused by methicillin-resistant S. aureus isolates has increased in recent years (Jones et al., 2004; Chambers and Deleo, 2009). Brain abscesses evolve through a series of well-defined stages, as revealed by MRI (magnetic resonance imaging) and CT (computed tomography) scans (Mathisen and Johnson, 1997; Gupta et al., 2010). Early cerebritis occurs from day 1 to 3 and is typified by neutrophil accumulation, tissue necrosis and significant oedema. Microglial and astrocyte activation is also evident at this stage and persists throughout infection. This is followed by the late cerebritis stage, occurring from days 4 to 9 and characterized by prominent macrophage and lymphocytic infiltrates. The capsule stage occurs from days 7 to 14 and is associated with the formation of a well-vascularized abscess wall to sequester the lesion and protect surrounding normal brain parenchyma from additional damage (Flaris and Hickey, 1992; Kielian, 2004; Aldrich and Kielian, 2011).
Previous studies from our laboratory have demonstrated that IL-1 is among the most rapidly induced cytokines following CNS (central nervous system) S. aureus infection, with enhanced mRNA expression observed within 1 h after bacterial exposure (Kielian and Hickey, 2000; Garg et al., 2009). IL-1 (interleukin-1) is a pluripotent cytokine implicated in various neurodegenerative, infectious and inflammatory diseases of the CNS (Jacobs et al., 1991; Zwijnenburg et al., 2003; Shaftel et al., 2008). The IL-1 family consists of 11 members that include cytokines, co-receptors, decoy receptors, binding proteins, and inhibitory receptors (Dinarello, 2009). Among them, IL-1α and IL-1β have been widely studied and participate in a variety of physiological and pathological processes (Dinarello, 2009). IL-1β is synthesized as an inactive 31 kDa precursor, which is processed by the inflammasome via caspase 1 into its active secreted form (Schroder and Tschopp, 2010; Davis et al., 2011). In contrast with pro-IL-1β, pro-IL-1α is biologically active but can also be processed into its mature form (Dinarello, 2009). Both IL-1α and IL-1β transduce signals by binding to IL-1RI (IL-1 receptor type I), leading to activation of the adaptor protein MyD88 (myeloid differentiation primary response gene 88), NF-κB (nuclear factor-κB) nuclear translocation and transcription of numerous inflammatory genes. Several mechanisms exist for negatively regulating IL-1RI signalling, including IL-1RII that binds both IL-1α and IL-1β and acts as a decoy receptor, and IL-1Ra that serves as an endogenous competitive inhibitor of IL-1α and IL-1β by binding but not activating IL-1RI (Dinarello, 2009).
Microglia are a major source of IL-1β in response to S. aureus, which is modulated via the co-ordinate actions of TLR (Toll-like receptor)/MyD88 signalling and NLRP3 (Nod-like receptor protein 3) inflammasome activation (Kielian et al., 2005a; Esen and Kielian, 2006; Hanamsagar et al., 2011). Yet despite this information, the functional importance of IL-1 in the context of acute CNS S. aureus infection remains incomplete. To address the collective actions of IL-1α and IL-1β during brain abscess formation, we investigated the role of the IL-1 system in the genesis of protective host immunity and regulation of astrocyte hemichannel activity using IL-1RI KO (knockout) mice. Another objective was to build upon the known biology of MyD88-dependent pathways to advance our knowledge of which cascade(s) plays a dominant role in protection during acute CNS bacterial infection. In this regard, IL-1RI signalling was found to be critical, since IL-1RI KO animals were extremely sensitive to S. aureus and most mice succumbed to infection within 18–24 h following bacterial exposure. Even an attempt to extend the survival period of IL-1RI KO mice by infection with a 1-log lower bacterial inoculum did not considerably improve infection outcome, with few animals surviving beyond 72 h following S. aureus exposure. The enhanced mortality rate of IL-1RI KO mice was typified by attenuated cytokine/chemokine expression and immune cell influx early after infection (i.e. 12 and 18 h), which resulted in significantly elevated bacterial burdens. A recent study from our laboratory demonstrated that S. aureus infection leads to the transient opening of hemichannels in astrocytes (Karpuk et al., 2011), which here we attribute to IL-1RI action. Taken together, our data suggest an important role for IL-1RI signalling in eliciting innate immune responses, astrocyte hemichannel activity, and bacterial containment during early CNS S. aureus infection. These studies advance our understanding of the hierarchy of MyD88-dependent pathways and, in the context of earlier work with MyD88 and TLR2 KO animals, demonstrates that IL-1RI action surpasses individual MyD88-dependent TLR signalling pathways in terms of essentiality for protection during acute CNS bacterial infection.
MATERIALS AND METHODS
Mice
IL-1RI KO mice (C57BL/6 background) were purchased from Jackson Laboratory and age- and sex-matched C57BL/6 mice were used as WT (wild-type) controls. All animals were bred and housed in an Association for Assessment and Accreditation of Laboratory Animal Care International-accredited animal facility at the University of Nebraska Medical Center, provided with food and water ad libitum, and housed under 12 h light/12 h dark cycles. Brain abscess studies were performed with mice 8–10 weeks of age.
Experimental brain abscess model
Brain abscesses were induced as previously described by intracerebral injection of a methicillin-resistant S. aureus USA300 isolate (CAV1002) that was recovered from an otherwise healthy individual that died from a brain abscess (Kielian et al., 2007; Sifri et al., 2007). Briefly, a 1 cm longitudinal incision was made in the scalp under avertin anaesthesia to expose the underlying skull sutures and facilitate the identification of bregma. A rodent stereotaxic apparatus equipped with a Cunningham mouse adaptor (Stoelting) was used to implant S. aureus-encapsulated beads into the striatum, using the following co-ordinates relative to bregma: +1.0 mm rostral, +2.0 mm lateral and −3.0 mm deep from the surface of the brain. A burr hole was made and a 10 μl Hamilton syringe fitted with a 26-gauge needle was used to slowly deliver 2 μl of S. aureus-laden beads [103–104 cfu (colony forming unit)] into the brain parenchyma. The needle remained in place for 2.5 min following injection to minimize bead efflux and potential leakage into the meninges. The incision was closed using Vetbond (3M) and mice were regularly monitored throughout the course of infection. Any animal that displayed a severe moribund state was immediately killed. The animal use protocol, approved by the University of Nebraska Medical Center Animal Care and Use Committee, is in accord with the National Institutes of Health guidelines for the use of rodents.
