Abstract
The receptor for advanced glycation end products (RAGE) plays an important role in host defense against bacterial infection. In the present experiments, we investigated the mechanisms by which RAGE contributes to the ability of neutrophils to eradicate bacteria. Wild-type (RAGE+/+) neutrophils demonstrated significantly greater ability to kill Eschericia coli compared with RAGE−/− neutrophils. After intraperitoneal injection of E. coli, increased numbers of bacteria were found in the peritoneal fluid from RAGE−/− as compared with RAGE+/+ mice. Exposure of neutrophils to the protypical RAGE ligand AGE resulted in activation of nicotinamide adenine dinucleotide phosphate (NADPH) oxidase and enhanced killing of E. coli, and intraperitoneal injection of AGE enhanced bacterial clearance during peritonitis. However, incubation of neutrophils with high mobility group box 1 protein (HMGB1), which also binds to RAGE, diminished E. coli-induced activation of NADPH oxidase in neutrophils and bacterial killing both in vitro and in vivo. Deletion of the COOH-terminal tail of HMGB1, a region necessary for binding to RAGE, abrogated the ability of HMGB1 to inhibit bacterial killing. Incubation of neutrophils with HMGB1 diminished bacterial or AGE-dependent activation of NADPH oxidase. The increase in phosphorylation of the p40phox subunit of NADPH oxidase that occurred after culture of neutrophils with E. coli was inhibited by exposure of the cells to HMGB1. These results showing that HMGB1, through RAGE-dependent mechanisms, diminishes bacterial killing by neutrophils as well as NADPH oxidase activation provide a novel mechanism by which HMGB1 can potentiate sepsis-associated organ dysfunction and mortality.
Keywords: receptor for advanced glycation end products, nicotinamide adenine dinucleotide phosphate oxidase, peritonitis, sepsis, inflammation, Eschericia coli
neutrophils play central roles in acute inflammatory and innate immune responses through producing anti-bacterial peptides, cytokines, and other proinflammatory mediators, including reactive oxygen intermediates, and contributing to the formation of extracellular traps (8, 22, 33). Nicotinamide adenine dinucleotide phosphate (NADPH) oxidase, a high-output, superoxide-generating system, is an important source of the reactive oxygen intermediates produced by activated neutrophils. Deficiency in NADPH oxidase is associated with diminished ability of neutrophils to effectively kill bacteria. There is increased susceptibility to infection with Staphylococci and other extracellular bacteria in patients with chronic granulomatous disease, a condition associated with a hereditary defect of NADPH oxidase (14, 15, 17, 18).
The receptor for advanced glycation end products (RAGE) recognizes a diverse spectrum of ligands, including nonenzymatically modified glycoproteins such as advanced glycation end products (AGE) (31, 32), as well as high mobility group box 1 protein (HMGB1) and calgranulin (calcium binding cellular factors, S100B) (19, 20). Cellular activation through RAGE engagement is involved in regulating cellular functions, such as metabolic homeostasis and chemotaxis, and also participates in pathological conditions associated with dysregulated inflammation including diabetes, atherosclerosis, arthritis, and cancer progression (23, 28, 36). Cellular pathways downstream of RAGE engagement lead to activation of mitogen-activated protein kinase signaling and nuclear factor-κB, with expression of pro-inflammatory cytokines, chemokines, and adhesion molecules (9, 43). Transgenic mice deficient in RAGE expression develop less severe lung and liver injury in sepsis models (23, 29). Blockade of interactions between RAGE and its ligands, achieved through systemic administration of soluble RAGE, results in improved outcome from sepsis (37). However, inhibition of RAGE engagement has potent immunosuppressive effects as shown by increased bacterial growth in experimental models of peritonitis (39). The apparent discrepancy between the effects of RAGE in experimental sepsis CLP versus peritonitis is not determined, and in particular, the mechanism by which RAGE contributes to the eradication of bacteria is not known. However, previous studies have shown that RAGE engagement can lead to activation of NADPH oxidase, an event associated with enhanced bacterial killing by neutrophils, macrophages, and other cell populations (12, 33).
In the present experiments, we investigated the mechanisms by which RAGE contributes to the ability of neutrophils to eradicate bacteria under both in vitro and in vivo settings. We found that RAGE activation facilitated bacterial killing by neutrophils, likely through mechanisms involving activation of NADPH oxidase. Surprisingly, although both AGE and HMGB1 are known RAGE ligands, they evoked opposite effects on bacterial killing. Our results revealed that unlike prototypical RAGE ligands, such as AGE, HMGB1 through downregulating activation of NADPH oxidase can inhibit the bactericidal ability of neutrophils. Such findings provide new insights into the mechanisms by which RAGE and HMGB1 may contribute to inflammation and organ dysfunction during severe infection and sepsis.
MATERIALS AND METHODS
Mice.
Male C57BL/6 mice (wild-type) were purchased from the National Cancer Institute, Frederick, MD. Mice deficient in RAGE (RAGE−/−) were a gift from Dr. A. Bierhaus (University of Heidelberg, Heidelberg, Germany). Mice with mutation of p47phox (C57BL/6J-Ncf1m1J/J) were obtained from Jackson Laboratory (Bar Harbor, ME). Mice were housed and studied at the University of Alabama at Birmingham using Institutional Animal Care and Use Committee-approved protocols. Experiments were performed using 8- to 10-wk-old mice.
Reagents.
RPMI 1640 was purchased from BioWhittaker (Walkersville, MD). FBS and penicillin-streptomycin were obtained from Gemini Bioproducts (Calabasas, CA). Hanks balanced salt solution (HBSS) was purchased from Invitrogen (Grand Island, NY). Custom antibody mixtures (Abs) and negative selection columns for neutrophil isolation were from StemCell Technologies (Vancouver, Canada). AGE-modified bovine serum albumin was from BioVision (Mountain View, CA). S100B was purchased from R&D (Minneapolis, MN). Recombinant HMGB1 and mutant HMGB1 lacking the COOH-terminal tail (ΔC-HMGB1) were prepared as previously described (5, 34). Cytochrome c and antibody to actin were purchased from Sigma-Aldrich (St. Louis, MO). Anti-phospho-p40phox (Thr154) antibody was purchased from Cell Signaling (Danvers, MA).
