Abstract
Doxorubicin, a commonly prescribed chemotherapeutic agent, causes skeletal muscle wasting in cancer patients undergoing treatment and increases mitochondrial reactive oxygen species (ROS) production. ROS stimulate protein degradation in muscle by activating proteolytic systems that include caspase-3 and the ubiquitin-proteasome pathway. We hypothesized that doxorubicin causes skeletal muscle catabolism through ROS, causing upregulation of E3 ubiquitin ligases and caspase-3. We tested this hypothesis by exposing differentiated C2C12 myotubes to doxorubicin (0.2 μM). Doxorubicin decreased myotube width 48 h following exposure, along with a 40–50% reduction in myosin and sarcomeric actin. Cytosolic oxidant activity was elevated in myotubes 2 h following doxorubicin exposure. This increase in oxidants was followed by an increase in the E3 ubiquitin ligase atrogin-1/muscle atrophy F-box (MAFbx) and caspase-3. Treating myotubes with SS31 (opposes mitochondrial ROS) inhibited expression of ROS-sensitive atrogin-1/MAFbx and protected against doxorubicin-stimulated catabolism. These findings suggest doxorubicin acts via mitochondrial ROS to stimulate myotube atrophy.
Keywords: skeletal muscle, cancer cachexia, oxidative stress, reactive oxygen species
in patients, muscle wasting is a primary component of cancer cachexia that exacerbates illness and promotes death (6). Cancer chemotherapy can exacerbate this problem. Patients receiving the anthracycline compound doxorubicin experience loss of mass, with reductions in the sizes of type 1 and type 2 muscle fibers (5, 44). Our previous work in a rodent model of chemotherapy documented the loss of skeletal muscle mass, along with a reduced cross-sectional area following doxorubicin administration (16, 17).
The mechanism of doxorubicin-induced catabolism is undefined but could occur via reactive oxygen species (ROS). Doxorubicin stimulates ROS production in both cardiac (12) and skeletal muscle (17) via effects on mitochondrial electron transport (7). Elevated ROS can lead to muscle protein degradation via increased expression and/or activation of proteolytic systems including caspase-3 and the ubiquitin-proteasome pathway (3, 4, 21). Understanding the role that ROS play in chemotherapy-induced skeletal muscle catabolism is important for the development of therapies to reduce skeletal muscle wasting in patients (3).
For this study, we hypothesized that doxorubicin causes skeletal muscle catabolism through mitochondrial ROS, thereby upregulating redox-sensitive E3 ubiquitin ligases [atrogin-1/muscle atrophy F-box (MAFbx) and MuRF1] and activating proteases (caspase-3). Experiments were conducted using mature C2C12 myotubes treated with clinically relevant concentrations of doxorubicin (10, 36). Catabolism endpoints included myotube width, total protein, and sarcomeric actin and myosin protein. Mitochondrial involvement was tested using SS31, a cell-permeable small peptide that concentrates in the mitochondrial inner membrane, improving mitochondrial function and reducing superoxide production in numerous tissues, including skeletal muscle (1, 49).
METHODS
Cell culture.
C2C12 myoblasts were purchased from the American Type Culture Collection (Manassas, VA) and grown for 4 days (10% fetal bovine serum, DMEM). On reaching 80% confluence, cells were serum restricted (2% heat-inactivated horse serum, DMEM) and grown for 5 additional days. On the fifth day following serum restriction, mature myotubes were treated with phosphate-buffered saline (PBS; control) or 0.1–2 μM doxorubicin (Sigma, St. Louis, MO). For intervention experiments, myotubes were treated with the cell-permeable antioxidant SS31 (1 μM; W.M. Keck Foundation Biotechnology Resource Laboratory, Yale University, New Haven, CT). Myotubes were harvested for biochemical analyses at various time points following doxorubicin exposure.
Cell integrity.
Myotube membrane integrity was assessed using the fluorescent stain Sapphire700 (LI-COR, Lincoln, NE). After doxorubicin exposure, Sapphire700 (1:200) mixed with fresh medium was placed on the cells and incubated for 15 min. The medium was removed, and plates were scanned for fluorescence using the Odyssey infrared imaging system (LI-COR).
Myotube width.
To measure myotube width, four brightfield images per dish were captured, randomized, and coded. Two diagonal lines were drawn across each image. Width was measured where the diagonal lines transected myotubes. A blinded subject made the measurements using Metamorph image acquisition software (Metamorph 6.2R6; Molecular Devices, Downingtown, PA).
Western blot analysis.
After treatment, myotubes were washed with PBS and scraped into 2× sample loading buffer (120 mM Tris, pH 7.5; 200 mM DTT; 20% glycerol; 4% SDS; 0.002% bromphenol blue). Lysates were sonicated on ice and then heated at 98°C for 3 min. Equal amounts of protein were loaded into each lane of 4–15% SDS-polyacrylamide gels (Criterion precast gels; Bio-Rad, Hercules, CA) and transferred to reduced-fluorescence polyvinylidene difluoride membranes (Immobilon-FL; Millipore, Bedford, MA). Membranes with transferred proteins were blocked overnight at room temperature in Odyssey blocking buffer (LI-COR). Primary antibodies were incubated overnight at room temperature in Odyssey blocking buffer mixed 1:1 with PBS plus 0.2% Tween. Secondary antibodies were incubated for 45 min in Odyssey-PBS plus 0.2% Tween plus 0.01% SDS. Myosin (1:2,000) and actin (1:2,000) antibodies were purchased from Sigma. To detect actin fragmentation, an indicator of caspase-3 activity (14), samples were run on a 15% SDS-polyacrylamide gel and both intact (42 kDa) and fragmented (14 kDa) actin were measured simultaneously. Atrogin-1/MAFbx (1:1,000) and MuRF1 (1:1,000) antibodies were obtained from ECM Biosciences (Versailles, KY). The caspase-3 (1:1,000) antibody was obtained from Cell Signaling Technologies (Danvers, MA). The α-II spectrin antibody (1:500) was obtained from Millipore. Fluorescent secondary antibodies were used for detection (goat anti-mouse Alexa-680, Molecular Probes-Invitrogen; goat anti-rabbit IRD800, Rockland Immunochemicals, Gilbertsville, PA). Fluorescence intensity data were quantified using the Odyssey infrared imaging system (LI-COR) and normalized for total protein using Simply Blue stain (Invitrogen, Carlsbad, CA).
