Abstract
Extracellular matrix accumulation contributes to the progression of chronic kidney disease. Many growth factors including insulin-like growth factor-I (IGF-I) enhance matrix protein accumulation. Proximal tubular epithelial cells (PTCs) synthesize matrix proteins. NADPH oxidases are major sources of reactive oxygen species (ROS), important signaling molecules that mediate biological responses in a variety of cells and tissue. We investigated the mechanism by which IGF-I regulates fibronectin accumulation in PTCs and the role of a potential redox-dependent signaling pathway. IGF-I induces an increase in NADPH-dependent superoxide generation, enhances the release of hydrogen peroxide, and increases the expression of NADPH oxidase 4 (Nox4) in PTCs. IGF-I also stimulates phosphorylation of Akt, and inhibition of Akt or its upstream activator phosphatidylinositol 3-kinase attenuates IGF-I-induced fibronectin accumulation. Expression of dominant negative Akt also inhibits IGF-I-induced expression of fibronectin, indicating a role for this kinase in fibronectin accumulation. Expression of dominant negative adenovirus Nox4 inhibits IGF-I-induced NADPH oxidase activity, Akt phosphorylation, and fibronectin protein expression. Moreover, transfection of small interfering RNA targeting Nox4 decreases Nox4 protein expression and blocks IGF-I-induced Akt phosphorylation and the increase in fibronectin, placing Nox4 and ROS upstream of Akt signaling pathway. To confirm the role of Nox4, PTCs were infected with adenovirus construct expressing wild-type Nox4. Ad-Nox4, but not control Ad-green fluorescent protein, upregulated Nox4 expression and increased NADPH oxidase activity as well as fibronectin expression. Taken together, these results provide the first evidence for a role of Nox4 in IGF-I-induced Akt phosphorylation and fibronectin expression in tubular epithelial cells.
Keywords: extracellular matrix, growth factor signaling, kidney, reactive oxygen species, redox signaling, NADPH oxidase 4, insulin-like growth factor
kidney diseases that eventuate in fibrosis of the tubular/interstitial compartment are characterized by increased expression and release of growth factors and cytokines (1, 2, 3). Glucose or transforming growth factor-β (TGF-β) stimulates proximal tubular epithelial cells (PTCs), resulting in an increased production of matrix proteins including collagens and fibronectin (FN) (36, 37, 43). Insulin-like growth factor-I (IGF-I) and IGF-I receptor are upregulated in the kidney in disease states, and in particular in diabetes (19, 20, 33, 40). Moreover, the increased levels of IGF-I in the tubular fluid of animals with proteinuria enhances collagen production in cultured proximal tubular epithelial cells (32). The IGF-I receptor is a ligand-activated tyrosine kinase that signals through phosphatidylinositol 3-kinase (PI3K)/Akt as well as ERK1/ERK2 MAPK-dependent pathways to elicit its cellular responses (26, 34, 42, 46). Proximal tubular epithelial cells express the IGF-I receptor and IGF-I activates PI3K and enhances the phosphorylation of Akt to induce protein synthesis and hypertrophy of proximal tubular epithelial cells (46). Whether IGF-I enhances fibronectin accumulation and the mechanism by which IGF-I enhances matrix protein accumulation in proximal tubular cells have not been well defined.
Reactive oxygen species (ROS) have been implicated in growth factor-induced signaling pathways through regulation of the redox state of the target cells (5, 6, 10). In the kidney, ROS are primarily produced by NADPH oxidases of the Nox family (6, 9, 11, 25, 45). There is a wide array of evidence that Nox-derived ROS modulate vascular and renal cell function, including extracellular matrix protein synthesis, in response to G protein-coupled as well as ligand-activated tyrosine kinase receptors (5, 6, 8, 10, 11).
In this study, we explored the effect of IGF-I on fibronectin accumulation and the potential role of Akt and ROS generated by NADPH oxidases in mediating the effect of IGF-I. We demonstrate that IGF-I activates the NADPH oxidase-dependent ROS generation and rapidly upregulates the Nox4 isoform, resulting in Akt phosphorylation. Nox4-dependent ROS production and Akt phosphorylation lead to accumulation of the extracellular matrix protein, fibronectin, in proximal tubular epithelial cells. Our data also suggest that both transcriptional and translational mechanisms are implicated in the regulation of fibronectin by IGF-I in renal proximal tubular cells. Thus, alteration of the redox state in pathological conditions in which IGF-I is implicated (such as diabetic kidney disease) may contribute to matrix accumulation.
MATERIALS AND METHODS
Cell culture.
Rat PTCs were maintained in Dulbecco's modified Eagle's medium containing 5 mM glucose (DMEM) plus 10% fetal bovine serum in a 5% CO2 atmosphere at 37°C. In some experiments, the data were confirmed in primary cultures of rat tubular epithelial cells and in immortalized mouse (MCTs) and human proximal tubular epithelial cell line (HK2). Cells were used between passages 6 and 15. Before addition of agents, the medium was changed to DMEM plus 0.1% FBS and cultured for 24 to 48 h. IGF-I was used at a concentration of 250 ng/ml. Note that this concentration corresponds to circulating levels of IGF-I (21) as described previously (26).
Quantitative real-time PCR analysis.
Quantitative real-time PCR analysis (qRT-PCR) was performed as previously described (17, 18). PTCs were grown to 90–95% confluency in 60-mm dishes and were serum starved for 24 h. Cells were then treated with IGF-I (250 ng/ml) for 12, 24, and 48 h, respectively. After cells were washed twice with PBS, RNA was extracted using RNAzole bee method. Real-time PCR gene expression analysis (RT2qPCR primer assay kit, SA Biosciences, Frederick, MD) was performed for fibronectin mRNA using the ΔΔCt method (where Ct is threshold cycle number). Fibronectin mRNA expression was quantified using a Realplex mastercycler (Eppendorf, Westbury, NY) with SYBR green dye and RT2qPCR primers (SA Biosciences). Fibronectin mRNA was normalized to GAPDH mRNA levels.
Fibronectin promoter activity.