Isolation of neutrophils.
Mouse neutrophils were purified from bone marrow cell suspensions essentially as described previously (45). In brief, bone marrow cells were incubated with 30 μl of Ab cocktail specific to the cell surface markers F4/80, CD4, CD45R, CD5, and TER119 for 15 min at 4°C. Anti-biotin tetrameric Ab complexes (100 μl) were then added to the cells and incubated for 15 min at 4°C followed by incubation with 60 μl of colloidal magnetic dextran iron particles for 15 min at 4°C. The cell suspension was then placed into a column surrounded by a magnet. The T cells, B cells, red blood cells, monocytes, and macrophages were captured in the column, allowing the neutrophils to pass through as a result of negative selection. Cells were then washed with RPMI 1640 with or without FBS (5%). Neutrophil purity, as determined by Wright-Giemsa-stained cytospin preparations, was consistently greater than 98%.
In vitro killing activity assay.
Neutrophils (0.5 × 106) were incubated with ampicillin-resistant Escherica coli DH5α (1 × 106 CFU) in RPMI 1640 medium (1 ml) without serum for 90 min at 37°C. Next, 20 μl of cell/bacterial suspension was incubated with 480 μl Triton X-100 (0.1%) for 10 min to lyse neutrophils. Serial dilutions were then plated on agar plates with ampicillin and incubated overnight at 37°C. The number of bacterial colonies on agar plates was determined using colony counter software (Bio-Rad, Hercules, CA). Of note, incubation of bacteria with 0.1% Triton X-100 for 10 min had no significant effect on bacterial viability.
In vivo killing activity assay.
The efficiency of bacterial eradication in vivo was performed as previously described (30). In brief, mice were subjected to intraperitoneal administration of 200 μl ampicillin-resistant E. coli (104/ml saline) for 3 h. Peritoneal lavage fluid was acquired using 10 ml RPMI 1640 medium without serum, and the total number of cells and neutrophils were determined. The number of surviving bacteria was determined by incubation of the peritoneal lavage (95 μl) with 1% Triton X-100 (5 μl) for 10 min to lyse cells, and then serial dilutions were incubated on agar plates overnight at 37°C. Bacterial colonies were counted using colony counter software (Bio-Rad).
Assay for NADPH activity.
NADPH oxidase activity was measured using a standard cytochrome c reduction assay as previously described (25, 26). Briefly, neutrophils (5 × 105/ml) were incubated with cytochrome c (10 μM) in the presence or absence of E. coli (1 × 106), AGE (100 μg), HMGB1, or ΔC-HMGB1 (300 ng) in 1 ml of HBSS. The rate of cytochrome c reduction was recorded (λ = 550 nm, ϵM = 21 mM−1·cm−1) using a spectrophotometer (UV-2501PC Shimadzu; Shimadzu, Japan) for 15 min.
Imaging of DCF fluorescence.
Intracellular level of reactive oxygen species (ROS) was determined using the redox-sensitive probes DCFH-DA in conjunction with fluorescent microscopy (41, 44, 46). Briefly, neutrophils (2.5 × 106/well) were incubated with DCFH-DA (10 μM) in a four-well chambered coverglass (Nalge, Naperville, IL) for 60 min and then treated with E. coli (106 /well) for an additional 30 min. Fluorescent microscopic images were acquired using double bidirectional scans of live neutrophils with a Leica DMIRBE inverted epifluorescence/Nomarski microscope outfitted with Leica TCS NT laser confocal optics.
Imaging of cell surface RAGE.
Neutrophils were treated as indicated in the figure legends and incubated with paraformaldehyde (4%) in PBS for 20 min at room temperature. Cells were then washed with PBS and incubated with bovine serum albumin (3%) in PBS for 45 min, followed by incubation with specific antibodies to RAGE (Santa Cruz Biotechnology, Santa Cruz, CA) overnight at 4°C. Next, cells were washed with PBS and incubated with AlexaFluor 488-labeled antibody (Invitrogen, Carlsbad, CA) for 90 min at room temperature. After the cells were washed with PBS, they were mounted with emulsion oil solution containing DAPI to visualize nuclei. Microscopy was performed using a confocal laser scanning microscope (model LSM 710 confocal microscope; Carl Zeiss MicroImaging) provided by the High Resolution Imaging Facility at the University of Alabama at Birmingham.
Western blot analysis.
Western blot analysis was performed as previously described (46). Briefly, equal amounts of protein were resolved using 8 or 12% SDS-PAGE and transferred onto PVDF membranes (polyvinylidenedifluoride membrane, Immobilon-P; Millipore, Billerica, MA). To measure the amount of total and phosphorylated proteins, membranes were probed with specific antibodies followed by detection with horseradish peroxidase-conjugated goat anti-rabbit IgG. Bands were visualized by enhanced chemiluminescence (ECL Plus, Amersham) and quantified by AlphaEase FC software (Alpha Innotech, San Leandro, CA).
Statistical analysis.
Data are presented as means ± SD for each experimental group. One-way ANOVA followed by analysis with the Tukey-Kramer test was performed for comparisons between multiple groups, and Student's t-test was used for comparisons between two groups. A value of P < 0.05 was considered significant.
RESULTS
Activation of RAGE enhances the ability of neutrophils to eradicate bacteria in vitro and in vivo.
