Abstract
Collecting duct (CD)-derived endothelin-1 (ET-1) acting via endothelin B (ETB) receptors promotes Na+ excretion. Compromise of ET-1 signaling or ETB receptors in the CD cause sodium retention and increase blood pressure. Activity of the epithelial Na+ channel (ENaC) is limiting for Na+ reabsorption in the CD. To test for ETB receptor regulation of ENaC, we combined patch-clamp electrophysiology with CD-specific knockout (KO) of endothelin receptors. We also tested how ET-1 signaling via specific endothelin receptors influences ENaC activity under differing dietary Na+ regimens. ET-1 significantly decreased ENaC open probability in CD isolated from wild-type (WT) and CD ETA KO mice but not CD ETB KO and CD ETA/B KO mice. ENaC activity in WT and CD ETA but not CD ETB and CD ETA/B KO mice was inversely related to dietary Na+ intake. ENaC activity in CD ETB and CD ETA/B KO mice tended to be elevated under all dietary Na+ regimens compared with WT and CD ETA KO mice, reaching significance with high (2%) Na+ feeding. These results show that the bulk of ET-1 inhibition of ENaC activity is mediated by the ETB receptor. In addition, they could explain the Na+ retention and elevated blood pressure observed in CD ET-1 KO, CD ETB KO, and CD ETA/B KO mice consistent with ENaC regulation by ET-1 via ETB receptors contributing to the antihypertensive and natriuretic effects of the local endothelin system in the mammalian CD.
Keywords: sodium transport, endothelin-1, hypertension
renal sodium excretion is matched to sodium intake through feedback signaling to maintain blood pressure within a normal range. Renal sodium excretion is fine tuned in the aldosterone-sensitive distal nephron (ASDN). Here, the activity of the epithelial Na+ channel (ENaC) is limiting for sodium reabsorption (reviewed in Refs. 7, 16, 24). ENaC is localized to the apical membrane of principal cells in this nephron segment. Decreases in blood pressure lead to an increase in plasma aldosterone levels as the renin-angiotensin-aldosterone system (RAAS) becomes activated. This increases ENaC activity to minimize sodium excretion in counter to falling blood pressure. Thus ENaC is a critical end-effector in a key homeostatic control system regulating blood pressure. As such, gain- and loss-of-function mutations in ENaC and upstream regulators of this channel cause disease with abnormal blood pressure associated with dysfunctional renal sodium excretion (2, 15, 20).
Strong evidence exists that other systems function in parallel with the RAAS to control ENaC activity to set renal sodium excretion to an appropriate level considering sodium intake and blood pressure. Some of these parallel systems involve paracrine/autocrine-acting signals that are intrinsic to the distal nephron. Signaling by endothelin (ET) peptide hormones in the ASDN is one such system (17, 18).
The ASDN is recognized to express all components of this intrinsic regulatory system, expressing endothelin peptides and receptors, as well as final effectors that set sodium excretion (reviewed in Refs. 17 and 18). Current thinking is that an increase in urine flow as sensed by cells in the distal nephron stimulates production and release of autocrine/paracrine-acting endothelin peptides (21). These peptides, acting locally, exert a natriuretic and diuretic effect decreasing sodium and water reabsorption in the ASDN to increase urinary excretion of sodium and volume. This influence on renal excretion affects systemic sodium balance and blood pressure. Specific knockout of the gene producing ET-1 in the ASDN increases blood pressure in a sodium-dependent manner and compromises renal sodium excretion and a normal pressure-natriuresis response (1, 8, 26).
There are two types of endothelin receptors: ETA and ETB receptors (reviewed in Ref. 18). Both are canonical seven transmembrane G protein-coupled receptors able to signal through several different Gα subunits. Endothelin receptors are dimeric capable of forming homo- and heterodimers. Homodimers of each receptor type, and possibly the heterodimeric receptor, have distinct functional and pharmacological properties and have differential ligand preferences and downstream effectors. In contrast to the ETA receptor that preferentially binds ET-1 over other endothelin peptides, the ETB receptor does not discriminate between mature endothelin peptides binding and conveying a signal for ET-1, ET-2, and ET-3 equally well. The ETB receptor is the predominant endothelin receptor expressed in the ASDN (reviewed in Refs. 17 and 18). There also though is some level of ETA receptor expression here as shown by recent functional studies. In the ASDN, the ETB receptor is coupled to diverse signaling cascades and effectors including serine/threonine and tyrosine kinases, such as protein kinase A (PKA), mitogen-activated protein kinase (MAPK)/extracellular-regulated kinase (ERK) kinase1/2 (MEK1/2), MAPK1/2, and Src kinases, Ca2+ signaling proteins, and the nitric oxide synthase NOS1 (17, 18). It is unclear yet which signaling cascade is most important for inhibitory control of sodium reabsorption in the ASDN by endothelin peptides with experimental evidence existing for involvement of both Src-MAPK1/2 and NOS1 signaling (3, 5, 12, 23, 26).
