Abstract
RATIONALE
±3,4-methylenedioxymethamphetamine (MDMA, ‘ecstasy’) reportedly produces unique subjective effects, including increased sociability, feelings of closeness with others, and reduced interpersonal defensiveness. Despite their apparent importance in recreational and potential psychotherapeutic use of MDMA, the defining characteristics and neurobiological mechanisms of these interpersonal effects are poorly understood.
MATERIALS AND METHODS
We investigated acute effects of MDMA on self-reported sociability, and neuronal activation in response to socially threatening (angry and fearful faces) and socially rewarding (happy faces) stimuli. Assessment of social threat response focused on amygdala activation, whereas assessment of social reward focused on ventral striatum activation. Healthy volunteers (N=9) reporting past ecstasy use completed three experimental sessions, receiving MDMA (0.75mg/kg, and 1.5mg/kg), and placebo (PBO) under double-blind conditions. During peak drug effects, participants underwent functional magnetic resonance imaging while viewing standardized images depicting emotional facial expressions including angry, fearful, happy and neutral expressions. They also completed standardized self-report measures of sociability.
RESULTS
MDMA (1.5mg/kg) increased self-reported sociability compared to MDMA (0.75mg/kg) and PBO. MDMA (1.5mg/kg) attenuated left amygdala response to angry facial expressions compared to PBO, but MDMA did not affect amygdala reactivity to fearful expressions. MDMA (0.75mg/kg) enhanced ventral striatum response to happy expressions relative to PBO.
CONCLUSIONS
These data present the first evidence that MDMA may increase sociability in humans both by diminishing responses to threatening stimuli and enhancing responses to rewarding social signals.
Keywords: MDMA, ecstasy, social reward, social threat, sociability
INTRODUCTION
±3,4-methylenedioxymethamphetamine (MDMA, ‘ecstasy’) is reported to have a unique subjective profile, including ‘empathogenic’ effects such as increased sociability and decreased defensiveness (Parrott 2007). These properties are cited by users as a motivator to use ecstasy (Sumnall et al. 2006), and they may also underpin the purported effectiveness of MDMA as an adjunct to psychotherapy. Advocates for psychotherapeutic MDMA use argue that the drug increases psychological openness to the therapeutic process (Parrott 2007).
Despite generating widespread interest, the purported empathogenic effects of MDMA are poorly understood. Nevertheless, there is some evidence that MDMA induces social behavior in animals, and increases sociability ratings in humans. In rats, moderate MDMA doses increase social interaction (Morley et al. 2005; Thompson et al. 2007). Notably, MDMA increases rats’ tendency to lie adjacent to each other, which is thought to indicate sociability (Morley et al. 2005; Thompson et al. 2007). These behavioral changes involve release of oxytocin (OT), a neuropeptide implicated in attachment and pair bonding, with OT receptor antagonism attenuating MDMA’s prosocial effects in rodents (Thompson et al. 2007). In humans, MDMA doses ranging from 1 to 2 mg/kg increase self-reported extroversion (e.g. Liechti et al. 2000a; Vollenweider et al. 1999), friendliness (Tancer and Johanson 2007; Tancer and Johanson 2003), sociability (Tancer et al. 2003), talkativeness (Tancer et al. 2007; Tancer et al. 2003) and closeness to others (Kolbrich et al. 2008), although some studies have failed to observe these effects (Harris et al. 2002; Johanson et al. 2006; Liechti and Vollenweider, 2000b).
Previous imaging studies suggest possible brain mechanisms for MDMA’s social effects. Studies using functional Magnetic Resonance Imaging (fMRI) to measure blood oxygenated level dependent (BOLD) signal, an indirect indicator of neural activation, show the amygdala to be particularly important in socioemotional processing, especially processing of threat-related social information (Whalen et al. 1998; Zald 2003). In normal individuals, exposure to threat-related social signals such as fearful and angry facial expressions increases amygdala activity (Whalen et al. 1998; Zald, 2003); this effect is heightened in individuals with social anxiety (Phan et al. 2006). Administration of Δ9-tetrahydrocannabinoid (THC), the main psychoactive constituent of cannabis, reduces amygdala response to social signals of threat, an action that may underlie anxiolytic effects of THC (Phan et al. 2008). OT administration also reduces amygdala activation in response to threatening social stimuli (Kirsch et al. 2005), and patients with Williams-Beuren syndrome, characterized by hypersociability, demonstrate attenuated amygdala responses to social threat (Meyer-Lindenberg et al. 2005). Thus, to the extent that MDMA increases sociability, it may be expected to reduce amygdala responses to threat-related social signals.
