Abstract
BACKGROUND:
Predictors of new and long-term respiratory symptoms for rural residents are not well defined.
OBJECTIVE:
To identify early predictors of respiratory symptoms in a rural community population.
METHODS:
The study population consisted of 871 adults living in the rural community of Humboldt, Saskatchewan, who participated in two cross-sectional respiratory studies conducted in 1993 and 2003. Questionnaire information obtained at both time points included respiratory symptoms (cough, phlegm and wheeze), history of allergy, smoking, and information regarding home and farm environments. Transitional modelling, in which measurement in a longitudinal sequence is described as a function of previous outcomes, was used to predict later outcomes of cough, phlegm and wheeze. Asymptomatic individuals in 1993 were assessed to determine factors associated with the development of symptoms during the study period.
RESULTS:
The prevalences of cough, phlegm and wheeze in 1993 were 16.1%, 18.1% and 25.5%, respectively. Change in symptoms over time was significant for cough, phlegm and wheeze. The adjusted ORs (95% CI) from separate transitional models for each respiratory outcome in 1993 that predicted the same symptom in 2003 were 6.32 (4.02 to 9.95) for cough, 14.36 (9.01 to 22.89) for phlegm and 6.40 (4.40 to 9.32) for wheeze. For asymptomatic individuals in 1993, home dampness, allergic reaction to inhaled allergens and cigarette smoking were major risk factors associated with respiratory symptoms that were reported in 2003.
CONCLUSION:
The presence of previous respiratory symptoms, allergies and environmental exposures can predict the occurrence of future respiratory symptoms in adults.
Keywords: Cough, Longitudinal respiratory symptoms, Phlegm, Predictors, Wheeze
Abstract
HISTORIQUE :
Les prédicteurs des nouveaux symptômes respiratoires et de ces symptômes à long terme sont mal définis chez les habitants des régions rurales.
OBJECTIF :
Déterminer les prédicteurs précoces de symptômes respiratoires dans la population d’une collectivité rurale.
MÉTHODOLOGIE :
La population à l’étude se composait de 871 habitants adultes de la collectivité rurale de Humboldt, en Saskatchewan, qui avaient participé à deux études respiratoires transversales en 1993 et en 2003. L’information relative au questionnaire obtenue à ces deux moments incluait les symptômes respiratoires (toux, mucosités et respiration sifflante), les antécédents d’allergies, le tabagisme et les renseignements sur les environnements de la maison et de la ferme. La modélisation transitionnelle, qui décrit une mesure dans une séquence longitudinale en fonction des issues antérieures, a permis de prédire les issues de toux, de mucosités et de respiration sifflante plus tard. En 1993, les personnes asymptomatiques ont été évaluées pour déterminer les facteurs associés à l’apparition de symptômes pendant la période de l’étude.
RÉSULTATS :
En 1993, la prévalence de toux, de mucosités et de respiration sifflante s’élevait à 16,1 %, 18,1 % et 25,5 %, respectivement. L’évolution des symptômes de toux, de mucosités et de respiration sifflante était importante au fil du temps. Les RRR (95 % IC) de modèles transitionnels distincts à l’égard de chaque issue respiratoire en 1993 qui prédisaient le même symptôme en 2003 correspondaient à 6,32 (4,02 à 9,95) à l’égard de la toux, à 14,36 (9,01 à 22,89) à l’égard des mucosités et à 6,40 (4,40 à 9,32) à l’égard de la respiration sifflante. Chez les personnes asymptomatiques en 1993, l’humidité du domicile, les réactions allergiques aux allergènes inhalés et le tabagisme étaient des facteurs de risque majeurs de symptômes respiratoires déclarés en 2003.
CONCLUSION :
La présence des symptômes respiratoires antérieurs, les allergies et l’exposition environnementale permettent de prédire l’occurrence de futurs symptômes respiratoires chez les adultes.
Chronic cough and wheeze affects between 25% and 35% of the adult Canadian population (1). However, the progression of symptoms and their determinants, which can provide information about the nature of respiratory disease over time, have not been explored in Canadian populations. The study of the longitudinal predictors of respiratory health in general populations of rural residents is limited (2). Certain associations between environmental exposures and respiratory symptoms have been previously reported (3–11). Factors reported to determine respiratory symptoms in general populations of adults include smoking (8,12), age (10,13), sex (1,8,13), atopy (10) and damp housing (4). In Canada, longitudinal studies of respiratory symptoms in rural populations have primarily explored the health effects of grain dust in occupationally exposed workers (5,9,14).
Previous studies examining longitudinal changes in respiratory symptoms have focused on examining current symptoms and their association with previous exposures. However, there are limited reports of the nature of persistent symptoms and their predictors as well as the predictors of new onset of symptoms (9,10).