A reporter plasmid consisting of the fibronectin promoter adjacent to a firefly luciferase reporter gene was used to determine the transcriptional activity of the fibronectin promoter in PTCs. Cells were grown in 12-well plates to 60%-70% confluence. Plasmids were then transfected using GeneJuice (Novagen, Madison, WI). Before transfection, cells were washed twice with PBS and media were replaced with 1 ml of OPTI-MEM I (Invitrogen). Precomplex of the DNA with GeneJuice in Opti-MEM was mixed and incubated at room temperature for 15 min. GeneJuice was added to the complex of DNA and Plus reagent and incubated for 15 min at room temperature. DNA with GeneJuice complexes was added to each well and incubated at 37°C with 5% CO2. After incubation for 6 h, media were replaced to complete media. After 24 h of transfection, cells were incubated in serum-free media for 24 h followed by treatment with IGF-I for 12, 24, and 48 h time points. Cells were harvested, washed twice with PBS, and lysed in 0.1 ml of lysis buffer. Luciferase activity was determined using the Luciferase Reporter Assay System by a luminometer according to the manufacturer's instructions (Promega, Madison, WI) and normalized by protein content.
NADPH oxidase assay.
NADPH oxidase activity was measured by the lucigenin-enhanced chemiluminescence method as described previously (24, 25). Briefly, cultured cells were homogenized in lysis buffer (20 mM KH2PO4, pH 7.0, 1 mM EGTA, 1 mM phenylmethylsulfonyl fluoride, 10 μg/ml aprotinin, and 0.5 μg/ml leupeptin) using a Dounce homogenizer (100 strokes on ice). To start the assay, 100-μl aliquots of homogenates were added to 900 μl of 50 mM phosphate buffer, pH 7.0, containing 1 mM EGTA, 150 mM sucrose, 5 μM lucigenin, and 100 μM NADPH. Photon emission in terms of relative light units was measured in a luminometer every 30 s for 5 min. There was no measurable activity in the absence of NADPH. Superoxide anion production was expressed as relative chemiluminescence (light) units (RLU)/mg protein. Protein content was measured using the Bio-Rad protein assay reagent. Means ± SE were calculated for each set of samples.
Hydrogen peroxide detection.
Extracellular hydrogen peroxide (H2O2) was detected using the Amplex Red Assay Kit as previously described (9, 28) and following the manufacturer's instructions.
Western blot analysis.
For immunoblotting, proteins were separated using SDS-PAGE and transferred to nitrocellulose membranes. The membranes were blocked with 5% low-fat milk in TBS-Tween and then incubated with a rabbit polyclonal Nox4 antibody (7, 9, 10, 25), a rabbit polyclonal anti-fibronectin antibody (1:1,000; catalog no. F3648, Sigma), or a mouse monoclonal anti-GAPDH (1:2,500; Sigma). Phospho-antibodies [Akt Ser473, 4E-BP1 Thr37/46, and S6 kinase (S6K) Thr389], and their unphosphorylated counterparts were obtained from Cell Signaling Technologies and used at a dilution of 1:1,000. The appropriate horseradish peroxidase-conjugated secondary antibodies were added, and bands were visualized by enhanced chemiluminescence. Densitometric analysis was performed using ImageJ software (National Institutes of Health, Bethesda, MD).
Small interfering RNA knockdown.
For the RNA interference experiments, a SMART-pool consisting of four short or small interfering RNA (siRNA) duplexes specific for rat Nox4 was obtained from Dharmacon. The SMARTpool of siRNAs was introduced into the cells by transfection using Oligofectamine as described (8, 9, 18). Scrambled (nontargeting) siRNAs served as controls to validate the specificity of the siRNAs. Scrambled control and siRNA for Nox4 were used at a concentration of 400 nM.
Adenovirus infection.
Cells were grown to 80% confluency and infected with an adenovirus expressing either dominant negative (DN) Akt or green fluorescent protein (GFP) vector as control, full-length active wild-type Nox4 (Ad-Nox4), or truncated dominant negative Nox4 (Ad-Nox4ΔFAD/ΔNAD) as described previously (7, 8, 9, 18, 35). Expression was determined by Western blotting and was maximal at 48 h postinfection. Ad-Nox4 and Ad-Nox4ΔFAD/ΔNAD were a generous gift from Dr. B. Goldstein (Merck).
Statistical analyses.
Quantitative data are presented as means ± SE for at least three experiments. Statistical analysis was based on Student's t-test for comparison of two groups and one-way analysis of variance for multiple group comparisons. P < 0.05 was used to indicate statistical significance.
RESULTS
IGF-I enhances Nox4-dependent ROS generation.
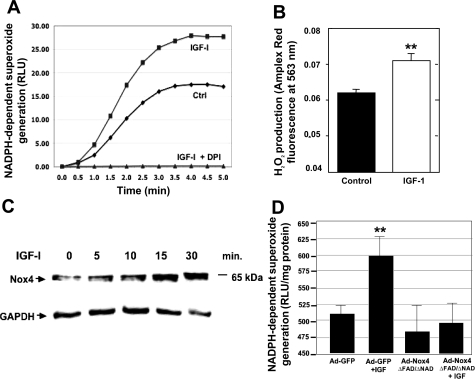
To determine the effect of IGF-I on ROS generation, we measured NADPH-dependent superoxide generation (NADPH oxidase activity). Treatment of PTCs with IGF-I results in enhanced superoxide generation compared with cells treated with buffer alone (Fig. 1A). Preincubation of the cells with diphenyleneiodonium (DPI), a compound that inhibits flavin-containing oxidases, abrogated IGF-I-induced superoxide generation (Fig. 1A). Dismutation of superoxide (O2·−), spontaneously or enzymatically, by superoxide dismutase (SOD) produces H2O2. Using Amplex Red as a detector of H2O2, we find that the production of H2O2 was also enhanced in the presence of IGF-I (Fig. 1B). Taken together, these data suggest that IGF-I-induced ROS generation is most likely mediated by NADPH oxidases. Given that the Nox catalytic subunit Nox4 is highly expressed in the kidney, we examined the effects of IGF-I on Nox4 protein expression. Cells were incubated with IGF-I, and Nox4 expression was determined by Western blotting. IGF-I increases Nox4 protein expression in a time-dependent manner as early as 5 min of treatment and is sustained for at least 30 min (Fig. 1C), indicating a role for Nox4 in IGF-I-induced ROS generation. To confirm the role of Nox4 in IGF-I-mediated ROS generation, an adenovirus dominant-negative (DN) form of Nox4, which lacks the FAD and heme-binding domains (Ad-Nox4 ΔFAD/ΔNAD), was utilized. Infection of the cells with Ad-DN-Nox4, but not Ad-GFP control, effectively blocked IGF-I-induced NADPH oxidase activity (Fig. 1D). Together, these findings indicate that IGF-I upregulates Nox4 protein expression and that Nox4 mediates IGF-I-induced ROS generation in PTCs.