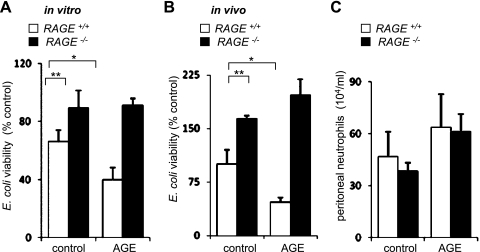
In initial experiments, we determined the ability of cultured control (RAGE+/+) and RAGE-deficient (RAGE−/−) neutrophils to kill bacteria. As shown in Fig. 1A, there was significantly greater killing of E. coli (∼30%) within 2 h after incubation with wild-type neutrophils compared with that found after coculture with RAGE-deficient neutrophils. The importance of RAGE for bacterial killing was established by inclusion of the prototypical RAGE ligand AGE in the neutrophil cultures. Exposure of wild-type, but not RAGE−/−, neutrophils to AGE resulted in enhanced killing of bacteria (Fig. 1A). Next, we determined whether deficiency in RAGE has similar effects in vivo. Mice were given E. coli (106) intraperitoneally, and then the number of viable bacteria was determined in peritoneal lavage fluid obtained 3 h later. As shown in Fig. 1B, RAGE-deficient mice had decreased ability to eradicate bacteria compared with RAGE+/+ mice.
Fig. 1.
Effects of advanced glycation end products (RAGE) deficiency on bacterial killing in vitro and in vivo. A: bone marrow neutrophils (0.5 × 106 / ml) obtained from RAGE+/+ or receptor for RAGE-deficient mice (RAGE−/−) were pretreated with AGE (0 or 100 μg/ml) for 15 min and then incubated with Eschericia coli (106/ml) for an additional 90 min. Means ± SD values were obtained from three independent experiments. **P < 0.01 comparing RAGE+/+ with RAGE−/− or *P < 0.05 comparing control RAGE+/+ with AGE-treated RAGE+/+ neutrophils. The numbers of surviving bacteria were calculated as the percentage of E. coli numbers obtained after culture without neutrophils. B and C: wild-type (WT) (RAGE+/+) or RAGE−/− mice were subjected to intraperitoneal injection of AGE (0 or 300 μg) and E. coli (104) with peritoneal lavage 3 h later. The numbers of viable E. coli (B) and total numbers of neutrophils (C) obtained from peritoneal lavages are shown. The number of E. coli (9.1 ± 1.8 × 104 CFU/ml) obtained from peritoneal lavage of RAGE+/+ mice was used as a control (100% viability) to calculate percent viability of E. coli recovered from AGE-treated and from RAGE-deficient mice. Values are means ± SD (n = 3). **P < 0.01 comparing RAGE+/+ with RAGE−/− or *P < 0.05 comparing control RAGE+/+ with AGE-treated RAGE+/+ mice.
Intraperitoneal injection of the RAGE ligand AGE enhanced bacterial killing during peritonitis by wild-type mice, but AGE had minimal effect in RAGE-deficient mice. Similar numbers of neutrophils were present in peritoneal lavages after E. coli infection in RAGE+/+ or RAGE−/− mice (Fig. 1C), indicating that the diminished bacterial killing found in RAGE−/− mice was not due to decreased recruitment of neutrophils into the peritoneum but rather to alterations in cellular response.
Exposure to HMGB1 decreases the ability of neutrophils to kill bacteria.
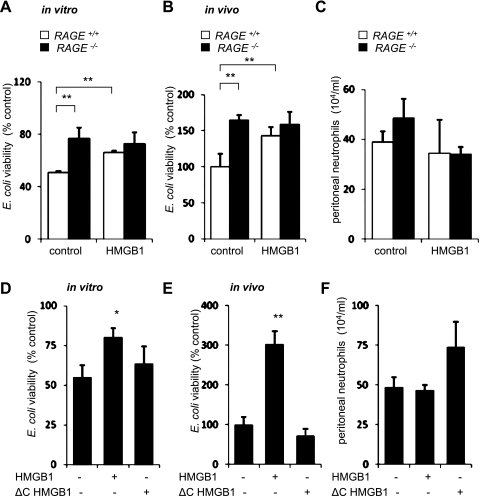
Because activation of RAGE with AGE enhanced killing of bacteria by neutrophils, we determined whether such anti-bacterial effects were also induced by HMGB1, which has been shown to bind with high affinity to RAGE (1). However, unlike the potentiating effects of AGE on bacterial killing, culture of RAGE+/+neutrophils with HMGB1 was associated with increased survival of bacteria compared with incubation of neutrophils with E. coli alone (Fig. 2A). In these experiments, the inhibitory effects of HMGB1 on bacterial killing occurred at a concentration of 300 ng/ml, which is similar to HMGB1 levels previously reported in the lungs and circulation in critically ill patients with sepsis or acute lung injury (3).
Fig. 2.
Effects of high mobility group box 1 protein (HMGB1) on bacterial killing. A: neutrophils (0.5 × 106/ml) were incubated with HMGB1 (0 or 300 ng/ml) for 15 min and then cultured with E. coli (106/ml) for 90 min, or E. coli were incubated without neutrophils. Means ± SD values were obtained from three independent experiments. **P < 0.01 comparing RAGE+/+ with RAGE−/− or comparing HMGB1-treated RAGE+/+ with RAGE+/+ neutrophils. In B and C, mice were given E. coli (104 ip) with or without HMGB1 (0 or 300 ng ip), and then peritoneal lavages were obtained 3 h later. The percentage of viable bacteria (B) and total numbers of neutrophils (C) in peritoneal lavage are shown. The number of E. coli (10.4 ± 1.9 × 104 CFU/ml) obtained from peritoneal lavage of RAGE+/+ mice not given HMGB1 was used as a control (100% viability). Values are means ± SD (n = 3). **P < 0.01 compared with untreated RAGE+/+ controls. D–F: deletion of the COOH-terminal region of HMGB1 (ΔC-HMGB1) diminishes the ability of HMGB1 to inhibit bacterial killing. D: number of E. coli obtained after 90 min of culture with neutrophils that were pretreated with HMGB1 or ΔC-HMGB1 (0 or 300 ng/ml) for 15 min. Values are means ± SD (n = 3). *P < 0.05 comparing untreated to HMGB1-treated cells. E and F: percentages of remaining viable bacteria (E) and numbers of neutrophils (F) in peritoneal lavages that were obtained 3 h after mice received E. coli (104 ip) with or without full-length HMGB1 or mutant ΔC-HMGB1. The number of E. coli (9.2 ± 1.4 × 104 CFU/ml) obtained from peritoneal lavage of mice not exposed to HMGB1 or ΔC-HMGB1 was used as a control (100% viability). Values are means ± SD (n = 3). *P < 0.05 compared with untreated cells.