Relative quantification real-time PCR.
Reverse transcription was performed using Muloney murine leukemia virus reverse transcriptase and random hexamers (Promega, Madison, WI) plus 20 mg of total RNA isolated from myotubes with TRIzol (Invitrogen). Primers for atrogin-1/MAFbx (forward and reverse: 5′-ATGCACACTGGTGCAGAGAG-3′ and 5′-TGTAAGCACACAGGCAGGTC-3′), MuRF1 (5′-ACGAGAAGAAGAGCGAGC-3′ and 5′-CTTGGCACTTGAGAGGAA-3′), and GAPDH (5′-CATGGCCTTCCGTGTTCCTA-3′ and 5′-GCGGCACGTCAGATCCA) were purchased from Invitrogen. PCR was performed using the Applied Biosystems 7500 Real-Time PCR system. Targets were amplified from 50 ng of cDNA using SYBR Green Master Mix reagent (stage 1: 1 cycle, 50°C, 2 min; stage 2: 1 cycle, 95°C, 10 min; stage 3: 40 cycles, 95°C, 15 s, 60°C, 1 min; Applied Biosystems). Reactions were performed in duplicate or triplicate for each cDNA sample. The abundance of target mRNA relative to GAPDH mRNA was determined using the comparative cycle threshold method (19, 30).
Cytosolic oxidant activity.
As described previously (17, 39), the fluorochrome probe 2′,7′-dichlorofluorescein diacetate (DCFH-DA; Molecular Probes, Eugene, OR) was used to measure oxidant activity. After exposure to doxorubicin, the medium was removed and the cytosol of mature C2C12 myotubes was loaded with DCFH-DA (10 μM) in PBS at 37°C for 15 min. Accumulation of the oxidized derivative (DCF; 480-nm excitation, 520-nm emissions) was measured with the use of an epifluorescence microscope (Labophot-2; Nikon Instruments, Melville, NY), a charge-coupled device camera (Series 72; Dage-MTI, Michigan City, IN), and a computer-controlled shutter in the excitation light pathway. Images were analyzed for the mean emission density from the six brightest myotubes using a commercial data acquisition and analysis software (Metamorph 6.2R6).
Statistical analyses.
Data are means ± SE. Differences were assessed using Student's t-test for the comparison of two means or one-way ANOVA for comparisons of multiple means. When an overall statistical significance was detected by the one-way ANOVA, a post hoc Dunnett's test was performed for comparisons with the control. Statistical significance was accepted when P < 0.05. Statistical calculations were performed using commercial software (Prizm 5.0a; GraphPad Software, La Jolla, CA; Microsoft Excel; Microsoft, Redmond, WA).
RESULTS
Catabolic effects of doxorubicin.
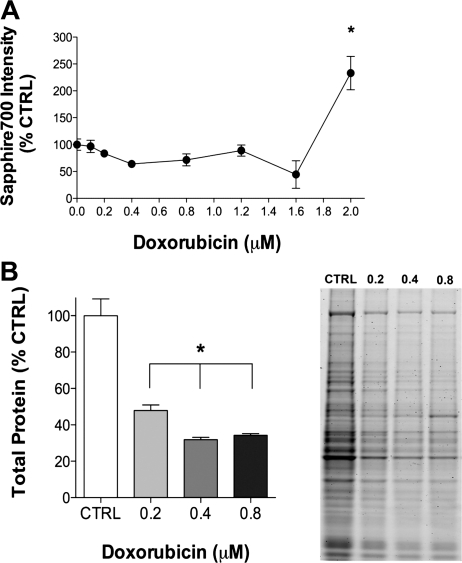
We established a dose-response curve for doxorubicin in C2C12 myotubes by assessing cell integrity using the vital stain Sapphire700 (Fig. 1). Only the highest concentration of doxorubicin (2 μM) promoted the loss of cell integrity, an indicator of cell death, 24 h following exposure (Fig. 1A). To assess loss of total protein in response to doxorubicin, we treated cells with three lower concentrations of doxorubicin (0.2–0.8 μM). Compared with controls, total protein was depressed ∼60% following exposure to all three concentrations (Fig. 1B).
Fig. 1.
Increasing concentrations of doxorubicin cause loss of cell integrity and total protein. A: averaged data for cell integrity (Sapphire700) 24 h following exposure to varying concentrations of doxorubicin (n = 3/group). B: averaged data for total protein 48 h following doxorubicin exposure (n = 3/group) and a representative gel showing total protein stain. Data are means ± SE. *P < 0.05 vs. control. Ctrl, control.