The inhibitory actions of ET-1 on ASDN sodium reabsorption are mitigated by amiloride (1), a blocker of ENaC, suggesting that this channel is an important final effector transmitting the natriuretic actions of locally produced endothelin peptides. Consistent with this, exogenous ET-1 decreases ENaC activity in isolated, split-open ASDN from rat and the activity of this channel in an heterologous expression system and an amphibian cell model of the distal nephron: polarized A6 cells grown in culture (3, 5, 12). Pharmacological inhibitors of ETB but not ETA receptors and specific gene deletion of the ETB but not ETA receptor in the ASDN decrease sodium excretion (9–11). Specific agonism of ETB receptors increases sodium excretion (23, 30). Moreover, knockout of the ETB receptor and combined deletion of both the ETA and ETB receptors specifically in the ASDN result in elevations in blood pressure (9, 10). Such findings point to the ETB receptor as conveying the bulk of inhibitory endothelin signaling in the ASDN. Consistent with this, specific antagonism of the ETB receptor abrogates the inhibitory actions of ET-1 on ENaC in the isolated, split-open rat ASDN (3).
By using knockout (KO) mice engineered to have differential deletion of the genes encoding the different endothelin receptors specifically in the ASDN, we definitively test here whether the ETB or ETA receptor conveys the bulk of the inhibitory endothelin signal to ENaC. Moreover, these studies also are the first to test directly if ENaC is a final effector of ET-1 signaling in the murine ASDN expanding understanding of conservation of this regulatory pathway in mammals. Downstream effectors that transduce inhibitory ET-1 signaling to ENaC in the murine ASDN also are probed here. The most important findings of the current study, though, pertain to investigation of the relation between sodium intake and ENaC activity in mice with differential knockout of endothelin receptors in the ASDN. Mice lacking the ETB receptor have elevated ENaC activity with the channel being less responsive to changes in dietary sodium intake. A consequence of this is that in the absence of inhibitory regulation by the ETB receptor, ENaC activity is inappropriately elevated, particularly in the presence of high sodium intake, because normal feedback regulation of the channel is compromised. Dysregulation such as this is expected to disrupt normal sodium excretion manifesting as a sodium-sensitive change in blood pressure. Thus the current findings are consistent with inhibitory ET-1 regulation of ENaC via ETB receptors having a natriuretic effect with loss of this regulation being prohypertensive.
METHODS
All chemicals and materials were from Sigma (St. Louis, MO) unless noted otherwise. Animal use and welfare adhered to the National Institutes of Health “Guide for the Care and Use of Laboratory Animals” following protocols reviewed and approved by the Institutional Laboratory Animal Care and Use Committees of the University of Texas Health Science Center at San Antonio and the University of Utah Health Sciences Center. Generation and description of CD-specific ETA, ETB, and ETA/B receptor KO mice has been published previously (1, 8–11, 26). In brief, CD-specific KO mice were generated by mating males homozygous for a loxP-flanked (floxed) exon essential for coding a specific endothelin receptor to females heterozygous for the same floxed exon plus heterozygous for CD-specific Cre recombinase expression driven by the aquaporin 2 promotor. Only offspring homozygous for the floxed exon and heterozygous for AQP2-Cre were used as CD-specific KO. Genotyping has been described in detail previously (1, 8–11, 26). For experiments, mice were maintained on a nominally Na+-free diet (<0.01% Na+; Harlan TEKLAD TD.90228), regular diet containing 0.32% Na+ (Harlan TEKLAD TD.7912), or a high-Na+ diet (2% Na+; Harlan TEKLAD TD.92034) 1 wk before experimentation.
Isolation of the ASDN containing connecting tubule (CNT) and CD suitable for electrophysiology has been described previously (3, 4, 22, 27). Briefly, mice were euthanized by CO2 administration followed by cervical dislocation with kidneys immediately removed. Kidneys were cut into thin slices (<1 mm) with slices placed into ice-cold physiological saline solution buffered with HEPES (pH 7.4). The ASDN was identified as merging of the CNT into the CD and was mechanically isolated from cortical sections of kidney slices by microdissection using watchmaker forceps under a stereomicroscope. Isolated ASDN was allowed to settle onto 5 × 5 mm coverglass coated with poly-l-lysine. Coverglass containing ASDN was placed in a perfusion chamber mounted on an inverted Nikon Eclipse TE2000 microscope and superfused with room temperature HEPES-buffered (pH 7.4) saline solution. ASDN were split open with two sharpened micropipettes controlled with different micromanipulators to gain access to the apical membrane and were used within 1–2 h of isolation.