MDMA might also increase sociability by enhancing the rewarding value of positive social interactions. Positive social stimuli act as a natural reward; rodents demonstrate conditioned preferences for places previously paired with a social partner (Douglas et al. 2004; Thiele et al. 2008), and in humans, positive social signs such as smiling faces activate reward circuitry (Tsukiura and Cabeza 2008). The ventral striatum (VS) is central to reward processing (Knutson and Cooper 2005), and is activated by a range of rewarding stimuli including drugs of abuse (Gilman et al. 2008). Increased salience of rewarding social stimuli, reflected in enhanced VS activation in response to positive social cues, would be expected to lead to increased social approach behavior, and hence sociability.
This study employed fMRI after acute MDMA administration to assess possible mechanisms for the effects of MDMA on sociability in humans. Initially, we investigated whether controlled laboratory administration of MDMA enhances self-reported sociability. We then aimed to assess whether MDMA altered neural processing of threatening and rewarding social stimuli, focusing on amygdala and VS respectively. We hypothesized that a) MDMA would increase self-reported sociability; b) MDMA would decrease amygdala reactivity in response to threatening (angry and fearful) facial expressions; and c) MDMA would enhance VS responses to rewarding (happy) facial expressions.
MATERIALS AND METHODS
Participants
Recruitment used on-line advertisements and word of mouth. Nine healthy, right-handed volunteers (18–29 years) reporting ecstasy use ≥ twice participated. Exclusion was based on: lifetime psychotic or bipolar disorder, past year other Axis 1 psychiatric disorder (DSM-IV, APA, 1994); medical/neurological illness assessed with medical exam, electrocardiogram and clinical interview; Body Mass Index outside healthy range (18–30); prior adverse ecstasy response; and MRI counter-indication. All participants provided written informed consent and were fully debriefed at completion, as approved by University of Chicago Institutional Review Board and in accordance with the Declaration of Helsinki.
Protocol
We employed a 3-session, within-participants, double-blind design. Participants received MDMA (0.75mg/kg and 1.5mg/kg) in ascending order with randomised placebo (PBO); sessions were scheduled ≥ 6 days apart. Participants abstained from cannabis for 7 days, alcohol for 24 hours, and all other recreational drugs for 48 hours prior to sessions. Compliance was verified with urine (Rapid Check 9 Panel Multi-Drug Screen, Craig Medical, Vista, CA), saliva (Oratect III, Branan Medical Corporation, Irvine, CA) and breathalyzer (Alco-sensor III, Intoximeters, St. Louis, MO) screens. Female participants provided a negative pregnancy test at each session (Aimstrip, Craig Medical, Vista, CA). Participants did not eat for 2 hours before sessions.
Drug sessions commenced at noon. After baseline measurements including cardiovascular and subjective measures, participants ingested an opaque gelatin capsule (size 00) containing either MDMA (0.75mg/kg or 1.5mg/kg) with lactose, or PBO (lactose). Doses in this low-to-moderate range have been safely administered to humans previously (Dumont and Verkes 2006). Doses as low as 0.5 mg/kg produce significant, although modest, increases in euphoria (Harris et al. 2002), whereas our moderate dose (1.5mg/kg) is within the recreational dose range (see Cole et al. 2002), and produces robust subjective effects (see Dumont et al. 2006). After capsule ingestion, participants remained in the hospital for at least 4.5 hours, during which they underwent regular cardiovascular checks and completed subjective questionnaires in a comfortable laboratory environment. Approximately 45 minutes after capsule administration, participants were escorted to the Brain Research Imaging Centre within the hospital where they underwent imaging. Participants then returned to the study room where they remained until session completion.
