Abstract
BACKGROUND:
Sputum cell counts have identified inflammatory subtypes of bronchitis in relatively small numbers of subjects with asthma, chronic obstructive pulmonary disease (COPD) and chronic cough in research studies. The prevalence of different subtypes of bronchitis in routine clinical practice, however, has not been reported.
OBJECTIVE:
To examine the heterogeneity of bronchitis and its relationship to the severity of airflow obstruction.
METHODS:
A retrospective cross-sectional survey based on a computerized database of spontaneous or induced sputum cell counts examined in a large university tertiary respiratory outpatient clinic.
RESULTS:
The database contained 4232 consecutive sputum records from 2443 patients with chronic cough (39%), asthma (37%), asthma with COPD (9%), COPD (13%) and bronchiectasis (3%). Total and differential cell counts were obtained from 86% of successful sputum samples. Induced sputum provided more viable samples than spontaneous expectorate. Approximately one-third of patients with asthma and one-fifth of patients with COPD experience eosinophilic bronchitis. Asthmatic patients with moderate to severe airflow obstruction had a greater number of sputum eosinophils. There was a significantly higher number of total cell counts and percentage of neutrophils in the sputum of COPD patients with moderate and severe airflow obstruction than in those with mild airflow obstruction.
CONCLUSION:
There is heterogeneity in the cellularity of sputum in various airway diseases. Patients with clinically stable airway diseases may have high sputum cell counts. During exacerbations, more patients may experience neutrophilic bronchitis. Severity of airflow obstruction is associated with eosinophilic bronchitis in patients with asthma, and neutrophilic bronchitis in patients with nonasthmatic COPD.
Keywords: Asthma, Bronchitis heterogeneity, Clinical practice, COPD, Sputum cell counts
Abstract
HISTORIQUE :
Dans les études de recherche, la numération cellulaire des expectorations permet de déceler des sous-types de bronchite chez un nombre relativement peu nombreux de sujets atteints d’asthme, de maladie pulmonaire obstructive chronique (MPOC) ou de toux chronique. La prévalence de divers sous-types de bronchite n’a toutefois pas fait l’objet de comptes rendus en pratique clinique.
OBJECTIF :
Examiner l’hétérogénéité de la bronchite et son lien avec la gravité de l’obstruction des voies respiratoires.
MÉTHODOLOGIE :
Sondage transversal rétrospectif fondé sur une base de données informatisée de la numération cellulaire des expectorations spontanées ou induites au sein du grand service de consultations externes d’un centre universitaire de soins tertiaires.
RÉSULTATS :
La base de données contenait 4 232 dossiers consécutifs d’expectorations provenant de 2 443 patients atteints de toux chronique (39 %), d’asthme (37 %), d’asthme accompagné de MPOC (9 %), de MPOC (13 %) et de bronchiectasie (3 %). Les numérations cellulaires totales et différentielles provenaient de 86 % des échantillons d’expectorations réussis. Les expectorations induites fournissaient des échantillons plus viables que les expectorations spontanées. Environ le tiers des patients atteints d’asthme et le cinquième de ceux ayant une MPOC ont souffert d’une bronchite à éosinophiles. Les patients asthmatiques présentant une obstruction modérée à grave des voies respiratoires affichaient un plus grand nombre d’éosinophiles dans les expectorations. La numération cellulaire totale et le pourcentage de neutrophiles étaient considérablement plus élevés chez les patients atteints de MPOC présentant une obstruction modérée à grave des voies respiratoires que chez ceux présentant une obstruction bénigne des voies respiratoires.
CONCLUSION :
On constate une hétérogénéité dans la cellularité des expectorations de diverses maladies respiratoires. Les patients dont la maladie respiratoire est stable sur le plan clinique peuvent présenter une numération cellulaire élevée des expectorations. Pendant les exacerbations, plus de patients peuvent souffrir de bronchite à éosinophiles. La gravité de l’obstruction des voies respiratoires s’associe à une bronchite à éosinophiles chez les patients atteints d’asthme, et à une bronchite à neutrophiles chez les patients atteints d’une MPOC non asthmatique.