ENaC activity in principal cells of murine ASDN was quantified in cell-attached patches of the apical membrane made under voltage-clamp conditions (−Vp = −60 mV) using standard procedures (3, 4, 22, 27). Current recordings were made in a still bath with experimental reagents added directly to the recording chamber. Recording pipettes had resistances of 8–12 MΩ. Typical bath and pipette solutions were (in mM) 150 NaCl, 5 mM KCl, 1 CaCl2, 2 MgCl2, 5 glucose, and 10 HEPES (pH 7.4); and 140 LiCl, 2 MgCl2, and 10 HEPES (pH 7.4), respectively. For each experimental condition, ASDN from at least four different mice were assayed. Gap-free single channel current data from gigaohm seals were acquired (and subsequently analyzed) with an Axopatch 200B (Axon) or EPC-9 (HEKA) patch-clamp amplifier interfaced via a Digidata 1322A (Axon) to a PC running the pClamp 9.2 suite of software (Axon). Currents were low-pass filtered at 100 Hz with an eight-pole Bessel filter (Warner). Unitary current (i) was determined, as normal, from all-point amplitude histograms fitted with single- or multi-Gaussian curves using the standard 50% threshold criterion to differentiate between events. Events were inspected visually before acceptance. Channel activity, defined as NPo, was calculated using the following equation: NPo = (t1 + 2t2 + . . . + ntn), where N and Po are the number of ENaC in a patch and the mean open probability of these channels, respectively, and tn is the fractional open time spent at each of the observed current levels. Po was calculated by dividing NPo by the number of active channels within a patch as defined by all-point amplitude histograms. For calculating Po in paired experiments, N was fixed as the greatest number of active channels observed in control or experimental conditions. In such paired patch-clamp experiments, N cannot change in response to the experimental maneuver (e.g., ET-1), so any detected effect must be an effect on Po. The error associated with calculating Po increases as this variable moves away from 0.5 and approaches 0 or unity. To assure reliable calculation of Po, we measured Po with standard and accepted tools using long recording times (>1 min) and patches containing five or fewer channels. This approach, which provides the most confidence other than using seals with only one channel, is routinely used to determine Po (14, 25).
All summarized data are reported as means ± SE. Data from before and after treatment within the same experiment were compared with the paired t-test. Data from different experiments were compared with WT control with a t-test or the sodium-free group with a one-way ANOVA using the Dunnett posttest. P ≤ 0.05 was considered significant. For presentation, current data from some cell-attached patches were subsequently software filtered at 50 Hz and slow baseline drifts were corrected.
RESULTS
ET-1 decreases ENaC activity in WT and CD ETA KO but not CD ETB KO and CD ETA/B KO mice.
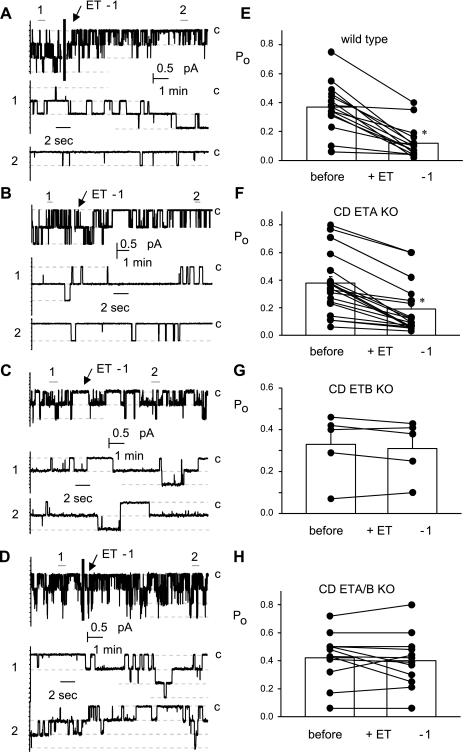
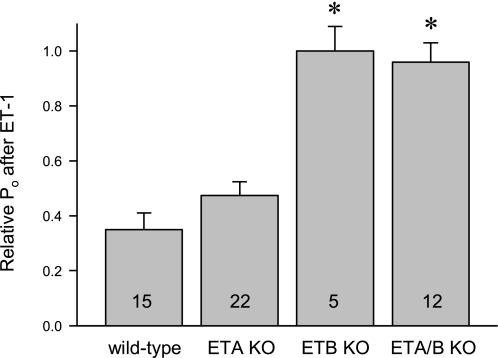
The representative current traces from paired patch-clamp experiments displayed in Fig. 1 show the effects of 20 nM ET-1 on ENaC activity in ASDN from WT (A), CD ETA KO (B), CD ETB KO (C), and CD ETA/B KO (D) mice. These cell-attached patches were clamped to −60 mV. At this voltage and with our bath and pipette solutions inward Na+ current is downward. The effect of ET-1 on ENaC Po is acute and reversible. The summary graphs to the right demonstrate that ET-1 significantly decreases ENaC Po in ASDN from WT (E) and CD ETA KO (F) mice but not CD ETB KO (G) and CD ETA/B KO (H) mice. Consequently, the relative Po, as shown in Fig. 2, of ENaC in CD ETB KO and ETA/B KO mice is significantly greater in the presence of ET-1 compared with that in WT and CD ETA KO mice.