Subjective measures included a Drug Effects Questionnaire (DEQ; Johanson and Uhlenhuth 1980), a 7-item Visual Analogue Scale (VAS; Folstein and Luria 1973), and the Profile of Mood States (POMS; McNair and Droppleman 1971). The DEQ requires participants to rate on a visual analogue scale the extent to which they 1) feel any drug effect (from ‘none at all’ to ‘a lot’); 2) like the drug effect; 3) feel high; and 4) would like more drug. The VAS comprised the following: stimulated, anxious, sedated, down, elated, sociable, and nauseated. Participants rated whether each adjective described how they were feeling (from ‘not at all’ to ‘extremely’). The POMS is a 72-item adjective checklist rated on a 5-point Likert Scale. The POMS yields 8 subscores, including a Friendliness scale. DEQ and VAS were administered at 0, 30, 60, 135, 210 and 240 minutes post capsule with cardiovascular measurements. POMS was administered at 0, 60 and 240 minutes, with the middle time coinciding with onset of peak drug effect (Cami et al. 2000). Based on hypotheses, subjective outcome measures were: 1) VAS Sociability; and 2) POMS Friendliness. We also report DEQ Feel Drug scores to confirm non-specific subjective MDMA effects.
FMRI data collection commenced approximately 85 minutes after capsule administration (PBO: 80.8 ± 6.6 mins; 0.75mg/kg MDMA: 82.1 ± 12.6 mins; 1.5mg/kg MDMA: 91.4 ±10.5 mins) to coincide with peak drug effects (Cami et al. 2000). Participants underwent a facial Emotion Recognition (ER) task, employing stimuli from the Ekman and Friesen (1976) series. These stimuli have recruited amygdala in previous pharmacological fMRI studies (e.g. Anderson et al. 2007). There were four ER runs per session, each including six blocks of faces (happy, neutral, angry, fearful, disgusted and sad, each presented in a separate block; see Figure 1; based on emotion- and region-specific hypotheses, disgusted and sad face block data were not employed in these analyses). Using a button box with their right hand, participants rated whether stimuli showed an unpleasant, neutral or pleasant expression. A block of radios was also presented; responses to radio stimuli were not utilized in these analyses. Each run contained novel stimuli and presentation order of emotions was pseudo-randomized across runs. Face blocks were interleaved with equal length fixation blocks; for fixation participants rated the shading of each screen. Each run consisted of 14–20 second blocks; 6 emotional face, 1 radio, and 7 fixation blocks. Total task time (4 runs) was 18 minutes, 40 seconds.
Figure 1.

Schematic of Emotional Recognition stimuli employed during fMRI.
Participants also performed a motor-visual task to enable examination of MDMA’s effects on brain response to non-emotional stimuli. This task involved alternating 20 second blocks during which participants saw a flashing checkerboard while pressing a single button every second, or a gray fixation screen while remaining still. Participants viewed 6 checkerboard and 8 gray blocks over two runs, with a total task time of 4 minutes, 40 seconds.
Imaging: Acquisition and Analyses
Imaging data were collected with a 3T GE magnetic resonance scanner. BOLD functional images were collected from 30 axial, 5-mm-thick slices using T2*-sensitive gradient echo reverse spiral acquisition sequences (repetition, 2000 ms; echo, 25ms; 64 X 64 matrix; 24 cm field of view; flip angle, 77). Functional runs were followed by a high-resolution, T1-weighted volumetric anatomical scan for anatomical localization.
