Abstract
Comparison of diverse orthologs is a powerful tool to study the structure and function of channel proteins. We investigated the response of human, killifish, pig, and shark cystic fibrosis transmembrane conductance regulator (CFTR) to specific inhibitors of the channel: CFTRinh-172, glibenclamide, and GlyH-101. In three systems, including organ perfusion of the shark rectal gland, primary cultures of shark rectal gland tubules, and expression studies of each ortholog in cRNA microinjected Xenopus laevis oocytes, we observed fundamental differences in the sensitivity to inhibition by these channel blockers. In organ perfusion studies, shark CFTR was insensitive to inhibition by CFTRinh-172. This insensitivity was also seen in short-circuit current experiments with cultured rectal gland tubular epithelial cells (maximum inhibition 4 ± 1.3%). In oocyte expression studies, shark CFTR was again insensitive to CFTRinh-172 (maximum inhibition 10.3 ± 2.5% at 25 μM), pig CFTR was insensitive to glibenclamide (maximum inhibition 18.4 ± 4.4% at 250 μM), and all orthologs were sensitive to GlyH-101. The amino acid residues considered responsible by previous site-directed mutagenesis for binding of the three inhibitors are conserved in the four CFTR isoforms studied. These experiments demonstrate a profound difference in the sensitivity of different orthologs of CFTR proteins to inhibition by CFTR blockers that cannot be explained by mutagenesis of single amino acids. We believe that the potency of the inhibitors CFTRinh-172, glibenclamide, and GlyH-101 on the CFTR chloride channel protein is likely dictated by the local environment and the three-dimensional structure of additional residues that form the vestibules, the chloride pore, and regulatory regions of the channel.
Keywords: shark rectal gland, Xenopus laevis oocytes, forskolin, isobutylmethylxanthine, two-electrode voltage clamp, cystic fibrosis transmembrane conductance regulator
cystic fibrosis (CF) results from mutations in the gene encoding the cystic fibrosis transmembrane conductance regulator (CFTR), an epithelial chloride channel (25) that is expressed in secretory and absorptive epithelia in the airways, pancreas, intestine, testis, and other tissues. The disease is characterized by chronic lung infection, pancreatic insufficiency and male infertility, with progressive deterioration of lung function and death (42). CFTR is a member of the ATP-binding cassette family of membrane proteins (13) but is unique within this family in functioning as an ion channel rather than a transporter protein.1
CFTR is composed of two regions of six transmembrane domains (TMDs), two nucleotide-binding domains (NBDs), and a cytosolic regulatory region (R domain) that contains multiple sites for cAMP-dependent phosphorylation (1, 51). Transport of ions through pore-forming transmembrane α-helices is controlled by the NBDs, which interact with ATP to form a dimer (61). This ATP-driven dimerization of CFTR's cytoplasmic nucleotide-binding domains is directly linked to the opening of the ion channel in the transmembrane domains.
CFTR was cloned more than two decades ago (6, 45), but the atomic structure of the protein remains unclear as only low-resolution structures of CFTR are available (46, 65). Obtaining a high-resolution structure of CFTR holds promise for targeted therapy of CF.
Ion permeation through ion channels is influenced by charged amino acid side chains at the entrance of the channel pore (20). These residues attract oppositely charged ions from the solution, increasing their effective local concentration, while repelling ions of like charge (38, 53). Functional evidence suggests that permeant anions bind to several discrete sites within the CFTR channel pore (12, 30, 33, 54, 57). These binding sites attract chloride ions into the CFTR pore and coordinate ion-ion interactions that are necessary for rapid ion movement through the pore (17, 18). Site-directed amino acid mutagenesis studies implicate the positively charged amino acid side chains of K95 (14) and R334 (19, 53). R347 in TM6 may not interact directly with permeating anions but instead forms a salt bridge with D924, thus stabilizing the pore (11).
Inhibitors of the CFTR channel have been employed as tools to investigate the role of key amino acids in the CFTR channel pore. Chloride ion-binding sites within the CFTR pore serve as sites at which substances bind to occlude the pore and inhibit chloride permeation through the channel (15, 16, 37, 69). A diverse group of organic anions inhibit chloride transport by this mechanism (9, 23, 48). Those that have been studied extensively include the sulfonylurea glibenclamide (50, 67, 69) and the glycine hydrazide GlyH-101 (39). Glibenclamide and GlyH-101 act as open channel blockers, glibenclamide blocking intracellularly and GlyH-101 extracellularly. Another well-studied inhibitor, the thiazolidone CFTRinh-172 (8, 32, 58, 59), does not function as an open channel blocker but rather affects channel gating (27). Despite several site-directed mutagenesis studies (8, 21, 29), the location and number of these inhibitor binding-sites in CFTR remain unclear.
The three agents tested are not specific inhibitors of the CFTR channel. Glibenclamide inhibits other ATP-binding cassette (ABC) transporters including the sulfonylurea receptor and P-glycoprotein (4) and calcium-activated Cl− channels in mammalian cardiac myocytes (64). GlyH-101 inhibits other anion channels and transporters such as TMEM16A (10) and the SLC26 anion exchangers SLC26a3, -a6, a9, and -a11 (3, 56). CFTRinh-172 inhibits sodium transport in sweat glands (62). Both CFTRinh-172 and GlyH-101 affect mitochondrial function, independent of their action on CFTR (24).
Studies comparing protein orthologs have been a powerful tool for examining the structure and function of CFTR (31, 41, 43, 44). The evolutionary distance between orthologs, on one hand, and the conservation of certain motifs on the other, permit the study of structure-function relationships without site-directed mutagenesis and thus without manipulating the protein under examination. In this work we investigate the response of four different CFTR orthologs to multiple inhibitors of the channel. We investigated human- (45), killifish- (52), pig- (41), and shark CFTR (22, 34). Using three different systems, we observe fundamental differences among orthologs in the inhibition by different CFTR channel blockers.
MATERIALS AND METHODS
In vitro perfusion of shark rectal glands.
Rectal glands were obtained from dogfish sharks, Squalus acanthias, of either sex weighing 2–6 kg that were euthanized by pithing using the approved method of the Mount Desert Island Biological Laboratory and its Institutional Animal Care and Use Committee (approval no. 10-03). Glands were removed from the shark, cannulated, and perfused with elasmobranch Ringer's solution for 30 min to reach basal levels of chloride secretion (∼250 μeq·h−1·g−1) as previously described (2, 5, 28, 44). At 30 min, forskolin (1 μM) and IBMX (100 μM) were added to the perfusate to achieve maximal rates of chloride secretion measured at 1-min intervals. After 20 min of stimulated secretion, 10 μM CFTRinh-172 was added to the perfusion solution for an additional 30 min in the presence of forskolin and IBMX (50–70 min) and then removed (70–90 min). Control glands were treated identically but were perfused only with forskolin and IBMX from 30–90 min. Results are expressed as mean micro equivalents of chloride secreted per hour per gram wet weight (μeq·h−1·g−1) ± SE.
Primary cell cultures of shark rectal gland tubules and measurements of transepithelial chloride transport as short-circuit current in primary culture monolayers: inhibition with CFTRinh-172, glibenclamide, and GlyH-101.