Fig. 1.
Endothelin-1 (ET-1) decreases epithelial Na+ channel (ENaC) open probability (Po) in wild-type (WT) and collecting duct (CD) endothlin A (ETA) knockout (KO) but not CD ETB and CD ETA/B KO mice. Representative gap-free current traces of ENaC in cell-attached patches made on the apical membranes of principal cells in split-open ASDN from WT (A) and CD ETA (B), ETB (C), and ETA/B KO (D) mice. The activity of ENaC before and after addition of 20 nM ET-1 is shown. Dashed lines show the respective current levels with C denoting the closed state. Areas under the bars labeled 1 (before) and 2 (after) are shown below at an expanded time scale. Summary graphs documenting the effects of ET-1 on ENaC Po from paired experiments similar to those in A–D are shown to the right: WT (E) and CD ETA (F), ETB (G), and ETA/B KO (H). *Significant decrease (P < 0.05) compared with before addition of ET-1.
Fig. 2.
ENaC Po in the presence of exogenous ET-1 is greater in CD ETB and CD ETA/B KO mice but not CD ETA KO mice compared with that in WT mice. Summary graph showing the relative Po of ENaC after ET-1 treatment in aldosterone-sensitive distal nepfron (ASDN) from WT mice compared with that in CD ETA, ETB, and ETA/B KO mice. Number of experiments in each group indicated. *Significantly greater compared with WT.
Src and MAPK activity are required for ET-1-dependent decreases in ENaC activity.
We showed previously in rat ASDN that Src and MAPK signaling are required for ET-1-dependent decreases in ENaC activity (3). Confirming this, both kinases also are required for ET-1-dependent regulation of ENaC in the murine ASDN (results not shown). In contrast to in their absence where ET-1 rapidly and markedly decreases ENaC Po, in the presence of the Src inhibitor PP2 (1.0 μM) or the MEK1/2 inhibitor PD98059 (10 μM), ET-1 failed to decrease ENaC Po. Such findings are consistent with conservation of mechanism across species.
Inverse relation between ENaC activity and dietary Na+ intake is blunted in CD ETB KO and CD ETA/B KO but not CD ETA KO mice.
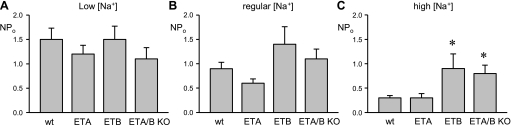
The activity of ENaC is known to be inversely related to dietary Na+ feeding decreasing as animals are fed diets containing greater amounts of Na+ (4, 22, 27, 29). The summary graph shown in Fig. 3A (see also Table 1) of results from patch-clamp experiments measuring ENaC NPo in ASDN from WT mice maintained with differing dietary Na+ regimens reveals this inverse relation. As demonstrated using an identical patch-clamp strategy, ENaC NPo in CD ETA KO (Fig. 3B), but not CD ETB KO (Fig. 3C) or CD ETA/B KO (Fig. 3D) mice, also is inversely related to dietary Na+ with activity in the prior group when maintained on a high Na+ regimen being significantly less than that with nominally Na+-free conditions. In contrast, specific gene deletion of ETB or both endothelin receptors in the CD disrupted this relation with ENaC activity not being different between Na+-free and high Na+ conditions. A consequence of this, as shown in Fig. 4 (see also Table 1), is that at all levels of dietary Na+ feeding ENaC activity in CD ETB and CD ETA/B KO mice but not CD ETA KO mice tends to be greater than that in WT mice reaching significance under high Na+ regimens.
Fig. 3.
ENaC in CD ETB and ETA/B KO mice is less responsive to changes in dietary Na+ intake. Summary graphs of the activity of ENaC in ASDN isolated from WT (A) and CD ETA (B), ETB (C), and ETA/B (D) KO mice maintained with different dietary Na+ regimens. Number of experiments in each group indicated. *Significantly less compared with sodium-free (<0.01% [Na+]) condition.
Table 1.