The present analysis aimed to identify the predictors of persistent respiratory symptoms and the predictors of new onset of symptoms studied longitudinally in a rural population. We report on the findings of two cross-sectional studies, conducted 10 years apart, involving adults living in a rural Saskatchewan community.
METHODS
Populations
Two successive cross-sectional community studies of adults were conducted in 1993 and 2003 in the rural community of Humboldt, Saskatchewan. According to figures from the 2001 census, the community of Humboldt had a population of approximately 5200, who were primarily of Caucasian background (15). Humboldt is mainly an agricultural community. Wheat, canola, flax, pea, rye, lentils, canary seeds and barley are the primary crops grown in the area. Beef cattle production, in addition to other livestock, are significant for the surrounding community. In 1993, there were 1998 adult subjects 18 to 74 years of age who participated in the survey, resulting in a response rate of 85.9% (16). In 2003, there were 2090 adult residents 18 to 79 years of age, who represented 71% of those contacted (17). For both studies, participants were identified by means of a community canvass as previously described (16,17). All participants brought self-administered completed questionnaires to a health screening centre located in the community. The study protocols were similar between study time points (16–17). However, these two cross-sectional studies were not designed to have a longitudinal component at the planning stage. There were 871 subjects who participated in both the 1993 and 2003 studies and, on both occasions, completed a questionnaire that included identical questions for respiratory symptoms, smoking history, and socioeconomic and lifestyle variables. Of 1794 eligible subjects studied in 1993, 923 could not be located in the 2003 study. Before initiation of both the 1993 and 2003 studies, approval from the Biomedical Research Ethics Board of the University of Saskatchewan (Saskatoon, Saskatchewan) was obtained.
Questionnaire
Respiratory health was assessed using the American Thoracic Society adult respiratory questionnaire (18). Cough was determined by a positive response (ie, ‘yes’) to any of the following questions: “Do you usually have a cough?”, “Do you usually cough at all on getting up, or first thing in the morning?”, “Do you usually cough at all during the rest of the day? or at night?”. Phlegm was determined by a positive response to any of the following questions: “Do you usually bring up phlegm from your chest?”, “Do you usually bring up phlegm at all on getting up, or first thing in the morning?”, “Do you usually bring up phlegm at all during the rest of the day? or at night?” Wheeze of any nature was determined by a positive response to any of the following questions: “Does your chest ever sound wheezy or whistling: When you have a cold?” (wheeze/cold), “Occasionally apart from a cold?” (wheeze/no cold). The questionnaire also identified environmental and family characteristics including damp housing (report of any damage caused by dampness such as wet spots on walls or floors in the home) and grain dust exposure (history of grain farming or handling grains such as wheat, durham, oats, barley, flax, rapeseed, rye, mustard, alfalfa or legumes). Three types of smoking history were assessed including current smoker (smoking in the past year) or ex-smoker (no current smoking and a past history of smoking at least 20 packs) or nonsmoker (all others). Ever smoker was defined as combination of current and ex-smokers. An allergic reaction to inhaled allergens was determined by a positive answer to the question “Have you ever had an allergic reaction to inhaled allergen (eg, pollen, dust [plant or animal], animal fur or smoke)?”.
Statistical analysis
χ2 tests were used to determine the univariate association of respiratory symptoms and predictors of grain dust exposure, home dampness, allergic reaction to inhaled allergens and cigarette smoking. The McNemar test for paired samples was used to examine the significance of change in prevalence (19). The longitudinal data analysis was conducted using transitional models (20) in which the measurement Yij represents the status (presence or absence) of respiratory outcome of ith individual at jth time point. Yij can be described as a function of previous outcome or history hij = (Yi1, …,Yij-1) and covariates xij, in which hij represents the status (presence or absence) of respiratory outcomes of ith individual at (j-1) previous time points (1, 2, …, j-1) and xij represent the covariates of ith individual at (j-1) previous time points (1, 2,…, j-1). Useful transitional models were Markov chains in which the conditional distribution of yij given hij depends only on the q prior observations yij-1,…,yij-q. The integer q is referred to as the model order. In the present analysis, a multivariate logistic regression model was considered for binary respiratory outcome that comprises a first-order Markov chain because there was one previous outcome. Here, three separate models for three respiratory outcomes were considered: cough, phlegm and wheeze. ORs and 95% CIs were used to express the strength of the associations. SAS version 9.03 (SAS Institute, USA) (21) for Windows (Microsoft Corporation, USA) was used for statistical analysis. Data from among the group of participants who were free from symptoms of cough, phlegm and wheeze in 1993 were further analyzed using a separate multivariate logistic regression model for each outcome.