Obstructive airway diseases include different components such as symptoms, variable airflow limitation, chronic airflow limitation, airway hyper-responsiveness, airway inflammation, emphysema and bronchiectasis (1). These components can coexist in various combinations that are not mutually exclusive. Airway inflammation (bronchitis) plays a central role in airway diseases. It is responsible for symptoms, variable airflow limitation through the release of bronchoconstrictor mediators, and chronic airway limitation through airway remodelling and structural changes. Clinical evaluation based only on symptoms and spirometry is not sufficient to assess airway inflammation in patients with moderate-to-severe asthma (2) because there is a weak association between airway inflammation and symptoms and spirometry (3,4). Examination of sputum cell counts is a useful way to identify different types of inflammation (bronchitis), which could be eosinophilic bronchitis (EB), neutrophilic bronchitis (NB), both or neither (1). The prevalence of different types of bronchitis in routine clinical practice is not known. Our institute has been using quantitative sputum cell counts in the management of difficult airway diseases for the past 15 years, and has a database of records for the past five years. The objective of the present study was to describe our experience of using sputum cell counts in clinical practice within an academic out-patient clinic, and to demonstrate the variability of inflammatory subtypes in different airway diseases and its relation to severity of airflow obstruction.
METHODS
Design
The present study was designed as a retrospective cross-sectional survey, based on records housed in a computerized database of spontaneous or induced sputum cell counts examined from January 2004 to January 2008 at the Firestone Institute for Respiratory Health (Hamilton, Ontario). The database was created using FileMaker Pro version 10.0 (FileMaker Inc, USA) and contained the following patient information: age, sex, address, postbronchodilator spirometry, sputum cell counts, physician diagnosis, indication for the test and medication. All patients who registered in the clinic for whom sputum examination was requested by a physician were included in the database. This included patients who provided spontaneous sputum and those for whom sputum induction was requested including those who could not expectorate during induction. The database was used to identify the inflammatory subtypes of bronchitis of patients with stable or exacerbated airway diseases. The study was approved by the Research Ethics Board of St Joseph’s Healthcare, Hamilton, Ontario.
Patients
The patients were those referred for the examination of sputum cell counts from the outpatient clinic at the Firestone Institute for Respiratory Health. They were clinically stable or experienced an exacerbation of physician-diagnosed asthma, asthma with chronic obstructive pulmonary disease (COPD), COPD without asthma, chronic cough or bronchiectasis. All patients were scheduled for sputum induction; however, if the patient was able to produce a spontaneous sample, it was accepted and the appointment for induction was cancelled.
Study definitions
The subtypes of bronchitis were classified as EB, NB, mixed granulocytic, trivial neutrophilia of unknown significance or normal cell counts (paucigranulocytic). EB was defined as a percentage of sputum eosinophils of 1.1% or greater (5). NB was defined as a total cell count of 9.7 million cells/g of sputum or greater and proportion of neutrophils of 64% or greater (5). Mixed granulocytic bronchitis was defined as a total cell count of 9.7 million cells/g of sputum or greater, and proportion of eosinophils and neutrophils of 1.1% or greater and 64% or greater, respectively. Trivial neutrophilia of unknown significance was defined as an isolated rise in total cell count of 9.7 million cells/g of sputum or greater, or proportion of sputum neutrophils of 64% or greater. A normal cell count was defined as a total cell count of less than 9.7 million cells/g of sputum and proportion of neutrophils less than 64%, and eosinophils less than 1.1% (5). The presence of moderate or many macrophage smokers’ inclusions was used to indicate that the patient was a current or former smoker. A diagnosis of asthma was based on information provided by the referring physician, which included evidence of reversible airflow limitation (an increase in forced expiratory volume in 1 s [FEV1] of 15% or greater and 200 mL or greater from the prebronchodilator value) or airway hyper-responsiveness (a provocative concentration of methacholine causing a 20% or greater fall in FEV1 of less than 8 mg/mL). Asthma severity was classified according to the Global Initiative for Asthma criteria (6) and based on both severity of airflow obstruction and corticosteroid treatment at the time of sputum collection. COPD was indicated by a postbronchodilator FEV1/vital capacity of less than 70% (7). Severity of airflow obstruction in patients with COPD was classified according to the Global Initiative for Chronic Obstructive Lung Disease criteria (8). A diagnosis of asthma with COPD was based on a compatible clinical history with evidence of reversible airflow limitation, airway hyper-responsiveness and a postbronchodilator FEV1/forced vital capacity of less than 70%. Chronic cough was defined as cough lasting at least eight weeks with no overt clinical or radiological evidence of lung disease (9). Identification of patients with bronchiectasis was based on documentation of chest radiographic and/or high-resolution computed tomography scan evidence in the medical record. An exacerbation was defined as an increase in cough, dyspnea, sputum volume or purulence, or a fall in FEV1 by at least 20% that, in the opinion of the physician, required an adjustment to therapy (10). A successful test was one in which suitable nonsquamous total and differential cell counts could be obtained.