ENaC activity with differing dietary Na+ regimens in wild-type and CD-specific ET receptor KO mice
| Dietary Na+ | NPo | N | Po | f |
|---|---|---|---|---|
| Wild type | ||||
| Low | 1.5 ± 0.23 | 3.3 ± 0.40 | 0.41 ± 0.03 | 0.57 (27/47) |
| Regular | 0.9 ± 0.13* | 2.8 ± 0.25 | 0.29 ± 0.03* | 0.47 (32/68) |
| High | 0.3 ± 0.05* | 1.6 ± 0.15* | 0.16 ± 0.02* | 0.39 (26/66) |
| CD ETA KO | ||||
| Low | 1.2 ± 0.18 | 3.0 ± 0.25 | 0.38 ± 0.03 | 0.61 (35/57) |
| Regular | 0.6 ± 0.09* | 1.8 ± 0.19* | 0.27 ± 0.03* | 0.55 (28/51) |
| High | 0.3 ± 0.09* | 1.7 ± 0.18* | 0.17 ± 0.03* | 0.40 (18/45) |
| CD ETB KO | ||||
| Low | 1.5 ± 0.27 | 3.1 ± 0.44 | 0.42 ± 0.04 | 0.63 (21/33) |
| Regular | 1.4 ± 0.36 | 3.4 ± 0.69 | 0.32 ± 0.04 | 0.66 (19/29) |
| High | 0.9 ± 0.30† | 2.3 ± 0.27 | 0.22 ± 0.03* | 0.44 (17/39) |
| CD ETA/B KO | ||||
| Low | 1.1 ± 0.23 | 2.4 ± 0.41 | 0.43 ± 0.04 | 0.57 (12/21) |
| Regular | 1.1 ± 0.20 | 3.3 ± 0.48 | 0.30 ± 0.03 | 0.58 (19/33) |
| High | 0.8 ± 0.17† | 2.4 ± 0.34 | 0.34 ± 0.05† | 0.48 (11/23) |
Low, regular, and high dietary Na+ is <0.01, 0.32 and 2.0% Na+, respectively. f, frequency (patches with at least one active channel/total number of viable seals for that condition).
CD, collecting duct; ET, endothelin; KO, knockout; Nand Po, number of epithelial Na+ channel (ENaC) in a patch and the mean open probability of these channels, respectively.
Significantly lower versus low Na+ feeding within the same genotype, ANOVA with Dunnett's Test;
Significantly higher versus wild-type under identical conditions, t-test.
Fig. 4.
ENaC activity in the presence of high dietary Na+ is elevated in CD ETB and ETA/B KO mice. Summary graphs of the activity of ENaC in the ASDN isolated from WT and CD ETA, ETB. and ETA/B KO mice maintained with low- (A), regular- (B). and high-[Na+] diets (C). *Significantly greater compared with WT.
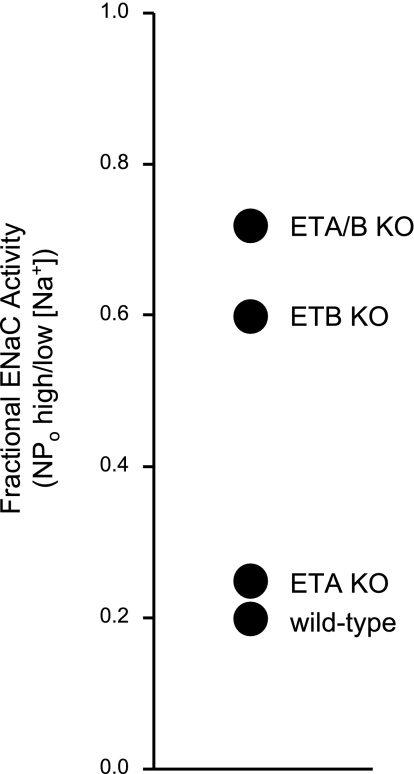
Specific deletion of the ETB receptor in the CD decreases the ability of ENaC to respond to changes in dietary Na+ intake locking channel activity high.
The results presented above demonstrate that ENaC in mice engineered to lack the ETB receptor specifically in the CD do not respond to ET-1 and have greater activity in the presence of ET-1. Moreover, the absence of ET-1 regulation of ENaC results in the channel being less able to respond to changes in dietary Na+ intake: ENaC activity in CD ETB and CD ETA/B KO but not CD ETA KO mice is greater compared with WT mice with high Na+ intake and the activity of ENaC in the latter but not former is inversely related to dietary Na+ intake. As we have reported previously (22, 27), a useful comparison to enable better appreciation of how the activity of ENaC changes as a function of dietary Na+ intake is to calculate fractional ENaC activity by dividing the activity the channel has under high-Na+ feeding conditions by that with nominally Na+-free conditions. The greater fractional ENaC activity becomes the less able the channel is to respond to changes in Na+ intake. A high fractional ENaC activity predicts a decrease in renal Na+ excretion and associated increases in Na+ retention and blood pressure. Figure 5 reports fractional ENaC activity for WT, CD ETA KO, CD ETB KO, and CD ETA/B KO mice. Fractional ENaC activity is elevated in the latter two groups consistent with these animals having compromised renal Na+ excretion and elevated blood pressure, particularly in the presence of high Na+ intake (9, 10). These results could explain the Na+ retention and elevated blood pressure observed in CD ET-1 KO, CD ETB KO, and CD ETA/B KO mice consistent with ENaC regulation by ET-1 via ETB receptors contributing to the antihypertensive and natriuretic effects of the local endothelin system in the mammalian CD (1, 8, 9, 10, 26).