RESULTS
There were 871 subjects who participated in both cross-sectional studies conducted in 1993 and 2003. Of these, 359 (41.2%) were men and 512 (58.8%) were women. The mean (± SD) age of the participants at baseline was 46.8±13.4 years. Table 1 shows the characteristics of the 871 subjects comprising the study population who were studied both at baseline in 1993 and on a second occasion in 2003. The proportion of individuals who reported grain dust exposure (history of grain farming or grain handling) was 17.9% in 1993 and 18.7% in 2003. The proportion of individuals living in damp houses was 10.8% and 8.6% in the two studies, respectively. Of the respondents who said ‘yes’ to home dampness in 1993, 25.5% continued to have home dampness in 2003. There was no change in the proportion of subjects who claimed a history of allergic reaction to inhaled allergens during the two time points. There were fewer current smokers in 2003 compared with 1993; however, the proportion of ex-smokers increased by 6.3% in 2003 (Table 1). In the 2003 Humboldt study (17), 52.1% of persons studied were never smokers and 47.9% were ever smokers (current or ex-smokers). Although this was a moderate increase of ever smokers in 2003 from 1993 (46.1% to 47.9%), it followed the national smoking pattern (22) for ever smokers and never smokers.
TABLE 1.
Baseline characteristics of the study population in 1993 and in 2003 (n=871)
| Characteristic | Baseline (1993) | Follow-up (2003) |
|---|---|---|
| Age range, years | ||
| 20–29 | 94 (10.8) | – |
| 30–39 | 217 (24.9) | 86 (9.9) |
| 40–49 | 156 (17.9) | 224 (25.7) |
| 50–59 | 200 (23.0) | 155 (17.8) |
| 60–69 | 204 (23.4) | 194 (22.3) |
| 70–79 | – | 212 (24.3) |
| Grain dust exposure | ||
| Yes | 156 (17.9) | 163 (18.7) |
| No | 715 (82.1) | 708 (81.3) |
| Living in a damp house | ||
| Yes | 94 (10.8) | 75 (8.6) |
| No | 777 (89.2) | 796 (91.4) |
| Allergic reaction to inhaled allergens | ||
| Yes | 270 (31.0) | 262 (30.1) |
| No | 601 (69.0) | 609 (69.9) |
| Smoking status | ||
| Never smoker | 470 (53.9) | 454 (52.1) |
| Ex-smoker | 282 (32.4) | 337 (38.7) |
| Current smoker | 119 (13.7) | 80 (9.2) |
Data presented as n (%)
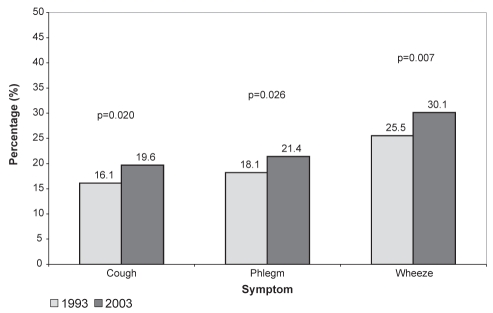
Figure 1 illustrates the prevalence of respiratory symptoms in 1993 and 2003. There was a significant change in prevalence from 1993 to 2003 for cough (0.35% increase per year; P=0.02), wheeze (0.46% increase per year; P=0.007) and phlegm (0.33% increase per year; P=0.026). The effects of aging on the prevalences of symptoms and smoking were assessed (data not shown). Older subjects experienced more symptoms, as did ex-smokers and current smokers.
Figure 1).
Prevalence of respiratory symptoms in 1993 and 2003
Table 2 presents the results of the bivariate χ2 analysis of association conducted to examine each symptom in 2003 separately with reported cough, phlegm and wheeze in 1993 and with other exposures (allergic reaction to inhaled allergens, exposure to home dampness, grain dust and smoking) in 1993. In 1993, cough, phlegm and wheeze were significantly associated with a report of cough, phlegm or wheeze in 2003. There was a strong association of grain dust exposure in 1993 with both cough and phlegm in 2003. Allergic reaction to inhaled allergens, dampness in the home and smoking status in 1993 were all significantly associated with increased prevalence of wheeze in 2003. In addition, smoking status in 1993 was associated with cough and phlegm in 2003.
TABLE 2.