Quantification of bacterial titres and pro-inflammatory mediator expression in brain abscess homogenates
At the appropriate time points post-infection, mice were killed with an overdose of inhaled isoflurane and perfused transcardially with ice-cold PBS. Brain abscesses were dissected within 1–2 mm of the lesion margins and immediately disrupted in 500 μl of homogenization buffer [1×PBS supplemented with a protease inhibitor cocktail tablet (Roche) and RNase inhibitor (Promega)] on ice. Serial 10-fold dilutions of brain tissue homogenates were plated on trypticase soy-agar plates supplemented with 5% sheep blood (Hemostat Laboratories) and are expressed as log10 cfu/g wet tissue weight. The remaining homogenate was centrifuged at 14000 rev./min for 20 min at 4°C and supernatants were stored at −80°C until cytokine/chemokine analysis. To compare pro-inflammatory mediator expression profiles between IL-1RI KO and WT mice, a custom-designed mouse multi-analyte microbead array was utilized according to the manufacturer's instructions (MILLIPLEX; Millipore). This array allows for the simultaneous detection of 19 individual inflammatory molecules in a single 75 μl sample, including IL-1α, IL-1β, TNFα (tumour necrosis factor α), IFNγ (interferon γ), IL-6, IL-9, IL-10, IL-12p40, IL-12p70, IL-15, IL-17, CXCL1 (CXC chemokine ligand 1)/KC (keratinocyte chemoattractant), CXCL2/MIP-2 (macrophage inflammatory protein-2), CXCL9/MIG (monokine induced by IFNγ), CXCL10/IP-10 (IFNγ-induced protein 10), CCL2 (CC chemokine ligand 2)/MCP-1 (monocyte chemoattractant protein-1), CCL3/MIP-1α, CCL4/MIP-1β and CCL5/RANTES (regulated upon activation, normal T-cell expressed and secreted). Results were analysed using a Bio-Plex workstation (Bio-Rad) and adjusted based on the amount of total protein extracted from brain tissue homogenates for normalization using a colorimetric Bio-Rad DC Protein Assay kit as specified by the manufacturer (Bio-Rad).
Immunofluorescence staining and confocal microscopy
Brain abscess tissues from IL-1RI KO and WT mice were collected at either 18 h or 3 days post-infection. Animals were perfused via the right heart ventricle with 4% (w/v) PFA (paraformaldehyde) in 0.1 M phosphate buffer, pH 7.4, for tissue fixation. Subsequently, brain tissues were post-fixed in cold 4% PFA for 1 h and cryoprotected in 30% sucrose prior to freezing and embedding in OCT (optimal cutting temperature compound, Sakura Finetek USA Inc.) for cryostat sectioning. Serial sections of brain tissues (10 μm) were prepared and processed for immunofluorescence staining using Abs (antibodies) specific for Iba-1 (Biocare Medical) to assess microglial/macrophage reactivity, caspase 1 (Millipore), and activated caspase 3 (Cell signalling Technology) with staining detected with a biotinylated anti-rabbit IgG (Jackson Immunoresearch Laboratories) and either streptavidin-Alexa Fluor® 488 or -Alexa Fluor® 594 conjugates (Molecular Probes). Confocal images were acquired using a Zeiss 510 META laser scanning microscope (Carl Zeiss) with tissues stained with secondary Abs only as a negative control.
Flow cytometry analysis
To determine whether IL-1RI deficiency impacted neutrophil or macrophage recruitment following CNS S. aureus infection, cells were quantified by FACS analysis as previously described (Garg et al., 2009; Holley and Kielian, 2012). Briefly, cells were stained with the following Abs to differentiate between neutrophils (Ly-6G+, F4/80− and CD45high), macrophages (Ly-6G−, F4/80+ and CD45high), and microglia (Ly-6G−, F4/80+ and CD45low−intermediate). All Abs were purchased from BD Biosciences. Cells were analysed using a BD LSRII with compensation set based on the staining of each individual fluorochrome alone and correction for autofluorescence with unstained cells. Controls included cells stained with isotype control Abs to assess the degree of non-specific staining. Analysis was performed using BD FACSDiva™ software with cells gated on the total leucocyte population. Results are presented as the percentage of neutrophils, macrophages and microglia recovered from IL-1RI KO animals compared with WT mice (set to 100%), with normalization to adjust for the recovery of different cell numbers from the two mouse strains.
Acute brain slice preparation and quantification of astrocyte hemichannel activity
To determine whether astrocyte hemichannel activity elicited during acute brain abscess developed was linked with IL-1RI activity, hemichannel opening was assessed by EtBr (ethidium bromide) uptake assays as previously described (Karpuk et al., 2011). Briefly, IL-1RI KO and WT mice were killed by cervical dislocation and immediately decapitated. The brain was quickly removed and bathed in ice-cold ACSF (artificial cerebral spinal fluid; in mM: 124 NaCl, 26 NaHCO3, 3 KCl, 2 MgCl2, 2 CaCl2, 0.4 ascorbic acid and 10 glucose) for slice preparation. Next, horizontal slices (300–400 μm thick) were cut using a Leica VT1000S vibrating blade microtome (Leica Microsystems) and immediately placed in ACSF at 32°C. After a 20–30 min incubation period at 32°C, brain slices were held in ACSF at room temperature for at least 1 h before use in experiments. All incubation solutions were equilibrated and continuously bubbled with carbogen (95% O2 and 5% CO2). Slices were incubated with CTB (CellTracker Blue; 2 μM; Molecular Probes) for 30 min prior to EtBr uptake assays to facilitate astrocyte identification.
To determine the impact of IL-1RI signalling on astrocyte hemichannel activity, Z-stack images using appropriate filters for CTB and EtBr visualization were captured before and during EtBr (2.5 μM) application in the bath solution at regions immediately bordering the brain abscess margins extending to 1 mm from the primary lesion as described below. A minimum of five images were acquired in every region over a series of time points spanning 30 min after EtBr application. The cell bodies of CTB+ astrocytes were marked as a ROI (region of interest), whereupon the maximal fluorescent intensity of EtBr in each ROI was plotted against image acquisition time to calculate slope coefficients using a linear regression algorithm (Karpuk et al., 2011). Simultaneously, background slope coefficients were calculated from the same images as an average EtBr fluorescence intensity in three to five ROIs in which no EtBr staining or CTB expression was observed. The rate of EtBr uptake was calculated by subtracting background slope coefficients from cellular slope coefficients and is reported in arbitrary units per minute.
Definition of striatal regions analysed for hemichannel activity measurements
All CTB+ astrocytes analysed for hemichannel studies were located in the striatum, where measurements were initiated at the brain abscess border and extended outward for a distance of up to 1 mm. The brain abscess border was identified as the junction between necrotic and live tissue in which intense CTB astrocytes were visible. All distance calculations were performed using AxioVision software (Carl Zeiss).
Statistical analysis
Significant differences between experimental groups were determined by one-way ANOVA followed by Pairwise Multiple Comparison (Tukey Test) or Student's t test. For all analyses, a P<0.05 was considered statistically significant.