Fig. 1.
A: Insulin-like growth factor-I (IGF-I; 250 ng/ml) induces the NADPH-dependent production of superoxide in primary isolated rat tubular epithelial cells (PTCs). This effect is strongly inhibited by diphenyleneiodonium (DPI; 10 nM). NADPH-dependent superoxide production was measured using the lucigenin-based chemiluminescence assay and values are expressed as relative light units (RLU). Ctrl, control. B: exposure of rat PTCs to 250 ng/ml IGF-I for 1 h enhanced hydrogen peroxide production. Hydrogen peroxide was assessed using Amplex Red method. C: IGF-I rapidly increases NADPH oxidase (Nox) 4 protein expression in primary isolated rat PTCs. D: Nox4 is required for IGF-I-induced reactive oxygen species production. Rat PTCs were infected with either an adenovirus expressing dominant negative Nox4 (Ad-Nox4 ΔFAD/ΔNAD) or Ad-green fluorescent protein (GFP) as a control. After 48 h, cells were then treated with IGF-I (250 ng/ml) for 10 min and then assayed for NADPH-dependent superoxide production using the lucigenin-based chemiluminescence assay. Values are means ± SE (n = 3); **P < 0.01.
IGF-I increases fibronectin expression through a PI3K- and Akt-dependent mechanism.
We and others have previously shown that IGF-I activates PI3K and enhances Akt phosphorylation in proximal tubular epithelial cells and that this pathway mediates protein synthesis and cell hypertrophy (46). However, the effect of IGF-I on fibronectin (FN) expression and the role of ROS in mediating the enhancing effect of IGF-I on matrix protein expression have not been investigated. To identify putative Nox4-dependent redox-sensitive signaling pathways activated by IGF-I, PTCs were treated with IGF-I. As shown in Fig. 2A, IGF-I induced phosphorylation of Akt as early as 5 min, an effect that was sustained for up to 60 min. There was no change in total Akt protein expression. Similar findings were observed in mouse proximal tubular epithelial cells (data not shown). To determine whether IGF-I induces fibronectin accumulation through a PI3K/Akt-dependent mechanism, we used pharmacologic inhibitors of PI3K or Akt. Treatment of PTCs with IGF-I increased fibronectin accumulation, which was reduced when cells were preincubated with the PI3K inhibitor LY294002 or the Akt inhibitor Akt-X (Fig. 2B). The role of Akt in IGF-I-mediated FN expression was further examined by infecting the cells with an adenovirus vector expressing a dominant-negative mutant of Akt (Ad-DN-Akt). GFP adenovirus was used as a control. Infection with Ad-DN-Akt prevented IGF-I-induced FN expression compared with infection with Ad-GFP alone (Fig. 2C), further implicating Akt phosphorylation in IGF-I-induced FN expression.
Fig. 2.
A: IGF-I rapidly induces Akt phosphorylation and activation. Akt is phosphorylated (p-Akt) on serine 473 within 5 min in response to treatment with 250 ng/ml IGF-I. Western blot analysis was performed on 30 μg of total cellular lysates prepared from primary isolated rat PTCs. B: IGF-I (250 ng/ml)-induced fibronectin (FN) expression is inhibited by chemical inhibitors of phosphatidylinositol 3-kinase (PI3K) (LY294002, 20 μM) or Akt (Akt inhibitor X, 2.5 μM). C: dominant negative Akt adenovirus (Ad-DN-Akt) abolishes IGF-I (250 ng/ml)-induced expression of fibronectin in PTCs. Adenovirus encoding GFP (Ad-GFP) was used as a control. Histograms represent the intensity of the fibronectin expression bands to the GAPDH band quantified by densitometry. Values are means ± SE (n = 3); increased **P < 0.01, or reduced compared with IGF-I induced, #P < 0.05.
Nox4 regulates IGF-I induced Akt phosphorylation in proximal tubular cells.
Because IGF-I increases Nox4 protein expression and enhances NADPH-dependent ROS generation concomitant with Akt phosphorylation, we investigated the involvement of Nox4 in IGF-I-induced Akt phosphorylation. PTCs were infected with dominant-negative Nox4 (Ad-Nox4 ΔFAD/ΔNAD) or Ad-GFP control. Following stimulation with IGF-I, phosphorylation of Akt was examined by Western blot analysis. Infection of the cells with Ad-DN-Nox4 blocked IGF-I-stimulated phosphorylation of Akt compared with GFP control (Fig. 3A). To further define the role for Nox4 in IGF-I-induced Akt phosphorylation, we employed a Nox4 knockdown strategy. Cells transfected with siRNA Nox4 (siNox4) but not scrambled control (Scr) exhibited reduced phosphorylation of Akt in the presence of IGF-I (Fig. 3B). Knockdown of Nox4 protein expression was confirmed by Western blot analysis.
Fig. 3.
Nox4 is required for IGF-I-induced Akt phosphorylation. A: PTCs were infected with either an adenovirus expressing dominant negative Nox4 (Ad-Nox4 ΔFAD/ΔNAD) or Ad-GFP as a control. After 48 h, cells were then treated with IGF-I (250 ng/ml) for 10 min. Dominant negative Nox4 adenovirus abolished IGF-I-induced Akt phosphorylation at serine 473 as assessed by Western blot analysis. B: small interfering RNA (siRNA) against Nox4 results in a significant reduction of Nox4 protein levels (middle) and a concomitant reduction in IGF-I-induced Akt phosphorylation (top). Scr, scrambled siRNA. Histograms (bottom) represent the intensity of the p-Akt bands to the total Akt or GAPDH, respectively, and values are expressed as a percentage of control.