Bivariate analyses comparing cough, phlegm and wheeze in 2003 with environmental characteristics and history of cough, phlegm and wheeze in 1993
| Variables in 1993 |
Cough in 2003* |
Phlegm in 2003† |
Wheeze in 2003‡ |
|||
|---|---|---|---|---|---|---|
| Yes | No | Yes | No | Yes | No | |
| Cough§ | ||||||
| Yes | 73 (52.1) ** | 67 (47.7) | – | – | – | – |
| No | 98 (13.4) | 633 (86.6) | – | – | – | – |
| Phlegm§ | ||||||
| Yes | – | – | 98 (62.0)** | 60 (38.0) | – | – |
| No | – | – | 88 (12.3) | 625 (87.7) | – | – |
| Wheeze§ | ||||||
| Yes | – | – | – | – | 138 (62.2)†† | 84 (37.8) |
| No | – | – | – | – | 124 (19.1) | 525 (80.9) |
| Grain dust exposure¶ | ||||||
| Yes | 41 (26.3)** | 115 (73.7) | 52 (33.3)†† | 104 (66.7) | 56 (35.9) | 100 (64.1) |
| No | 130 (18.2) | 585 (81.8) | 134 (18.7) | 581 (81.3) | 206 (28.8) | 509 (71.2) |
| Sex¶ | ||||||
| Male | 75 (20.9) | 284 (79.1) | 100 (27.9)†† | 259 (72.1) | 123 (34.3)** | 236 (65.7) |
| Female | 96 (18.8) | 416 (81.2) | 86 (16.8) | 426 (83.2) | 139 (27.1) | 373 (72.9) |
| Allergic reaction to inhaled allergens¶ | ||||||
| Yes | 57 (21.1) | 213 (78.9) | 57 (21.1) | 213 (78.9) | 120 (44.4)†† | 150 (55.6) |
| No | 114 (19.0) | 487 (81.0) | 129 (21.5) | 472 (78.5) | 142 (23.6) | 459 (76.4) |
| Dampness in house¶ | ||||||
| Yes | 24 (25.5) | 70 (74.5) | 22 (23.4) | 72 (76.6) | 40 (42.5)†† | 54 (57.5) |
| No | 147 (18.9) | 630 (81.1) | 164 (21.1) | 613 (78.9) | 222 (28.6) | 555 (71.4) |
| Smoking status¶ | ||||||
| Ever smoker | 104 (25.9)†† | 297 (74.1) | 114 (28.4)†† | 287 (71.6) | 143 (35.7)†† | 258 (64.3) |
| Never smoker | 65 (14.1) | 397 (85.9) | 69 (14.9) | 393 (85.1) | 117 (25.3) | 345 (74.7) |
Data presented as n (%).
Comparing cough in 2003 with environmental characteristics and history of cough in 1993;
Comparing phlegm in 2003 with environmental characteristics and history of phlegm in 1993;
Comparing wheeze in 2003 with environmental characteristics and history of wheeze in 1993;
P values calculated using McNemar’s test;
P values calculated using χ2 test of association;
P<0.05;
P<0.01
Table 3 summarizes the results from separate multivariate transitional models (OR [95% CIs]) adjusted for age, sex and other exposures used for each respiratory symptom in 1993 to predict the same symptom in 2003. The OR was 6.32 (95% CI 4.02 to 9.95) for cough, 14.36 (95% CI 9.01 to 22.89) for phlegm and 6.40 (95% CI 4.40 to 9.32) for wheeze. Besides previous symptoms, reported history of allergic reaction to inhaled allergens, exposure to home dampness, grain dust exposure and participating in smoking in 1993 predicted respiratory symptoms in 2003.
TABLE 3.
Separate age- and sex-adjusted multivariate transition models for respiratory outcomes in 2003
| Variables in 1993 |
Self-reported symptoms and models in 2003 |
||
|---|---|---|---|
| Cough (model I) | Phlegm (model II) | Wheeze (model III) | |
| Cough | 6.32 (4.02–9.95)† | – | – |
| Phlegm | – | 14.36 (9.01–22.89)† | – |
| Wheeze | – | – | 6.40 (4.40–9.32)† |
| Dampness in home* | 2.01 (1.12–3.60)‡ | 1.66 (0.87–3.14) | 1.51 (0.89–2.56) |
| Allergic reaction to inhaled allergens* | 0.85 (0.55–1.32) | 0.75 (0.47–1.20) | 2.37 (1.64–3.41)† |
| Grain dust exposure* | 1.83 (1.09–3.05)‡ | 1.81 (1.07–3.05)‡ | 1.21 (0.76–1.92) |
| Smoking status* (ever smoker) | 1.87 (1.24–2.81)§ | 1.96 (1.28–2.98)§ | 1.37 (0.96–1.95) |
Data presented as OR (95% CI).
Reference category is ‘No’;
P<0.001;
P<0.05;
P<0.01
Furthermore, a subgroup of asymptomatic participants who reported no cough, phlegm or wheeze in 1993 were selected to examine the associations of the exposure related to each symptom reported in 2003. Of the 871 subjects in the study, 83.9% were cough free, 81.9% were phlegm free and 74.5% were wheeze free in 1993. By 2003, 13.4% of the subjects had developed cough, 12.3% had developed phlegm and 19.1% had developed wheeze. Adjusting for age and sex, home dampness and smoking status (ever smoker) in 1993 were significant determinants for the development of cough in 2003 (Table 4). Smoking status was a significant determinant for the development of phlegm and wheeze (Table 4). Allergic reaction to inhaled allergens in 1993 was also a significant factor for the presence of wheeze in 2003.