RESULTS
IL-1RI signalling is essential for survival during acute CNS S. aureus infection
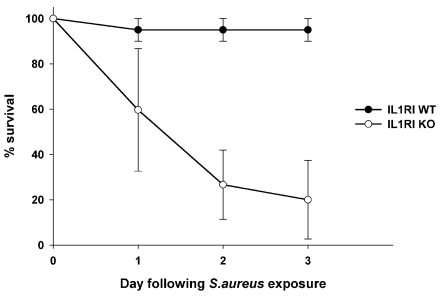
Since IL-1 expression is rapidly induced following CNS S. aureus infection (Kielian and Hickey, 2000; Garg et al., 2009), we examined the functional impact of combined IL-1α and IL-1β activity using a well-developed experimental brain abscess model in IL-1RI KO mice. Importantly, although prior work from our laboratory established the critical role of MyD88-dependent signalling for survival during early CNS infection (Kielian et al., 2007), it remained unclear whether this was predominately mediated by TLR or IL-1RI activity, which was another objective of the current study. IL-1RI KO animals were highly susceptible to intracerebral S. aureus, with approximately 40% of mice succumbing to infection within 24 h, whereas survival did not extend beyond day 3 post-infection (Figure 1). In contrast, WT animals exhibited minimal mortality with the standard S. aureus infectious inoculum used in these studies (i.e. 7×103–104 cfu). In subsequent experiments, IL-1RI KO mice were infected with 1-log lower bacteria (i.e. 103 cfu) in an attempt to extend the survival period; however, mortality rates were still high and animals did not survive beyond day 3 post-infection. Due to the heightened sensitivity of IL-1RI KO mice to CNS S. aureus infection, animals were killed at 12 and 18 h following infection in all subsequent experiments to avoid survival bias. These findings clearly reveal that IL-1RI activity is essential for overcoming acute insults immediately following CNS S. aureus exposure.
Figure 1. IL-1RI signalling is critical for survival during acute CNS S. aureus infection.
IL-1RI KO and WT mice (n = 8–10 per group) received intracerebral injections of S. aureus (7×103 cfu), whereupon survival was monitored over a 3-day period. Results represent the percentage survival from three independent experiments (means±S.E.M.).
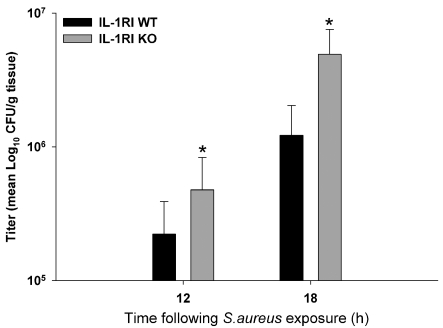
One possibility to explain the rapid mortality rate observed in IL-1RI KO mice was the inability to contain infection following IL-1RI loss. To investigate this possibility, viable bacterial burdens associated with brain abscesses of IL-1RI KO and WT mice were evaluated. Bacterial titres were significantly increased in IL-1RI KO animals at both 12 and 18 h post-infection compared with WT mice (Figure 2). Interestingly, although IL-1RI signalling proceeds via a MyD88-dependent pathway, our previous report did not reveal any differences in bacterial burdens between MyD88 KO and WT animals (Kielian et al., 2007), revealing a novel mechanism whereby signalling emanating from IL-1RI activity influences S. aureus containment within the CNS.
Figure 2. IL-1RI loss leads to increased bacterial burdens during early CNS S. aureus infection.
IL-1RI KO and WT mice (n = 8–10 per group) received intracerebral injections of S. aureus (7×103 cfu). Animals were killed at either 12 or 18 h post-infection, whereupon bacterial burdens were quantified and normalized to tissue wet weight (g). Significant differences between IL-1RI KO and WT mice are denoted by asterisks (*P<0.05).
IL-1RI deficiency leads to impaired inflammatory mediator induction in vivo
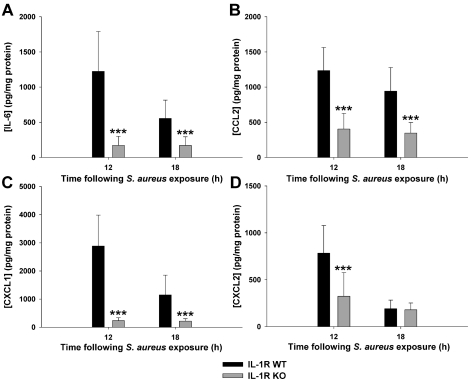
A wide variety of pro-inflammatory cytokines and chemokines are rapidly induced in response to CNS S. aureus infection (Stenzel et al., 2005; Garg et al., 2009). Based on the importance of IL-1RI activity in dictating survival and bacterial burdens during acute brain abscess development, we next determined whether this was associated with alterations in pro-inflammatory mediator induction. Interestingly, IL-1RI deficiency resulted in a selective reduction in cytokine and chemokine expression (i.e. IL-6, CCL2, CXCL1 and CXCL2; Figures 3A–3D respectively), whereas other inflammatory mediators were not affected (i.e. IL-1β, IL-15, CCL3 and CXCL9; results not shown). These results reveal a selective autocrine/paracrine role for IL-1α and IL-1β signalling via IL-1RI to target the expression of specific cytokines and chemokines during acute CNS S. aureus infection. This differs from our earlier report in MyD88 KO animals, which displayed global defects in inflammatory mediator release (Kielian et al., 2007), which is likely explained by the more widespread induction of cytokines/chemokines emanating from the loss of both TLR and IL-1RI cascades.
Figure 3. IL-1RI signalling is important for eliciting cytokine and chemokine production in response to CNS S. aureus challenge.
IL-1RI KO and WT mice (n = 8–10 per group) received intracerebral injections of S. aureus (7×103 cfu) and were killed at either 12 or 18 h post-infection. Supernatants from infected hemispheres were evaluated for IL-6 (A), CCL2 (B), CXCL1 (C) and CXCL2 (D) expression by MILLIPLEX analysis with results normalized to total protein concentrations to correct for difference in tissue sampling size. Results are representative of three independent experiments and significant differences between IL-1RI KO and WT mice are denoted by asterisks (***P<0.001).
IL-1RI signalling regulates innate immune cell influx during early brain abscess development
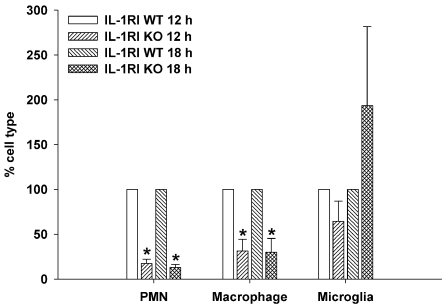
The significant reductions in neutrophil and monocyte chemokines observed in brain abscesses of IL-1RI KO mice led us to investigate whether this would translate into altered peripheral immune cell recruitment into the brain. Indeed, this was the case, as both neutrophil and macrophage infiltrates were significantly decreased in IL-1RI KO mice at both 12 and 18 h post-infection compared with WT animals (Figure 4). The inability to recruit sufficient numbers of these professional phagocytes may contribute, in part, to the inability to control S. aureus burdens in IL-1RI KO mice during early infection.
Figure 4. IL-1RI signalling is critical for neutrophil and macrophage recruitment following S. aureus infection.
IL-1RI KO and WT mice (n = 8–10 per group) received intracerebral injections of S. aureus (7×103 cfu) and were killed at either 12 or 18 h post-infection, whereupon neutrophils, macrophages and microglia were quantified by FACS analysis. Results are expressed as the percentage of each cell population normalized to WT values from three independent experiments (means±S.E.M.) with significant differences between IL-1RI KO and WT animals denoted by asterisks (*P<0.05).