Procedures
Sputum induction and examination for total and differential cell counts were performed by the methods described by Pizzichini et al (11). Spirometry was performed according to American Thoracic Society standards, before or 10 min after salbutamol 200 μg (12). Reference values were taken from Crapo et al (13).
Analysis
Normally distributed data were summarized as arithmetic mean and SD. Variables with a non-normal distribution were summarized as median with interquartile range. Categorical variables were presented as a percentage. One-way ANOVA and Kruskal-Wallis tests were used for the between-group comparisons of numerical variables with normal and non-normal distributions, respectively. Results were considered to be statistically significant at P<0.05. Statistical analysis was performed using SPSS Graduate Pack version 15.0 (IBM Corporation, USA) for Windows (Microsoft Corporation, USA).
RESULTS
Clinical feasibility
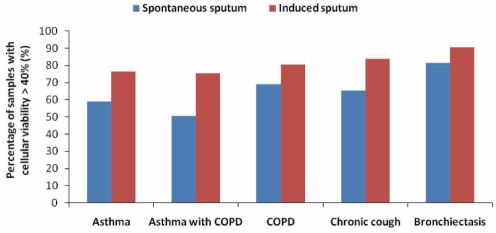
The sputum database contained 4232 consecutive records from 2443 patients with airway diseases. Forty-three per cent of the patients underwent more than one sputum examination. A total of 1116 patients had asthma, of which 19% were associated with COPD (8.7% of the total study population). Thirteen per cent of patients had COPD without asthma, and 38.6% of patients had chronic cough as the primary diagnosis (Table 1). Sputum was successfully obtained in 81% of the tests. Total and differential cell counts were achieved in 86% of these sputum samples. The percentage of samples with a cellular viability of greater than 40% was higher in induced sputum (75.3% to 90.5%) than in spontaneous sputum (50.7% to 81.5%) collected from patients with various airway diseases (Figure 1).
TABLE 1.
Patient characteristics
|
Primary diagnosis |
|||||
|---|---|---|---|---|---|
| Asthma | Asthma with COPD | COPD | Chronic cough | Bronchiectasis | |
| Patients, n (%) | 903 (37) | 213 (8.7) | 308 (12.6) | 943 (38.6) | 76 (3.1) |
| Age, years | 47±18 | 63±14 | 66±12 | 56±16 | 63±14 |
| Male sex, % | 44 | 54 | 57 | 39 | 32 |
| Current or former smoker, % | 13 | 19 | 22 | 10 | 5 |
| FEV1, % predicted | 88±19 | 58±17 | 61±20 | 92±18 | 82±28 |
| Post salbutamol FEV1/VC, % | 76±11 | 57±12 | 55±15 | 79±10 | 71±11 |
| Inhaled corticosteroids*, μg/day (median [IQR]) | 800 (2000) | 1600 (1200) | 1000 (2000) | 0 (5000) | 0 (800) |
| Patients receiving systemic corticosteroid†, % | 14 | 25 | 17 | 6 | 23 |
Data presented as mean ± SD unless otherwise indicated.
Dose calculated as beclomethasone dipropionate equivalents, in which 1 μg beclomethasone = 1 μg budesonide = 0.5 μg fluticasone;
Systemic corticosteroid included prednisone or methylprednisone. COPD Chronic obstructive pulmonary disease; FEV1 Forced expiratory volume in 1 s; IQR Interquartile range; VC Vital capacity
Figure 1).