Isolated tubules of shark rectal gland (SRG) epithelial cells were prepared under sterile conditions as previously described (60), and rectal gland tubules were plated onto Corning Transwell polyester membrane inserts (pore size 0.4 μm, membrane diameter 6.5 mm, catalog no. CLS3470, Sigma Aldrich, St. Louis, MO). Confluent primary culture monolayers were mounted in a modified Ussing chamber and bathed with a solution containing (in mM) 270 NaCl, 4 KCl, 3 MgCl2, 2.5 CaCl2, 8 NaHCO3, 1 KH2PO4, 350 urea, and 5 glucose at pH 7.5. The chamber was kept at 20°C and was constantly gassed with 99% O2 and 1% CO2. The voltage-clamp and data acquisition equipment was designed and constructed by W. Van Driessche (Catholic University, Louvain, Belgium) and has been described in detail previously (7). Following stabilization of the basal short-circuit current (Isc), a steep apical-to-basolateral Cl− gradient was imposed by replacing the basolateral perfusate by a solution containing (in mM) 270 Na-gluconate, 4 K-gluconate, 3 MgCl2, 2.5 CaCl2, 8 NaHCO3, 1 KH2PO4, 350 urea, and 5 glucose at pH 7.5. Subsequently, nystatin was added basolaterally (0.72 mg/ml) to measure apical Cl− absorption from a 1,000-fold concentrated stock solution in DMSO followed by brief sonication. After stabilization of the Isc, chloride current across apical CFTR channels was stimulated maximally by the addition of C-type natriuretic peptide (CNP) (50 nM) and forskolin (10 μM) to the basolateral side. Percent inhibition by CFTRinh-172 (10 μM), glibenclamide (300 μM), and GlyH-101 (90 μM) was determined by the ratio of Isc measured at the peak of stimulation and Isc at a steady state after addition of the inhibitors to both the apical and basolateral solution. Results are expressed as percent inhibition ± SE.
Synthesis of human CFTR, killifish CFTR, pig CFTR, and shark CFTR cRNA.
CFTR orthologs were obtained from the laboratories of Raymond Frizzell [University of Pittsburgh; human CFTR (hCFTR)], Michael Welsh [University of Iowa; killifish CFTR (kfCFTR) and pig CFTR (pCFTR)], and John Riordan [University of North Carolina; shark CFTR (sCFTR)] and were cloned into T7 expression vectors. Human, shark, and pig CFTR were cloned into pcDNA3.1, whereas killifish CFTR was cloned into pGEMTeasy. Expression vectors were grown in 150-ml cultures of TOP10 Escherichia coli (Invitrogen, Carlsbad, CA), maxiprep was performed using Pure Yield Maxi prep Systems (Promega, Madison, WI), and the full-length construct was sequenced to confirm the integrity of the CFTR open reading frames. CFTR DNA (12 μg) was linearized with XhoI and purified by PCR purification (Qiagen, Alameda, CA). Capped cDNA was synthesized using T7 RNA polymerase and in vitro transcription following the instructions of T7 in vitro transcription system (Ambion, Austin, TX). The reaction products were precipitated using lithium-chloride precipitation and measured with the Agilent Bioanalyzer system (Agilent, Santa Clara, CA).
Oocyte preparation and expression of hCFTR, kfCFTR, pCFTR, and sCFTR.
Ovarian lobules of mature female Xenopus laevis (Xenopus I, Dexter, MI) were obtained, and mature stage V and VI oocytes were selected for two-electrode voltage clamping as described previously (5, 63). After 12 h the oocytes were injected with 10 ng cRNA/50 nl or an equivalent volume of water and then stored for 1–2 days in modified Barth solution at 18°C as described previously (5).
Two-electrode voltage clamping: inhibition of hCFTR, kfCFTR, pCFTR, and sCFTR with CFTRinh-172, glibenclamide, and GlyH-101.
Electrophysiological recordings were performed 1–2 days after injection of the cRNA. Electrodes, pulled on a micropipette puller (Sutter Instruments, Novato, CA), were filled with 3 M KCl and had input resistances between 0.6 and 1.3 MΩ. During two-electrode voltage clamping, oocytes were clamped at a holding potential of −30 mV and current-voltage (I-V) curves were obtained by taking ramps from −120 to +60 mV at a rate of 100 mV/s with the use of a two-electrode voltage clamp (TEV-200, Dagan Instruments, Foster City, CA). Reversal potentials were determined and the conductance was calculated using the current at the clamped voltage of −20 mV and the current at the clamped voltage of +20 mV. During the experiments, oocytes were perfused with ND96 containing 1.8 mM CaCl2. I-V ramps were taken under basal conditions and during stimulation by forskolin (10 μM) and IBMX (1 mM). When the stimulation reached a steady state, the inhibitors were added, beginning with the lowest concentration. IBMX and forskolin was continually perfused during the different inhibitor concentrations. Once currents with one concentration of the inhibitor reached a steady state, the next higher concentration of the same inhibitor was used (Fig. 4). Identical concentrations of inhibitors were used on the four orthologs. All concentrations of inhibitors were greater than the IC50 for human CFTR. The inhibitors examined were as follows: CFTRinh-172 (Sigma) and GlyH-101 (obtained from Cystic Fibrosis Foundation Therapeutics) at concentrations of 5, 10, and 20 μM and glibenclamide (Sigma) at concentrations of 50, 100, and 200 μM. Inhibition was determined by the ratio of the conductance measured at the steady state of forskolin/IBMX stimulation and the conductance obtained at the steady state of inhibition by the dose of the specific inhibitor. Data were analyzed with pCLAMP software (Axon Instruments, Sunnyvale, CA). Results are expressed as micro siemens (μS) ± SE. P values were obtained using Student's t-test, comparing the percent inhibition of the different orthologs to the percent inhibition of human CFTR at the same concentration of the inhibitor.
Fig. 4.
Representative recordings of conductance from Xenopus oocytes expressing different CFTR orthologs. Recordings begin with addition of forskolin/IBMX (10 μM/1 mM) to stimulate conductance. The sequential application of the three increasing concentrations of each inhibitor is indicated by 1 for the lowest, 2 for the medium, and 3 for the highest concentration. A: forskolin/IBMX (10 μM/1 mM) and CFTRinh-172 (5, 10, and 20 μM). B: forskolin/IBMX and glibenclamide (50, 100, and 200 μM). C: forskolin/IBMX and GlyH-101 (5, 10, and 20 μM).
Dose-response experiments for CFTRinh-172, inhibition of sCFTR- vs. hCFTR-injected oocytes, and for glibenclamide, inhibition of pCFTR- vs. hCFTR-injected oocytes.
Dose-response experiments for the most significant findings (sCFTR insensitivity to CFTRinh-172 compared with hCFTR and pCFTR insensitivity to glibenclamide compared with hCFTR) were performed by testing additional concentrations of each inhibitor. Those for CFTRinh-172 were 1, 5, 8, 10, 15, 20, and 25 μM and for glibenclamide were 20, 50, 80, 100, 150, 200, and 250 μM, reaching water solubility limits for both inhibitors with the maximum concentration used. IC50 values were calculated using the PRISM 5 software (GraphPad, La Jolla, CA).
All reagents and chemicals were purchased from Sigma Aldrich unless otherwise noted.
RESULTS
In vitro perfusion of SRGs.
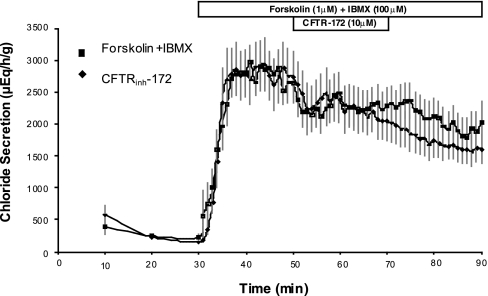
Experiments with CFTRinh-172 were first carried out in the perfused SRG. After 30 min of low basal chloride secretion, when the rectal gland is stimulated by forskolin (1 μM) + IBMX (100 μM), chloride secretion abruptly increased 15- to 30-fold from basal values of 100–250 μeq·h−1·g−1 to values of 2,400 to 3,000 μeq·h−1·g−1 (Fig. 1). In experiments where CFTRinh-172 (10 μM) was added from 50–70 min to the perfusate of stimulated glands receiving forskolin and IBMX, there was no effect of this channel blocker compared with control glands perfused with forskolin and IBMX only (Fig. 1).