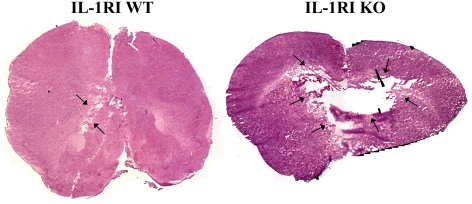
IL-1RI deficiency causes extensive tissue damage during acute brain abscess development
We envisioned that the rapid rate at which IL-1RI KO mice succumbed to CNS S. aureus infection may be explained, in part, by excessive necrosis. To investigate this possibility, histological examination of brain abscess tissues was performed. Indeed, IL-1RI KO mice exhibited larger lesions compared with WT animals, the latter of which only presented with mild cerebritis (Figure 5). In IL-1RI KO mice, lesions extended beyond the infected area and invaded the contralateral hemisphere (Figure 5). Although tissue destruction during brain abscess development proceeds primarily via necrosis, apoptotic cell death has also been reported (Stenzel et al., 2005). To evaluate whether apoptotic cell death was altered in IL-1RI KO mice, tissue sections were stained with an Ab specific for activated caspase 3. The amount of activated caspase 3 staining was similar between IL-1RI KO and WT mice (results not shown), indicating that the IL-1RI pathway has no impact on apoptotic cell death during early brain abscess development in this model system.
Figure 5. IL-1RI deficiency exacerbates tissue damage during acute brain abscess development.
Representative H&E (haematoxylin and eosin) stained images of brain sections from WT and IL-1RI KO mice at 18 h following S. aureus infection were created by stitching consecutive microscopic fields of view (lesions delineated by arrows). Black areas represent regions devoid of signal during the imaging stitching program. Results are representative of six individual animals per group.
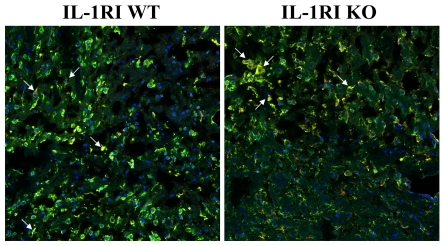
IL-1 is produced in an inactive form and requires proteolytic processing by the inflammasome for its maturation and secretion (Schroder and Tschopp, 2010; Davis et al., 2011). Since microglia/macrophages are a major source of IL-1 during brain abscess development, we next determined whether the loss of IL-1RI signalling would lead to alterations in cellular activation and/or caspase 1 levels. Interestingly, microglial/macrophage activation was reduced in IL-1RI KO mice compared with WT animals; however, no differences in caspase 1 immunoreactivity were observed (Figure 6). The majority of caspase 1 staining was associated with Iba-1+ microglia/macrophages, confirming these cells as a main source of IL-1 during CNS S. aureus infection (Figure 6).
Figure 6. Microglial/macrophage activation during acute brain abscess development is less pronounced in IL-1RI KO mice.
IL-1RI KO and WT mice received intracerebral injections of S. aureus (103 cfu) to extend survival of the former. Tissues were collected from animals at day 3 following infection for immunofluorescence staining and confocal microscopy for Iba-1 (green) and caspase 1 (red; magnification ×20). Arrows denote Iba-1+ microglia/macrophages that express caspase 1. Results are representative of tissues collected from six individual animals per group.
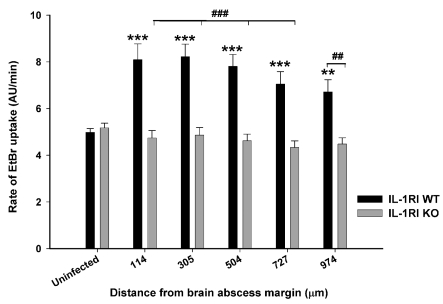
IL-1RI action is critical for opening astrocyte hemichannels within the abscess pro-inflammatory milieu
A recent study from our laboratory demonstrated that the inflammatory milieu created during brain abscess development led to the opening of astrocyte hemichannels concomitant with inhibition of gap junction communication (Karpuk et al., 2011). Interestingly, hemichannel activity was only observed during acute infection (i.e. day 3) and was no longer detected at day 7 when the intensity of the pro-inflammatory milieu begins to subside (Baldwin and Kielian, 2004; Aldrich and Kielian, 2011). Previous reports from other groups have shown that IL-1β is capable of inducing astrocyte hemichannel activity (Retamal et al., 2007; Orellana et al., 2009); however, these studies were performed in cultured astrocytes and, as such, do not take into account the complex cellular interactions that are represented in brain slices. In addition, no studies to date have assessed the duality of IL-1α and IL-1β action on astrocyte hemichannel activity, a question that we addressed in the current report with the use of acute brain slices from IL-1RI KO mice. As expected, hemichannel activity, as demonstrated by EtBr uptake, was significantly elevated in brain slices from WT animals harbouring brain abscesses (Figure 7). Importantly, open hemichannels were not detected in IL-1RI KO mice in any regions surrounding the brain abscess margins (Figure 7), demonstrating that S. aureus infection triggers astrocyte hemichannel activity via an IL-1RI-dependent manner. To our knowledge, this is the first report where astrocyte hemichannel activity has been attributed to IL-1RI action in brain slices that possess the complex three-dimensional interactions with multiple cell types, which is more reminiscent of in vivo pathology. The functional consequences of astrocyte hemichannel activity in the context of CNS bacterial infection remain to be determined.
Figure 7. IL-1RI signalling is critical for hemichannel opening during acute brain abscess development.
IL-1RI KO and WT mice received intracerebral injections of S. aureus (103 cfu), whereupon acute brain slices were prepared at day 3 post-infection to monitor hemichannel activity by EtBr uptake assays at the indicated distance from the brain abscess margin (μm). Values represent the means±S.D. from uninfected (six slices, 491 cells analysed) or brain abscess (seven slices, 676 cells analysed) tissues of WT mice, as well as uninfected (six slices, 444 cells analysed) or brain abscess (12 slices, 744 cells analysed) tissues from IL-1RI KO animals. Significant differences between uninfected and infected slices are denoted by asterisks (**P<0.01; ***P<0.001), whereas distinctions between WT and IL-1RI KO slices are indicated by hatched signs (##P<0.01, ###P<0.001).