Fig. 5.
The ability of ENaC to respond to changes in dietary Na+ is compromised in CD ETB and ETA/B KO mice. Fractional ENaC activity (activity observed with a high-Na+ diet divided by that with a Na+-free diet) for WT and CD ETA, ETB, and ETA/B KO mice.
DISCUSSION
The current results are explained by a local endothelin signaling system intrinsic to the ASDN playing a key role in modulating ENaC activity to facilitate matching of renal sodium excretion to sodium intake in the pursuit of maintaining blood pressure within a normal range. Our findings expand understanding of the cellular mechanisms underpinning such regulation. We show that ET-1 decreases the activity of ENaC in the murine ASDN. Consistent with that reported previously by us for ET-1 regulation of ENaC in the rat ASDN (3), Src and MEK1/2 kinases also are necessary for this regulation in the mouse. The ETB, but not necessarily the ETA receptor, is required for inhibitory regulation. The activity of ENaC in mice engineered to lack the ETB receptor specifically in the CD is greater in the presence of ET-1. Because of feedback regulation, the activity of ENaC normally is inversely related to dietary sodium intake. Deletion of the ETB receptor specifically in the ASDN compromises this relation to where ENaC is inappropriately active in the presence of high sodium intake. A consequence of this is that mice lacking the ETB receptor in the ASDN are unable to have a full response to changes in sodium balance, to include ETB receptor-dependent decreases in ENaC activity. Thus ENaC activity is inappropriately elevated in these knockout mice particularly in the face of a positive sodium balance.
ET-1 decreases ENaC activity.
The collecting duct produces more ET-1 than any other segment in the nephron (reviewed in 18). In the current studies, ET-1 significantly decreased the activity, specifically open probability, of ENaC in isolated, split-open murine ASDN in a rapid and reversible manner. This observation agrees with previous findings where ET-1 decreased the open probability of ENaC in rat ASDN, A6 cells, and NIH 3T3 cells stably expressing the channel (3, 5, 12). ET-1 is known to decrease sodium reabsorption in the isolated rat and rabbit collecting ducts (19, 28). Direct medullary infusion of an endothelin receptor agonist, moreover, increases sodium excretion (23). The consistency of this regulation across species implies that inhibitory regulation of ENaC by ET-1 in the ASDN is important to all mammals. Supporting this, knockout of the ET-1 gene specifically in the collecting duct compromises the pressure-natriuresis response, impairs sodium excretion, elevates blood pressure, and leads to salt sensitivity all of which are returned to normal by the ENaC blocker amiloride (1, 6, 26).
Inhibitory ET-1 actions on ENaC are mediated by the ETB receptor.
The current findings are most consistent with a receptor-mediated cascade coupling ET-1 to ENaC rather than a direct effect for the peptide hormone was introduced in our experiments outside the recording pipette, which contained the channel under study. Moreover, we find that specific deletion in the ASDN of either the ETB receptor or both the ETA and ETB receptors, but not the ETA receptor alone, abolishes the inhibitory effects of ET-1 on ENaC. This definitively establishes the ETB receptor as conveying the bulk of inhibitory ET-1 signaling to ENaC in the ASDN. This is in agreement with expression profiles and binding studies showing that the ETB receptor predominates compared with the ETA receptor in the ASDN (reviewed in Refs. 17 and 18). It also agrees with the identification using pharmacological tools of the ETB receptor as mediating ET-1 inhibition of ENaC in the isolated, split-open rat ASDN and A6 cells (3, 5). In addition, infusion of a specific ETB receptor antagonist directly into the medulla reduces the natriuretic response to volume expansion in rats without changing renal or medullary blood flow (13). Activation of ETB receptors with direct infusion of an agonist into the medulla, on the other hand, increases sodium excretion but only in the presence of a functional ETB receptor (23). Rats lacking the ETB receptor, moreover, are particularly prone to deoxycorticosterone acetate- and salt-induced hypertension, which is corrected by amiloride (6). Specific gene deletion in the ASDN of either the ETB receptor alone or both the ETB and ETA receptors, but not the ETA receptor alone, elevates blood pressure on a normal sodium diet, which further increases during high-sodium intake (9–11). These KO mice, moreover, have reduced sodium excretion after acute sodium loading with amiloride again correcting both the increase in blood pressure and impaired sodium excretion. The current results considered in the context of these earlier findings strongly point to locally derived ET-1 in the ASDN acting in an autocrine/paracrine manner through ETB receptors to exert an antihypertensive and natriuretic effect that when compromised impairs normal renal sodium excretion and consequently, elevates blood pressure.
Compromise of ET-1 regulation disrupts the inverse relation between dietary Na+ intake and ENaC activity.