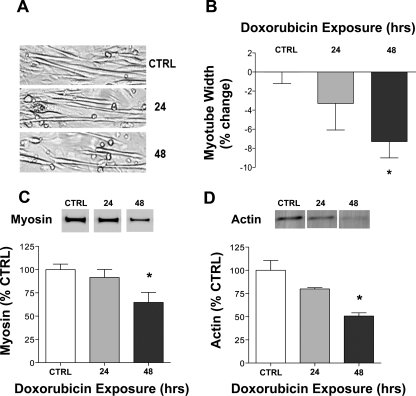
In all further experiments, we used 0.2 μM doxorubicin, a concentration that did not alter cell integrity 48 h following exposure (doxorubicin: 99 ± 6% vs. control: 100 ± 9%, n = 6/group) and is lower than circulating concentrations found in patients (10, 36). We show the catabolic effects of 0.2 μM doxorubicin on myotubes after 48 h of exposure in Fig. 2. Myotube width was decreased 8%, along with a 40–50% reduction in both myosin and sarcomeric actin.
Fig. 2.
Doxorubicin exposure reduces myotube width and decreases myosin and actin protein levels. A: representative images of myotubes 24 and 48 h following exposure to doxorubicin (0.2 μM) or an equal volume of vehicle (control). B: averaged data for myotube width (n = 4/group). Averaged data for myosin (C; n = 6/group) and actin (D; n = 3/group) protein are shown relative to total protein. Data are means ± SE. *P < 0.05 vs. control. Representative Western blots may not be from contiguous lanes.
Cystolic oxidant activity following doxorubicin exposure.
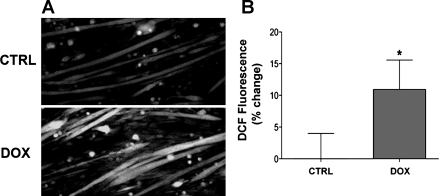
Doxorubicin stimulates oxidant production in cardiac and skeletal muscle (13, 17), a response that is closely linked with muscle catabolism (33). Figure 3 shows that doxorubicin also increased cytosolic oxidant activity in myotubes 2 h following exposure. This appears to be a delayed effect, because doxorubicin did not alter oxidant activity 15 min following exposure (doxorubicin: 95 ± 6% vs. control: 100 ± 3%; n = 6/group).
Fig. 3.
Doxorubicin simulates cytosolic oxidant activity in C2C12 myotubes. Images depict representative fluorescence (A) and averaged data for dichlorofluoroscein (DCF) fluorescence (B) in intact myotubes following 2 h of exposure to doxorubicin or an equal volume of vehicle (control; n = 18/group). Data are means ± SE. *P < 0.05 vs. control. Dox, doxorubicin.
Doxorubicin effects on atrogin-1/MAFbx and MuRF1.
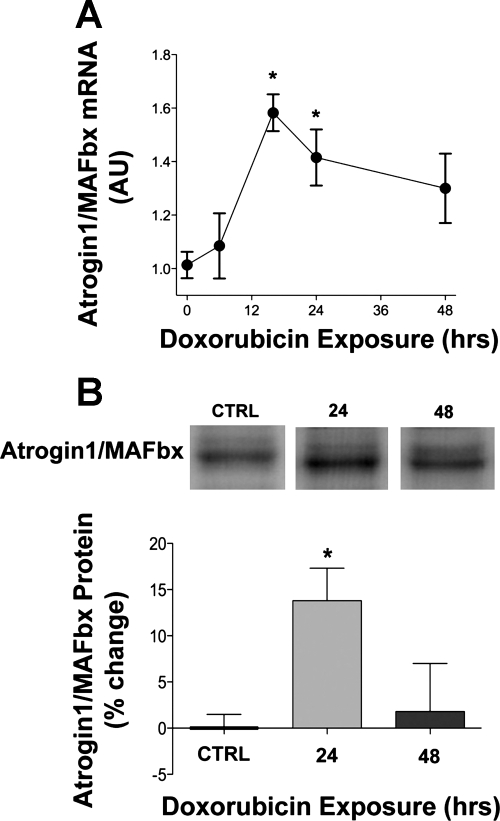
The E3 ubiquitin ligases atrogin-1/MAFbx and MuRF1 mediate skeletal muscle catabolism (27, 34). Figure 4 depicts atrogin-1/MAFbx mRNA and protein levels in myotubes following doxorubicin exposure. Doxorubicin stimulated a late rise in atrogin-1/MAFbx mRNA levels in myotubes (Fig. 4A), along with an increase in protein (Fig. 4B). Regulators of atrogin-1/MAFbx expression, p38 MAPK and Akt, were not altered by doxorubicin; we detected no changes in total protein or phosphorylation state of either kinase (data not shown).
Fig. 4.
Doxorubicin stimulates atrogin-1/MAFbx mRNA and protein. A: data depict atrogin-1/muscle atrophy F-box (MAFbx) mRNA in myotubes 6, 16, 24, and 48 h after doxorubicin exposure (n = 6/group at all time points). B: averaged data for atrogin-1/MAFbx protein 24 and 48 h following doxorubicin exposure (n = 13 for vehicle and 24 h; n = 4 for 48 h). Data are means ± SE. *P < 0.05 vs. control. Representative Western blots may not be from contiguous lanes. AU, arbitrary units.
MuRF1 mRNA levels were not altered at any time point following doxorubicin exposure (4 h: 0.77 ± 0.05 AU; 6 h: 0.40 ± 0.01 AU; 16 h: 0.37 ± 0.02 AU; 24 h: 0.59 ± 0.02 AU; control: 1 ± 0.05; n = 3/group). Doxorubicin did not alter MuRF1 protein levels (6 h: 95 ± 5% of control; 16 h: 102 ± 6%; 24 h: 105 ± 6%; 48 h: 97 ± 3%; control: 100 ± 6%; n = 3/group). This is consistent with our findings that I-κB degradation, a marker of NF-κB activation, was not altered following doxorubicin exposure (data not shown).