Fig. 1.
In vitro perfusion of shark rectal glands. At 30 min, 1 μM forskolin and 100 μM IBMX were added to the perfusate in all glands. CFTRinh-172 (10 μM) was added from 50–70 min in experimental glands (n = 5), and the effect on chloride secretion was not statistically different compared with controls (n = 18). Values are mean chloride secretion in μeq·h−1·g−1 ± SE.
Measurements of transepithelial chloride currents in SRG tubular epithelial cells: inhibition with CFTRinh-172, glibenclamide, and GlyH-101.
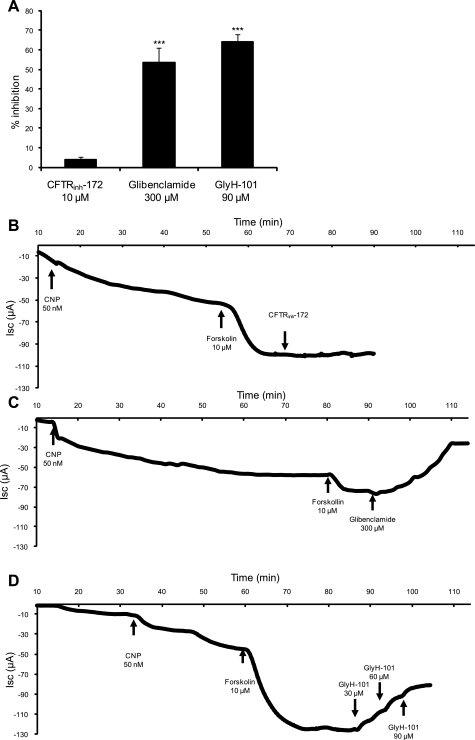
Experiments with cultured monolayers of SRG epithelial cells confirmed the insensitivity of rectal gland chloride secretion to CFTRinh-172 observed in perfusion experiments (Fig. 2). Nystatin permeabilization of the basolateral membrane was applied to examine CFTR-mediated chloride currents driven by an inwardly directed Cl− concentration gradient across the apical membrane. After stimulation with CNP and forskolin, CFTRinh-172 inhibited to only 4.0 ± 1.3% (n = 8), glibenclamide to 54 ± 7.4% (n = 3), and GlyH-101 to 64 ± 3.9% (n = 4) of the stimulated current. (Fig. 2A): Representative tracings for each inhibitor are shown in Fig. 2, B–D.
Fig. 2.
Inhibition of short-circuit current (Isc) by CFTR inhibitors in primary cultures of shark rectal gland (SRG) tubular epithelial cells. A: summary of mean % inhibition with 10 μM CFTRinh-172 (n = 8), 300 μM glibenclamide (n = 3), and 90 μM GlyH-101 (n = 4). After stimulation with C-type natriuretic peptide (CNP) and forskolin, CFTRinh-172 inhibited to only 4.0 ± 1.3%, glibenclamide to 54 ± 7.42%, and GlyH-101 to 64 ± 3.9%. The inhibition by glibenclamide and GlyH-101 is highly significant (***P < 0.001) compared with CFTRinh-172. Values represent means ± SE. B–D: representative Isc recordings with CFTRinh-172 (B), glibenclamide (C), and GlyH-101 (D).
Conductance of hCFTR-, kfCFTR-, pCFTR-, and sCFTR- injected oocytes and uninjected and water-injected controls under basal and stimulated conditions.
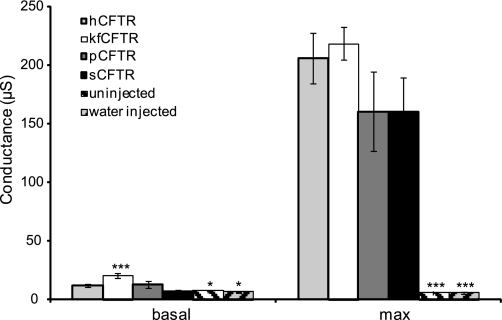
Conductance of oocytes was measured in 122 oocytes injected with either hCFTR (n = 35), kfCFTR (n = 18), pCFTR (n = 24), sCFTR (n = 21), uninjected (n = 12) or water-injected (n = 12). hCFTR, kfCFTR, pCFTR, and sCFTR had basal conductances of 11.9 ± 1.4 μS, 20.5 ± 1.9 μS, 13 ± 3.3 μS, and 6.7 ± 1.3 μS, respectively. Uninjected and water-injected oocytes had lower baseline conductances of 7.2 ± 1.2 μS and 6.2 ± 0.6 μS (P < 0.05 compared with hCFTR) (Fig. 3, basal). Human-, shark-, and pig CFTR basal currents were under 5% of maximum stimulated currents. Killifish CFTR basal conductance was above 5% of maximum stimulated conductance and was significantly elevated compared with human CFTR, uninjected, and water-injected oocytes (P < 0.001). Elevated basal chloride conductance has previously been observed in primary cultures of killifish opercular epithelium (35).
Fig. 3.
Conductances of Xenopus oocytes expressing hCFTR (n = 35), killifish (kf)CFTR (n = 18), pig (p)CFTR (n = 24), shark (s)CFTR (n = 21), and uninjected (n = 12) or water-injected oocytes (n = 12). Conductance was calculated using the current at the clamped voltage of −20 mV and the current at the clamped voltage of + 20 mV. Uninjected and water-injected control oocytes had a significant lower basal conductance compared with hCFTR and a significant lower conductance after stimulation compared with hCFTR. Basal conductance of kfCFTR was significantly higher compared with hCFTR. The maximum stimulated steady-state conductances of the four CFTR orthologs did not differ significantly. Values are means ± SE. *P < 0.05; ***P < 0.001 compared with hCFTR.
After addition of 10 μM forskolin and 1 mM IBMX to the perfusate, hCFTR, kfCFTR, pCFTR, and sCFTR had similar steady-state conductances (206 ± 21.9 μS, 218.7 ± 14.3 μS, 160.1 ± 29.5 μS, and 160.7 ± 34 μS, respectively; Fig. 3 max). However, in uninjected and water-injected oocytes the conductances after stimulation with forskolin/IBMX were unchanged compared with baseline (5.9 ± 1.6 μS for uninjected and 5.8 ± 0.5 μS for water injected) (both P < 0.001 compared with hCFTR after stimulation).
Time course of the CFTR inhibitors CFTRinh-172, glibenclamide, and GlyH-101 on the four CFTR orthologs in representative oocyte experiments.
In each experiment (Fig. 4), oocyte chloride conductance increased as CFTR was stimulated by forskolin/IBMX. Three concentrations of each inhibitor were then added sequentially at time points indicated by the arrows. The mean conductance values for all studies with the three inhibitors in all orthologs are given in Figs. 5, 6, and 7. As illustrated in Fig. 4A, hCFTR was highly responsive to inhibition by CFTRinh-172, whereas pig and shark CFTR were insensitive to this inhibitor. In Fig. 4B, hCFTR and sCFTR were sensitive to increasing concentrations of glibenclamide, whereas killifish CFTR inhibition was intermediate and pig CFTR was insensitive to all concentrations of glibenclamide. In contrast (Fig. 4C), all four orthologs of CFTR were sensitive to the inhibitor GlyH-101.