DISCUSSION
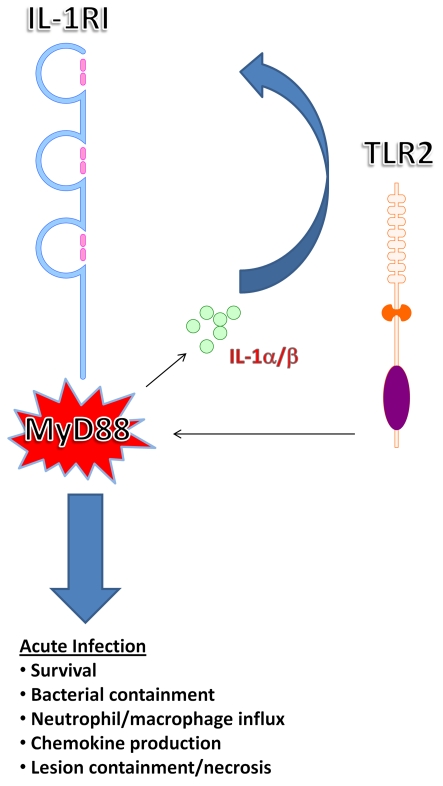
A major objective of the current study was to build upon the known biology of MyD88-dependent signalling to advance our knowledge of which pathway plays a dominant role in protection during acute CNS bacterial infection. Specifically, since previous studies from our laboratory have revealed a critical role for MyD88-dependent signalling in eliciting inflammatory mediator release during early brain abscess development (Kielian et al., 2007), we were interested in determining the contribution of IL-1RI in this process, since this receptor also utilizes MyD88 as a signalling adaptor (Takeuchi and Akira, 2010; Hanke and Kielian, 2011). Furthermore, comparisons in disease phenotypes with previous reports by us and others with TLR2 KO animals would enable an assessment of which MyD88-dependent pathway was playing the primary role during acute infection (Kielian et al., 2005b; Stenzel et al., 2008). Here, we demonstrate that IL-1RI signalling is crucial for survival during the acute phase of brain abscess development. This point is highlighted by the fact that IL-1RI KO mice still succumbed to infection even after exposure to a 1-log lower bacterial inoculum that is not capable of eliciting brain abscess formation in immunocompetent animals. These findings are reminiscent of the phenotype we reported previously in MyD88 KO mice (Kielian et al., 2007). When considered in the context of prior studies with TLR KO animals, we conclude that IL-1RI signalling transcends TLR-dependent pathways in terms of importance during acute infection based on several lines of evidence (Figure 8). First, although TLR2 KO mice are more susceptible to CNS S. aureus infection, these animals can survive for longer intervals if bacterial burdens are reduced, which is not the case for either IL-1RI or MyD88 KO mice. Secondly, we have found no evidence for TLR9 involvement in the genesis of CNS immune responses during brain abscess formation (T. Kielian, unpublished work). Thirdly, both IL-1RI and MyD88 KO animals exhibited exaggerated necrosis during acute brain abscess formation compared with WT mice, whereas lesion sizes were only modestly elevated during early infection in TLR2 KO animals (Stenzel et al., 2008). Fourthly, bacterial burdens were significantly elevated in IL-1RI KO mice, whereas this was not observed in TLR2 KO animals during the first week post-infection (Kielian et al., 2005b; Stenzel et al., 2008; Vidlak et al., 2011). Collectively, these observations support the conclusion that the IL-1RI/MyD88 pathway is essential for protection during acute brain abscess formation, whereas in the context of an intact IL-1RI response, TLR2-dependent pathways become more critical during later stages of infection. It is intriguing that IL-1RI signalling plays such a crucial role, since IL-1RI activity is secondary to IL-1α/IL-1β production triggered by TLR activation upon pathogen recognition. However, the plethora of effector molecules elicited by TLR pathways might explain why the CNS is more resilient to TLR2 compared with IL-1RI loss, although this remains to be determined.
Figure 8. IL-1RI/MyD88 pathways are more critical compared with TLR2 signalling during acute CNS S. aureus infection.
Studies with IL-1RI and MyD88 KO mice have established the essential nature of both molecules for survival, inflammatory mediator expression, and leucocyte recruitment early following CNS infection. In addition, the IL-1RI/MyD88 cascade is critical for regulating necrosis, whereas TLR2 loss is less involved during early infection.
Although bacterial burdens were elevated in IL-1RI KO animals, this did not translate into heightened pro-inflammatory mediator expression or immune cell influx into the infected CNS. Rather, neutrophil and macrophage entry into brain abscesses of IL-1RI KO mice was significantly attenuated, which coincided with significant reductions in neutrophil (CXCL1 and CXCL2) and macrophage (CCL2) chemokines. Another possibility to explain this impaired leucocyte recruitment is that the lack of IL-1 action prevents the induction of adhesion molecules at the BBB (blood–brain barrier) critical for leucocyte extravasation, although this was not examined in the current study. Impaired recruitment of professional phagocytes is a likely reason for elevated S. aureus burdens in IL-1RI KO animals. Indeed, prior studies from our laboratory and others have demonstrated the essential role of neutrophils in bacterial containment both within the CNS and peripheral sites of infection (Lo et al., 1998; Kielian et al., 2001; Navarini et al., 2009). However, additional mechanisms are clearly involved when considering the fact that IL-1RI KO animals do not survive beyond day 3 post-infection, whereas neutrophil depleted mice can live to at least day 5 after bacterial exposure (Kielian et al., 2001). Although bacterial burdens were significantly elevated in IL-1RI KO animals, this increase was rather modest, which suggests that alternative mechanism(s) are also likely responsible for the enhanced sensitivity of IL-1RI KO mice to CNS S. aureus infection. We demonstrated that the degree of apoptosis and cerebral oedema (results not shown) were similar between infected IL-1RI KO and WT animals, suggesting that these pathways are not involved in the heightened sensitivity of the former to brain abscess formation. Evidence exists that IL-1RI/MyD88 signalling regulates fibrotic reactions (Gasse et al., 2007; He et al., 2010). By extension, it would be interesting to determine whether IL-1RI action elicits a bordering function to restrict abscess expansion within the brain. Unfortunately, since IL-1RI KO mice do not survive past day 3 post-infection (even with significant reductions in the infectious inoculum) the impact of IL-1RI signalling on brain abscess wall formation cannot be addressed because fibrotic responses are not initiated until 7–10 days after bacterial exposure (Flaris and Hickey, 1992; Kielian et al., 2008; Aldrich and Kielian, 2011). Identifying the pathway(s) responsible for the inability of IL-1RI KO animals to survive acute S. aureus infection remains the topic of future studies, which are beyond the scope of the current report.