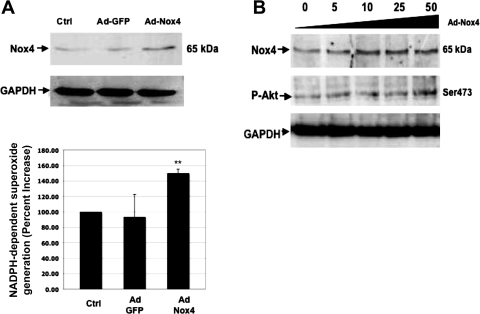
To confirm our hypothesis that Nox4-derived ROS are involved in the phosphorylation and activation of Akt, we used an adenoviral vector, which expresses full-length and active wild-type Nox4 (Ad-Nox4). Infection of PTCs with Ad-Nox4 increased the expression of Nox4 protein and enhanced NADPH-dependent superoxide generation compared with Ad-GFP-infected or noninfected control cells (Fig. 4A, top and bottom). To examine the role of Nox4 in Akt phosphorylation, PTCs were infected with increasing concentrations of full-length Ad-Nox4. Immunoblotting of the cell lysates shows a dose-dependent increase in Nox4 protein expression, which correlates with the increase in Akt phosphorylation (Fig. 4B). Taken together, the data indicate a role for Nox4 protein and ROS generation in the phosphorylation of Akt in PTCs.
Fig. 4.
Wild-type Nox4 overexpression results in increased NADPH-dependent superoxide production and phosphorylation of Akt. A: rat PTCs were infected with a full-length active wild-type Nox4 adenovirus (Ad-Nox4), and after 48 h, the lysates were assayed for Nox4 protein expression (A, top) and NADPH-dependent superoxide production (A, bottom). B: a titration of the wild-type Nox4 adenovirus shows a dose-dependent increase in Nox4 protein production concomitant with increased phosphorylation of Akt at serine 473. Ad-GFP was used as a control. Values are means ± SE; **P < 0.01.
Nox4 regulates IGF-I-induced fibronectin expression in proximal tubular cells.
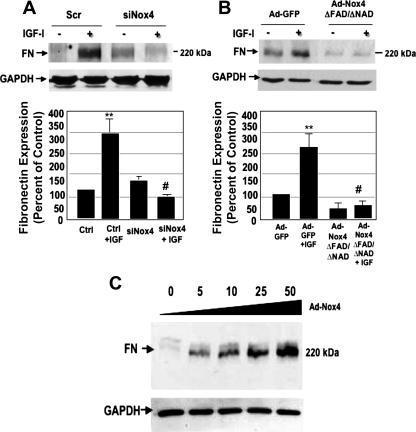
We next examined the direct contribution of Nox4 to FN accumulation in PTCs incubated with IGF-I. RNAi-mediated knockdown of Nox4 (siNox4) but not scrambled control blocked FN expression (Fig. 5A). Additionally, overexpression of adenoviral dominant-negative Nox4 (Nox4 ΔFAD/ΔNAD) but not GFP adenovirus control blocked IGF-I-induced FN expression (Fig. 5B). Conversely, titration of adenoviral wild-type Nox4 (Ad-Nox4) induces FN expression in a dose-dependent manner (Fig. 5C). Taken together, these data indicate that Nox4 is necessary and sufficient for IGF-I-induced FN accumulation.
Fig. 5.
Nox4-derived reactive oxygen species are required for IGF-I-induced Nox4 expression of fibronectin in PTCs. A: siRNA against Nox4 effectively abrogates the expression of fibronectin at 48 h post-IGF-I treatment relative to control (Scr) siRNA. B: infection of PTCs with an adenovirus expressing dominant negative Nox4 (Ad-Nox4 ΔFAD/ΔNAD) inhibits IGF-I-induced fibronectin expression. C: overexpression of full-length wild-type Nox4 adenovirus (Ad-Nox4) increases fibronectin expression in a dose-dependent manner. Histograms (bottom) represent the intensity of the fibronectin expression to GAPDH quantified by densitometry. Values are means ± SE (n = 3); increased **P < 0.01, or reduced compared with IGF-I induced, #P < 0.05.
Regulation of fibronectin expression by IGF-I is mediated through transcriptional and translational mechanisms.
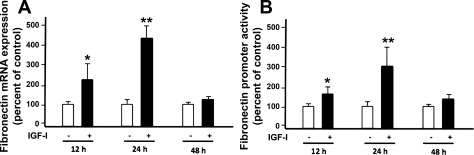
The effect of IGF-I on FN mRNA expression was assessed by qRT-PCR. We show that, in PTCs, IGF-I enhances the expression of fibronectin mRNA (Fig. 6A), peaking at 24 h posttreatment and returning to basal levels by 48 h. This indicates a potential role for transcription in the regulation of IGF-I-induced fibronectin expression. To confirm this, we transfected PTCs with a plasmid construct containing a minimal fibronectin promoter adjacent to the firefly luciferase gene or with the vector only as a control. We found that IGF-I causes an increase in luminescence, indicating enhanced FN promoter activity (Fig. 6B). It should be noted that increased FN promoter activity is concomitant with the increase in FN mRNA expression.
Fig. 6.
IGF-I increases fibronectin mRNA expression and fibronectin promoter activity at similar time points. A: PTCs were serum deprived for 24 h and then treated with IGF-I (250 ng/ml) for 12, 24, and 48 h, respectively. RNA was extracted using RNAzole bee method. RT-PCR was performed for fibronectin mRNA using ΔΔCt method. Fibronectin mRNA expression was quantified with SYBR green dye and RT2qPCR primers. Data represent the relative induction of fibronectin mRNA to GAPDH mRNA levels. Data are means and SE of three experiments. B: the reporter plasmid containing fibronectin promoter that drives the expression of the luciferase gene was transfected into the PTCs using GeneJuice. After 24 h of transfection, cells were incubated with serum-free media for 24 h followed by treatment with IGF-I (250 ng/ml) for indicated time points. Luciferase activity was determined and normalized by protein content. pCDNA3 was used as a control vector. Data are means and SE of three experiments. Data demonstrate that IGF-I significantly induces the fibronectin promoter activity at 12 and 24 h. Results are expressed as means ± SE. *P < 0.05 and **P < 0.01 compared with uninduced timed control.