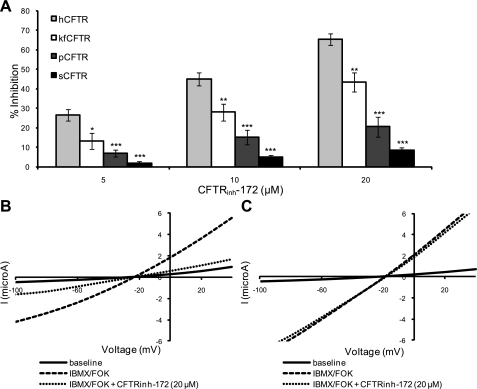
Fig. 5.
Comparison of the effects of CFTRinh-172 on different CFTR orthologs. A: summary of mean % inhibition of hCFTR (n = 16), kfCFTR (n = 5), pCFTR (n = 4), and sCFTR (n = 12) with 5, 10, and 20 μM CFTRinh-172. The orthologs h-, kf-, and pCFTR were inhibited by CFTRinh-172, although pCFTR was much less responsive (20.6 ± 5.1% at 20 μM). However, sCFTR was insensitive to CFTRinh-172 at all concentrations (1.9 ± 0.9% inhibition at 5 μM, 4.9 ± 1.2% at 10 μM, and 8.5 ± 1.5% at 20 μM). The lack of inhibition of sCFTR and pCFTR by CFTRinh-172 is highly significant compared with hCFTR (P < 0.001 at all concentrations). Values are means ± SE. *P < 0.05; **P < 0.01; ***P < 0.001 compared with hCFTR. B and C: representative current-voltage (I-V) plots from oocytes expressing hCFTR (B) and sCFTR (C). FOK, forskolin.
Fig. 6.
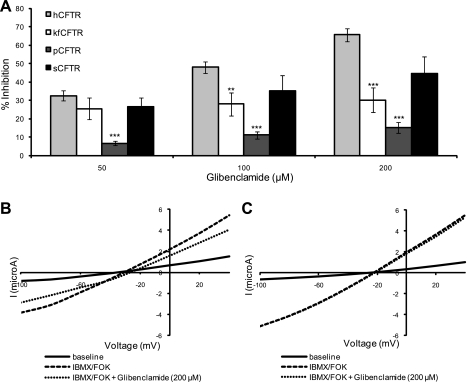
Comparison of the effects of glibenclamide on different CFTR orthologs. A: summary of mean % inhibition of hCFTR (n = 14), kfCFTR (n = 6), pCFTR (n = 16), and sCFTR (n = 4) with 50, 100, and 200 μM glibenclamide. pCFTR was insensitive to glibenclamide (6.8 ± 1.9% inhibition at 50 μM, 11.2 ± 1.8% at 100 μM, and 15.2 ± 3.0% at 200 μM). Furthermore, no change in the magnitude of block of kfCFTR was observed over the concentration range studied. All values are means ± SE. *P < 0.05; **P < 0.01; ***P < 0.001 compared with hCFTR. B and C: representative I-V plots from oocytes expressing h- (B) and pCFTR (C).
Fig. 7.
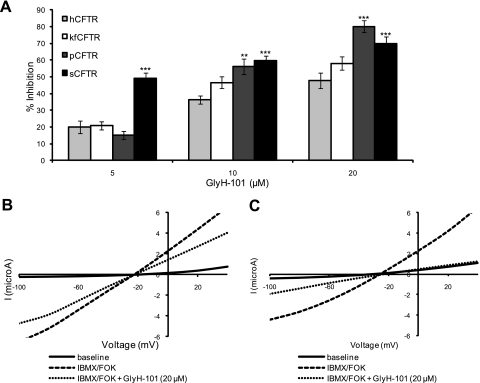
Comparison of the effects of GlyH-101 on different CFTR orthologs. A: summary of mean % inhibition of hCFTR (n = 5), kfCFTR (n = 7), pCFTR (n = 4), and sCFTR (n = 5) with 5, 10, and 20 μM of GlyH-101. All orthologs are responsive to GlyH-101. All values are means ± SE. *P < 0.05; **P < 0.01; ***P < 0.001 compared with hCFTR. B and C: representative I-V plots from oocytes expressing h- (B) and sCFTR (C).
Response to CFTRinh-172 in CFTR from diverse orthologs (hCFTR, kfCFTR, pCFTR, and sCFTR) in oocytes.
We compared the response of the four orthologs of CFTR to increasing concentrations of CFTRinh-172 (5, 10, 20 μM) (Fig. 5). CFTRinh-172 inhibited hCFTR significantly at each concentration (65.5 ± 2.9% at 20 μM). However, we observed less response to CFTRinh-172 with kfCFTR and pCFTR. pCFTR inhibition was significantly less than human at all concentrations examined (P < 0.001 compared with hCFTR). At the highest concentration (20 μM), inhibition of pCFTR was 20.6 ± 5.1% (Fig. 5A). In the Xenopus oocyte expression system, similar to the perfused SRG and cultured tubular cells, sCFTR was practically unresponsive to CFTRinh-172 (1.9 ± 0.9% inhibition at 5 μM, 4.9 ± 1.2% at 10 μM, 8.5 ± 1.5% at 20 μM). The failure of CFTRinh-172 to inhibit sCFTR is highly significant at all concentrations compared with hCFTR (Fig. 5A). I-V relationships for hCFTR and sCFTR are shown in Fig. 5, B and C. I-V ramps were taken at the end of each condition: baseline perfusion with frog Ringer's solution, stimulation with forskolin and IBMX, and inhibition with CFTRinh-172. The I-V plots illustrate that hCFTR and sCFTR show similar currents at baseline and after stimulation. However, they differ markedly in the currents seen after addition of CFTRinh-172.
Response to glibenclamide in CFTR from diverse orthologs (hCFTR, kfCFTR, pCFTR, and sCFTR).
Glibenclamide inhibited hCFTR significantly (32.6 ± 2.8% inhibition at 50 μM, 48 ± 3.1% at 100 μM, and 65.8 ± 3.6% at 200 μM) (Fig. 6). Unlike the response to CFTRinh-172, sCFTR was responsive to glibenclamide (26.6 ± 4.8% inhibition at 50 μM, 35.1 ± 8.5% at 100 μM, and 44.6 ± 9.4% at 200 μM) (Fig. 6A). Similar to the results with CFTRinh-172, kfCFTR was also less responsive to glibenclamide at higher concentrations (30.2 ± 6.7% at 200 μM, P < 0.001 compared with hCFTR).
The most striking observation with glibenclamide was the markedly impaired response of pCFTR to this inhibitor (6.8 ± 1.2% inhibition at 50 μM, 11.2 ± 1.8% at 100 μM, and 15.2 ± 3.0% at 200 μM) (Fig. 6A). Glibenclamide was unable to inhibit conductance in oocytes injected with pCFTR compared with hCFTR (P < 0.001 for each concentration of inhibitor). The I-V plots for hCFTR and pCFTR are shown in Fig. 6, B and C. Despite similar basal and stimulated currents, hCFTR was significantly inhibited by glibenclamide (200 μM), while pCFTR remained practically unchanged.
Response to GlyH-101 in CFTR from diverse orthologs.