The ability of HMGB1 to inhibit bacterial killing was also found under in vivo conditions, where increased numbers of bacteria were present in peritoneal lavages of mice given HMGB1 at the same time as E. coli (Fig. 2B). Of note, similar numbers of peritoneal neutrophils were recovered after intraperitoneal E. coli administration with or without HMGB1 (Fig. 2C).
To determine whether the inhibitory effect of HMGB1 on bacterial killing is related to its ability to bind RAGE, neutrophils were incubated with ΔC-HMGB1, which is unable to interact with RAGE (1, 5). As shown in Fig. 2D, unlike full-length HMGB1, which diminished bacterial killing by neutrophils, inclusion of ΔC-HMGB1 in the neutrophil cultures had no effect on bacterial killing.
Increased numbers of neutrophils compared with control and HMGB1-treated mice were found in peritoneal lavages from mice treated with ΔC-HMGB1 intraperitoneally (Fig. 2E). However, administration of ΔC-HMGB1 had no effect on intra-abdominal clearance of E. coli, unlike the inhibitory effects of full-length HMGB1 (Fig. 2F). These in vitro and in vivo results suggest that interaction with RAGE is responsible for the inhibitory effects of HMGB1 on bacterial killing.
Exposure of neutrophils to HMGB1 inhibits activation of NADPH oxidase.
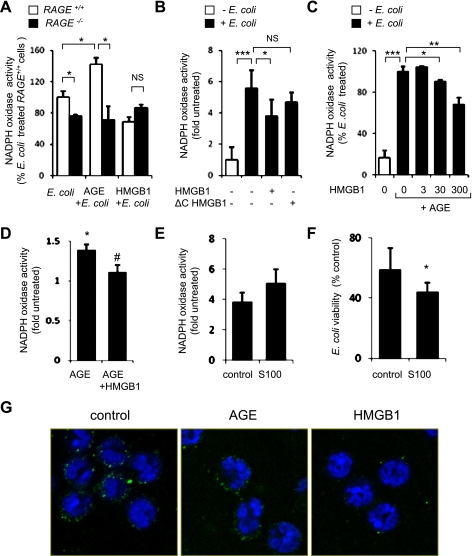
Activation of NADPH oxidase and production of ROS by neutrophils are essential for the efficient eradication of extracellular bacteria (33). Because interaction with AGE and HMGB1 had differing effects on bacterial killing by neutrophils, we determined whether such differences were related to the extent of NADPH oxidase activation. As shown in Fig. 3A, exposure of neutrophils to E. coli or AGE rapidly induced NADPH oxidase activity. In contrast, HMGB1 diminished bacterial or AGE-dependent activation of NADPH oxidase. Whereas incubation of neutrophils with HMGB1 dose dependently decreased AGE-induced NADPH oxidase activation (Fig. 3C), ΔC-HMGB1 (Fig. 3B) or heat-inactivated HMGB1 (data not shown) did not alter the ability of neutrophils to activate NADPH oxidase. Simultaneous addition of HMGB1 (300 ng/ml) and AGE (100 μg/ml) into the neutrophil cultures resulted in decreased activation of NADPH oxidase compared with stimulation of neutrophils with AGE alone (Fig. 3D). Of note, the participation of RAGE in the activation of NADPH oxidase and in killing of bacteria was confirmed when neutrophils were incubated with S100B, an additional RAGE ligand (19), S100B ligand (Fig. 3, E and F). Although similar amounts of RAGE were detected on the cell membrane of control and AGE-treated neutrophils, a marked decrease in RAGE expression was found after neutrophils were cultured with HMGB1 (Fig. 3G).
Fig. 3.
Effects of AGE, HMGB1, S100B, and ΔC-HMGB1 on nicotinamide adenine dinucleotide phosphate (NADPH) oxidase activity in E. coli-stimulated neutrophils. A: NADPH oxidase activity was determined in WT (RAGE+/+) and RAGE-deficient (RAGE−/−) neutrophils after incubation of the cells with AGE (0 or 100 μg/ml) or HMGB1 (0 or 300 ng/ml) for 15 min followed by culture with E. coli (106/ml) for an additional 15 min. Values are means ± SD (n = 3). *P < 0.05, NS, not significant. B: NADPH oxidase activity in neutrophils treated with HMGB1 or ΔC-HMGB1. Cells were cultured with HMGB1 (0 or 300 ng/ml) or ΔC-HMGB1 (0 or 300 ng/ml) for 15 min and then exposed to E. coli (0 or 106/ml) for 15 min. Values are means ± SD (n = 3). *P < 0.05, ***P < 0.001, NS, not significant. C: neutrophils were treated with HMGB1 (0, 3, 30, or 300 ng/ml) for 15 min and then incubated with AGE (0 or 100 μg/ml) and E. coli (106/ml) for a further 15 min. NADPH oxidase activity was then measured. Values are means ± SD (n = 3). *P < 0.05, **P < 0.01, ***P < 0.001. D: NADPH oxidase activity was measured in neutrophils treated with AGE (0 or 100 μg/ml) or simultaneously with AGE (100 μg/ml) and HMGB1 (300 ng/ml) for 15 min. Values are mean ± SD (n = 3). *P < 0.05 compared with untreated cells or #P < 0.05 compared with neutrophils treated with AGE alone. E: NADPH oxidase activity was determined after incubation of neutrophils (RAGE+/+) with S100B (0 or 50 μg/ml) for 15 min. Values are means ± SD (n = 3). F: ability of neutrophils to eradicate E. coli was determined after exposure of neutrophils (0.5 × 106/ml) to S100B (0 or 50 μg/ml) for 15 min followed by inclusion of E. coli (0 or 106/ml) in the cultures for an additional 120 min. Values are means ± SD (n = 3). *P < 0.05 compared S100B treated with control. G: representative images show expression of RAGE on the cell surface of control neutrophils and neutrophils treated with HMGB1 or AGE for 2 h. Green, RAGE; blue, nuclei.