TABLE 4.
Age- and sex-adjusted multivariate models for respiratory symptoms in 2003 associated with risk factors reported in 1993 among participants who were asymptomatic in 1993
| Variables in 1993 |
Self-reported symptoms and models in 2003 |
||
|---|---|---|---|
| Cough (n=731) (model I) | Phlegm (n=713) (model II) | Wheeze (n=649) (model III) | |
| Dampness in home* | 2.05 (1.10–3.83)† | 1.86 (0.92–3.73) | 1.41 (0.74–2.69) |
| Allergic reaction to inhaled allergens* | 1.29 (0.80–2.08) | 0.98 (0.58–1.66) | 2.33 (1.50–3.58)‡ |
| Grain dust exposure* | 1.49 (0.84–2.67) | 1.62 (0.89–2.96) | 1.18 (0.68–2.04) |
| Smoking status* (ever smoker) | 2.04 (1.30–3.21)§ | 2.02 (1.25–3.26)§ | 1.69 (1.10–2.57)† |
Data presented as OR (95% CI).
Reference category is ‘No’;
P<0.05;
P<0.001;
P<0.01
DISCUSSION
Our findings demonstrated that respiratory symptoms of cough, phlegm and wheeze were present in a considerable proportion of persons in a rural population, and that these symptoms tended to be long lasting, with moderate increases in the prevalence of these symptoms over the 10-year study period (1993 to 2003). Our results also showed that the presence of symptom(s) in 1993 was the strongest risk factor for the prevalence of symptoms in 2003 and, that after adjusting for age and sex, personal factors (ever smoking and allergy history), the environmental factors of home dampness and previous grain dust exposure, predicted the prevalence of symptoms 10 years later. In addition, home dampness, cigarette smoking and allergic reaction to inhaled allergens were significant predictors of the development of symptoms 10 years later.
Damp housing has been identified as a risk factor for respiratory symptoms including wheeze, wheeze with shortness of breath, and cough in cross-sectional studies (23–29). In one longitudinal study of respiratory symptoms (4), it was found to be associated with new wheeze and cough. Dales et al (23) recently reported an association between mould or dampness in the home and the presence of cough, wheeze and current asthma. Similarly, Park et al (24) found a relationship between fungal growth in buildings and respiratory symptoms. We recently reported that the prevalences of chronic wheeze, wheeze with shortness of breath and allergies were higher for women reporting damp housing compared with those who did not report damp housing (26). Studies conducted in Finland (27) and the Netherlands (28) also found that cough and phlegm were strongly associated with living in a damp or mouldy home. A review by Bornehag et al (29) reported that dampness in buildings appeared to increase the risk of symptoms such as cough, wheeze and the condition of asthma. A recent meta-analysis (30) suggested that building dampness and mould were associated with increased prevalence of respiratory and asthma-related health outcomes. In the current study, home dampness – which was used as a surrogate measure for certain housing conditions – was predictive of respiratory symptoms. The findings in the present report provide additional evidence for the relationship between the built environment and adverse respiratory health outcomes.
Grain dust exposure is a known risk factor for respiratory symptoms (5,31–33). Our previous work (5) showed that among grain elevator workers, grain dust was a risk factor for respiratory symptoms, such as wheeze, phlegm, cough and dyspnea, and that grain dust control was an effective method of reducing the prevalence of respiratory symptoms. The prevalence of respiratory symptoms, such as cough, sputum, wheeze, and shortness of breath with wheeze, were significantly more prevalent among wheat flour millers than among control subjects in Nigeria (31). Schwartz et al (32) found that grain workers who were exposed to higher concentrations of airborne dust and endotoxin had a significantly higher prevalence of chronic respiratory symptoms (cough, phlegm and wheeze) than those who were not exposed to higher concentrations. We previously reported that smoking and grain farming were significant predictors of wheeze in both men and women (33). An increasing trend in the risk of asthma and/or wheeze was observed with increases in the duration of farming, indicating a dose-response relationship (33). While previous studies have identified its importance in occupational environments, the current report identifies its importance in the rural farming environment.