The percentage of sputum samples with a cellular viability of greater than 40% among induced or spontaneous sputum samples in patients with different airway diseases. COPD Chronic obstructive pulmonary disease
Subtypes of bronchitis
Stable disease:
Although patients were considered to be stable by their physicians, only 18% of the sputum examinations showed normal cell counts. Forty-two per cent of sputum samples in patients with asthma and 45% of samples from patients with asthma and associated COPD showed EB either in isolation or associated with raised levels of neutrophils. The majority of patients with nonasthmatic COPD showed NB (34%); EB was also not uncommon (18%) (Table 2).
TABLE 2.
The percentage of inflammatory subtypes of bronchitis in stable and exacerbated airway diseases
| Physician diagnosis |
Stable disease |
Exacerbated disease |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total tests, n |
Inflammatory subtypes of bronchitis, % |
Total tests, n |
Inflammatory subtypes of bronchitis, % |
|||||||||
| EB | NB | EBNB | Trivial NB | Normal cell counts | EB | NB | EBNB | Trivial NB | Normal cell counts | |||
| Asthma | 664 | 36 | 14 | 6 | 16 | 28 | 115 | 35 | 21 | 13 | 16 | 15 |
| COPD | 529 | 18 | 34 | 7 | 30 | 11 | 171 | 18 | 46 | 8 | 18 | 10 |
| Asthma and COPD | 175 | 35 | 19 | 10 | 21 | 15 | 60 | 24 | 33 | 12 | 24 | 7 |
| Chronic cough | 510 | 19 | 25 | 5 | 24 | 27 | 95 | 14 | 37 | 13 | 23 | 13 |
| Bronchiectasis | 575 | 8 | 51 | 18 | 17 | 6 | 54 | 6 | 66 | 17 | 11 | 0 |
The total number of tests for a disease category (example, for asthma: 664+115=779) is less than the total number of patients in Table 1 because Table 2 includes data only from patients in whom sputum collection was successful. COPD Chronic obstructive pulmonary disease; EB Eosinophilic bronchitis; EBNB Combined eosinophilic and neutrophilic bronchitis; NB Neutrophilic bronchitis
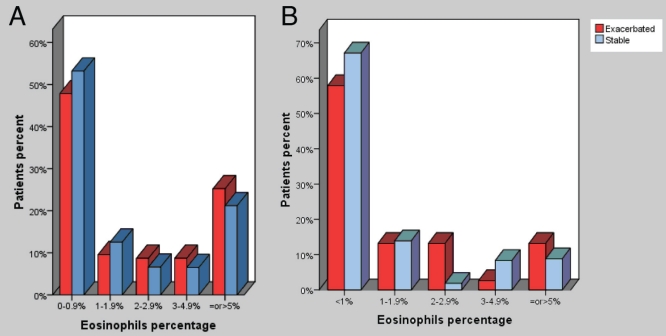
Figure 2A shows the distribution of patients with EB when different cut-off values were used to define eosinophilia. Twenty-eight per cent of patients with asthma and 16% of patients with COPD had eosinophil counts of 3% or higher.
Figure 2).
A The distribution of patients with asthma (stable and during exacerbations) with eosinophilic bronchitis when different cut-off values were used to define eosinophilia. B The distribution of patients with chronic obstructive pulmonary disease (stable and during exacerbations) with eosinophilic bronchitis when different cut-off values were used to define eosinophilia
Exacerbated disease:
Forty-eight per cent of sputum samples in patients with asthma, and 36% of samples in patients with asthma and associated COPD showed EB either in isolation or associated with NB. In patients with a primary diagnosis of COPD, the exacerbations were mostly associated with NB alone (46%), or associated with eosinophilia (8%) and EB alone (18%). Twenty-two per cent of sputum samples examined during asthma (with or without COPD) exacerbations and 10% of samples collected during COPD exacerbations did not show bronchitis (Table 2). Figure 2B shows the distribution of patients with EB when different cut-off values were used to define eosinophilia.
Sputum cell counts in subtypes of bronchitis
The central tendency and dispersion of cells in the sputum of different inflammatory subtypes of bronchitis are summarized in Table 3. The average total cell count was much lower in the sputum of patients with EB (5.3×106/g) than that in those with NB (34.4×106/g). The median percentage of eosinophils and mean percentage of neutrophils, respectively, were 4.7% and 51.1% in the sputum of patients with EB, 0% and 88.4% in the sputum of patients with NB, and 3.3% and 82.1% in the sputum of patients with mixed granulocytic bronchitis. A predominance of macrophages (63.6%) was shown in sputum with normal cell counts.