In contrast to CFTRinh-172 and glibenclamide, hCFTR, kfCFTR, sCFTR, and pCFTR were each responsive to GlyH-101 (Fig. 7). At the higher concentrations of GlyH-101 used (10 and 20 μM), inhibition of p- and sCFTR was significantly greater than hCFTR (P < 0.001 for each compared with hCFTR). The I-V plots for hCFTR and sCFTR are shown in Fig. 7, B and C. Despite similar levels of current before and after stimulation with IBMX/forskolin, sCFTR was much more inhibited by 20 μM of GlyH-101 compared with hCFTR (P < 0.001). In agreement with Muanprasat et al. (39), we observed inward rectification of hCFTR currents at lower concentrations of GlyH-101 (5 and 10 μM) but not at higher concentrations (20 μM).
Dose response to CFTRinh-172 in sCFTR- vs. hCFTR-injected oocytes and to glibenclamide in pCFTR- vs. hCFTR-injected oocytes.
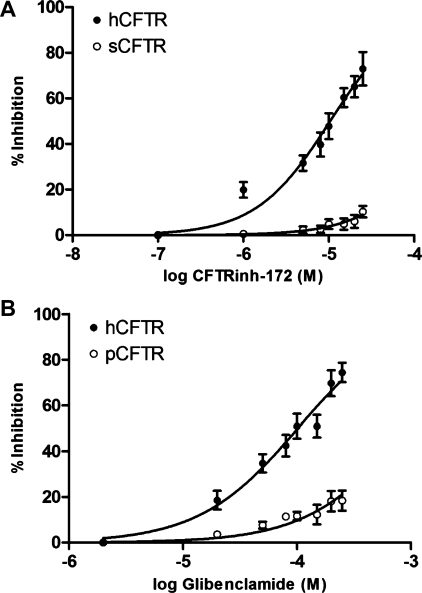
We carried out further dose-response experiments and calculated IC50 values for the most significant findings of our study (sCFTR insensitivity to CFTRinh-172 and pCFTR insensitivity to glibenclamide). In oocytes expressing hCFTR, the dose response with CFTRinh-172 revealed an IC50 of 10.4 μM and a maximal inhibition of 73.3 ± 7.4%. In contrast, in sCFTR- injected oocytes, maximal inhibition by CFTRinh-172 was only 10.3 ± 2.5% with a theoretical IC50 of 250 μM (Fig. 8A). For glibenclamide, in oocytes expressing hCFTR, dose-response experiments displayed an IC50 of 102 μM and a maximal inhibition of 74.4 ± 4.2%. However, pCFTR-injected oocytes showed maximal inhibition of only 18.4 ± 4.4% with a theoretical IC50 of 930 μM (Fig. 8B).
Fig. 8.
Logarithmic dose response demonstrating relative insensitivity of sCFTR (n = 7) vs. hCFTR (n = 11) to inhibition by CFTRinh-172 (A) and relative insensitivity of pCFTR (n = 11) vs. hCFTR (n = 8) to inhibition by glibenclamide (B). All values are means ± SE. The mean maximum inhibition by CFTRinh-172 was 73 ± 7.4% (IC50 = 10.3 μM) for hCFTR and 10.3 ± 2.5% (IC50 = 251 μM) for sCFTR. The mean maximum inhibition by glibenclamide was 74.4 ± 4.2% (IC50 = 102 μM) for hCFTR and 18.4 ± 4.4% (IC50 = 930 μM) for pCFTR.
DISCUSSION
In summary, our experiments demonstrate the following: 1) The thiazolidone inhibitor CFTRinh-172 does not inhibit shark CFTR in any of the three preparations examined, including homologous expression in the intact perfused shark rectal gland, primary monolayer cultures of rectal gland epithelial cells, and heterologous expression in Xenopus oocytes. In the oocyte, CFTRinh-172 does inhibit human, pig, and killifish CFTR, although this inhibition is significantly less in pig CFTR compared with humans. 2) In the oocyte, the sulfonylurea glibenclamide does not inhibit pig CFTR. Furthermore, there was no change in the magnitude of block of kfCFTR over the concentration range studied. 3) The glycine hydrazide GlyH-101 is a universal inhibitor of CFTR, blocking the channel in all four orthologs, but with inhibition of the pig and shark protein being significantly greater than human.
These experiments examined well-established inhibitors of CFTR (36, 48), using comparison of four wild-type orthologs rather than mutagenesis to explore the charged vestibule model of the CFTR protein. Green and Andersen (20) envisioned the general features of channel structure as evolutionary solutions to the limitations on conduction imposed by diffusion (20). They suggested that rapid ion translocation across membranes is enhanced if the narrow region of the channel pore, responsible for selectivity and binding, is flanked by vestibules designed to increase the capture radius at the channel mouth and to concentrate permeant ions using electrostatic forces. This model is widely used to explain structure-function relationships of CFTR (49, 53) and consists of three parts: an outer vestibule, pointing towards the outside of the cell membrane; a narrow region, representing the rate limiting selectivity filter; and an inner vestibule that opens to the cytoplasm. Since CFTR transports negatively charged chloride ions, positively charged amino acids adjacent to the rate limiting section of the pore are thought to enhance chloride permeation (36).
The three negatively charged inhibitors studied are proposed to bind at different sites of the channel, GlyH-101 to the outer vestibule and glibenclamide to the inner vestibule (49). CFTRinh-172 does not function as an open channel blocker since there is no rectification of current during block (32). Several different mechanisms of block have been proposed (24, 27).
Multiple site-directed mutagenesis studies have been performed to elucidate specific binding sites of the CFTR inhibitors. Caci et al. (8) proposed R347 as important for blockade of CFTR by CFTRinh-172. Gupta et al. (21) observed that two mutations in the 6th transmembrane region, F337A and T338A, significantly weakened glibenclamide block. Linsdell et al. (29) identified K95 as an important residue in the channel pore inner vestibule for binding of glibenclamide. St. Aubin et al. (55) identified R303, a second important residue of the inner vestibule region. They conclude that larger open channel blockers might bind to R303, whereas smaller molecules can penetrate deeper into the pore and bind to K95.
For GlyH-101, no site-directed mutagenesis studies have been performed. Since GlyH-101 block shows inward rectification, it is likely that GlyH-101 binds in the external pore vestibule (39). Several residues in the external mouth of the CFTR pore have been proposed as anion binding sites; however, none have been examined for binding of GlyH-101: R334 (15, 18), R104, R117, and K335 (68). Norimatsu et al. (40) used homology modeling of CFTR to identify potential GlyH-101 binding sites F337 and T338, which were previously found in mutagenesis studies to be important for glibenclamide block (21).
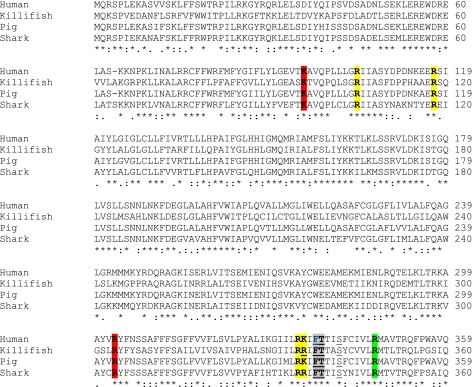
When the amino acid sequence alignment of the four CFTR orthologs is examined (Fig. 9), we found that each of the residues described above is conserved in all four orthologs of CFTR. We emphasize that this conservation of residues includes both those altered in mutagenesis studies for CFTRinh-172 and glibenclamide binding and those proposed in homology modeling for GlyH-101. Thus, these single amino acids alone cannot explain the variations in response to inhibitors seen in our studies.