The effects of smoking on the development of respiratory symptoms such as cough, phlegm and wheeze (25,33–37) are well known. According to the Canadian Tobacco Use Monitoring Survey (22), in 2003, 21% of Canadians 15 years of age and older were current smokers, 53% were never smokers and 26% were ex-smokers. Results from the Humboldt study in 2003 followed the national smoking pattern for ever smokers and never smokers. However, the percentage of current smokers was lower in Humboldt (9.2%) compared with the Canadian national average (21%). Some of the decline in smoking prevalence seen in the current study may be in response to a community bylaw banning smoking in public places that was implemented before the second study.
Recently conducted cross-sectional studies (25,34,36–37) reported that smoking was the most important risk factor associated with wheeze, cough or phlegm. A population-based prospective study from Hong Kong (35) showed that second-hand smoke exposure in the workplace is significantly associated with frequent cough and phlegm when observed both cross-sectionally and prospectively. Initiation of smoking between time points of studies was associated with increased cough or wheezing in smokers compared with nonsmokers (3,8,10,38). A longitudinal study of changes in respiratory status in young adults (3) reported that smoking was significantly associated with new onset of wheeze and asthma. Similar to these findings, our study reports that previous cigarette smoking is associated with a risk of developing or experiencing persistent respiratory symptoms of cough, phlegm and wheeze.
Our study also showed that reported previous allergic reaction to inhaled allergens strongly predicted wheeze. This is consistent with reports from three other studies examining predictors of respiratory symptoms. In a cross-sectional study, Potts et al (25) also observed that wheeze and productive cough were associated with specific allergens such as cats and house dust mites. Skin test reactivity was associated with wheeze without a cold eight years later in one study (11), and in predicting asthma symptoms (including wheeze) 17 years later in another (7).
One of the strengths of our study was the large longitudinal sample of 871 respondents, with resulting robust statistical power. On the other hand, the study examined only two time points that were 10 years apart. Therefore, the increases in prevalence could be the result of a period-cohort effect (39) with an aging population. Also, the use of symptom information, rather than diagnosed disease potentially could limit the period cohort effect due to changes in diagnosing patterns that might have occurred with the use of diagnosed disease such as asthma or chronic obstructive pulmonary disease. Information regarding the date of development of symptoms for participants who were symptom free in 1993 and reported symptoms in 2003 was not available. However, assessing the exposure history in 1993, rather than in 2003, reduces the potential for induction bias associated with limited information on exact occurrence of symptoms and exposure history at the second survey time point. The lack of objective measurement of environmental exposures and atopy, and reliance on self-report by questionnaire responses to dampness, smoking, grain exposure and allergy history are two possible sources of misclassification and responder bias. To limit responder bias, both time points used similar questionnaires (ie, questions used for the present analysis were identical at both time points) and identical procedures for collecting data. Questions assessing respiratory symptoms were the standardized questions validated by the American Thoracic Society (18). Sears et al (40) showed that repeated use of the same respiratory questionnaire over time is not subject to responder bias.
The current study demonstrated that reported respiratory symptoms persist over time and that earlier exposures (both domestic and farm related) are predictors for later respiratory symptoms.
Acknowledgments
This work was funded in part by a postdoctoral fellowship to Dr Karunanayake from the CIHR Strategic Initiative in Health Research ‘Public Health and the Agricultural Rural Ecosystem’ (PHARE) Program (CIHR grant # STP 53906), and the Canadian Centre for Health and Safety in Agriculture Founding Chairs Fellowship Program. The authors express their appreciation to the people of Humboldt, Saskatchewan, for their continued cooperation with the 1993 and 2003 studies, and to members of the local organizing committees, for their assistance with the studies.
Footnotes
FUNDING: The 1993 Humboldt study was funded by the Saskatchewan Health Services Utilization and Research Committee, and the 2003 Humboldt study was funded by the Canadian Institutes of Health Research (Grant no: MOP-57907).
CONFLICTS OF INTERESTS: The authors have no conflicts of interest to declare.