Doxorubicin effects on protease activity.
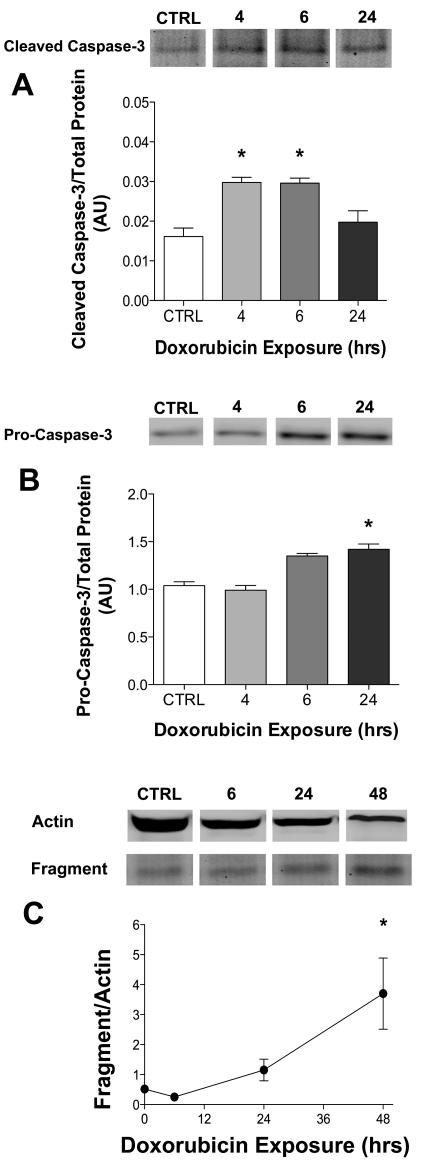
Protease activity is elevated in cardiac muscle following doxorubicin exposure (23), a possible mediator of the loss of myofilament proteins. We observed an increase in cleaved (active) caspase-3 at 4–6 h after doxorubicin exposure (Fig. 5A). This was followed by an upregulation of procaspase-3 at the 24-h time point (Fig. 5A). This increase in caspase-3 is supported by an increase in actin fragmentation, an indicator of caspase-3 activity (2, 14), 48 h following doxorubicin exposure (Fig. 5C). We determined calpain activity by measuring the 145-kDa fragment of α-II spectrin, a characteristic marker of calpain activation (40, 41). There was no change in the α-II spectrin fragment in myotubes exposed to doxorubicin (4 h: 96 ± 3% of control, 6 h: 92 ± 2%; 24 h: 77 ± 3%; control: 100 ± 5%; n = 4/group).
Fig. 5.
Cleaved caspase-3 (19 kDa) and procaspase-3 (43 kDa) are elevated in myotubes following doxorubicin exposure. Averaged data for cleaved caspase-3 (A) and procaspase-3 (B) protein (n = 3/group) relative to total protein in myotubes exposed to doxorubicin. C: averaged data for actin fragment relative to total actin (n = 3/group). Data are means ± SE. *P < 0.05 vs. control. Representative Western blots may not be from contiguous lanes.
SS31 protects against doxorubicin-induced catabolism.
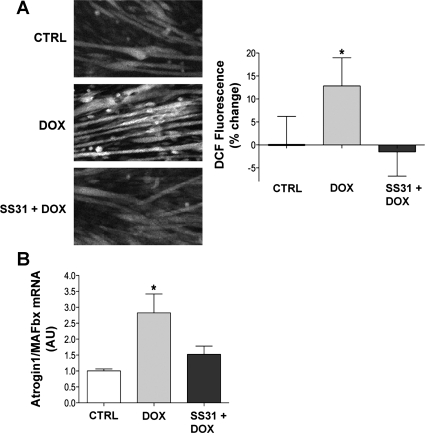
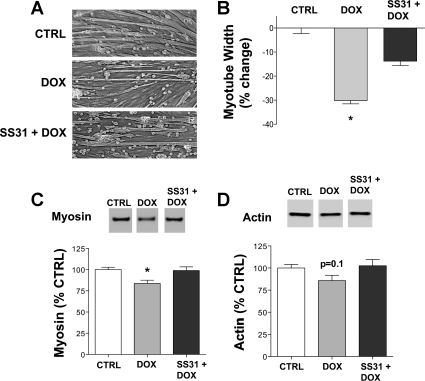
The antioxidant SS31 localizes to mitochondria, lessening ROS production and oxidative damage (49). In myotubes, SS31 blocked the doxorubicin-induced increase in cytosolic oxidant activity after 2 h (Fig. 6A). SS31 also blunted the increase in atrogin-1/MAFbx mRNA (Fig. 6B), the decrease in myotube width (Fig. 7B), and the loss of myosin (Fig. 7C) and actin (Fig. 7D) caused by doxorubicin.
Fig. 6.
SS31 abolishes doxorubicin-induced cytosolic oxidant activity and atrogin-1/MAFbx. A: images depict representative fluorescence (left) and averaged data for DCF fluorescence (right) in intact myotubes following 2 h of exposure to doxorubicin and SS31 (n = 16/group). B: data depict atrogin-1/MAFbx mRNA levels in myotubes 24 h after doxorubicin and SS31 exposure (n = 3/group). Data are means ± SE. *P < 0.05 vs. control.