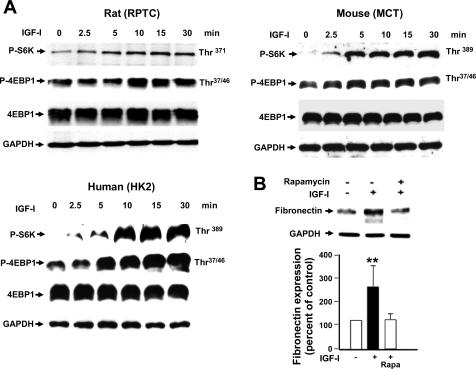
Extracellular matrix protein expression may also be regulated by translational mechanism (36, 43), and we sought to determine whether mammalian target of rapamycin (mTOR) translational pathway plays a role in IGF-I-induced fibronectin expression. The ribosomal S6 kinase (S6K) and the cap-dependent eukaryotic initiation factor binding protein 4E-BP1 are both downstream effectors of mTOR signaling. Our data indicate that IGF-I increases the phosphorylation of mTOR substrates S6K and 4E-BP1 in a time-dependent manner (Fig. 7A). This observation was confirmed in rat PTCs (Fig. 7A, top left), mouse PTCs (top right), and human PTCs (bottom). Furthermore, pretreatment of rat PTCs with the specific mTOR inhibitor rapamycin abrogated IGF-I-induced fibronectin expression (Fig. 7B). These results suggest that regulation of FN by IGF-I also requires the activation of mTOR translational pathway.
Fig. 7.
IGF-I activates the mammalian target of rapamycin (mTOR) pathway leading to fibronectin expression. A: mTOR substrates S6 kinase (S6K) and eukaryotic initiation factor binding protein 4E-BP1 are phosphorylated within 5 min in response to treatment with 250 ng/ml IGF-I. 30 μg of RIPA lysates were separated by SDS-PAGE and probed in rat, mouse, and human proximal tubular epithelial cells, as described in materials and methods. B: IGF-I-induced fibronectin expression is inhibited by a selective inhibitor of mTOR [25 nM rapamycin (Rapa)] in rat PTCs. Histogram represents the intensity of the fibronectin expression to GAPDH quantified by densitometry. Values are means ± SE (n = 3); **P < 0.01.
DISCUSSION
In this study, we provide strong evidence that in proximal tubular epithelial cells, IGF-I enhances fibronectin accumulation through ROS generated by the NADPH oxidase isoform Nox4 and enhanced phosphorylation of Akt. The role of Nox4 in the enhanced Akt phosphorylation and fibronectin accumulation was documented by the following observations. First, IGF-I enhances NADPH oxidase-dependent ROS generation and upregulates Nox4. In cells treated with IGF-I, inhibition of Nox4 by dominant negative Nox4 or siRNA targeting Nox4 inhibits NADPH oxidase activity and Akt phosphorylation and decreases fibronectin expression. Moreover, overexpression of Nox4 increases Akt phosphorylation and fibronectin accumulation. Collectively, the data indicate that Nox4 is an important mediator of the effect of IGF-I to enhance Akt phosphorylation and fibronectin accumulation in proximal tubular epithelial cells.
The growth hormone/IGF-I axis contributes to the pathophysiological manifestations of renal disease (19, 20, 33, 46), and proximal tubular epithelial cells are important targets that release matrix proteins such as laminins, collagens, and fibronectin (33, 36, 44). Increased filtration of circulating IGF-I in proteinuric diseases and its upregulation in diabetic nephropathy increases the local concentration of IGF-I in the proximal tubules and contributes to fibrosis (41). The cellular mechanisms by which IGF-I exerts its biological activities are not completely defined. IGF-I signals through multiple signaling pathways to exert specific biological effects including protein synthesis, cell migration and proliferation, and matrix accumulation in various cells including renal cells (26, 38, 44, 46, 47). In proximal tubular epithelial cells, IGF-I induces cell hypertrophy and protein synthesis at least partially through a PI3K-dependent mechanism (46). Here we find that IGF-I induces the accumulation of the extracellular matrix protein fibronectin in a PI3K/Akt-dependent manner. This is in contrast to the finding in mesangial cells where IGF-I enhances fibronectin accumulation in a calcineurin-dependent mechanism and independent of Akt or ERK (26). This was supported by the finding that inhibition of calcineurin by the administration of cyclosporine A reduced fibronectin accumulation in the glomeruli but not in cortical tissue in a rodent model of diabetes (24). In human lens epithelial cells, for example, IGF-I counteracts the effect of TGF-β on fibronectin expression (14), whereas in vascular smooth muscle cells, IGF-I increases the expression of fibronectin (47). It is likely that IGF-I regulates matrix protein expression in a cell type-specific manner. In proximal tubular epithelial cells, we find that IGF-I increases fibronectin expression through phosphorylation of Akt since inhibition of Akt by pharmacological or genetic means prevents the effect of IGF-I. It should be noted that the regulation of extracellular matrix by IGF-I in cells such as glomerular mesangial cells or vascular smooth muscle cells has been well investigated. Only a few reports describe the profibrotic action of the growth factor in renal epithelial cells.