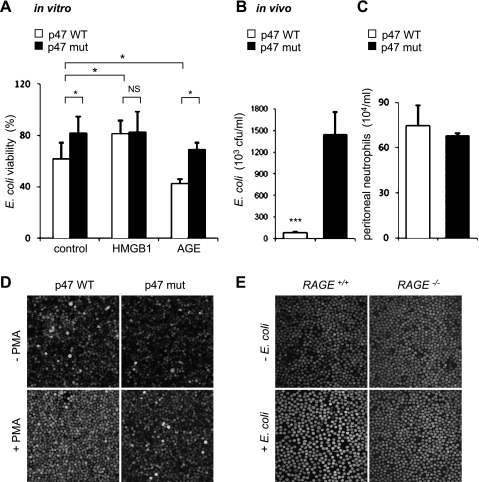
In additional experiments (Fig. 4), we confirmed that activation of NADPH oxidase by PMA potentiated, whereas inactivation of NADPH oxidase due to mutation of the p47phox subunit diminished ROS formation and bacterial killing by neutrophils under both in vitro and in vivo conditions. Of note, exposure to E. coli resulted in marked increase of ROS formation in RAGE+/+ compared with RAGE−/− neutrophils (Fig. 4E). These results suggest that activation of RAGE followed by ROS production promoted killing of bacteria.
Fig. 4.
Inactivation of NADPH oxidase diminishes eradication of bacteria in vitro and in vivo. A: effects of HMGB1 or AGE on the ability of WT and p47phox mutant neutrophils to kill bacteria. Neutrophils (WT or p47 mutant) were incubated with HMGB1 (0 or 300 ng/ml) for 15 min or with AGE (0 or 100 μg/ml) for 15 min followed by culture with E. coli (106/ml) for an additional 15 min. NADPH oxidase activity was then measured. Values are means ± SD (n = 3). *P < 0.05. B and C: wild-type (C57BL/6) mice and mice deficient in NADPH oxidase activity (C57BL/6J-Ncf1m1J/J) were subjected to intraperitoneal administration of E. coli (104), and then the numbers viable E. coli (p47 WT 8 ± 7 × 104 CFU/ml and p47 mutant 1.45 ± 0.31 × 104 CFU/ml) (B) and total number of neutrophils (C) determined in peritoneal lavages were obtained 3 h later. Values are means ± SD (n = 3). ***P < 0.001 compared with WT mice. D and E: representative images show the level of DCF fluorescence in p47phox WT or mutant neutrophils treated with PMA (0 or 50 nM) for 30 min (D) or after exposure RAGE+/+ or RAGE−/− neutrophils to E. coli (E).
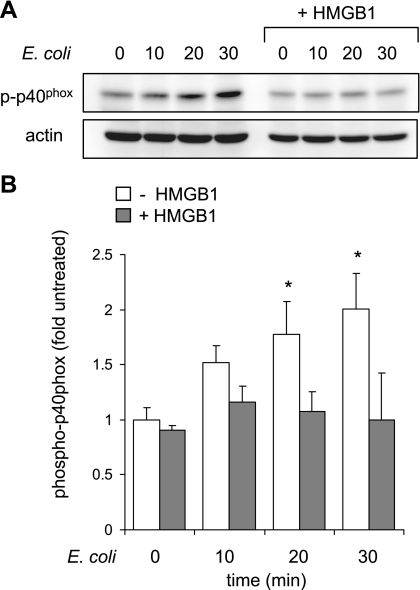
Activation of NADPH oxidase requires phosphorylation and assembly of several subunits, including p40phox. Phosphorylation of p40phox has been shown to be essential step for activation of NADPH oxidase in neutrophils (10). Because HMGB1 inhibited NADPH oxidase activation by AGE or bacteria, we determined the phosphorylation status of the p40phox subunit of NOX2 in neutrophils exposed to HMGB1. As shown in Fig. 5, the increase in p40phox phosphorylation that occurred after culture of neutrophils with E. coli was inhibited by exposure of the cells to HMGB1. These results suggest that the ability of HMGB1 to inhibit NADPH oxidase activation may be a potential mechanism by which HMGB1 diminishes bacterial killing both in vitro and in vivo.
Fig. 5.
HMGB1 diminishes p40phox phosphorylation in E. coli-stimulated neutrophils. Representative Western blots show p40phox phosphorylation and actin levels obtained from neutrophils treated with E. coli (106) for 0, 10, 20, or 30 min. Cells were cultured with HMGB1 (0 or 300 ng/ml) for 15 min before addition of E. coli. Values are means ± SD (n = 3). *P < 0.05 comparing neutrophils cultured with E. coli alone to control (untreated) neutrophils or to neutrophils treated with HMGB1 and E. coli.
DISCUSSION
In the present studies, we found that activation of RAGE plays a central role in the eradication of bacteria by neutrophils under in vitro and in vivo conditions. However, unlike AGE or S100B, two RAGE ligands, which increased bacterial killing by neutrophils in culture and in an experimental model of peritonitis, and HMGB1, which has also been shown to interact with RAGE, had opposite effects, diminishing bacterial killing under both in vitro and in vivo conditions. The involvement of RAGE in bacterial killing is consistent with previous studies using experimental models of peritonitis that showed enhanced bacterial growth in the peritoneum as well as systemic spread of bacteria in RAGE-deficient mice (39).
Although signaling pathways, including activation of mitogen-activated protein kinases and nuclear factor-κB, are known to be activated upon engagement of RAGE (7, 21), little is known about the mechanisms by which RAGE facilitates bacterial eradication. Previous studies demonstrated that engagement of RAGE on neutrophil like cell lines resulted in activation of NADPH oxidase and production of ROS (27). The present experiments showed that engagement of RAGE on neutrophils by AGE also resulted in NADPH activation and contributed to bacterial killing.