Data from three Ekman and three motor-visual runs were excluded for excessive movement (>3mm displacement or >3° rotation in any one direction). All other data met stability criteria with minimum motion correction. Data were analysed using SPM2 (Welcome Department of Cognitive Neurology, http://www.fil.ion.ucl.ac.uk/spm); a similar approach is described in Phan et al. (2008). Images were spatially realigned to correct for head motion, warped to an EPI template in Montreal Neurologic Institute (MNI) space, resampled to 2mm3 voxels, and smoothed with an 8mm3 kernel. The general linear model was applied to the time series, convolved with the canonical hemodynamic response function (Friston et al. 1995) with a 128 s high-pass filter. Condition effects were modelled with box-car regressors representing the occurrence of each block type, and effects were estimated at each voxel, for each participant. Individual contrast maps (Statistical Parametric Maps, SPMs) were then analysed at the second level in a random effects statistical model (Holmes and Friston 1998). We assessed for significant differences in amygdala activation (anger versus neutral and fear versus neutral) and in VS (happy versus neutral) between drug conditions using repeated-measures ANOVAs. First, we conducted whole-brain ANOVA, to examine main effects of drug on emotion-related brain reactivity in the a priori regions of interest (ROIs); we set a whole-brain, voxel-wise significance threshold of p < 0.005 uncorrected. Second, we followed up significant main effects with paired t-tests, based on our directionally-specific hypotheses, with the same whole-brain, voxel-wise significance threshold of p < 0.005 uncorrected. Third, we examined if these activation clusters reached significance after correction for multiple comparisons across a small volume (“SVC”) using anatomically-based volumes/ROIs. These statistical thresholds have recently been applied to fMRI studies of amygdala-striatum function (Del-Ben et al. 2005; Hariri et al. 2002; McBride et al. 2006). Fourth, we clarified the direction and extent of activation changes by extracting BOLD signal responses (parameter estimates, β weights) from 10mm diameter spheres around peak activations from amygdala (for anger and fear) and VS (for happy). Extracted estimates were entered into a repeated-measures ANOVA, followed by paired t-tests as necessary (significance set at p < .05).
The amygdala ROI was defined by a 10mm diameter sphere around MNI coordinates (±24,−2,−24), based on our prior fMRI study employing emotional faces (Fitzgerald et al. 2006). VS ROI was defined by a 10mm diameter sphere surrounding MNI coordinates (±6,10,−6) based on previous research on reward-related VS activation (Schott et al. 2008). To assess effects of MDMA on regions other than amygdala and VS, we analysed activation of primary visual (V1) and motor cortex (M1) during the motor-visual task (checkboard versus gray). M1 and V1 were defined anatomically by atlas (Walter et al. 2003); significance for ANOVAs and follow-up tests was set liberally at p < 0.005, uncorrected across ROIs.
In addition, for generation of novel hypotheses for future research, we assessed effects of MDMA on whole-brain activation in response to each emotion contrast (Anger > Neutral, Fear > Neutral, Happy > Neutral) and to the motor-visual task (Motor-Visual > Rest). Activations were thresholded at p < .001, uncorrected, with a minimum cluster size of 80mm3.
Cardiovascular, Subjective and Behavioral Data Analyses
For cardiovascular measures and DEQ Feel Drug scores, peak change from pre-capsule measures were analysed using repeated-measures ANOVAs, followed by paired t-tests as necessary. For subjective sociability, two-way repeated measures ANOVAs assessed for interactions between drug and time on change from baseline scores. Two-way ANOVAs investigated main effects of drug, and interactions between drug and facial emotion type, on accuracy and reaction time for the ER task. Significant main effects were followed up with paired t-tests. Interactions were followed up with simple main effects with effects of drug examined at each time/emotion; t-tests assessed significant simple main effects as required. Alpha was set at p < .05 for initial ANOVAs. An adjusted alpha level of p < .01 was employed for post-hoc analyses.
RESULTS
Participants’ mean age (±S.D.) was 24.0 (3.2). Two of nine participants were female and six identified as Caucasian. Lifetime ecstasy use ranged from 2 to 300 occasions. In the month before the study, five participants reported daily cigarette use, five reported using cannabis at least once, and eight used alcohol. All participants reported cannabis use, eight had used stimulants (e.g. cocaine), and seven reported hallucinogen use (e.g. psilocybin) in their lifetime (see Table 1).
Table 1.