Reactive oxygen species, including those derived from NADPH oxidases of the Nox family, have been implicated in receptor tyrosine kinase signaling including signals activated by IGF-I (16, 29, 38, 48). In the kidney, NADPH oxidases of the Nox family and specifically the isoform Nox4 are major sources of superoxide anion and H2O2 (5, 6, 9, 23, 25). Although the role of Nox4 as a major source of ROS in the kidney is known (9, 25), the present study provides the first evidence of the importance of this oxidase in the propagation of IGF-I redox signaling in tubular epithelial cells. Our observation that IGF-I elicits an increase in Nox4 protein expression is important since, in contrast to other enzymes of the Nox family, Nox4 is constitutively active (5, 6, 39). Therefore, an increase in the expression of the catalytic unit itself is translated to an increase in ROS generation. The regulation of Nox4 activity through control of its expression levels was previously reported in vascular and renal cells upon stimulation by various agonists such as angiotensin II (8), TGF-β (10), and high-glucose environment (9). These observations are consistent with a previous report showing that IGF-I regulates Nox4 protein expression without change in its mRNA levels in cardiac cells (38). Our data suggest that IGF-I acutely modulates oxidative stress in PTCs via alteration of Nox4 levels. Our studies demonstrating that inhibition of Nox4 by siRNA and the dominant negative Nox4 adenovirus abrogate both Akt phosphorylation and fibronectin accumulation places this oxidase upstream of Akt phosphorylation. The observation that overexpression of Nox4 enhances fibronectin accumulation indicates that this oxidase is essential for the accumulation of this matrix protein. The present work identifies for the first time Nox4 as a proximal activator of Akt in the redox pathway linking IGF-I receptor to fibronectin accumulation in renal tubular epithelial cells. Although it is known that Nox4 is present in epithelial cells including kidney epithelium (12, 22, 49), its role has not been well characterized in these cells. To our knowledge, apart from one report (45), no data exist regarding the function of Nox4 and the redox signaling pathways engaged by the oxidase in renal epithelial cells.
The proximal tubular epithelium is an important contributor to interstitial fibrosis, which ultimately leads to kidney failure. Interstitial fibrosis is characterized in part by the accumulation of matrix proteins such as collagens and fibronectin in the renal tubulo-interstitial compartment. Fibronectin is typically the first protein to appear in an interstitial scar and serves as both a recruiter of fibroblasts and a scaffold for the deposition of other matrix proteins. Our results support the concept that Nox4-dependent oxidative stress is involved in fibrotic processes as it was proposed in lung (12, 31), heart (4), or kidney (25) fibrosis. The present work provides additional rational for the consideration of Nox4 as a primary target for the design of novel therapeutic intervention in fibrotic disease in general and kidney fibrosis in particular.
The mechanism by which IGF-I acts through Nox4 to enhance FN accumulation remains to be determined. We have evidence suggesting that IGF-I may regulate fibronectin expression through both translational as well as transcriptional pathways. On one hand, we show that IGF-I regulates FN mRNA levels and FN promoter activity, indicating that transcriptional mechanisms are implicated in the action of the growth factor. On the other hand, the fact that IGF-I activates the mTOR translational pathway (as evidenced by enhanced 4E-BP1 and S6K phosphorylation) together with the finding that the mTOR inhibitor rapamycin prevents IGF-I-induced FN expression demonstrate that translational mechanisms also play a key role in the control of FN accumulation. This is consistent with our observation that PI3K and Akt, two known upstream activators of mTOR, are implicated in the modulation of FN expression in PTCs.
In conclusion, we describe for the first time that Nox4 is upregulated in response to IGF-I and mediates IGF-I-induced FN expression through an Akt-dependent mechanism in proximal tubular epithelial cells. Collectively, the data support the idea that oxidative stress contributes to matrix accumulation in diseases characterized by upregulation of IGF-I or its receptor such as diabetic kidney disease. Importantly, our observations also suggest that mTOR inhibition together with antioxidants targeting Nox4 may represent a potential therapy to reduce fibrosis in kidney disease. IGF-I treatment was reported to ameliorate diabetic neuropathy in animals (50, 51). However, our data showing that IGF-I contributes to renal cell injury suggest that therapeutic interventions proposing IGF-I administration in diabetes should be taken with caution. This is supported by the fact that, similar to the kidney, IGF-I has been shown to be deleterious in diabetic retinopathy (14, 30). Therefore, pathways that are commonly implicated in diabetes complications such as Nox4 and mTOR signaling may represent a more appropriate target for the development of therapeutic interventions.
GRANTS
Support for these studies was provided by the following sources: Veterans Administration Merit Review grant and National Institutes of Health Grant DK-R01-078971 (H. E. Abboud) and Career Development Award and National Institutes of Health Grant CA131272 (K. Block), Juvenile Diabetes Research Foundation regular research grant, and National Institutes of Health Grant DK-079996 (Y. Gorin).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: D.N. and B.B. performed experiments; D.N. analyzed data; D.N. and H.E.A. interpreted results of experiments; D.N. and B.B. prepared figures; D.N. drafted manuscript; K.B., Y.C.G., and H.E.A. conception and design of research; K.B., Y.C.G., and H.E.A. edited and revised manuscript; H.E.A. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Andrea Barrentine and Sergio Garcia for technical assistance.