Another interesting finding was that only a subset of mediators was affected by the loss of IL-1RI signalling, including IL-6, CXCL1, CXCL2 and CCL2. One possible explanation is that the expression of these molecules is directly triggered by IL-1RI action. Alternatively, signals emanating from IL-1RI may increase cytokine/chemokine mRNA t½. Indeed, IL-1 has been shown to stabilize CXCL1 and CCL2 expression (Tebo et al., 2000; Thibeault et al., 2001). This select action of IL-1RI signalling differs from our earlier report in MyD88 KO animals, which displayed global defects in inflammatory mediator release (Kielian et al., 2007), which is likely explained by the more widespread induction of cytokines/chemokines emanating from the loss of both TLR and IL-1RI cascades. Therefore these findings build upon the known biology of MyD88-dependent pathways in the context of CNS bacterial infection to assign a crucial role for IL-1RI activity. IL-1 and TNFα often interact to regulate effector responses and previous studies from our laboratory have documented that autocrine/paracrine TNFα regulates TLR2 expression in microglia and astrocytes following S. aureus exposure (Syed et al., 2007; Phulwani et al., 2008). Therefore it was important to assess whether TNFα levels were altered in IL-1RI KO animals. TNFα protein was not detectable at early intervals post-infection in either IL-1RI KO or WT mice (i.e. 12 and 18 h; results not shown). The fact that numerous other pro-inflammatory mediators were readily observed during this timeframe suggests that TNFα may not play a key role in the acute CNS response to S. aureus. This possibility is supported by our previous findings demonstrating that TNFα KO mice did not exhibit dramatic alterations in bacterial burdens or other immune parameters (Kielian et al., 2004). However, the role of TNFα is not straightforward, since another group showed a critical role for TNFα in bacterial containment, survival and immune infiltrates in the experimental brain abscess model (Stenzel et al., 2005). Surprisingly, in this study the authors reported that TNFα KO mice exhibited exaggerated neutrophil and macrophage infiltrates throughout the course of infection, which differs from our current study in IL-1RI KO animals. Therefore although we cannot disregard a role for TNFα in regulating the observed impairments in bacterial clearance and immune parameters in IL-1RI KO mice, the inability to detect the cytokine suggests that it may not represent a major regulatory pathway, at least in the context of early infection.
Recently, several neuroinflammatory diseases have been associated with perturbations in astrocyte hemichannel activity, which has been suggested to contribute to neuronal loss (Orellana et al., 2009; Karpuk et al., 2011; Orellana et al., 2011; Takeuchi et al., 2011). It is presumed that dysregulated astrocyte hemichannel activity leads to the disruption of homoeostatic ion and neurotransmitter gradients (i.e. K+ and glutamate respectively) via the bi-directional trafficking of molecules through open hemichannels (Giaume et al., 2010). As a consequence, the protective functions of astrocytes are compromised, leading to impaired neuronal physiology and cell death. A recent study from our laboratory demonstrated that astrocyte hemichannels were open immediately adjacent to the abscess margins and coincided with intense astrocyte and microglial/macrophage activation (Karpuk et al., 2011). In addition, hemichannel activity was only observed during acute but not late infection (i.e. days 3 and 7 post-infection respectively) and was dictated by the physical proximity to the inflammatory nidus. Both IL-1β and TNFα have been implicated in inducing astrocyte hemichannel opening in vitro (Retamal et al., 2007; Froger et al., 2010). Since it is unclear which cytokine is most critical for inducing astrocyte hemichannel activity in the context of an inflammatory response, we evaluated hemichannel opening in acute brain slices from IL-1RI KO mice. Importantly, the use of slices maintains cellular networks relatively intact, which is more reflective of in vivo events compared with studying purified astrocytes in isolation. Interestingly, astrocyte hemichannel activity during acute brain abscesses was nearly abolished in IL-1RI KO mice, suggesting that IL-1α/IL-1β actions are the main driving signals for hemichannel opening in brain slices, where the complete cellular repertoire is present. Therefore strategies that interfere with IL-1RI signalling may be used to manipulate hemichannel activity and inflammation within the CNS, as we more fully appreciate the impact of open hemichannels on CNS homoeostasis and function. It remains to be determined whether hemichannel activity during acute brain abscess formation is beneficial or detrimental, which also remains an open question in other models of CNS pathology and inflammation (Thompson et al., 2006; Kielian, 2008; Karpuk et al., 2011; Orellana et al., 2011). Strictly based on the correlation between high mortality rates in IL-1RI KO animals and hemichannel closure, it might be concluded that hemichannel activity plays a protective role during infection. However, this possibility remains highly speculative and the lack of currently available methods to specifically manipulate hemichannel activity without impacting gap junction channels (formed by the joining of two hemichannels on adjacent cells) makes this issue currently difficult to address (Spray et al., 2006; Kielian, 2008).
Although an earlier report from our laboratory revealed that brain abscess pathogenesis was more severe in mice lacking IL-1β, this study did not address the potential redundant actions of IL-1α in the context of CNS S. aureus infection (Kielian et al., 2004). Importantly, IL-1RI KO mice in the current study were more sensitive to CNS S. aureus infection compared with IL-1β KO animals in our earlier report (Kielian et al., 2004). In addition, IL-1RI KO mice displayed reductions in several pro-inflammatory mediators, including CXCL2, which was not previously observed in IL-1β KO animals (Kielian et al., 2004). However, another factor may be the result of bacterial strain differences, since the current study utilized a S. aureus isolate of important clinical origins (i.e. recovered from an otherwise healthy patient who died of a brain abscess), whereas our earlier report utilized a laboratory isolate that has been shown to possess a mutation in the stress response regulator sigma factor β (Kullik et al., 1998; Kielian et al., 2004). However, both isolates are high toxin producers and our recent studies have demonstrated that S. aureus α- and γ-toxins are involved in regulating IL-1β processing and release in primary microglia (Hanamsagar et al., 2011). Collectively, these findings advance our knowledge about the biology of IL-1 function by demonstrating that the co-ordinate actions of IL-1α and IL-1β are essential for eliciting protective immunity during acute bacterial infection and that IL-1α can partially compensate for the loss of IL-1β. By extension, it is possible to envision augmenting IL-1RI activity during CNS infection to expedite pathogen clearance and reduce long-term neurological deficits for patients recovering from brain abscesses.
In summary, these studies reveal a novel role for signals emanating from the IL-1RI during early S. aureus infection within the CNS. In fact, this pathway is so crucial that survival cannot be extended by reducing the infectious dose to levels that are not capable of eliciting a brain abscess in immunocompetent animals (i.e. 103 cfu). Based on its importance in regulating numerous aspects of the host CNS immune response (i.e. cytokine/chemokine expression and cellular infiltrates) as well as astrocyte hemichannel opening, manipulating IL-1RI action may prove to be an attractive means to expedite bacterial clearance from the infected brain, which could result in fewer long-term neurological impairments for patients recovering from these devastating CNS infections.
ACKNOWLEDGEMENT
We thank Amanda Angle for excellent technical assistance.
Footnotes
This work was supported by the National Institutes of Health National Institute of Neurological Disorders and Stroke [grant numbers R01 NS053487 and NS040730 (to T.K.)].