TABLE 3.
Sputum viability (%) and differential cell counts in different subtypes of bronchitis
| Inflammatory subgroups of bronchitis | Viability |
Differential cell count*, 106/g (%) |
||||||
|---|---|---|---|---|---|---|---|---|
| Total cells | Neutrophils | Eosinophils* | Lymphocytes | Macrophages | Bronchial epithelial cells | |||
| EB (n=640) | Absolute value | 5.3 (5.5) | 2.7 (2.7) | 0.2 (0.5) | 0.1 (0.1) | 1.6 (2.1) | 0 (0) | |
| % | 62.5 (18.1) | 51.1 (23.9) | 4.7 (10.7) | 1.1 (1.4) | 35.3 (22.2) | 0.5 (1.3) | ||
| NB (n=921) | Absolute value | 34.4 (32.7) | 31.4 (31.8) | 0 (0.1) | 0.1 (2.7) | 2.8 (2.7) | 0 (0) | |
| % | 82 (16.3) | 88.4 (8.4) | 0 (0.3) | 0.5 (0.8) | 10.8 (8.1) | 0 (0.2) | ||
| EBNB (n=280) | Absolute value | 26.2 (21.3) | 22 (19.6) | 0.7 (0.9) | 0.2 (0.2) | 2.8 (2.5) | 0 (0.1) | |
| % | 78.5 (16.5) | 82.1 (8.3) | 3 (3.3) | 0.6 (0.7) | 12.3 (7.3) | 0.1 (0.5) | ||
| Trivial NB (n=614) | Absolute value | 6.7 (8.5) | 4.5 (3.9) | 0 (0) | 0.1 (0.6) | 2.1 (5.1) | 0 (0.1) | |
| % | 68.4 (18.1) | 75.8 | 0 (0.5) | 0.9 (1.9) | 22.6 (15.7) | 0.3 (0.9) | ||
| Normal cell counts (n=493) | Absolute value | 3.2 (2.3) | 1.2 (1.2) | 0 (0) | 0 (0.1) | 2 (1.5) | 0 (0.1) | |
| % | 62.7 (18.3) | 33.8 (18.7) | 0 (0.3) | 1 (1.3) | 63.6 (19) | 1 (2.7) | ||
Data for all cells (except eosinophils) presented as mean (SD).
Data for eosinophils are presented as median (interquartile range). EB Eosinophilic bronchitis; EBNB Combined eosinophilic and neutrophilic bronchitis; NB Neutrophilic bronchitis
Stability of phenotypes in stable disease
Sputum was available on two or more occasions in 128 patients with asthma and in 80 patients with COPD (without asthma). In patients with asthma, overall, the same phenotype was observed for a second time only in 23% of patients. Clinically relevant EB (3% or higher) tended to recur only in 18% of patients compared with NB, which recurred and/or persisted in 8%. In patients with COPD, overall, the same phenotype was observed a second time only in 14%.
Sputum cell counts in relation to severity of airflow obstruction
Spirometry data (postbronchodilator FEV1 and vital capacity) were available for 1074 patients with asthma and 265 patients with COPD. After adjusting for the dose of inhaled corticosteroids, asthmatic patients with moderate to severe airflow obstruction (FEV1 of lower than 79% predicted) had higher sputum eosinophil counts than in patients with mild airflow obstruction. No differences were observed in total cell counts and the percentage of neutrophils between categories. In contrast, there was a significantly higher number of total cells and percentage of neutrophils in the sputum of COPD patients with moderate and severe chronic airflow obstruction than in those with mild airflow obstruction, whereas no significant difference was found in the percentage of eosinophils (Tables 4 and 5).
TABLE 4.
Cell counts in relation to airflow obstruction in patients with asthma (n=1074)
| Cells |
Forced expiratory volume in 1 s (FEV1) |
||
|---|---|---|---|
| ≥80% predicted (n=693) | 60%–79% predicted (n=299) | <60% predicted (n=82) | |
| Eosinophils, % (median [IQR]) | 0.5 (2.3) | 1.5 (7.2)* | 2.2 (7.25)* |
| Neutrophils, % (mean ± SD) | 51.6±28.6 | 58.5±26.9 | 62.7±27.4 |
| Total cell count, ×106/g (mean ± SD) | 8.9±14.1 | 10.9±18.0 | 13.1±22.9 |
Compared with mild asthma (FEV1 ≥80% predicted), P<0.05. IQR Interquartile range
TABLE 5.