Fig. 9.
Sequence alignment (1–360) of h-, kf-, p-, and sCFTR. Colors indicate amino acids considered important by mutagenesis for binding of CFTRinh-172 (green) and glibenclamide (red). Residues that have been shown to be important for the outer vestibule region and therefore could play a role in binding of GlyH-101 are shown in yellow. Residues that have been suggested to be important for binding of both GlyH-101 by homology modeling and glibenclamide by mutagenesis are indicated in gray. The pore amino acids are underlined. Asterisk symbol (*) indicates that the amino acids are identical in all sequences in the alignment; colon sign (:) indicates that conserved substitutions are observed; period sign (.) indicates that semiconserved substitutions are observed. All residues that are proposed to be important for inhibitor binding are conserved throughout the different orthologs and cannot explain the different bioelectric properties of the four CFTR orthologs. Alignments were generated using ClustalW and the Biological Workbench programs.
Considering that R347 alone does not explain the striking differences in CFTRinh-172 potency among different CFTR orthologs, we conclude, in agreement with Caci et al. (8), that that the binding site of CFTRinh-172 likely lies elsewhere in the CFTR protein and that R347 has primarily an allosteric effect. Indeed, the R347D mutation alters ATPase activity in the NBDs, suggesting that R347 might be involved in conformational coupling between the TMDs and the NBDs (26). Furthermore, the observation of fundamental differences in the response to CFTRinh-172 in CFTR orthologs sharing high similarity in sequence and function, in the same expression system, argues that CFTRinh-172 binds directly to the CFTR protein and does not act primarily on subcellular structures like mitochondria as proposed previously (24). Our results offer new information favoring a complex mechanism of CFTRinh-172 inhibition with the inhibitor acting directly on the channel.
The insensitivity of pCFTR to glibenclamide observed by Liu et al. (31) in primary cultures and in our oocyte experiments also cannot be explained by differences in single amino acid residues between the orthologs. There are two conflicting mechanisms proposed for the glibenclamide block of conductance (21). Single inhibitor binding sites (47, 50) and multiple binding sites (21, 36, 66) have been proposed. Our results are consistent with the later model favoring a complex mechanism of block involving multiple binding sites.
Given the many differences between orthologs observed in these experiments that cannot be explained by targeting single amino acids, we conclude that study of wild-type channels from diverse species offers critical new information. We believe that the potency of the inhibitors CFTRinh-172, glibenclamide, and Gly-H101 on the CFTR chloride channel protein is dictated by the three-dimensional structure of additional residues that contribute to the inhibitor binding sites, the vestibules, and the chloride channel pore.
GRANTS
This work was supported by National Institutes of Health (NIH) Grants DK 34208, National Institute of Environmental Health Sciences P30-ES 3828 and American Heart Association Grant 92011310 to J. N. Forrest and NIH-National Center for Research Resources Idea Networks of Biomedical Research Excellence (INBRE) Grant P20RR016463.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
M.S., K.S., M.B.B., and J.N.F. conception and design of research; M.S., K.S., and M.B.B. performed experiments; M.S., K.S., and J.N.F. analyzed data; M.S., K.S., M.B.B., and J.N.F. interpreted results of experiments; M.S., K.S., and M.B.B. prepared figures; M.S., K.S., M.B.B., and J.N.F. drafted manuscript; M.S., K.S., and J.N.F. edited and revised manuscript; M.S., K.S., M.B.B., and J.N.F. approved final version of manuscript.
ACKNOWLEDGMENTS
We gratefully thank Dr. Hugo De Jonge, Dr. Denry Sato, Christine Chapline, Anna Kufner, Brendan Vosburgh, and Catherine Kelley for excellent technical help and assistance in these studies. We thank Dr. David Dawson and Jan-Philipp Machtens for helpful discussions. We also acknowledge Robert Bridges Ph.D., Rosalind Franklin University of Medicine and Science, and the Cystic Fibrosis Foundation Therapeutics for providing GlyH-101.
Footnotes
This article is the topic of an Editorial Focus by David N. Sheppard (51a).
REFERENCES
- 1. Akabas MH. Cystic fibrosis transmembrane conductance regulator. Structure and function of an epithelial chloride channel. J Biol Chem 275: 3729–3732, 2000 [DOI] [PubMed] [Google Scholar]
- 2. Aller SG, Lombardo ID, Bhanot S, Forrest JN., Jr Cloning, characterization, and functional expression of a CNP receptor regulating CFTR in the shark rectal gland. Am J Physiol Cell Physiol 276: C442–C449, 1999 [DOI] [PubMed] [Google Scholar]
- 3. Bertrand CA, Zhang R, Pilewski JM, Frizzell RA. SLC26A9 is a constitutively active, CFTR-regulated anion conductance in human bronchial epithelia. J Gen Physiol 133: 421–438, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bessadok A, Garcia E, Jacquet H, Martin S, Garrigues A, Loiseau N, Andre F, Orlowski S, Vivaudou M. Recognition of sulfonylurea receptor (ABCC8/9) ligands by the multidrug resistance transporter P-glycoprotein (ABCB1): functional similarities based on common structural features between two multispecific ABC proteins. J Biol Chem 286: 3552–3569, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bewley MS, Pena JT, Plesch FN, Decker SE, Weber GJ, Forrest JN., Jr Shark rectal gland vasoactive intestinal peptide receptor: cloning, functional expression, and regulation of CFTR chloride channels. Am J Physiol Regul Integr Comp Physiol 291: R1157–R1164, 2006 [DOI] [PubMed] [Google Scholar]
- 6. Buchwald M, Tsui LC, Riordan JR. The search for the cystic fibrosis gene. Am J Physiol Lung Cell Mol Physiol 257: L47–L52, 1989 [DOI] [PubMed] [Google Scholar]
- 7. Butterworth MB, Edinger RS, Johnson JP, Frizzell RA. Acute ENaC stimulation by cAMP in a kidney cell line is mediated by exocytic insertion from a recycling channel pool. J Gen Physiol 125: 81–101, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Caci E, Caputo A, Hinzpeter A, Arous N, Fanen P, Sonawane N, Verkman AS, Ravazzolo R, Zegarra-Moran O, Galietta LJ. Evidence for direct CFTR inhibition by CFTR(inh)-172 based on Arg347 mutagenesis. Biochem J 413: 135–142, 2008 [DOI] [PubMed] [Google Scholar]
- 9. Cai Z, Lansdell KA, Sheppard DN. Inhibition of heterologously expressed cystic fibrosis transmembrane conductance regulator Cl− channels by non-sulphonylurea hypoglycaemic agents. Br J Pharmacol 128: 108–118, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Caputo A, Caci E, Ferrera L, Pedemonte N, Barsanti C, Sondo E, Pfeffer U, Ravazzolo R, Zegarra-Moran O, Galietta LJ. TMEM16A, a membrane protein associated with calcium-dependent chloride channel activity. Science 322: 590–594, 2008 [DOI] [PubMed] [Google Scholar]