As mentioned above and reported previously, the activity of ENaC in the ASDN is under feedback control resulting in an inverse relation between the activity of this channel and sodium intake (22, 27, 29). In addition, the preponderance of experimental findings in humans and rodents suggest that alterations in fluid volume status and sodium intake provoke corresponding changes in renal endothelin levels and urinary ET-1 excretion (reviewed in Refs. 17 and 18). Baseline and volume expansion-associated urinary ET-1 excretion is reduced in mice with CD-specific knockout of the ET-1 gene (1). The current findings that the activity of ENaC in ASDN from CD ETB and CD ETA/B receptor KO mice lacks a tight association with changes in sodium intake being significantly elevated in the presence of high sodium intake compared with similarly treated control animals identifies the local endothelin system intrinsic to the ASDN as playing a role in feedback regulation of ENaC and as such, ultimately renal sodium excretion. That deoxycorticosterone acetate exacerbates the blood pressure and impaired sodium excretion phenotype in animals lacking the ETB receptor suggests that local control by the endothelin system functions in parallel with the RAAS (6).
ENaC activity is elevated in the absence of inhibitory ET-1-ETB receptor signaling, and the channel is unable to respond appropriately to changes in dietary Na+ intake.
Loss of inhibitory endothelin regulation of sodium excretion, as shown with pharmacological tools and specific gene deletion in the ASDN of ET-1, the ETB receptor, or both the ETA and ETB receptors, is prohypertensive and sensitive to amiloride (reviewed in 18). The current results reveal the mechanism of this. Loss of inhibitory regulation by endothelin via ETB receptors impairs feedback control of ENaC in response to changes in dietary sodium intake. As a result ENaC is inappropriately active, particularly in the presence of a sodium load. This compromises the ability of the kidney to excrete sodium and volume impairing the appropriate natriuretic response to a positive sodium balance leading to an elevation in blood pressure, which increases in response to further increases in sodium.
GRANTS
This research was supported by the National Heart, Lung, and Blood Institute P01 HL-095499 grant.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: V.B., E.M., D.E.K., and J.D.S. conception and design of research; V.B. and E.M. performed experiments; V.B. and E.M. analyzed data; V.B., E.M., and J.D.S. prepared figures; V.B., E.M., D.E.K., and J.D.S. approved final version of manuscript; E.M., D.E.K., and J.D.S. interpreted results of experiments; J.D.S. drafted manuscript; J.D.S. edited and revised manuscript.
REFERENCES
- 1. Ahn D, Ge Y, Stricklett PK, Gill P, Taylor D, Hughes AK, Yanagisawa M, Miller L, Nelson RD, Kohan DE. Collecting duct-specific knockout of endothelin-1 causes hypertension and sodium retention. J Clin Invest 114: 504–511, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bonny O, Hummler E. Dysfunction of epithelial sodium transport: From human to mouse. Kidney Int 57: 1313–1318, 2000 [DOI] [PubMed] [Google Scholar]
- 3. Bugaj V, Pochynyuk O, Mironova E, Vandewalle A, Medina JL, Stockand JD. Regulation of the epithelial Na+ channel by endothelin-1 in rat collecting duct. Am J Physiol Renal Physiol 295: F1063–F1070, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bugaj V, Pochynyuk O, Stockand JD. Activation of the epithelial Na+ channel in the collecting duct by vasopressin contributes to water reabsorption. Am J Physiol Renal Physiol 297: F1411–F1418, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gallego MS, Ling BN. Regulation of amiloride-sensitive Na+ channels by endothelin-1 in distal nephron cells. Am J Physiol Renal Fluid Electrolyte Physiol 271: F451–F460, 1996 [DOI] [PubMed] [Google Scholar]
- 6. Gariepy CE, Ohuchi T, Williams SC, Richardson JA, Yanagisawa M. Salt-sensitive hypertension in endothelin-B receptor-deficient rats. J Clin Invest 105: 925–933, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Garty H, Palmer LG. Epithelial sodium channels: function, structure, and regulation (Review). Physiol Rev 77: 359–396, 1997 [DOI] [PubMed] [Google Scholar]
- 8. Ge Y, Ahn D, Stricklett PK, Hughes AK, Yanagisawa M, Verbalis JG, Kohan DE. Collecting duct-specific knockout of endothelin-1 alters vasopressin regulation of urine osmolality. Am J Physiol Renal Physiol 288: F912–F920, 2005 [DOI] [PubMed] [Google Scholar]