Cell counts in relation to airflow obstruction in patients with chronic obstructive pulmonary disease (COPD) (n=265)
| Cells |
Forced expiratory volume in 1 s (FEV1) |
|||
|---|---|---|---|---|
| ≥80% predicted (n=32) | 50%–79% predicted (n=128) | 30%–49% predicted (n=75) | <30% predicted (n=30) | |
| Eosinophils, % (median [IQR]) | 0.3 (1.0) | 0.5 (1.7) | 0.6 (1.3) | 0.3 (0.83) |
| Neutrophils, % (mean ± SD) | 61.4 ±27.4 | 73.1±21.7* | 75.5±19.9* | 90.1±12.0† |
| Total cell count, ×106/g (mean ± SD) | 5.3±5.6 | 14.5±20.6* | 18.8±25.3* | 24.1±27.6* |
Compared with mild COPD (FEV1 ≥80% predicted), P<0.05;
Compared with mild COPD (FEV1 ≥80% predicted), P<0.01. IQR Interquartile range
DISCUSSION
The present report is the largest description of sputum cell counts obtained from patients with airway diseases in clinical practice. The present study demonstrated that it is feasible to implement quantitative cell counts in sputum as a clinical test, which could be successfully achieved in the majority of patients and provide evidence that there is heterogeneity in the cellularity of sputum in various airway diseases. Patients with airway diseases, even when considered clinically stable, may have uncontrolled bronchitis. However, phenotypes based on cross-sectional studies are not stable. Patients with asthma and more severe airflow obstruction generally have an EB, while more severe airflow obstruction is associated with an NB among patients with nonasthmatic COPD.
Our study confirms previous observations from small numbers of research subjects and clinical reports that there is heterogeneity of bronchitis in airway diseases. This is relevant for several reasons. First, bronchitis has many causes and, therefore, it is not surprising that the nature of bronchitis depends on the cause. The pattern of bronchitis is independent of the physiological abnormality that defines disease (14). Thus, patients with asthma could have NB and patients with COPD could develop EB. Some patients with bronchiectasis may develop EB, for example, when associated with fungal sensitization. Identification of bronchitis type helps to plan investigations to determine etiology in patients with asthma (15). Second, patients may experience uncontrolled bronchitis even when symptoms are deemed by a clinician to be stable or controlled. This confirms our previous observations in a small study that clinical judgment of the presence and type of bronchitis is imprecise (16). Third, the type of bronchitis defines the response to treatment. While EB is a predictor of response to treatment with inhaled corticosteroids, NB is usually a predictor of nonresponse (10). Thus, the presence of an EB when patients are clinically controlled suggests that further increases in the dose of inhaled corticosteroids would reduce the risk of future exacerbations. The presence of non-EB in more than one-half of COPD exacerbations and one-third of asthma exacerbations suggest that these exacerbations may not require treatment with corticosteroids. This, however, needs more rigorous examination. Fourth, the prevalence of EB in patients with chronic cough is comparable with previous reports (17). Fifth, our experience confirms that it is feasible to implement examination of sputum cell counts in an outpatient clinical practice. Eighty-six per cent of successful clinical samples provided reliable cell counts. In our clinic, the results and an interpreted report are available to the referring physician within 24 h of collection. Although spontaneously expectorated sputum provides cell counts comparable with induced sputum, induced sputum has the advantage of better cell viability (18). Patients with nonasthmatic COPD underwent sputum examination more often than patients with asthma, both to optimize their treatment and probably because they experienced more infective exacerbations (Tables 2 and 3). Finally, the presence of eosinophilia was associated with airflow obstruction in asthma and neutrophilia, and was associated with airflow obstruction in patients with nonasthmatic COPD, suggesting causal roles for these cells in airflow obstruction in these respective abnormalities.