Activation of NADPH oxidase in neutrophils is known to play a key role in host defense against bacteria. In the present studies, we found that culture of neutrophils with E. coli resulted in activation of NADPH oxidase in wild-type but less activation was found in RAGE-deficient cells. The mechanism for RAGE-dependent activation of NADPH oxidase is not well delineated, although stimulation of RAGE is known to activate mitogen-activated protein kinase pathways, including extracellular regulated kinase 1/2 (ERK1/2), which have been shown to participate in the activation of NADPH oxidase (11, 27).
Under basal conditions, the NADPH oxidase subunits p40phox or p47phox are disassociated in the cytoplasm (4, 16, 40). Phosphorylation and subsequent assembly of p40phox and p47phox to form the functional NADPH oxidase enzyme occurs after cellular activation. In the neutrophil-like H-90 cell line, the RAGE ligand S100B has been shown to prime the cells for enhanced fMLP-induced respiratory burst as a result of ERK1/2 activation and p47phox translocation to the cellular membrane (27). In the present experiments, we directly demonstrated that RAGE ligation with AGE induced NADPH oxidase activation in neutrophils, and also that exposure of neutrophils to HMGB1 not only decreased the activation of NADPH oxidase produced by exposure to E. colinbut also reduced E. coli-induced p40phox phosphorylation.
Previous studies have shown that HMGB1 binds with high affinity to RAGE (13, 42). We therefore expected that exposure of neutrophils to HMGB1 would result in activation of NADPH oxidase and enhanced killing of bacteria in a manner similar to that found after incubation of neutrophils with AGE, a prototypical RAGE ligand. However, this was not a case, indicating that HMGB1 competitively inhibited the bactericidal effects of RAGE ligation. The requirement for interaction between HMGB1 and RAGE to produce the HMGB1-associated decrease in bacterial killing was shown in two ways. First, HMGB1 lacking the RAGE binding COOH-terminal tail was without effect on bacterial killing. Second, the ability of HMGB1 to diminish neutrophil-associated bacterial killing was absent when RAGE−/− neutrophils were incubated with HMGB1 and E. coli, and no effect of HMGB1 on peritoneal clearance of bacteria was found under in vivo conditions in RAGE−/− mice. An important question raised by these experiments is how AGE and HMGB1, two RAGE ligands, not only can have opposite effects on activation of NADPH oxidase and bacterial killing but also can compete with each other for effect. While the ability of HMGB1 to bind to RAGE is well established, it is presently unknown whether the region of RAGE interacting with HMGB1 is the same as that which is utilized by AGE and other RAGE ligands. Moreover, we have shown that exposure to HMGB1 diminished the amount of RAGE on the cell membrane, likely affecting ability of other ligands to stimulate RAGE.
RAGE and its ligands are implicated in a variety of inflammation-related pathological states, including sepsis, diabetes, atherosclerosis, cystic fibrosis, and cancer (23, 28, 36). For example, mice deficient in RAGE have been shown to be resistant to LPS-induced organ injury and mortality but show diminished ability to control bacterial proliferation in E. coli peritonitis (23, 38, 39). Diabetic mice lacking RAGE have diminished atherosclerosis (35). The apparent discrepancy between the beneficial effects of the absence of RAGE on sterile inflammatory processes and the detrimental properties of RAGE deficiency on bacterial eradication during peritonitis highlights the importance of achieving an appropriate balance of inflammatory activation in pathophysiological processes in which infection plays a major role.
Therapies directed against HMGB1 have been shown to be beneficial in a wide variety neutrophil-associated inflammatory conditions including acute lung injury, sepsis, and ischemia-reperfusion-induced tissue injury (1). Potential mechanisms for the effects of such interventions have been postulated to reflect their ability to diminish HMGB1-associated cellular activation and to reverse the inhibitory effects of HMGB1 on the clearance of activated neutrophils from proinflammatory foci (1, 5, 24). The present results, showing that HMGB1, through RAGE-dependent mechanisms, diminishes bacterial killing by neutrophils as well as NADPH oxidase activation provides a novel mechanism by which HMGB1 can potentiate sepsis-associated organ dysfunction and mortality. In particular, in the setting of severe infection, increased extracellular concentrations of HMGB1 appear to be capable of inhibiting neutrophil-dependent antibacterial defense mechanisms, thereby allowing bacterial proliferation and dissemination which then contribute to organ injury. Additional studies will be necessary to establish the importance of this putative pathway in clinical situations where appropriate antibacterial therapy is being utilized.
GRANTS
This work was supported in part by Fondation MONAHAN and Fondation Bettencourt-Schueller to J.-M. Tadié and National Institutes of Health Grants HL-76206 to E. Abraham and GM-87748 to J. Zmijewski.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: J.-M.T., J.W.Z., and E.A. conception and design of research; J.-M.T., H.-B.B., S.B., and J.W.Z. performed experiments; J.-M.T., H.-B.B., J.W.Z., and E.A. analyzed data; J.-M.T., H.-B.B., J.W.Z., and E.A. interpreted results of experiments; J.-M.T. and J.W.Z. prepared figures; J.-M.T., J.W.Z., and E.A. drafted manuscript; J.-M.T., J.W.Z., and E.A. edited and revised manuscript; J.-M.T., H.-B.B., S.B., J.W.Z., and E.A. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Celeste P. Bell for technical support.