REFERENCES
- 1. Abboud HE. Growth factors and the mesangium. J Am Soc Nephrol 2: S185–S189, 1992 [DOI] [PubMed] [Google Scholar]
- 2. Abboud HE. Growth factors in glomerulonephritis. Kidney Int 43: 252–267, 1993 [DOI] [PubMed] [Google Scholar]
- 3. Abboud HE. Growth factors and diabetic nephrology: an overview. Kidney Int Suppl 60: S3–S6, 1997 [PubMed] [Google Scholar]
- 4. Ago T, Kuroda J, Pain J, Fu C, Li H, Sadoshima J. Upregulation of Nox4 by hypertrophic stimuli promotes apoptosis and mitochondrial dysfunction in cardiac myocytes. Circ Res 106: 1253–1264, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Barnes JL, Gorin Y. Myofibroblast differentiation during fibrosis: role of NAD(P)H oxidases. Kidney Int 79: 944–956, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bedard K, Krause KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev 87: 245–313, 2007 [DOI] [PubMed] [Google Scholar]
- 7. Block K, Gorin Y, Hoover P, Williams P, Chelmicki T, Clark RA, Yoneda T, Abboud HE. NAD(P)H oxidases regulate HIF-2alpha protein expression. J Biol Chem 282: 8019–8026, 2007 [DOI] [PubMed] [Google Scholar]
- 8. Block K, Eid A, Griendling KK, Lee DY, Wittrant Y, Gorin Y. Nox4 NAD(P)H oxidase mediates Src-dependent tyrosine phosphorylation of PDK-1 in response to angiotensin II: role in mesangial cell hypertrophy and fibronectin expression. J Biol Chem 283: 24061–24076, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Block K, Gorin Y, Abboud HE. Subcellular localization of Nox4 and regulation in diabetes. Proc Natl Acad Sci USA 106: 14385–14390, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bondi CD, Manickam N, Lee DY, Block K, Gorin Y, Abboud HE, Barnes JL. NAD(P)H oxidase mediates TGF-beta1-induced activation of kidney myofibroblasts. J Am Soc Nephrol 21: 93–102, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Brown DI, Griendling KK. Nox proteins in signal transduction. Free Radic Biol Med 47: 1239–1253, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Carnesecchi S, Deffert C, Donati Y, Basset O, Hinz B, Preynat-Seauve O, Guichard C, Arbiser JL, Banfi B, Pache JC, Barazzone C, Krause KH. A key role for NOX4 in epithelial cell death during development of lung fibrosis. Antioxid Redox Signal 15: 607–619, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chantelau E, Meyer-Schwickerath R, Klabe K. Downregulation of serum IGF-1 for treatment of early worsening of diabetic retinopathy: a long-term follow-up of two cases. Ophthalmologica 224: 243–246, 2010 [DOI] [PubMed] [Google Scholar]
- 14. Chung SH, Jung SA, Cho YJ, Lee JH, Kim EK. IGF-1 counteracts TGF-beta-mediated enhancement of fibronectin for in vitro human lens epithelial cells. Yonsei Med J 48: 949–954, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Clemmons D, Maile L, Xi G, Shen X, Radhakrishnan Y. Igf-I signaling in response to hyperglycemia and the development of diabetic complications. Curr Diabetes Rev 7: 235–245, 2011 [DOI] [PubMed] [Google Scholar]
- 16. Edderkaoui M, Nitsche C, Zheng L, Pandol SJ, Gukovsky I, Gukovskaya AS. NADPH oxidase activation in pancreatic cancer cells is mediated through Akt-dependent up-regulation of p22phox. J Biol Chem 286: 7779–7787, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Eid AA, Gorin Y, Fagg BM, Maalouf R, Barnes JL, Block K, Abboud HE. Mechanisms of podocyte injury in diabetes: role of cytochrome P450 and NADPH oxidases. Diabetes 58: 1201–1211, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Eid AA, Ford BM, Block K, Kasinath BS, Gorin Y, Ghosh-Choudhury G, Barnes JL, Abboud HE. AMP-activated protein kinase (AMPK) negatively regulates Nox4-dependent activation of p53 and epithelial cell apoptosis in diabetes. J Biol Chem 285: 37503–37512, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. El Nahas AM, Sayed-Ahmed N. Insulin-like growth factor I and the kidney: friend or foe ? Exp Nephrol 1: 205–217, 1993 [PubMed] [Google Scholar]
- 20. Flyvbjerg A. Role of growth hormone, insulin-like growth factors (IGFs) and IGF-binding proteins in the renal complications of diabetes. Kidney Int Suppl 60: S12–S19, 1997 [PubMed] [Google Scholar]
- 21. Friedrich N, Alte D, Völzke H, Spilcke-Liss E, Lüdemann J, Lerch MM, Kohlmann T, Nauck M, Wallaschofski H. Reference ranges of serum IGF-1 and IGFBP-3 levels in a general adult population: results of the Study of Health in Pomerania (SHIP). Growth Horm IGF Res 18: 228–237, 2008 [DOI] [PubMed] [Google Scholar]
- 22. Geiszt M, Kopp JB, Várnai P, Leto TL. Identification of renox, an NAD(P)H oxidase in kidney. Proc Natl Acad Sci USA 97: 8010–8014, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gill PS, Wilcox CS. NADPH oxidases in the kidney. Antioxid Redox Signal 8: 1597–1607, 2006 [DOI] [PubMed] [Google Scholar]
- 24. Gorin Y, Ricono JM, Kim NH, Bhandari B, Choudhury GG, Abboud HE. Nox4 mediates angiotensin II-induced activation of Akt/protein kinase B in mesangial cells. Am J Physiol Renal Physiol 285: F219–F229, 2003 [DOI] [PubMed] [Google Scholar]
- 25. Gorin Y, Block K, Hernandez J, Bhandari B, Wagner B, Barnes JL, Abboud HE. Nox4 NAD(P)H oxidase mediates hypertrophy and fibronectin expression in the diabetic kidney. J Biol Chem 280: 39616–39626, 2005 [DOI] [PubMed] [Google Scholar]
- 26. Gooch JL, Tang Y, Ricono JM, Abboud HE. Insulin-like growth factor-I induces renal cell hypertrophy via a calcineurin-dependent mechanism. J Biol Chem 276: 42492–42500, 2001 [DOI] [PubMed] [Google Scholar]
- 27. Gooch JL, Barnes JL, Garcia S, Abboud HE. Calcineurin is activated in diabetes and is required for glomerular hypertrophy and ECM accumulation. Am J Physiol Renal Physiol 284: F144–F154, 2003 [DOI] [PubMed] [Google Scholar]
- 28. Gooch JL, Gorin Y, Zhang BX, Abboud HE. Involvement of calcineurin in transforming growth factor-beta-mediated regulation of extracellular matrix accumulation. J Biol Chem 279: 15561–15570, 2004 [DOI] [PubMed] [Google Scholar]