The present study had several limitations. First, it had the inherent problems and biases associated with a retrospective study design. Second, diagnostic categories were established based on historical physician diagnoses. Third, we did not have accurate information regarding the burden of cigarette smoking. This was inferred from the presence of inclusions within macrophages (19), which may have contributed to underestimation of the prevalence of cigarette smoking. Finally, cross-sectional study only provides a snapshot of cell counts at a single time point – the nature of bronchitis, however, changes over time depending on the various causes (20,21). Indeed, this was demonstrated in the small proportion of patients who underwent more than one sputum examination when they were clinically stable. The eosinophilic phenotype in patients with severe asthma who require prednisone to maintain control are likely to be more stable than the neutrophilic phenotype (22,23) considering that there are many determinants of airway neutrophilia – infections being the most notable. The lack of phenotype stability seriously questions cross-sectional studies that categorize patients based on examination of bronchitis on one occasion. We do not have longitudinal follow-up data on the patients reported in the present study to assess responses to specific therapies.
SUMMARY
The present study demonstrated that there is heterogeneity in the cellularity of sputum in various airway diseases. Patients with clinically stable airway diseases may have uncontrolled bronchitis that could influence the severity of airflow obstruction. It is feasible to implement sputum cell counting in clinical practice in a tertiary centre. We recommend measuring bronchitis during the initial evaluation of patients with airflow obstruction and during each exacerbation.
Acknowledgments
The authors are grateful to Dr Chris Allen, McMaster University (Hamilton, Ontario), for creating the sputum database at the Firestone Institute for Respiratory Health.
Footnotes
DISCLOSURE: Dr D’silva was supported by a Merck Canada Inc fellowship and Dr Hassan was supported by an Egyptian Ministry of Higher Education fellowship. Dr Nair holds a Canada Research Chair in Airway Inflammometry.
REFERENCES
- 1.Hargreave FE, Parameswaran K. Asthma, COPD and bronchitis are just components of airway disease. Eur Respir J. 2006;28:264–7. doi: 10.1183/09031936.06.00056106. [DOI] [PubMed] [Google Scholar]
- 2.Louis R, Lau LC, Bron AO, Roldaan AC, Radermecker M, Djukanovic R. The relationship between airways inflammation and asthma severity. Am J Respir Crit Care Med. 2000;161:9–16. doi: 10.1164/ajrccm.161.1.9802048. [DOI] [PubMed] [Google Scholar]
- 3.Sont JK, Han J, van Krieken JM, et al. Relationship between the inflammatory infiltrate in bronchial biopsy specimens and clinical severity of asthma in patients treated with inhaled steroids. Thorax. 1996;51:496–502. doi: 10.1136/thx.51.5.496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Crimi E, Spanevello A, Neri M, Ind PW, Rossi GA, Brusasco V. Dissociation between airway inflammation and airway hyperresponsiveness in allergic asthma. Am J Respir Crit Care Med. 1998;157:4–9. doi: 10.1164/ajrccm.157.1.9703002. [DOI] [PubMed] [Google Scholar]
- 5.Belda J, Leigh R, Parameswaran K, O’Byrne PM, Sears MR, Hargreave FE. Induced sputum cell counts in healthy adults. Am J Respir Crit Care Med. 2000;161:475–8. doi: 10.1164/ajrccm.161.2.9903097. [DOI] [PubMed] [Google Scholar]
- 6.Bateman ED, Hurd SS, Barnes PJ, et al. Global strategy for asthma management and prevention: GINA executive summary. Eur Respir J. 2008;31:43–78. doi: 10.1183/09031936.00138707. [DOI] [PubMed] [Google Scholar]