Fig. 7.
SS31 partially protects against doxorubicin-induced loss of myotube width and actin and myosin protein. A: representative images of myotubes 48 h following doxorubicin exposure with or without SS31. B: averaged data for myotube width (n = 6/group). Averaged data for myosin (C; n = 16/group) and actin (D; n = 19/group) protein are shown relative to total protein 48 h following doxorubicin and SS31 exposure. Data are means ± SE. *P < 0.05 vs. control. Representative Western blots may not be from contiguous lanes.
DISCUSSION
Our data show that doxorubicin acts directly on differentiated C2C12 myotubes to decrease myotube width, total protein, and myofilament proteins. These changes are preceded by a rise in mitochondrial ROS and other procatabolic mediators: atrogin-1/MAFbx and caspase-3. By blocking the increase in ROS using SS31, responses to doxorubicin-stimulated changes were blunted. These findings identify a causal role for oxidant-mediated signaling in doxorubicin-induced catabolism.
Doxorubicin and muscle catabolism.
In the clinic, loss of muscle mass is an unfortunate reality for cancer patients (6). This catabolic state persists while patients are undergoing treatment, affecting quality of life and long-term physical capacity (24). The loss of muscle mass is evident in patients receiving the chemotherapeutic agent doxorubicin (5, 44), an effect documented in rodent models of chemotherapy (15–17, 31). We observed a similar catabolic response in C2C12 myotubes following direct doxorubicin exposure. Increasing concentrations of doxorubicin resulted in a dose-dependent loss of total protein that did not compromise cellular integrity. Even at the lowest doxorubicin concentration (0.2 μM), myotube width and myosin content were significantly decreased within 48 h. To our knowledge, the present study is the first to demonstrate that direct exposure to doxorubicin can reduce myotube size.
Doxorubicin-induced ROS.
Anthracyclines, a class of chemotherapy drugs, are known to have deleterious effects on noncancerous tissue via oxidative stress (18). Doxorubicin, a representative anthracycline, increases ROS in both cardiac and skeletal muscle (12, 17, 41). The mitochondria are thought to be the primary source of doxorubicin-induced oxidants (7, 48). Doxorubicin has a high affinity for the inner membrane of the mitochondria, where it accumulates and can be reduced by complex I of the electron transport chain. This reaction forms an unstable semiquinone, which is then oxidized, transferring an electron to oxygen to produce the superoxide anion (8, 9, 32). We found cytosolic oxidant activity was elevated in C2C12 myotubes by 2 h following doxorubicin exposure. Although the cytosolic oxidant activity assay we used does not delineate the compartmentalization of ROS, exposure of myotubes to SS31, a mitochondrial-targeted peptide, blunted the increase in oxidants caused by doxorubicin, suggesting mitochondria as a potential source.
The fluorescent probe used to detect oxidants in our experiments was DCFH-DA. This probe is cell permeable, retained in the cell when cleaved by intracellular esterases and oxidized to the fluorescent DCF form in the presence of ROS. DCFH is oxidized by ROS and nitric oxide (NO) derivatives that include hydrogen peroxide (35), a by-product of the superoxide dismutase reaction involving superoxide anion. This reaction occurs quickly, resulting in the production of hydrogen peroxide, a relatively stable ROS that can escape into the cytosol and cause damage throughout the cell (42). Mitochondria are widely believed to constitute the primary source of superoxide anion in most tissues (45). There are three potential mechanisms by which SS31 can lessen mitochondrial ROS production. The chemical backbone of SS31 contains a tyrosine moiety, giving the molecule intrinsic antioxidant activity and the capability to directly scavenge hydrogen peroxide in both the matrix and intermembrane space, owing to its accumulation in the inner mitochondrial membrane (43). SS31 also promotes mitochondrial respiration, which indirectly decreases ROS production by increasing ATP synthesis and decreasing electron leak (47). Finally, SS31 prevents the loss of cytochrome c from the inner mitochondrial membrane (49). Cytochrome c is the electron carrier between complex III and IV of the electron transport chain, and the loss results in reduced ATP synthesis and increased electron leak, ultimately leading to ROS production (43).
Catabolic signaling following doxorubicin exposure.
The majority of muscle protein degradation occurs by the ubiquitin-proteasome pathway (26, 33). E3 ubiquitin ligases (atrogin-1/MAFbx, MuRF1) play a vital role in dictating the specificity of the system, marking muscle proteins for degradation (38). E3 ubiquitin ligases are upregulated in numerous catabolic states, including inflammation (11), starvation (22), and unloading (20). In cardiac muscle, doxorubicin enhances activity of the ubiquitin-proteasome pathway (25, 29) and upregulates the E3 ubiquitin ligase atrogin-1/MAFbx (46). Our findings are in agreement with the cardiac literature. Atrogin-1/MAFbx mRNA and protein levels were elevated in myotubes following doxorubicin exposure, suggesting catabolism through the proteasome pathway.
We observed no change in protein levels or phosphorylation state of Akt and p38 MAPK, upstream regulators of atrogin-1/MAFbx expression (27, 34, 46). The upregulation of atrogin-1/MAFbx occurred 16 h following doxorubicin exposure. This late rise could have prevented us from detecting a transient increase in p38 MAPK or Akt phosphorylation despite having measured both kinases at multiple time points (15 min to 6 h) following doxorubicin exposure.