Participants’ Drug Use Histories
| Mean | S.D | Range | |||
|---|---|---|---|---|---|
| Past Month Cigarettes/Daya | 10.20 | 5.93 | 5 – 20 | ||
| Past Month Alcohol Units/Weeka | 7.88 | 5.62 | 1 – 18 | ||
| Past Month THC Days Useda | 6.60 | 4.56 | 3 – 14 | ||
| Ecstasy Use – lifetime occasions | 63.89 | 94.92 | 2 – 300 | ||
| Ecstasy Use – age of first use | 17.44 | 1.94 | 15 – 20 | ||
| Ecstasy Use – months since use | 10.22 | 9.15 | 0.5 – 24 | ||
| Ecstasy Use in Past Year? | Yes | No | |||
| N | N | ||||
| 4 | 5 | ||||
| Lifetime Occasions of Use | Never | 1–10 | 11–50 | 51–100 | 100+ |
| N | N | N | N | N | |
| THC | 0 | 0 | 1 | 1 | 7 |
| Stimulantsb | 1 | 3 | 3 | 0 | 2 |
| Hallucinogensc | 2 | 2 | 2 | 1 | 2 |
| Opiatesd | 1 | 5 | 2 | 0 | 1 |
| Tranquillizerse | 4 | 4 | 1 | 0 | 0 |
Includes only participants reporting use of the substance in month prior to participation.
e.g. amphetamine, cocaine
e.g. lysergic acid diethylamide, psilocybin
e.g. heroin, opium, misused prescription opiates
MDMA increased peak systolic and diastolic blood pressure (BP) and heart rate (Table 2). Systolic BP was higher after MDMA (both doses) compared to PBO, and after MDMA (1.5mg/kg) compared to MDMA (0.75mg/kg). Diastolic BP only increased after MDMA (1.5mg/kg) compared to PBO, whereas HR increased on MDMA (both doses) compared to PBO, with no difference between the two active conditions. MDMA increased peak DEQ ratings of feeling any drug effect (DEQ Feel Drug; see Table 2). Feel Drug ratings were higher on MDMA (both doses) compared to PBO, and in the MDMA (1.5mg/kg) condition compared to MDMA (0.75mg/kg). There were no serious adverse effects of MDMA.
Table 2.
Effects of MDMA on Cardiovascular, Subjective and Behavioral Measures
| Placebo (PBO) | 0.75mg/kg MDMA (LOW) | 1.5mg/kg MDMA (MOD) | ANOVA | PBO vs. LOW | PBO vs. MOD | LOW vs. MOD | |
|---|---|---|---|---|---|---|---|
| Measure | Mean (S.D) | Mean (S.D.) | Mean (S.D.) | F (df) | t (df) | t (df) | t (df) |
| Systolic BP (mmHg)a | 4.8 (19.0) | 34.8 (13.5) | 48.1 (11.6) | 41.9* (2,16) | 5.9* (8) | 7.5* (8) | 3.9* (8) |
| Diastolic BP (mmHg)a | 3.6 (13.9) | 15.8 (13.4) | 26.0 (5.1) | 8.0* (2,16) | 2.2 (8) | 4.0* (8) | 1.8 (8) |
| Heart Rate (beats/minute)a | −5.3 (11.0) | 19.7 (16.2) | 35.3 (19.9) | 21.9* (2,16) | 4.1* (8) | 5.8* (8) | 2.9 (8) |
| DEQ Feel Druga | 14.5 (5.0) | 57.1 (4.6) | 91.8 (4.0) | 98.6* (2,16) | 7.1* (8) | 16.1* (8) | 6.1* (8) |
| VAS Sociability – Drug vs. Time | - | - | - | 3.1* (8,64) | - | - | - |
| VAS Sociability 135mins b | −6.3 (17.7) | −9.5 (13.5) | 19.7 (24.7) | 7.3* (2,16) | 0.5 (8) | 2.5 (8) p = .04 |
3.8* (8) |
| POMS Friendliness - Drug vs. Time | - | - | - | 1.3 (2,16) | - | - | - |
| ER Accuracy c | .89 (.04) | .91 (.01) | .86 (.02) | 0.9 (2,16) | |||
| ER Accuracy – Drug vs. Emotion Type | - | - | - | 1.2 (10,80) | |||
| ER RT d | 1345.7 (45.6) | 1168.9 (79.5) | 1292.3 (115.5) | 3.1 (2,16) | - | - | - |
| ER RT – Drug vs. Emotion Type | - | - | - | 0.6 (10,80) | - | - | - |
BP = blood pressure; DEQ = Drug Effects Questionnaire; VAS = Visual Analogue Scale; POMS = Profile of Mood States; ER = Emotional Recognition; RT = Reaction Time.
Peak change from pre-drug baseline.