- 11. Cotten JF, Welsh MJ. Cystic fibrosis-associated mutations at arginine 347 alter the pore architecture of CFTR. Evidence for disruption of a salt bridge. J Biol Chem 274: 5429–5435, 1999 [DOI] [PubMed] [Google Scholar]
- 12. Dawson DC, Smith SS, Mansoura MK. CFTR: mechanism of anion conduction. Physiol Rev 79: S47–S75, 1999 [DOI] [PubMed] [Google Scholar]
- 13. Dean M, Rzhetsky A, Allikmets R. The human ATP-binding cassette (ABC) transporter superfamily. Genome Res 11: 1156–1166, 2001 [DOI] [PubMed] [Google Scholar]
- 14. Ge N, Muise CN, Gong X, Linsdell P. Direct comparison of the functional roles played by different transmembrane regions in the cystic fibrosis transmembrane conductance regulator chloride channel pore. J Biol Chem 279: 55283–55289, 2004 [DOI] [PubMed] [Google Scholar]
- 15. Gong X, Burbridge SM, Lewis AC, Wong PY, Linsdell P. Mechanism of lonidamine inhibition of the CFTR chloride channel. Br J Pharmacol 137: 928–936, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gong X, Linsdell P. Coupled movement of permeant and blocking ions in the CFTR chloride channel pore. J Physiol 549: 375–385, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gong X, Linsdell P. Maximization of the rate of chloride conduction in the CFTR channel pore by ion-ion interactions. Arch Biochem Biophys 426: 78–82, 2004 [DOI] [PubMed] [Google Scholar]
- 18. Gong X, Linsdell P. Molecular determinants and role of an anion binding site in the external mouth of the CFTR chloride channel pore. J Physiol 549: 387–397, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gong X, Linsdell P. Mutation-induced blocker permeability and multiion block of the CFTR chloride channel pore. J Gen Physiol 122: 673–687, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Green WN, Andersen OS. Surface charges and ion channel function. Annu Rev Physiol 53: 341–359, 1991 [DOI] [PubMed] [Google Scholar]
- 21. Gupta J, Linsdell P. Point mutations in the pore region directly or indirectly affect glibenclamide block of the CFTR chloride channel. Pflügers Arch 443: 739–747, 2002 [DOI] [PubMed] [Google Scholar]
- 22. Hanrahan JWDF, Sansom S, Alon N, Jensen TJ, Riordan JR, Grzelczak Z. Low-conductance chloride channel activated by cAMP in the rectal gland of the shark Squalus acanthias and in cells heterologously expressing the shark CFTR gene. Bull Mt Desert Isl Biol Lab Salisb Cove Maine 32: 48–49, 1993 [Google Scholar]
- 23. Hwang TC, Sheppard DN. Molecular pharmacology of the CFTR Cl− channel. Trends Pharmacol Sci 20: 448–453, 1999 [DOI] [PubMed] [Google Scholar]
- 24. Kelly M, Trudel S, Brouillard F, Bouillaud F, Colas J, Nguyen-Khoa T, Ollero M, Edelman A, Fritsch J. Cystic fibrosis transmembrane regulator inhibitors CFTR(inh)-172 and GlyH-101 target mitochondrial functions, independently of chloride channel inhibition. J Pharmacol Exp Ther 333: 60–69, 2010 [DOI] [PubMed] [Google Scholar]
- 25. Kidd JF, Kogan I, Bear CE. Molecular basis for the chloride channel activity of cystic fibrosis transmembrane conductance regulator and the consequences of disease-causing mutations. Curr Top Dev Biol 60: 215–249, 2004 [DOI] [PubMed] [Google Scholar]
- 26. Kogan I, Ramjeesingh M, Huan LJ, Wang Y, Bear CE. Perturbation of the pore of the cystic fibrosis transmembrane conductance regulator (CFTR) inhibits its ATPase activity. J Biol Chem 276: 11575–11581, 2001 [DOI] [PubMed] [Google Scholar]
- 27. Kopeikin Z, Sohma Y, Li M, Hwang TC. On the mechanism of CFTR inhibition by a thiazolidinone derivative. J Gen Physiol 136: 659–671, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lehrich RW, Aller SG, Webster P, Marino CR, Forrest JN., Jr Vasoactive intestinal peptide, forskolin, and genistein increase apical CFTR trafficking in the rectal gland of the spiny dogfish, Squalus acanthias. Acute regulation of CFTR trafficking in an intact epithelium. J Clin Invest 101: 737–745, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Linsdell P. Location of a common inhibitor binding site in the cytoplasmic vestibule of the cystic fibrosis transmembrane conductance regulator chloride channel pore. J Biol Chem 280: 8945–8950, 2005 [DOI] [PubMed] [Google Scholar]
- 30. Linsdell P. Relationship between anion binding and anion permeability revealed by mutagenesis within the cystic fibrosis transmembrane conductance regulator chloride channel pore. J Physiol 531: 51–66, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Liu X, Luo M, Zhang L, Ding W, Yan Z, Engelhardt JF. Bioelectric properties of chloride channels in human, pig, ferret, and mouse airway epithelia. Am J Respir Cell Mol Biol 36: 313–323, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ma T, Thiagarajah JR, Yang H, Sonawane ND, Folli C, Galietta LJ, Verkman AS. Thiazolidinone CFTR inhibitor identified by high-throughput screening blocks cholera toxin-induced intestinal fluid secretion. J Clin Invest 110: 1651–1658, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mansoura MK, Smith SS, Choi AD, Richards NW, Strong TV, Drumm ML, Collins FS, Dawson DC. Cystic fibrosis transmembrane conductance regulator (CFTR) anion binding as a probe of the pore. Biophys J 74: 1320–1332, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Marshall J, Martin KA, Picciotto M, Hockfield S, Nairn AC, Kaczmarek LK. Identification and localization of a dogfish homolog of human cystic fibrosis transmembrane conductance regulator. J Biol Chem 266: 22749–22754, 1991 [PubMed] [Google Scholar]
- 35. Marshall WS, Bryson SE, Midelfart A, Hamilton WF. Low-conductance anion channel activated by cAMP in teleost Cl−-secreting cells. Am J Physiol Regul Integr Comp Physiol 268: R963–R969, 1995 [DOI] [PubMed] [Google Scholar]
- 36. McCarty NA. Permeation through the CFTR chloride channel. J Exp Biol 203: 1947–1962, 2000 [DOI] [PubMed] [Google Scholar]
- 37. McDonough S, Davidson N, Lester HA, McCarty NA. Novel pore-lining residues in CFTR that govern permeation and open-channel block. Neuron 13: 623–634, 1994 [DOI] [PubMed] [Google Scholar]
- 38. Moorhouse AJ, Keramidas A, Zaykin A, Schofield PR, Barry PH. Single channel analysis of conductance and rectification in cation-selective, mutant glycine receptor channels. J Gen Physiol 119: 411–425, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Muanprasat C, Sonawane ND, Salinas D, Taddei A, Galietta LJ, Verkman AS. Discovery of glycine hydrazide pore-occluding CFTR inhibitors: mechanism, structure-activity analysis, and in vivo efficacy. J Gen Physiol 124: 125–137, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Norimatsu Y, Ivetac A, Kirkham J, Frye L, Brewer M, Sansom M, Dawson DC. Identification of possible binding sites for the CFTR pore blocker, GlyH-101 (Abstract). Biophys J 98, Suppl 1: 323, 2010 [Google Scholar]