Other cellular proteases are involved in skeletal muscle catabolism (3). Calpains and caspases can release myofilament proteins, making them available to be degraded by the ubiquitin-proteasome pathway (3, 14, 37), and doxorubicin administration can increase the activity of both (41, 50). Our data suggest caspases are the proteases responsible for the release of myofilament proteins in myotubes exposed to doxorubicin. We observed both activation and an increase in the levels of cleaved caspase-3, with no change in calpain activity. Capsase-3 is specifically implicated in skeletal muscle protein degradation (37). Our study shows that procaspase-3 and cleaved (active) caspase-3 are increased in myotubes following doxorubicin exposure. Caspase-3 cleaves actin to produce a 14-kDa actin fragment, which serves as an indicator of activity (2, 14). We observed an increase in actin fragmentation 48 h following doxorubicin exposure, evidence of increased caspase-3 activity.
Oxidant-mediated catabolism.
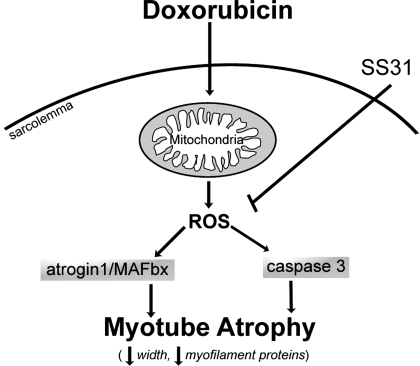
The disruption of redox balance (i.e., oxidative stress) can induce catabolic signaling and protein degradation in skeletal muscle (33). For example, direct exposure of myotubes to hydrogen peroxide increases expression of E3 ubiquitin ligases atrogin-1/MAFbx and MuRF1 (28). Doxorubicin stimulates oxidant activity in both myotubes and skeletal muscle and increases procatabolic signaling events (Fig. 8). The mitochondrion-targeted peptide SS31 abolished changes caused by doxorubicin, stabilizing cytosolic oxidant activity, preventing the rise in atrogin-1/MAFbx mRNA, and preserving myotube size. These findings strongly suggest that mitochondrial ROS play a causal role in doxorubicin-induced atrophy.
Fig. 8.
Summarized mechanism of doxorubicin-induced myotube atrophy. Model depicts intracellular pathways by which doxorubicin causes myotube atrophy based on data from the current findings. ROS, reactive oxygen species.
GRANTS
This study was supported by American Heart Association a Predoctoral Fellowship 09PRE2020088 (to L. A. Gilliam) and National Heart, Lung, and Blood Institute Training Grant T32 HL-086341 (to M. B. Reid; L. A. Gilliam, predoctoral scholar).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
L.A.G. and M.B.R. conception and design of research; L.A.G., J.S.M., E.W.P., J.D.S., A.S.W., and Z.R. performed experiments; L.A.G., J.S.M., E.W.P., J.D.S., A.S.W., and Z.R. analyzed data; L.A.G., J.S.M., and M.B.R. interpreted results of experiments; L.A.G. prepared figures; L.A.G. drafted manuscript; L.A.G. and M.B.R. edited and revised manuscript; L.A.G., J.S.M., E.W.P., J.D.S., A.S.W., Z.R., and M.B.R. approved final version of manuscript.
REFERENCES
- 1. Anderson EJ, Lusting ME, Boyle KE, Woodleigh TL, Kane DA, Lin CT, Price JW, 3rd, Kang L, Rabinovitch PS, Szeto HH, Houmard JA, Cortright RN, Wasserman DH, Neufer PD. Mitochondrial H2O2 emission and cellular redox state link excess fat intake to insulin resistance in both rodents and humans. J Clin Invest 119: 573–581, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Argadine HM, Hellyer NJ, Mantilla CB, Zhan WZ, Sieck GC. The effect of denervation on protein synthesis and degradation in adult rat diaphragm muscle. J Appl Physiol 107: 438–444, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Arthur PG, Grounds MD, Shavlakadze T. Oxidative stress as a therapeutic target during muscle wasting: considering the complex interactions. Curr Opin Clin Nutr Metab Care 11: 408–416, 2008 [DOI] [PubMed] [Google Scholar]
- 4. Bonetto A, Penna F, Muscaritoli M, Minero VG, Rossi Fanelli F, Baccino FM, Costelli P. Are antioxidants useful for treating skeletal muscle atrophy? Free Radic Biol Med 47: 906–916, 2009 [DOI] [PubMed] [Google Scholar]
- 5. Bonifati DM, Ori C, Rossi CR, Caira S, Fanin M, Angelini C. Neuromuscular damage after hyperthermic isolated limb perfusion in patients with melanoma or sarcoma treated with chemotherapeutic agents. Cancer Chemother Pharmacol 46: 517–522, 2000 [DOI] [PubMed] [Google Scholar]
- 6. Burckart K, Beca S, Urban RJ, Sheffield-Moore M. Pathogenesis of muscle wasting in cancer cachexia: targeted anabolic and anticatabolic therapies. Curr Opin Clin Nutr Metab Care 13: 410–416, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chandran K, Aggarwal D, Migrino RQ, Joseph J, McAllister D, Konorev EA, Antholine WE, Zielonka J, Srinivasan S, Avadhani NG, Kalyanaraman B. Doxorubicin inactivates myocardial cytochrome c oxidase in rats: cardioprotection by Mito-Q. Biophys J 96: 1388–1398, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chen Y, Jungsuwadee P, Vore M, Butterfield DA, Clair DK. Collateral damage in cancer chemotherapy: oxidative stress in nontargeted tissues. Mol Interv 7: 147–156, 2007 [DOI] [PubMed] [Google Scholar]