Change from pre-drug baseline.
Marginal means of accuracy scores by drug across all emotional facial expressions, presented with standard error.
Marginal means of reaction time scores (in msecs) across all emotional facial expressions, presented with standard error.
p = < 0.05 (p < 0.01 for posthoc tests).
MDMA significantly increased VAS ratings of Sociability (interaction of drug versus time), with a very large effect size (ήp2 = .28). Simple main effects analyses of drug condition at individual time points revealed that MDMA (1.5mg/kg) increased ratings at 135 min relative to MDMA (0.75mg/kg), and marginally increased ratings compared to PBO (see Table 2; Figure 2).
Figure 2.
MDMA effects on self-reported sociability. Left: Visual Analogue Scale Sociability. Right: Profile of Mood States Friendliness. Data are mean change from pre-drug baseline (± S.E.M.) as a function of minutes post-capsule. Asterisk denotes difference from MDMA (0.75mg/kg; p< 0.01); # denotes marginal difference from PBO (p = 0.04).
A similar pattern of results was found for POMS Friendliness (see Table 2; Figure 2), although the effect did not reach significance. However, the effect size of the main effect of drug was large (ήp2 > .23), thus the absence of a significant effect may be due to the sample size.
MDMA did not affect accuracy of Emotion Recognition or reaction time, and there was no interactive effect of drug by emotion on accuracy or reaction time.
FMRI analyses showed that MDMA attenuated amygdala activation to angry, but not fearful, faces. Repeated-measures ANOVA revealed a difference in left amygdala activation to angry versus neutral faces [peak MNI coordinates (−20, −2, −24); F(2,16) = 9.64, p< 0.005]. T-tests showed that left amygdala activation to angry versus neutral faces was greater on PBO than MDMA (1.5mg/kg: t(8) = 4.41, p < 0.005). After small volume correction (SVC) for multiple comparisons, this finding was significant at p = .052. Analyses of extracted BOLD signal responses from spheres about peak activation for left amygdala also revealed an overall effect of drug (F(2,16) = 7.24, p < .01), with amygdala activation (angry versus neutral) lower on MDMA (1.5mg/kg) than both PBO (t(8) = 3.34, p < .05) and MDMA (0.75mg/kg; t(8) = 2.44, p < .05; Figure 3). There was no effect of drug on amygdala reactivity to fear versus neutral faces.
Figure 3.

MDMA effects on amygdala activation. Left: Statistical t map overlaid on a canonical brain rendering (MNI coronal y-plane = −2) showing greater left amygdala reactivity to angry versus neutral faces in the PBO relative to the MDMA (1.5mg/kg) condition (PBO>MDMA). Cluster size is 280mm3 (35 contiguous voxels). Statistical t-score is shown at the bottom of the brain rendering. L = Left. Right: Left amygdala BOLD response (β weight in arbitrary units) angry versus neutral faces across drug conditions. Asterisk denotes difference from PBO (p < 0.05); # denotes difference from MDMA (0.75mg/kg; p < 0.05).
MDMA enhanced VS activation to happy faces. There was a significant effect of drug on right VS activation in response to happy versus neutral faces [peak MNI coordinates (6, 8, −10); F(2,16) = 9.75, p < 0.005]. T-tests indicated that VS activation to happy versus neutral faces was greater on MDMA (0.75mg/kg) than PBO (t(8) = 6.13, p < .001). After SVC, this finding was significant at p < .05. While not reaching significance, there was also a trend for VS activation to happy versus neutral faces to be greater on MDMA (1.5mg/kg) than PBO (t(8) = 2.72, p < .05); this trend did not survive SVC. Analyses of BOLD signal responses extracted from spheres about peak activation for VS happy versus neutral faces also indicated an overall drug effect (F(2,16) = 8.41, p < .005), with VS activation (happy versus neutral faces) greater in both MDMA conditions than PBO (0.75mg/kg: t(8) = 5.51, p < .005; 1.5mg/kg: t(8) = 2.44, p < .05; Figure 4).
Figure 4.