REFERENCES
- 1.Manfreda J, Becklake MR, Sears MR, et al. Prevalence of asthma symptoms among adults aged 20–44 years in Canada. Can Med Assoc J. 2001;164:995–1001. [PMC free article] [PubMed] [Google Scholar]
- 2.Ghosh S, Pahwa P, Rennie D, McDuffie HH. Opposing trends in the prevalence of health professional-diagnosed asthma by sex: A Canadian national population health survey study. Can Respir J. 2008;15:146–52. doi: 10.1155/2008/793913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Frank PI, Hazell ML, Morris JA, Linehan MF, Frank TL. A longitudinal study of changes in respiratory status in young adults, 1993–2001. Int J Tuberc Lung Dis. 2007;11:338–43. [PubMed] [Google Scholar]
- 4.Gunnbjörnsdóttir MI, Franklin KA, Norbäck D, et al. Prevalence and incidence of respiratory symptoms in relation to indoor dampness: The RHINE study. Thorax. 2006;61:221–5. doi: 10.1136/thx.2005.057430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pahwa P, McDuffie HH, Dosman JA. Longitudinal changes in prevalence of respiratory symptoms among Canadian grain elevator workers. Chest. 2006;129:1605–13. doi: 10.1378/chest.129.6.1605. [DOI] [PubMed] [Google Scholar]
- 6.Chinn S, Jarvis D, Burney P, et al. Increase in diagnosed asthma but not in symptoms in the European Community Respiratory Health Survey. Thorax. 2004;59:646–51. doi: 10.1136/thx.2004.021642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Toelle BG, Xuan W, Peat JK, Marks GB. Childhood factors that predict asthma in young adulthood. Eur Respir J. 2004;23:66–70. doi: 10.1183/09031936.03.00046903. [DOI] [PubMed] [Google Scholar]
- 8.Eagan TML, Bakke PS, Eide GE, Gulsvik A. Incidence of asthma and respiratory symptoms by sex, age and smoking in a community study. Eur Respir J. 2002;19:599–605. doi: 10.1183/09031936.02.00247302. [DOI] [PubMed] [Google Scholar]
- 9.Pahwa P, Senthilselvan A, McDuffie HH, Dosman JA. Predictors of onset of wheezing in grain elevator workers. Can Respir J. 1998;5:200–5. doi: 10.1155/1998/495410. [DOI] [PubMed] [Google Scholar]
- 10.Bodner CH, Ross S, Little J, et al. Risk factors for adult onset wheeze: A case control study. Am J Respir Crit Care Med. 1998;157:35–42. doi: 10.1164/ajrccm.157.1.9702062. [DOI] [PubMed] [Google Scholar]
- 11.Barbee RA, Halonen M, Kaltenborn WT, Burrows B. A longitudinal study of respiratory symptoms in a community population sample. Chest. 1991;99:20–6. doi: 10.1378/chest.99.1.20. [DOI] [PubMed] [Google Scholar]
- 12.Bodner C, Ross S, Douglas G, et al. The prevalence of adult onset wheeze: Longitudinal study. BMJ. 1997;374:792. doi: 10.1136/bmj.314.7083.792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brogger J, Bakke P, Eide GE, Johansen B, Andersen A, Gulsvik A. Long-term changes in adult asthma prevalence. Eur Respir J. 2003;21:468–72. doi: 10.1183/09031936.03.00056103. [DOI] [PubMed] [Google Scholar]
- 14.Becklake MR. Grain dust and lung health: Not just a nuisance dust. Can Res J. 2007;14:423–5. doi: 10.1155/2007/931094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Statistics Canada Population and dwelling counts, for Canada, provinces and territories, and census subdivisions (municipalities), 2006 and 2001 censuses – 100% data (table). Population and Dwelling Count Highlight Tables. 2007. 2006 Census. Statistics Canada Catalogue no. 97-550-XWE2006002. Ottawa, 2007. < http://www12.statcan.ca/english/census06/data/popdwell/Table.cfm?T=302&SR=1&S=6&O=D&RPP=25&PR=47&CMA=-1> (Accessed on December 8, 2009).
- 16.Chen Y, Rennie DC, Reeder BA. Age-related association between body mass index and blood pressure: The Humboldt Study. Int J Obes. 1995;19:825–31. [PubMed] [Google Scholar]
- 17.Chen Y, Rennie DC, Cormier Y, Dosman J. Sex specificity of asthma associated with objectively measured body mass index and waist circumference: The Humboldt Study. Chest. 2005;128:3048–54. doi: 10.1378/chest.128.4.3048. [DOI] [PubMed] [Google Scholar]
- 18.Ferris BG. Epidemiology Standardization Project (American Thoracic Society) Am Rev Respir Dis. 1978;118:1–120. [PubMed] [Google Scholar]
- 19.Bland M. An Introduction to Medical Statistics. Oxford: Oxford University Press; 2000. pp. 245–8. [Google Scholar]
- 20.Diggle PJ, Liang K-Y, Zeger SL. Analysis of Longitudinal Data. Oxford: Oxford Science Publications; 1995. p. 379. [Google Scholar]
- 21.SAS Institute . The SAS System for Windows, Release 9.03. SAS Institute; Cary, NC. USA: 2007. [Google Scholar]
- 22.Health Canada Canadian Tobacco Use Monitoring Survey (CTUMS): Smoking in Canada: An overview. 2003. < http://dsp-psd.pwgsc.gc.ca/Collection/H12-35-2003-1E.pdf> (Accessed on September 20, 2009).