- 7.Leigh R, Pizzichini MM, Morris MM, Maltais F, Hargreave FE, Pizzichini E. Stable COPD: Predicting benefit from high-dose inhaled corticosteroid treatment. Eur Respir J. 2006;27:964–71. doi: 10.1183/09031936.06.00072105. [DOI] [PubMed] [Google Scholar]
- 8.Rabe KF, Hurd S, Anzueto A, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2007;176:532–55. doi: 10.1164/rccm.200703-456SO. [DOI] [PubMed] [Google Scholar]
- 9.Irwin RS, Madison JM. The diagnosis and treatment of cough. N Engl J Med. 2000;343:1715–21. doi: 10.1056/NEJM200012073432308. [DOI] [PubMed] [Google Scholar]
- 10.Jayaram L, Pizzichini MM, Cook RJ, et al. Determining asthma treatment by monitoring sputum cell counts: Effect on exacerbations. Eur Respir J. 2006;27:483–94. doi: 10.1183/09031936.06.00137704. [DOI] [PubMed] [Google Scholar]
- 11.Pizzichini E, Pizzichini MM, Efthimiadis A, et al. Indices of airway inflammation in induced sputum: Reproducibility and validity of cell and fluid-phase measurements. Am J Respir Crit Care Med. 1996;154:308–17. doi: 10.1164/ajrccm.154.2.8756799. [DOI] [PubMed] [Google Scholar]
- 12.Miller MR, Hankinson J, Brusasco V, et al. for the ATS/ERS Task Force on Standardisation of spirometry Eur Respir J. 2005;26:319–38. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 13.Crapo RO, Morris AH, Gardner RM. Reference spirometric values using techniques and equipment that meet ATS recommendations. Am Rev Respir Dis. 1981;123:659–64. doi: 10.1164/arrd.1981.123.6.659. [DOI] [PubMed] [Google Scholar]
- 14.Hargreave FE, Nair P. The definition and diagnosis of asthma. Clin Exp Allergy. 2009;39:1652–8. doi: 10.1111/j.1365-2222.2009.03321.x. [DOI] [PubMed] [Google Scholar]
- 15.Pallan S, Mahony JB, O’Byrne PM, Nair P. Asthma management by monitoring sputum neutrophil count. Chest. 2008;134:628–30. doi: 10.1378/chest.08-0400. [DOI] [PubMed] [Google Scholar]
- 16.Parameswaran K, Pizzichini E, Pizzichini MM, Hussack P, Efthimiadis A, Hargreave FE. Clinical judgement of airway inflammation versus sputum cell counts in patients with asthma. Eur Respir J. 2000;15:486–90. doi: 10.1034/j.1399-3003.2000.15.10.x. [DOI] [PubMed] [Google Scholar]
- 17.Brightling CE, Ward R, Goh KL, Wardlaw AJ, Pavord ID. Eosinophilic bronchitis is an important cause of chronic cough. Am J Respir Crit Care Med. 1999;160:406–10. doi: 10.1164/ajrccm.160.2.9810100. [DOI] [PubMed] [Google Scholar]
- 18.Pizzichini MM, Popov TA, Efthimiadis A, et al. Spontaneous and induced sputum to measure indices of airway inflammation in asthma. Am J Respir Crit Care Med. 1996;154:866–9. doi: 10.1164/ajrccm.154.4.8887576. [DOI] [PubMed] [Google Scholar]
- 19.Agius RM, Rutman A, Knight RK, Cole PJ. Human pulmonary alveolar macrophages with smokers’ inclusions: Their relation to the cessation of cigarette smoking. Br J Exp Pathol. 1986;67:407–13. [PMC free article] [PubMed] [Google Scholar]
- 20.D’silva L, Cook RJ, Allen CJ, Hargreave FE, Parameswaran K. Changing pattern of sputum cell counts during successive exacerbations of airway disease. Respir Med. 2007;101:2217–20. doi: 10.1016/j.rmed.2007.05.010. [DOI] [PubMed] [Google Scholar]
- 21.Al-Samri MT, Benedetti A, Préfontaine D, et al. Variability of sputum inflammatory cells in asthmatic patients receiving corticosteroid therapy: A prospective study using multiple samples. J Allergy Clin Immunol. 2010;125:1161–3. e4. doi: 10.1016/j.jaci.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 22.van Veen IH, Ten Brinke A, Gauw SA, Sterk PJ, Rabe KF, Bel EH. Consistency of sputum eosinophilia in difficult-to-treat asthma: A 5-year follow-up study. J Allergy Clin Immunol. 2009;124:615–7. doi: 10.1016/j.jaci.2009.06.029. [DOI] [PubMed] [Google Scholar]
- 23.Nair P, Pizzichini MM, Kjarsgaard M, et al. Mepolizumab for prednisone-dependent asthma with sputum eosinophilia. N Engl J Med. 2009;360:985–93. doi: 10.1056/NEJMoa0805435. [DOI] [PubMed] [Google Scholar]