REFERENCES
- 1. Andersson U, Tracey KJ. HMGB1 is a therapeutic target for sterile inflammation and infection. Annu Rev Immunol 29: 139–162, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Angus DC, Yang L, Kong L, Kellum JA, Delude RL, Tracey KJ, Weissfeld L. Circulating high-mobility group box 1 (HMGB1) concentrations are elevated in both uncomplicated pneumonia and pneumonia with severe sepsis. Crit Care Med 35: 1061–1067, 2007 [DOI] [PubMed] [Google Scholar]
- 4. Babior BM. NADPH oxidase: an update. Blood 93: 1464–1476, 1999 [PubMed] [Google Scholar]
- 5. Banerjee S, Friggeri A, Liu G, Abraham E. The C-terminal acidic tail is responsible for the inhibitory effects of HMGB1 on efferocytosis. J Leukoc Biol 88: 973–979, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bierhaus A, Schiekofer S, Schwaninger M, Andrassy M, Humpert PM, Chen J, Hong M, Luther T, Henle T, Kloting I, Morcos M, Hofmann M, Tritschler H, Weigle B, Kasper M, Smith M, Perry G, Schmidt AM, Stern DM, Haring HU, Schleicher E, Nawroth PP. Diabetes-associated sustained activation of the transcription factor nuclear factor-kappaB. Diabetes 50: 2792–2808, 2001 [DOI] [PubMed] [Google Scholar]
- 8. Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS, Weinrauch Y, Zychlinsky A. Neutrophil extracellular traps kill bacteria. Science 303: 1532–1535, 2004 [DOI] [PubMed] [Google Scholar]
- 9. Chavakis T, Bierhaus A, Al-Fakhri N, Schneider D, Witte S, Linn T, Nagashima M, Morser J, Arnold B, Preissner KT, Nawroth PP. The pattern recognition receptor (RAGE) is a counterreceptor for leukocyte integrins: a novel pathway for inflammatory cell recruitment. J Exp Med 198: 1507–1515, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chessa TA, Anderson KE, Hu Y, Xu Q, Rausch O, Stephens LR, Hawkins PT. Phosphorylation of threonine 154 in p40phox is an important physiological signal for activation of the neutrophil NADPH oxidase. Blood 116: 6027–6036, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dang PM, Stensballe A, Boussetta T, Raad H, Dewas C, Kroviarski Y, Hayem G, Jensen ON, Gougerot-Pocidalo MA, El-Benna J. A specific p47phox -serine phosphorylated by convergent MAPKs mediates neutrophil NADPH oxidase priming at inflammatory sites. J Clin Invest 116: 2033–2043, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. De Coursey TE. During the respiratory burst, do phagocytes need proton channels or potassium channels, or both? Sci STKE 2004: pe21, 2004 [DOI] [PubMed] [Google Scholar]
- 13. Dumitriu IE, Baruah P, Valentinis B, Voll RE, Herrmann M, Nawroth PP, Arnold B, Bianchi ME, Manfredi AA, Rovere-Querini P. Release of high mobility group box 1 by dendritic cells controls T cell activation via the receptor for advanced glycation end products. J Immunol 174: 7506–7515, 2005 [DOI] [PubMed] [Google Scholar]
- 14. Erickson RW, Malawista SE, Garrett MC, Van Blaricom G, Leto TL, Curnutte JT. Identification of a thermolabile component of the human neutrophil NADPH oxidase. A model for chronic granulomatous disease caused by deficiency of the p67-phox cytosolic component. J Clin Invest 89: 1587–1595, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Geiszt M, Kapus A, Ligeti E. Chronic granulomatous disease: more than the lack of superoxide? J Leukoc Biol 69: 191–196, 2001 [PubMed] [Google Scholar]
- 16. Groemping Y, Rittinger K. Activation and assembly of the NADPH oxidase: a structural perspective. Biochem J 386: 401–416, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Heyworth PG, Cross AR, Curnutte JT. Chronic granulomatous disease. Curr Opin Immunol 15: 578–584, 2003 [DOI] [PubMed] [Google Scholar]
- 18. Hiraoka W, Vazquez N, Nieves-Neira W, Chanock SJ, Pommier Y. Role of oxygen radicals generated by NADPH oxidase in apoptosis induced in human leukemia cells. J Clin Invest 102: 1961–1968, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hofmann MA, Drury S, Fu C, Qu W, Taguchi A, Lu Y, Avila C, Kambham N, Bierhaus A, Nawroth P, Neurath MF, Slattery T, Beach D, McClary J, Nagashima M, Morser J, Stern D, Schmidt AM. RAGE mediates a novel proinflammatory axis: a central cell surface receptor for S100/calgranulin polypeptides. Cell 97: 889–901, 1999 [DOI] [PubMed] [Google Scholar]
- 20. Hori O, Brett J, Slattery T, Cao R, Zhang J, Chen JX, Nagashima M, Lundh ER, Vijay S, Nitecki D, Moser J, Stern D, Schmidt AM. The receptor for advanced glycation end products (RAGE) is a cellular binding site for amphoterin Mediation of neurite outgrowth and co-expression of rage and amphoterin in the developing nervous system. J Biol Chem 270: 25752–25761, 1995. [DOI] [PubMed] [Google Scholar]
- 21. Huttunen HJ, Fages C, Rauvala H. Receptor for advanced glycation end products (RAGE)-mediated neurite outgrowth and activation of NF-kappaB require the cytoplasmic domain of the receptor but different downstream signaling pathways. J Biol Chem 274: 19919–19924, 1999 [DOI] [PubMed] [Google Scholar]
- 22. Klebanoff SJ. Myeloperoxidase: friend and foe. J Leukoc Biol 77: 598–625, 2005 [DOI] [PubMed] [Google Scholar]
- 23. Liliensiek B, Weigand MA, Bierhaus A, Nicklas W, Kasper M, Hofer S, Plachky J, Grone HJ, Kurschus FC, Schmidt AM, Yan SD, Martin E, Schleicher E, Stern DM, Hammerling GG, Nawroth PP, Arnold B. Receptor for advanced glycation end products (RAGE) regulates sepsis but not the adaptive immune response. J Clin Invest 113: 1641–1650, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Liu G, Wang J, Park YJ, Tsuruta Y, Lorne EF, Zhao X, Abraham E. High mobility group protein-1 inhibits phagocytosis of apoptotic neutrophils through binding to phosphatidylserine. J Immunol 181: 4240–4246, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lorne E, Zmijewski JW, Zhao X, Liu G, Tsuruta Y, Park YJ, Dupont H, Abraham E. Role of extracellular superoxide in neutrophil activation: interactions between xanthine oxidase and TLR4 induce proinflammatory cytokine production. Am J Physiol Cell Physiol 294: C985–C993, 2008 [DOI] [PubMed] [Google Scholar]