- 41. Ostedgaard LS, Rogers CS, Dong Q, Randak CO, Vermeer DW, Rokhlina T, Karp PH, Welsh MJ. Processing and function of CFTR-DeltaF508 are species-dependent. Proc Natl Acad Sci USA 104: 15370–15375, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Pilewski JM, Frizzell RA. Role of CFTR in airway disease. Physiol Rev 79: S215–S255, 1999 [DOI] [PubMed] [Google Scholar]
- 43. Price MP, Ishihara H, Sheppard DN, Welsh MJ. Function of Xenopus cystic fibrosis transmembrane conductance regulator (CFTR) Cl channels and use of human-Xenopus chimeras to investigate the pore properties of CFTR. J Biol Chem 271: 25184–25191, 1996 [DOI] [PubMed] [Google Scholar]
- 44. Ratner MA, Decker SE, Aller SG, Weber G, Forrest JN., Jr Mercury toxicity in the shark (Squalus acanthias) rectal gland: apical CFTR chloride channels are inhibited by mercuric chloride. J Exp Zool A Comp Exp Biol 305: 259–267, 2006 [DOI] [PubMed] [Google Scholar]
- 45. Riordan JR, Rommens JM, Kerem B, Alon N, Rozmahel R, Grzelczak Z, Zielenski J, Lok S, Plavsic N, Chou JL, Drumm ML, Iannuzzi MC, Collins FS, Tsui LC. Identification of the cystic fibrosis gene: cloning and characterization of complementary DNA. Science 245: 1066–1073, 1989. [DOI] [PubMed] [Google Scholar]
- 46. Rosenberg MF, Kamis AB, Aleksandrov LA, Ford RC, Riordan JR. Purification and crystallization of the cystic fibrosis transmembrane conductance regulator (CFTR). J Biol Chem 279: 39051–39057, 2004 [DOI] [PubMed] [Google Scholar]
- 47. Schultz BD, DeRoos AD, Venglarik CJ, Singh AK, Frizzell RA, Bridges RJ. Glibenclamide blockade of CFTR chloride channels. Am J Physiol Lung Cell Mol Physiol 271: L192–L200, 1996 [DOI] [PubMed] [Google Scholar]
- 48. Schultz BD, Singh AK, Devor DC, Bridges RJ. Pharmacology of CFTR chloride channel activity. Physiol Rev 79: S109–144, 1999 [DOI] [PubMed] [Google Scholar]
- 49. Sheppard DN. CFTR channel pharmacology: novel pore blockers identified by high-throughput screening. J Gen Physiol 124: 109–113, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Sheppard DN, Robinson KA. Mechanism of glibenclamide inhibition of cystic fibrosis transmembrane conductance regulator Cl− channels expressed in a murine cell line. J Physiol 503: 333–346, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Sheppard DN, Welsh MJ. Structure and function of the CFTR chloride channel. Physiol Rev 79: S23–S45, 1999 [DOI] [PubMed] [Google Scholar]
- 51a. Sheppard DN. CFTR channel pharmacology: insight from a flock of clones. Focus on “Divergent CFTR orthologs respond differently to the channel inhibitors CFTRinh-172, glibenclamide, and GlyH-101.” Am J Physiol Cell Physiol (October 12, 2011). doi:10.1152/ajpcell.00376.2011 [DOI] [PubMed] [Google Scholar]
- 52. Singer TD, Tucker SJ, Marshall WS, Higgins CF. A divergent CFTR homologue: highly regulated salt transport in the euryhaline teleost F. heteroclitus. Am J Physiol Cell Physiol 274: C715–C723, 1998 [DOI] [PubMed] [Google Scholar]
- 53. Smith SS, Liu X, Zhang ZR, Sun F, Kriewall TE, McCarty NA, Dawson DC. CFTR: covalent and noncovalent modification suggests a role for fixed charges in anion conduction. J Gen Physiol 118: 407–431, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Smith SS, Steinle ED, Meyerhoff ME, Dawson DC. Cystic fibrosis transmembrane conductance regulator. Physical basis for lyotropic anion selectivity patterns. J Gen Physiol 114: 799–818, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. St Aubin CN, Zhou JJ, Linsdell P. Identification of a second blocker binding site at the cytoplasmic mouth of the cystic fibrosis transmembrane conductance regulator chloride channel pore. Mol Pharmacol 71: 1360–1368, 2007 [DOI] [PubMed] [Google Scholar]
- 56. Stewart AK, Shmukler BE, Vandorpe DH, Reimold F, Heneghan JF, Nakakuki M, Akhavein A, Ko S, Ishiguro H, Alper SL. SLC26 anion exchangers of guinea pig pancreatic duct: molecular cloning and functional characterization. Am J Physiol Cell Physiol 301: C289–C303, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Tabcharani JA, Rommens JM, Hou YX, Chang XB, Tsui LC, Riordan JR, Hanrahan JW. Multi-ion pore behaviour in the CFTR chloride channel. Nature 366: 79–82, 1993 [DOI] [PubMed] [Google Scholar]
- 58. Thiagarajah JR, Broadbent T, Hsieh E, Verkman AS. Prevention of toxin-induced intestinal ion and fluid secretion by a small-molecule CFTR inhibitor. Gastroenterology 126: 511–519, 2004 [DOI] [PubMed] [Google Scholar]
- 59. Thiagarajah JR, Song Y, Haggie PM, Verkman AS. A small molecule CFTR inhibitor produces cystic fibrosis-like submucosal gland fluid secretions in normal airways. FASEB J 18: 875–877, 2004 [DOI] [PubMed] [Google Scholar]
- 60. Valentich JD, Forrest JN., Jr Cl− secretion by cultured shark rectal gland cells. I. Transepithelial transport. Am J Physiol Cell Physiol 260: C813–C823, 1991 [DOI] [PubMed] [Google Scholar]
- 61. Vergani P, Lockless SW, Nairn AC, Gadsby DC. CFTR channel opening by ATP-driven tight dimerization of its nucleotide-binding domains. Nature 433: 876–880, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Wang XF, Reddy MM, Quinton PM. Effects of a new cystic fibrosis transmembrane conductance regulator inhibitor on Cl− conductance in human sweat ducts. Exp Physiol 89: 417–425, 2004 [DOI] [PubMed] [Google Scholar]
- 63. Weber GJ, Mehr AP, Sirota JC, Aller SG, Decker SE, Dawson DC, Forrest JN., Jr Mercury and zinc differentially inhibit shark and human CFTR orthologues: involvement of shark cysteine 102. Am J Physiol Cell Physiol 290: C793–C801, 2006 [DOI] [PubMed] [Google Scholar]
- 64. Yamazaki J, Hume JR. Inhibitory effects of glibenclamide on cystic fibrosis transmembrane regulator, swelling-activated, and Ca(2+)-activated Cl− channels in mammalian cardiac myocytes. Circ Res 81: 101–109, 1997 [DOI] [PubMed] [Google Scholar]
- 65. Zhang L, Aleksandrov LA, Zhao Z, Birtley JR, Riordan JR, Ford RC. Architecture of the cystic fibrosis transmembrane conductance regulator protein and structural changes associated with phosphorylation and nucleotide binding. J Struct Biol 167: 242–251, 2009 [DOI] [PubMed] [Google Scholar]
- 66. Zhang ZR, Cui G, Zeltwanger S, McCarty NA. Time-dependent interactions of glibenclamide with CFTR: kinetically complex block of macroscopic currents. J Membr Biol 201: 139–155, 2004 [DOI] [PubMed] [Google Scholar]
- 67. Zhang ZR, Zeltwanger S, McCarty NA. Steady-state interactions of glibenclamide with CFTR: evidence for multiple sites in the pore. J Membr Biol 199: 15–28, 2004 [DOI] [PubMed] [Google Scholar]
- 68. Zhou JJ, Fatehi M, Linsdell P. Identification of positive charges situated at the outer mouth of the CFTR chloride channel pore. Pflügers Arch 457: 351–360, 2008 [DOI] [PubMed] [Google Scholar]
- 69. Zhou Z, Hu S, Hwang TC. Probing an open CFTR pore with organic anion blockers. J Gen Physiol 120: 647–662, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]