- 29. Handayaningsih AE, Iguchi G, Fukuoka H, Nishizawa H, Takahashi M, Yamamoto M, Herningtyas EH, Okimura Y, Kaji H, Chihara K, Seino S, Takahashi Y. Reactive oxygen species play an essential role in IGF-I signaling and IGF-I-induced myocyte hypertrophy in C2C12 myocytes. Endocrinology 152: 912–921, 2011 [DOI] [PubMed] [Google Scholar]
- 30. Haurigot V, Villacampa P, Ribera A, Llombart C, Bosch A, Nacher V, Ramos D, Ayuso E, Segovia JC, Bueren JA, Ruberte J, Bosch F. Increased intraocular insulin-like growth factor-I triggers blood-retinal barrier breakdown. J Biol Chem 284: 22961–22969, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hecker L, Vittal R, Jones T, Jagirdar R, Luckhardt TR, Horowitz JC, Pennathur S, Martinez FJ, Thannickal VJ. NADPH oxidase-4 mediates myofibroblast activation and fibrogenic responses to lung injury. Nat Med 15: 1077–1081, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hirschberg R. Bioactivity of glomerular ultrafiltrate during heavy proteinuria may contribute to renal tubulo-interstitial lesions: evidence for a role for insulin-like growth factor I. J Clin Invest 98: 116–124, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lam S, van der Geest RN, Verhagen NA, van Nieuwenhoven FA, Blom IE, Aten J, Goldschmeding R, Daha MR, van Kooten C. Connective tissue growth factor and IGF-I are produced by human renal fibroblasts and cooperate in the induction of collagen production by high glucose. Diabetes 52: 2975–2983, 2003 [DOI] [PubMed] [Google Scholar]
- 34. Laviola L, Natalicchio A, Giorgino F. The IGF-I signaling pathway. Curr Pharm Des 13: 663–669, 2007 [DOI] [PubMed] [Google Scholar]
- 35. Mandal CC, Ganapathy S, Gorin Y, Mahadev K, Block K, Abboud HE, Harris SE, Ghosh-Choudhury G, Ghosh-Choudhury N. Reactive oxygen species derived from Nox4 mediate BMP2 gene transcription and osteoblast differentiation. Biochem J 433: 393–402, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Mariappan MM, Feliers D, Mummidi S, Choudhury GG, Kasinath BS. High glucose, high insulin, and their combination rapidly induce laminin-beta1 synthesis by regulation of mRNA translation in renal epithelial cells. Diabetes 56: 476–485, 2007 [DOI] [PubMed] [Google Scholar]
- 37. Mariappan MM, Shetty M, Sataranatarajan K, Choudhury GG, Kasinath BS. Glycogen synthase kinase 3 beta is a novel regulator of high glucose- and high insulin-induced extracellular matrix protein synthesis in renal proximal tubular epithelial cells. J Biol Chem 283: 30566–30575, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Meng D, Lv DD, Fang J. Insulin-like growth factor-I induces reactive oxygen species production and cell migration through Nox4 and Rac1 in vascular smooth muscle cells. Cardiovasc Res 80: 299–308, 2008 [DOI] [PubMed] [Google Scholar]
- 39. Nisimoto Y, Jackson HM, Ogawa H, Kawahara T, Lambeth JD. Constitutive NADPH-dependent electron transferase activity of the Nox4 dehydrogenase domain. Biochemistry 49: 2433–2442, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Rossert J, Terraz C, Dupont S. Regulation of type I collagen genes expression. Nephrol Dial Transplant 15, Suppl 6: 66–68, 2000 [DOI] [PubMed] [Google Scholar]
- 41. Rossert J, Terraz-Durasnel C, Brideau G. Growth factors, cytokines, and renal fibrosis during the course of diabetic nephropathy. Diabetes Metab 26, Suppl 4: 16–24, 2000 [PubMed] [Google Scholar]
- 42. Salminen A, Kaarniranta K. Insulin/IGF-1 paradox of aging: regulation via AKT/IKK/NF-kappaB signaling. Cell Signal 22: 573–577, 2010 [DOI] [PubMed] [Google Scholar]
- 43. Sataranatarajan K, Mariappan MM, Lee MJ, Feliers D, Choudhury GG, Barnes JL, Kasinath BS. Regulation of elongation phase of mRNA translation in diabetic nephropathy: amelioration by rapamycin. Am J Pathol 171: 1733–1742, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Schreiber BD, Hughes ML, Groggel GC. Insulin-like growth factor-1 stimulates production of mesangial cell matrix components. Clin Nephrol 43: 368–374, 1995 [PubMed] [Google Scholar]
- 45. Sedeek M, Callera G, Montezano A, Gutsol A, Heitz F, Szyndralewiez C, Page P, Kennedy CR, Burns KD, Touyz RM, Hébert RL. Critical role of Nox4-based NADPH oxidase in glucose-induced oxidative stress in the kidney: implications in type 2 diabetic nephropathy. Am J Physiol Renal Physiol 299: F1348–F1358, 2010 [DOI] [PubMed] [Google Scholar]
- 46. Senthil D, Choudhury GG, Abboud HE, Sonenberg N, Kasinath BS. Regulation of protein synthesis by IGF-I in proximal tubular epithelial cells. Am J Physiol Renal Physiol 283: F1226–F1236, 2002 [DOI] [PubMed] [Google Scholar]
- 47. Tamaroglio TA, Lo CS. Regulation of fibronectin by insulin-like growth factor-I in cultured rat thoracic aortic smooth muscle cells and glomerular mesangial cells. Exp Cell Res 215: 338–346, 1994 [DOI] [PubMed] [Google Scholar]
- 48. Vaquero EC, Edderkaoui M, Pandol SJ, Gukovsky I, Gukovskaya AS. Reactive oxygen species produced by NAD(P)H oxidase inhibit apoptosis in pancreatic cancer cells. J Biol Chem 279: 34643–34654, 2004 [DOI] [PubMed] [Google Scholar]
- 49. Weyemi U, Caillou B, Talbot M, Ameziane-El-Hassani R, Lacroix L, Lagent-Chevallier O, Al Ghuzlan A, Roos D, Bidart JM, Virion A, Schlumberger M, Dupuy C. Intracellular expression of reactive oxygen species-generating NADPH oxidase NOX4 in normal and cancer thyroid tissues. Endocr Relat Cancer 17: 27–37, 2010 [DOI] [PubMed] [Google Scholar]
- 50. Zhuang HX, Wuarin L, Fei ZJ, Ishii DN. Insulin-like growth factor (IGF) gene expression is reduced in neural tissues and liver from rats with non-insulin-dependent diabetes mellitus, and IGF treatment ameliorates diabetic neuropathy. J Pharmacol Exp Ther 283: 366–374, 1997 [PubMed] [Google Scholar]
- 51. Zhuang HX, Snyder CK, Pu SF, Ishii DN. Insulin-like growth factors reverse or arrest diabetic neuropathy: effects on hyperalgesia and impaired nerve regeneration in rats. Exp Neurol 140: 198–205, 1996 [DOI] [PubMed] [Google Scholar]