REFERENCES
- Aldrich A, Kielian T. Central nervous system fibrosis is associated with fibrocyte-like infiltrates. Am J Pathol. 2011;179:2952–2962. doi: 10.1016/j.ajpath.2011.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin AC, Kielian T. Persistent immune activation associated with a mouse model of Staphylococcus aureus-induced experimental brain abscess. J Neuroimmunol. 2004;151:24–32. doi: 10.1016/j.jneuroim.2004.02.002. [DOI] [PubMed] [Google Scholar]
- Chambers HF, Deleo FR. Waves of resistance: Staphylococcus aureus in the antibiotic era. Nat Rev Microbiol. 2009;7:629–641. doi: 10.1038/nrmicro2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis BK, Wen H, Ting JP. The inflammasome NLRs in immunity, inflammation, and associated diseases. Annu Rev Immunol. 2011;29:707–735. doi: 10.1146/annurev-immunol-031210-101405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinarello CA. Immunological and inflammatory functions of the interleukin-1 family. Annu Rev Immunol. 2009;27:519–550. doi: 10.1146/annurev.immunol.021908.132612. [DOI] [PubMed] [Google Scholar]
- Esen N, Kielian T. Central role for MyD88 in the responses of microglia to pathogen-associated molecular patterns. J Immunol. 2006;176:6802–6811. doi: 10.4049/jimmunol.176.11.6802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flaris NA, Hickey WF. Development and characterization of an experimental model of brain abscess in the rat. Am J Pathol. 1992;141:1299–1307. [PMC free article] [PubMed] [Google Scholar]
- Froger N, Orellana JA, Calvo CF, Amigou E, Kozoriz MG, Naus CC, Saez JC, Giaume C. Inhibition of cytokine-induced connexin43 hemichannel activity in astrocytes is neuroprotective. Mol Cell Neurosci. 2010;45:37–46. doi: 10.1016/j.mcn.2010.05.007. [DOI] [PubMed] [Google Scholar]
- Garg S, Nichols JR, Esen N, Liu S, Phulwani NK, Syed MM, Wood WH, Zhang Y, Becker KG, Aldrich A, Kielian T. MyD88 expression by CNS-resident cells is pivotal for eliciting protective immunity in brain abscesses. ASN NEURO. 2009;1(2):art:e00007. doi: 10.1042/AN20090004. doi:10.1042/AN20090004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasse P, Mary C, Guenon I, Noulin N, Charron S, Schnyder-Candrian S, Schnyder B, Akira S, Quesniaux VF, Lagente V, Ryffel B, Couillin I. IL-1R1/MyD88 signaling and the inflammasome are essential in pulmonary inflammation and fibrosis in mice. J Clin Invest. 2007;117:3786–3799. doi: 10.1172/JCI32285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giaume C, Koulakoff A, Roux L, Holcman D, Rouach N. Astroglial networks: a step further in neuroglial and gliovascular interactions. Nat Rev Neurosci. 2010;11:87–99. doi: 10.1038/nrn2757. [DOI] [PubMed] [Google Scholar]
- Gupta RK, Srivastava S, Saksena S, Rathore RK, Awasthi R, Prasad KN, Husain M, Pandey CM, Husain N. Correlation of DTI metrics in the wall and cavity of brain abscess with histology and immunohistochemistry. NMR Biomed. 2010;23:262–269. doi: 10.1002/nbm.1448. [DOI] [PubMed] [Google Scholar]
- Hanamsagar R, Torres V, Kielian T. Inflammasome activation and IL-1β/IL-18 processing are influenced by distinct pathways in microglia. J Neurochem. 2011;119:736–748. doi: 10.1111/j.1471-4159.2011.07481.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanke ML, Kielian T. Toll-like receptors in health and disease in the brain: mechanisms and therapeutic potential. Clin Sci. 2011;121:367–387. doi: 10.1042/CS20110164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He X, Mekasha S, Mavrogiorgos N, Fitzgerald KA, Lien E, Ingalls RR. Inflammation and fibrosis during chlamydia pneumoniae infection is regulated by IL-1 and the NLRP3/ASC inflammasome. J Immunol. 2010;184:5743–5754. doi: 10.4049/jimmunol.0903937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holley MM, Kielian T. Th1 and Th17 cells regulate innate immune responses and bacterial clearance during central nervous system infection. J Immunol. 2012;188:1360–1670. doi: 10.4049/jimmunol.1101660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs CA, Baker PE, Roux ER, Picha KS, Toivola B, Waugh S, Kennedy MK. Experimental autoimmune encephalomyelitis is exacerbated by IL-1 alpha and suppressed by soluble IL-1 receptor. J Immunol. 1991;146:2983–2989. [PubMed] [Google Scholar]
- Jones ME, Draghi DC, Karlowsky JA, Sahm DF, Bradley JS. Prevalence of antimicrobial resistance in bacteria isolated from central nervous system specimens as reported by U.S. hospital laboratories from 2000 to 2002. Ann Clin Microbiol Antimicrob. 2004;3:3. doi: 10.1186/1476-0711-3-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karpuk N, Burkovetskaya M, Fritz T, Angle A, Kielian T. Neuroinflammation leads to region-dependent alterations in astrocyte gap junction communication and hemichannel activity. J Neurosci. 2011;31:414–425. doi: 10.1523/JNEUROSCI.5247-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kielian T. Immunopathogenesis of brain abscess. J Neuroinflammation. 2004;1:16. doi: 10.1186/1742-2094-1-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kielian T. Glial connexins and gap junctions in CNS inflammation and disease. J Neurochem. 2008;106:1000–1016. doi: 10.1111/j.1471-4159.2008.05405.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kielian T, Hickey WF. Proinflammatory cytokine, chemokine, and cellular adhesion molecule expression during the acute phase of experimental brain abscess development. Am J Pathol. 2000;157:647–658. doi: 10.1016/S0002-9440(10)64575-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kielian T, Barry B, Hickey WF. CXC chemokine receptor-2 ligands are required for neutrophil-mediated host defense in experimental brain abscesses. J Immunol. 2001;166:4634–4643. doi: 10.4049/jimmunol.166.7.4634. [DOI] [PubMed] [Google Scholar]
- Kielian T, Bearden ED, Baldwin AC, Esen N. IL-1 and TNF-alpha play a pivotal role in the host immune response in a mouse model of Staphylococcus aureus-induced experimental brain abscess. J Neuropathol Exp Neurol. 2004;63:381–396. doi: 10.1093/jnen/63.4.381. [DOI] [PubMed] [Google Scholar]