- 26. McCord JM, Fridovich I. The utility of superoxide dismutase in studying free radical reactions. II. The mechanism of the mediation of cytochrome c reduction by a variety of electron carriers. J Biol Chem 245: 1374–1377, 1970 [PubMed] [Google Scholar]
- 27. Omori K, Ohira T, Uchida Y, Ayilavarapu S, Batista EL, Jr, Yagi M, Iwata T, Liu H, Hasturk H, Kantarci A, Van Dyke TE. Priming of neutrophil oxidative burst in diabetes requires preassembly of the NADPH oxidase. J Leukoc Biol 84: 292–301, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Park L, Raman KG, Lee KJ, Lu Y, Ferran LJ, Jr, Chow WS, Stern D, Schmidt AM. Suppression of accelerated diabetic atherosclerosis by the soluble receptor for advanced glycation endproducts. Nat Med 4: 1025–1031, 1998 [DOI] [PubMed] [Google Scholar]
- 29. Ramsgaard L, Englert JM, Manni ML, Milutinovic PS, Gefter J, Tobolewski J, Crum L, Coudriet GM, Piganelli J, Zamora R, Vodovotz Y, Enghild JJ, Oury TD. Lack of the receptor for advanced glycation end-products attenuates E. coli pneumonia in Mice. PLos One 6: e20132, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Renckens R, Roelofs JJ, Florquin S, de Vos AF, Pater JM, Lijnen HR, Carmeliet P, van 't Veer C, van der Poll T. Endogenous tissue-type plasminogen activator is protective during Escherichia coli-induced abdominal sepsis in mice. J Immunol 177: 1189–1196, 2006 [DOI] [PubMed] [Google Scholar]
- 31. Schmidt AM, Yan SD, Yan SF, Stern DM. The biology of the receptor for advanced glycation end products and its ligands. Biochim Biophys Acta 1498: 99–111, 2000 [DOI] [PubMed] [Google Scholar]
- 32. Schmidt AM, Yan SD, Yan SF, Stern DM. The multiligand receptor RAGE as a progression factor amplifying immune and inflammatory responses. J Clin Invest 108: 949–955, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Segal AW. How neutrophils kill microbes. Annu Rev Immunol 23: 197–223, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sha Y, Zmijewski J, Xu Z, Abraham E. HMGB1 develops enhanced proinflammatory activity by binding to cytokines. J Immunol 180: 2531–2537, 2008 [DOI] [PubMed] [Google Scholar]
- 35. Soro-Paavonen A, Watson AM, Li J, Paavonen K, Koitka A, Calkin AC, Barit D, Coughlan MT, Drew BG, Lancaster GI, Thomas M, Forbes JM, Nawroth PP, Bierhaus A, Cooper ME, Jandeleit-Dahm K. A receptor for advanced glycation end products (RAGE) deficiency attenuates the development of atherosclerosis in diabetes. Diabetes 57: 2461–2469, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Taguchi A, Blood DC, del Toro G, Canet A, Lee DC, Qu W, Tanji N, Lu Y, Lalla E, Fu C, Hofmann MA, Kislinger T, Ingram M, Lu A, Tanaka H, Hori O, Ogawa S, Stern DM, Schmidt AM. Blockade of RAGE-amphoterin signalling suppresses tumour growth and metastases. Nature 405: 354–360, 2000 [DOI] [PubMed] [Google Scholar]
- 37. van Zoelen MA, Achouiti A, Schmidt AM, Yang H, Florquin S, Tracey KJ, van der Poll T. Ligands of the receptor for advanced glycation end products, including high-mobility group box 1, limit bacterial dissemination during Escherichia coli peritonitis. Crit Care Med 38: 1414–1422, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. van Zoelen MA, Achouiti A, van der Poll T. The role of receptor for advanced glycation endproducts (RAGE) in infection. Crit Care 15: 208, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. van Zoelen MA, Schmidt AM, Florquin S, Meijers JC, de Beer R, de Vos AF, Nawroth PP, Bierhaus A, van der Poll T. Receptor for advanced glycation end products facilitates host defense during Escherichia coli-induced abdominal sepsis in mice. J Infect Dis 200: 765–773, 2009 [DOI] [PubMed] [Google Scholar]
- 40. Vignais PV. The superoxide-generating NADPH oxidase: structural aspects and activation mechanism. Cell Mol Life Sci 59: 1428–1459, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wrona M, Patel K, Wardman P. Reactivity of 2′,7′-dichlorodihydrofluorescein and dihydrorhodamine 123 and their oxidized forms toward carbonate, nitrogen dioxide, and hydroxyl radicals. Free Radic Biol Med 38: 262–270, 2005 [DOI] [PubMed] [Google Scholar]
- 42. Yang D, Chen Q, Yang H, Tracey KJ, Bustin M, Oppenheim JJ. High mobility group box-1 protein induces the migration and activation of human dendritic cells and acts as an alarmin. J Leukoc Biol 81: 59–66, 2007 [DOI] [PubMed] [Google Scholar]
- 43. Zen K, Chen CX, Chen YT, Wilton R, Liu Y. Receptor for advanced glycation endproducts mediates neutrophil migration across intestinal epithelium. J Immunol 178: 2483–2490, 2007 [DOI] [PubMed] [Google Scholar]
- 44. Zmijewski JW, Banerjee S, Bae H, Friggeri A, Lazarowski ER, Abraham E. Exposure to hydrogen peroxide induces oxidation and activation of AMP-activated protein kinase. J Biol Chem 285: 33154–33164, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Zmijewski JW, Lorne E, Zhao X, Tsuruta Y, Sha Y, Liu G, Siegal GP, Abraham E. Mitochondrial respiratory complex I regulates neutrophil activation and severity of lung injury. Am J Respir Crit Care Med 178: 168–179, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Zmijewski JW, Zhao X, Xu Z, Abraham E. Exposure to hydrogen peroxide diminishes NF-κB activation, IκB-α degradation, and proteasome activity in neutrophils. Am J Physiol Cell Physiol 293: C255–C266, 2007 [DOI] [PubMed] [Google Scholar]