- 23.Dales R, Liu L, Wheeler AJ, Gilbert NL. Quality of indoor residential air and health. Can Med Assoc J. 2008;179:147–52. doi: 10.1503/cmaj.070359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Park JH, Cox-Ganser J, Rao C, Kreiss K. Fungal and endotoxin measurements in dust associated with respiratory symptoms in a water-damaged office building. Indoor Air. 2006;16:192–203. doi: 10.1111/j.1600-0668.2005.00415.x. [DOI] [PubMed] [Google Scholar]
- 25.Potts JF, Rona RJ, Oyarzun MJ, Amigo H, Bustos P. Indoor risk factors for cough and their relation to wheeze and sensitization in Chilean young adults. Am J Public Health. 2008;98:680–6. doi: 10.2105/AJPH.2006.093302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rennie D, Chen Y, Lawson J, Dosman J. Differential effects of damp housing on respiratory health in women. J Am Med Women Assoc. 2005;60:46–51. [PubMed] [Google Scholar]
- 27.Pirhonen I, Nevalainen A, Husman T, Pekkanen J. Home dampness, moulds and their influence on respiratory infections and symptoms in adults in Finland. Eur Respir J. 1996;9:2618–22. doi: 10.1183/09031936.96.09122618. [DOI] [PubMed] [Google Scholar]
- 28.Brunkreef B. Damp housing and adult respiratory symptoms. Allergy. 1992;47:498–502. doi: 10.1111/j.1398-9995.1992.tb00672.x. [DOI] [PubMed] [Google Scholar]
- 29.Bornehag C, Blomquist G, Gyntelberg F, et al. Dampness in buildings and health. Indoor Air. 2001;11:72–86. doi: 10.1034/j.1600-0668.2001.110202.x. [DOI] [PubMed] [Google Scholar]
- 30.Fisk WJ, Lei-Gomez Q, Mendell MJ. Meta-analysis of the associations of respiratory health effects with dampness and mold in homes. Indoor Air. 2007;17:284–96. doi: 10.1111/j.1600-0668.2007.00475.x. [DOI] [PubMed] [Google Scholar]
- 31.Ijadunola KT, Erhabor GE, Onayade AA, Ijadunola MY, Fatusi AO, Asuzu MC. Prevalence of respiratory symptoms among wheat flour mill workers in Ibadan, Nigeria. Am J Ind Med. 2004;45:251–9. doi: 10.1002/ajim.10344. [DOI] [PubMed] [Google Scholar]
- 32.Schwartz DA, Thorne PS, Yagla SJ, et al. The role of endotoxin in grain dust-induced lung disease. Am J Respir Crit Care Med. 1995;152:603–8. doi: 10.1164/ajrccm.152.2.7633714. [DOI] [PubMed] [Google Scholar]
- 33.Senthilselvan A, Chen Y, Dosman JA. Predictors of asthma and wheezing in adults: Grain farming, sex, and smoking. Am Rev Respir Dis. 1993;148:667–70. doi: 10.1164/ajrccm/148.3.667. [DOI] [PubMed] [Google Scholar]
- 34.Mayer AS, Stoller JK, Vendal S, et al. Risk factors for symptom onset in PI*Z alpha-1 antitrypsin deficiency. Int J Chron Obstruct Pulmon Dis. 2006;1:485–92. doi: 10.2147/copd.2006.1.4.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ho SY, Lam TH, Chung SF, Lam TP. Cross-sectional and prospective associations between passive smoking and respiratory symptoms at the workplace. Ann Epidemiol. 2007;17:126–31. doi: 10.1016/j.annepidem.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 36.Sunyer J, Jarris D, Gotschi T, et al. Chronic bronchitis and urban air pollution in an international study. Occup Environ Med. 2006;63:836–43. doi: 10.1136/oem.2006.027995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Miedinger D, Chhajed PN, Karli C, Lupi GA, Leuppi JD. Respiratory symptoms and smoking behaviour in Swiss conscripts. Swiss Med Weekly. 2006;136:659–63. doi: 10.57187/smw.2006.11544. [DOI] [PubMed] [Google Scholar]
- 38.Jaakkola MS, Jaakkola JJ, Becklake MR, Ernst P. Effect of passive smoking on the development of respiratory symptoms in young adults: An 8-year longitudinal study. J Clin Epidemiol. 1996;49:581–6. doi: 10.1016/0895-4356(96)00004-2. [DOI] [PubMed] [Google Scholar]
- 39.Rothman KJ, Greenland S. Modern Epidemiology. Baltimore: Lippincott, Williams & Wilkins; 1998. pp. 130–3. [Google Scholar]
- 40.Sears MR, Lewis S, Herbison GP, et al. Comparison of reported prevalences of recent asthma in longitudinal and cross-sectional studies. Eur Respir J. 1997;10:51–4. doi: 10.1183/09031936.97.10010051. [DOI] [PubMed] [Google Scholar]