- 9. Davies KJ, Doroshow JH. Redox cycling of anthracyclines by cardiac mitochondria. I. Anthracycline radical formation by NADH dehydrogenase. J Biol Chem 261: 3060–3067, 1986 [PubMed] [Google Scholar]
- 10. Delgado G, Potkul RK, Treat JA, Lewandowski GS, Barter JF, Forst D, Rahman A. A phase I/II study of intraperitoneally administered doxorubicin entrapped in cardiolipin liposomes in patients with ovarian cancer. Am J Obstet Gynecol 160: 812–817; discussion 817–819, 1989 [DOI] [PubMed] [Google Scholar]
- 11. Dogra C, Changotra H, Wedhas N, Qin X, Wergedal JE, Kumar A. TNF-related weak inducer of apoptosis (TWEAK) is a potent skeletal muscle-wasting cytokine. FASEB J 21: 1857–1869, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Doroshow JH. Effect of anthracycline antibiotics on oxygen radical formation in rat heart. Cancer Res 43: 460–472, 1983 [PubMed] [Google Scholar]
- 13. Doroshow JH, Davies KJ. Comparative cardiac oxygen radical metabolism by anthracycline antibiotics, mitoxantrone, bisantrene, 4′-(9-acridinylamino)-methanesulfon-m-anisidide, and neocarzinostatin. Biochem Pharmacol 32: 2935–2939, 1983 [DOI] [PubMed] [Google Scholar]
- 14. Du J, Wang X, Miereles C, Bailey JL, Debigare R, Zheng B, Price SR, Mitch WE. Activation of caspase-3 is an initial step triggering accelerated muscle proteolysis in catabolic conditions. J Clin Invest 113: 115–123, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Falkenberg JH, Iaizzo PA, McLoon LK. Muscle strength following direct injection of doxorubicin into rabbit sternocleidomastoid muscle in situ. Muscle Nerve 25: 735–741, 2002 [DOI] [PubMed] [Google Scholar]
- 16. Gilliam LA, Ferreira LF, Bruton JD, Moylan JS, Westerblad H, Clair DK, Reid MB. Doxorubicin acts through tumor necrosis factor receptor subtype 1 to cause dysfunction of murine skeletal muscle. J Appl Physiol 107: 1935–1942, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gilliam LA, Moylan JS, Ann Callahan L, Sumandea MP, Reid MB. Doxorubicin causes diaphragm weakness in murine models of cancer chemotherapy. Muscle Nerve 43: 94–102, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gilliam LA, Clair DK. Chemotherapy-induced weakness and fatigue in skeletal muscle: the role of oxidative stress. Antioxid Redox Signal 15: 2543–2563, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Giulietti A, Overbergh L, Valckx D, Decallonne B, Bouillon R, Mathieu C. An overview of real-time quantitative PCR: applications to quantify cytokine gene expression. Methods 25: 386–401, 2001 [DOI] [PubMed] [Google Scholar]
- 20. Haddad F, Adams GR, Bodell PW, Baldwin KM. Isometric resistance exercise fails to counteract skeletal muscle atrophy processes during the initial stages of unloading. J Appl Physiol 100: 433–441, 2006 [DOI] [PubMed] [Google Scholar]
- 21. Jackman RW, Kandarian SC. The molecular basis of skeletal muscle atrophy. Am J Physiol Cell Physiol 287: C834–C843, 2004 [DOI] [PubMed] [Google Scholar]
- 22. Jagoe RT, Lecker SH, Gomes M, Goldberg AL. Patterns of gene expression in atrophying skeletal muscles: response to food deprivation. FASEB J 16: 1697–1712, 2002 [DOI] [PubMed] [Google Scholar]
- 23. Kavazis AN, Smuder AJ, Min K, Tumer N, Powers SK. Short-term exercise training protects against doxorubicin-induced cardiac mitochondrial damage independent of HSP72. Am J Physiol Heart Circ Physiol 299: H1515–H1524, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Knobel H, Havard Loge J, Brit Lund M, Forfang K, Nome O, Kaasa S. Late medical complications and fatigue in Hodgkin's disease survivors. J Clin Oncol 19: 3226–3233, 2001 [DOI] [PubMed] [Google Scholar]
- 25. Kumarapeli AR, Horak KM, Glasford JW, Li J, Chen Q, Liu J, Zheng H, Wang X. A novel transgenic mouse model reveals deregulation of the ubiquitin-proteasome system in the heart by doxorubicin. FASEB J 19: 2051–2053, 2005 [DOI] [PubMed] [Google Scholar]
- 26. Lecker SH, Solomon V, Mitch WE, Goldberg AL. Muscle protein breakdown and the critical role of the ubiquitin-proteasome pathway in normal and disease states. J Nutr 129: 227S–237S, 1999 [DOI] [PubMed] [Google Scholar]
- 27. Li W, Moylan JS, Chambers MA, Smith J, Reid MB. Interleukin-1 stimulates catabolism in C2C12 myotubes. Am J Physiol Cell Physiol 297: C706–C714, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Li YP, Chen Y, Li AS, Reid MB. Hydrogen peroxide stimulates ubiquitin-conjugating activity and expression of genes for specific E2 and E3 proteins in skeletal muscle myotubes. Am J Physiol Cell Physiol 285: C806–C812, 2003 [DOI] [PubMed] [Google Scholar]