- Kielian T, Esen N, Bearden ED. Toll-like receptor 2 (TLR2) is pivotal for recognition of S. aureus peptidoglycan but not intact bacteria by microglia. Glia. 2005a;49:567–576. doi: 10.1002/glia.20144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kielian T, Haney A, Mayes PM, Garg S, Esen N. Toll-like receptor 2 modulates the proinflammatory milieu in Staphylococcus aureus-induced brain abscess. Infect Immun. 2005b;73:7428–7435. doi: 10.1128/IAI.73.11.7428-7435.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kielian T, Phulwani NK, Esen N, Syed MM, Haney AC, McCastlain K, Johnson J. MyD88-dependent signals are essential for the host immune response in experimental brain abscess. J Immunol. 2007;178:4528–4537. doi: 10.4049/jimmunol.178.7.4528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kielian T, Syed MM, Liu S, Phulwani NK, Phillips N, Wagoner G, Drew PD, Esen N. The synthetic peroxisome proliferator-activated receptor-gamma agonist ciglitazone attenuates neuroinflammation and accelerates encapsulation in bacterial brain abscesses. J Immunol. 2008;180:5004–5016. doi: 10.4049/jimmunol.180.7.5004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kullik I, Giachino P, Fuchs T. Deletion of the alternative sigma factor sigmaB in Staphylococcus aureus reveals its function as a global regulator of virulence genes. J Bacteriol. 1998;180:4814–4820. doi: 10.1128/jb.180.18.4814-4820.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo WD, Chen R, Boue DR, Stokes BT. Effect of neutrophil depletion in acute cerebritis. Brain Res. 1998;802:175–183. doi: 10.1016/s0006-8993(98)00600-3. [DOI] [PubMed] [Google Scholar]
- Lu CH, Chang WN, Lin YC, Tsai NW, Liliang PC, Su TM, Rau CS, Tsai YD, Liang CL, Chang CJ, Lee PY, Chang HW, Wu JJ. Bacterial brain abscess: microbiological features, epidemiological trends and therapeutic outcomes. QJM. 2002;95:501–509. doi: 10.1093/qjmed/95.8.501. [DOI] [PubMed] [Google Scholar]
- Mathisen GE, Johnson JP. Brain abscess. Clin Infect Dis. 1997;25:763–779; quiz 780–761. doi: 10.1086/515541. [DOI] [PubMed] [Google Scholar]
- Navarini AA, Lang KS, Verschoor A, Recher M, Zinkernagel AS, Nizet V, Odermatt B, Hengartner H, Zinkernagel RM. Innate immune-induced depletion of bone marrow neutrophils aggravates systemic bacterial infections. Proc Natl Acad Sci USA. 2009;106:7107–7112. doi: 10.1073/pnas.0901162106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orellana JA, Saez PJ, Shoji KF, Schalper KA, Palacios-Prado N, Velarde V, Giaume C, Bennett MV, Saez JC. Modulation of brain hemichannels and gap junction channels by pro-inflammatory agents and their possible role in neurodegeneration. Antioxid Redox Signal. 2009;11:369–399. doi: 10.1089/ars.2008.2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orellana JA, Shoji KF, Abudara V, Ezan P, Amigou E, Saez PJ, Jiang JX, Naus CC, Saez JC, Giaume C. Amyloid beta-induced death in neurons involves glial and neuronal hemichannels. J Neurosci. 2011;31:4962–4977. doi: 10.1523/JNEUROSCI.6417-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phulwani NK, Esen N, Syed MM, Kielian T. TLR2 expression in astrocytes is induced by TNF-alpha- and NF-kappa B-dependent pathways. J Immunol. 2008;181:3841–3849. doi: 10.4049/jimmunol.181.6.3841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Retamal MA, Froger N, Palacios-Prado N, Ezan P, Saez PJ, Saez JC, Giaume C. Cx43 hemichannels and gap junction channels in astrocytes are regulated oppositely by proinflammatory cytokines released from activated microglia. J Neurosci. 2007;27:13781–13792. doi: 10.1523/JNEUROSCI.2042-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroder K, Tschopp J. The inflammasomes. Cell. 2010;140:821–832. doi: 10.1016/j.cell.2010.01.040. [DOI] [PubMed] [Google Scholar]
- Shaftel SS, Griffin WS, O'Banion MK. The role of interleukin-1 in neuroinflammation and Alzheimer disease: an evolving perspective. J Neuroinflammation. 2008;5:7. doi: 10.1186/1742-2094-5-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sifri CD, Park J, Helm GA, Stemper ME, Shukla SK. Fatal brain abscess due to community-associated methicillin-resistant Staphylococcus aureus strain USA300. Clin Infect Dis. 2007;45:e113–117. doi: 10.1086/522171. [DOI] [PubMed] [Google Scholar]
- Spray DC, Ye ZC, Ransom BR. Functional connexin 'hemichannels': a critical appraisal. Glia. 2006;54:758–773. doi: 10.1002/glia.20429. [DOI] [PubMed] [Google Scholar]
- Stenzel W, Soltek S, Miletic H, Hermann MM, Korner H, Sedgwick JD, Schluter D, Deckert M. An essential role for tumor necrosis factor in the formation of experimental murine Staphylococcus aureus-induced brain abscess and clearance. J Neuropathol Exp Neurol. 2005;64:27–36. doi: 10.1093/jnen/64.1.27. [DOI] [PubMed] [Google Scholar]
- Stenzel W, Soltek S, Sanchez-Ruiz M, Akira S, Miletic H, Schluter D, Deckert M. Both TLR2 and TLR4 are required for the effective immune response in Staphylococcus aureus-induced experimental murine brain abscess. Am J Pathol. 2008;172:132–145. doi: 10.2353/ajpath.2008.070567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syed MM, Phulwani NK, Kielian T. Tumor necrosis factor-alpha (TNF-alpha) regulates Toll-like receptor 2 (TLR2) expression in microglia. J Neurochem. 2007;103:1461–1471. doi: 10.1111/j.1471-4159.2007.04838.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi H, Mizoguchi H, Doi Y, Jin S, Noda M, Liang J, Li H, Zhou Y, Mori R, Yasuoka S, Li E, Parajuli B, Kawanokuchi J, Sonobe Y, Sato J, Yamanaka K, Sobue G, Mizuno T, Suzumura A. Blockade of gap junction hemichannel suppresses disease progression in mouse models of amyotrophic lateral sclerosis and Alzheimer's disease. PLoS ONE. 2011;6:e21108. doi: 10.1371/journal.pone.0021108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi O, Akira S. Pattern recognition receptors and inflammation. Cell. 2010;140:805–820. doi: 10.1016/j.cell.2010.01.022. [DOI] [PubMed] [Google Scholar]
- Tebo JM, Datta S, Kishore R, Kolosov M, Major JA, Ohmori Y, Hamilton TA. Interleukin-1-mediated stabilization of mouse KC mRNA depends on sequences in both 5′- and 3′-untranslated regions. J Biol Chem. 2000;275:12987–12993. doi: 10.1074/jbc.275.17.12987. [DOI] [PubMed] [Google Scholar]
- Thibeault I, Laflamme N, Rivest S. Regulation of the gene encoding the monocyte chemoattractant protein 1 (MCP-1) in the mouse and rat brain in response to circulating LPS and proinflammatory cytokines. J Comp Neurol. 2001;434:461–477. doi: 10.1002/cne.1187. [DOI] [PubMed] [Google Scholar]
- Thompson RJ, Zhou N, MacVicar BA. Ischemia opens neuronal gap junction hemichannels. Science. 2006;312:924–927. doi: 10.1126/science.1126241. [DOI] [PubMed] [Google Scholar]
- Vidlak D, Mariani MM, Aldrich A, Liu S, Kielian T. Roles of Toll-like receptor 2 (TLR2) and superantigens on adaptive immune responses during CNS staphylococcal infection. Brain Behav Immun. 2011;25:905–914. doi: 10.1016/j.bbi.2010.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwijnenburg PJ, van der Poll T, Florquin S, Roord JJ, Van Furth AM. IL-1 receptor type 1 gene-deficient mice demonstrate an impaired host defense against pneumococcal meningitis. J Immunol. 2003;170:4724–4730. doi: 10.4049/jimmunol.170.9.4724. [DOI] [PubMed] [Google Scholar]