- 9. Ge Y, Bagnall A, Stricklett PK, Strait K, Webb DJ, Kotelevtsev Y, Kohan DE. Collecting duct-specific knockout of the endothelin B receptor causes hypertension and sodium retention. Am J Physiol Renal Physiol 291: F1274–F1280, 2006 [DOI] [PubMed] [Google Scholar]
- 10. Ge Y, Bagnall A, Stricklett PK, Webb D, Kotelevtsev Y, Kohan DE. Combined knockout of collecting duct endothelin A and B receptors causes hypertension and sodium retention. Am J Physiol Renal Physiol 295: F1635–F1640, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ge Y, Stricklett PK, Hughes AK, Yanagisawa M, Kohan DE. Collecting duct-specific knockout of the endothelin A receptor alters renal vasopressin responsiveness, but not sodium excretion or blood pressure. Am J Physiol Renal Physiol 289: F692–F698, 2005 [DOI] [PubMed] [Google Scholar]
- 12. Gilmore ES, Stutts MJ, Milgram SL. SRC family kinases mediate epithelial Na+ channel inhibition by endothelin. J Biol Chem 276: 42610–42617, 2001 [DOI] [PubMed] [Google Scholar]
- 13. Guo X, Yang T. Endothelin B receptor antagonism in the rat renal medulla reduces urine flow rate and sodium excretion. Exp Biol Med (Maywood) 231: 1001–1005, 2006 [PubMed] [Google Scholar]
- 14. Hille B. Ion Channels of Excitable Membranes. Sunderland, MA: Sinauer, 2001 [Google Scholar]
- 15. Hummler E, Horisberger JD. Genetic disorders of membrane transport. V. The epithelial sodium channel and its implication in human diseases. Am J Physiol Gastrointest Liver Physiol 276: G567–G571, 1999 [DOI] [PubMed] [Google Scholar]
- 16. Kellenberger S, Schild L. Epithelial sodium channel/degenerin family of ion channels: a variety of functions for a shared structure. Physiol Rev 82: 735–767, 2002 [DOI] [PubMed] [Google Scholar]
- 17. Kohan DE. Endothelin, hypertension and chronic kidney disease: new insights. Curr Opin Nephrol Hypertens 19: 134–139, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kohan DE, Rossi NF, Inscho EW, Pollock DM. Regulation of blood pressure and salt homeostasis by endothelin. Physiol Rev 91: 1–77, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kurokawa K, Yoshitomi K, Ikeda M, Uchida S, Naruse M, Imai M. Regulation of cortical collecting duct function: Effect of endothelin. Am Heart J 125, Suppl: 582–588, 1993 [DOI] [PubMed] [Google Scholar]
- 20. Lifton RP, Gharavi AG, Geller DS. Molecular mechanisms of human hypertension. Cell 104: 545–556, 2001 [DOI] [PubMed] [Google Scholar]
- 21. Lyon-Roberts B, Strait KA, van PE, Kittikulsuth W, Pollock JS, Pollock DM, Kohan DE. Flow regulation of collecting duct endothelin-1 production. Am J Physiol Renal Physiol 300: F650–F656, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mironova E, Peti-Peterdi J, Bugaj V, Stockand JD. Diminished paracrine regulation of the epithelial Na+ channel by purinergic signaling in mice lacking connexin 30. J Biol Chem 286: 1054–1060, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Nakano D, Pollock JS, Pollock DM. Renal medullary ETB receptors produce diuresis and natriuresis via NOS1. Am J Physiol Renal Physiol 294: F1205–F1211, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rossier BC, Pradervand S, Schild L, Hummler E. Epithelial sodium channel and the control of sodium balance: interaction between genetic and environmental factors. Annu Rev Physiol 64: 877–897, 2002 [DOI] [PubMed] [Google Scholar]
- 25. Sakmann B, Neher E. Single-Channel Recording. New York: Plenum, 1983 [Google Scholar]
- 26. Schneider MP, Ge Y, Pollock DM, Pollock JS, Kohan DE. Collecting duct-derived endothelin regulates arterial pressure and Na excretion via nitric oxide. Hypertension 51: 1605–1610, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Stockand JD, Mironova E, Bugaj V, Rieg T, Insel PA, Vallon V, Peti-Peterdi J, Pochynyuk O. Purinergic inhibition of ENaC produces aldosterone escape. J Am Soc Nephrol 21: 1903–1911, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tomita K, Nonoguchi H, Terada Y, Marumo F. Effects of ET-1 on water and chloride transport in cortical collecting ducts of the rat. Am J Physiol Renal Fluid Electrolyte Physiol 264: F690–F696, 1993 [DOI] [PubMed] [Google Scholar]
- 29. Vallon V, Hummler E, Rieg T, Pochynyuk O, Bugaj V, Schroth J, Dechenes G, Rossier B, Cunard R, Stockand J. Thiazolidinedione-induced fluid retention is independent of collecting duct alphaENaC activity. J Am Soc Nephrol 20: 721–729, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Waselle L, Gerona RR, Vitale N, Martin TF, Bader MF, Regazzi R. Role of phosphoinositide signaling in the control of insulin exocytosis. Mol Endocrinol 19: 3097–3106, 2005 [DOI] [PubMed] [Google Scholar]