- 29. Liu J, Zheng H, Tang M, Ryu YC, Wang X. A therapeutic dose of doxorubicin activates ubiquitin-proteasome system-mediated proteolysis by acting on both the ubiquitination apparatus and proteasome. Am J Physiol Heart Circ Physiol 295: H2541–H2550, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−ΔΔCT) method. Methods 25: 402–408, 2001 [DOI] [PubMed] [Google Scholar]
- 31. McLoon LK, Falkenberg JH, Dykstra D, Iaizzo PA. Doxorubicin chemomyectomy as a treatment for cervical dystonia: histological assessment after direct injection into the sternocleidomastoid muscle. Muscle Nerve 21: 1457–1464, 1998 [DOI] [PubMed] [Google Scholar]
- 32. Monsuez JJ, Charniot JC, Vignat N, Artigou JY. Cardiac side-effects of cancer chemotherapy. Int J Cardiol 144: 3–15, 2010 [DOI] [PubMed] [Google Scholar]
- 33. Moylan JS, Reid MB. Oxidative stress, chronic disease, and muscle wasting. Muscle Nerve 35: 411–429, 2007 [DOI] [PubMed] [Google Scholar]
- 34. Moylan JS, Smith JD, Chambers MA, McLoughlin TJ, Reid MB. TNF induction of atrogin-1/MAFbx mRNA depends on Foxo4 expression but not AKT-Foxo1/3 signaling. Am J Physiol Cell Physiol 295: C986–C993, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Myhre O, Andersen JM, Aarnes H, Fonnum F. Evaluation of the probes 2′,7′-dichlorofluorescin diacetate, luminol, and lucigenin as indicators of reactive species formation. Biochem Pharmacol 65: 1575–1582, 2003 [DOI] [PubMed] [Google Scholar]
- 36. Piscitelli SC, Rodvold KA, Rushing DA, Tewksbury DA. Pharmacokinetics and pharmacodynamics of doxorubicin in patients with small cell lung cancer. Clin Pharmacol Ther 53: 555–561, 1993 [DOI] [PubMed] [Google Scholar]
- 37. Powers SK, Kavazis AN, DeRuisseau KC. Mechanisms of disuse muscle atrophy: role of oxidative stress. Am J Physiol Regul Integr Comp Physiol 288: R337–R344, 2005 [DOI] [PubMed] [Google Scholar]
- 38. Ranek MJ, Wang X. Activation of the ubiquitin-proteasome system in doxorubicin cardiomyopathy. Curr Hypertens Rep 11: 389–395, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Reid MB, Haack KE, Franchek KM, Valberg PA, Kobzik L, West MS. Reactive oxygen in skeletal muscle. I. Intracellular oxidant kinetics and fatigue in vitro. J Appl Physiol 73: 1797–1804, 1992 [DOI] [PubMed] [Google Scholar]
- 40. Serbest G, Burkhardt MF, Siman R, Raghupathi R, Saatman KE. Temporal profiles of cytoskeletal protein loss following traumatic axonal injury in mice. Neurochem Res 32: 2006–2014, 2007 [DOI] [PubMed] [Google Scholar]
- 41. Smuder AJ, Kavazis AN, Min K, Powers SK. Exercise protects against doxorubicin-induced oxidative stress and proteolysis in skeletal muscle. J Appl Physiol 110: 935–942, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Supinski GS, Callahan LA. Free radical-mediated skeletal muscle dysfunction in inflammatory conditions. J Appl Physiol 102: 2056–2063, 2007 [DOI] [PubMed] [Google Scholar]
- 43. Szeto HH, Schiller PW. Novel therapies targeting inner mitochondrial membrane-from discovery to clinical development. Pharm Res 28: 2669–2679, 2011 [DOI] [PubMed] [Google Scholar]
- 44. Tozer RG, Tai P, Falconer W, Ducruet T, Karabadjian A, Bounous G, Molson JH, Droge W. Cysteine-rich protein reverses weight loss in lung cancer patients receiving chemotherapy or radiotherapy. Antioxid Redox Signal 10: 395–402, 2008 [DOI] [PubMed] [Google Scholar]
- 45. Turrens JF. Mitochondrial formation of reactive oxygen species. J Physiol 552: 335–344, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Yamamoto Y, Hoshino Y, Ito T, Nariai T, Mohri T, Obana M, Hayata N, Uozumi Y, Maeda M, Fujio Y, Azuma J. Atrogin-1 ubiquitin ligase is upregulated by doxorubicin via p38-MAP kinase in cardiac myocytes. Cardiovasc Res 79: 89–96, 2008 [DOI] [PubMed] [Google Scholar]
- 47. Yang L, Zhao K, Calingasan NY, Luo G, Szeto HH, Beal MF. Mitochondria targeted peptides protect against 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine neurotoxicity. Antioxid Redox Signal 11: 2095–2104, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Yen HC, Oberley TD, Vichitbandha S, Ho YS, Clair DK. The protective role of manganese superoxide dismutase against adriamycin-induced acute cardiac toxicity in transgenic mice. J Clin Invest 98: 1253–1260, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Zhao K, Zhao GM, Wu D, Soong Y, Birk AV, Schiller PW, Szeto HH. Cell-permeable peptide antioxidants targeted to inner mitochondrial membrane inhibit mitochondrial swelling, oxidative cell death, and reperfusion injury. J Biol Chem 279: 34682–34690, 2004 [DOI] [PubMed] [Google Scholar]
- 50. Zima T, Tesar V, Richardson PJ, Mantle D, Preedy VR. Effects of doxorubicin (adriamycin) and [(+)-1,2-bis(3,5-dioxopiperazinyl-1-yl)]propane (ICRF-187) on skeletal muscle protease activities. Toxicol Appl Pharmacol 171: 135–140, 2001 [DOI] [PubMed] [Google Scholar]