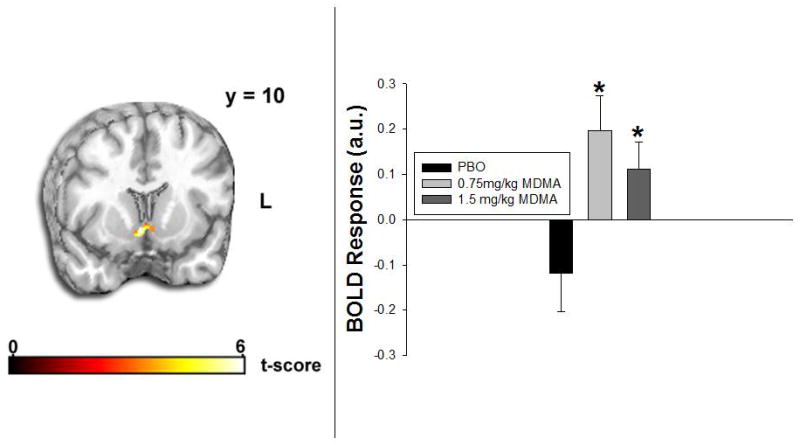
MDMA effects on ventral striatum activation. Left: Statistical t map overlaid on a canonical brain rendering (MNI coronal y-plane = 10) showing greater ventral striatum reactivity to happy versus neutral faces in the MDMA (0.75mg/kg) relative to the PBO condition (MDMA>PBO). Cluster size is 1520mm3 (190 contiguous voxels). Statistical t-score is shown at the bottom of the brain rendering. L = Left. Right: Ventral striatum BOLD response (β weight in arbitrary units) happy versus neutral faces across drug conditions. Asterisks denote difference from PBO (p< 0.05).
In the motorvisual task, MDMA decreased activation in V1 and M1. In V1, MDMA (1.5mg/kg) decreased activation relative to PBO ((6,−82, 6), t(8) = 3.81, p < .005, (12, −92, 16), t(8) = 3.72, p < .005). In M1, MDMA (1.5mg/kg) also reduced activation compared to PBO ((10, −12, 76), t(8) = 5.82, p < .005, (−12, −28, 74), t(8) = 4.77, p < .005, (66, −6, 14), t(8) = 3.80, p < .005, (16, −36, 70), t(8) = 3.54, p < .005). Drug effects on activations to emotional faces and motor-visual stimulation outside the a priori ROIs are presented in Table 3.
Table 3.
ANOVA-Derived Main Effect of Drug on Brain Response to Emotional Faces
| Contrast | Region | Laterality | Size (mm3) | Z Score | MNI Coordinates | ||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| Angry > Neutral | Middle Frontal Gyrus | R | 536 | 4.33 | 32 | 36 | 22 |
| R | 136 | 3.94 | 36 | 38 | 2 | ||
| R | 272 | 3.54 | 30 | 50 | −10 | ||
| L | 152 | 3.39 | −40 | 56 | 2 | ||
| Caudate | L | 384 | 4.11 | −20 | −14 | 24 | |
| Precuneus | L | 312 | 4.03 | −12 | −54 | 52 | |
| R | 280 | 3.37 | 18 | −50 | 50 | ||
| Precentral Gyrus | R | 456 | 4.02 | 14 | −22 | 78 | |
| R | 104 | 3.42 | 28 | −10 | 56 | ||
| Postcentral Gyrus | R | 280 | 3.98 | 12 | −34 | 82 | |
| Superior Frontal Gyrus | R | 344 | 3.94 | 18 | 8 | 56 | |
| Middle Temporal Gyrus | L | 784 | 3.74 | −34 | −66 | 14 | |
| R | 184 | 3.54 | 42 | −58 | 20 | ||
| Supplementary Motor Area | L | 848 | 3.73 | −16 | −14 | 60 | |
| R | 104 | 3.48 | 4 | 2 | 72 | ||
| Mid Cingulate | R | 400 | 3.72 | 18 | −20 | 38 | |
| L | 88 | 3.45 | −10 | −10 | 28 | ||
| Cerebellum | L | 168 | 3.72 | −8 | −72 | −30 | |
| Putamen | R | 144 | 3.39 | 24 | 8 | 6 | |
| Insula | L | 96 | 3.35 | −28 | 20 | 6 | |
| Amygdala | L | 80 | 2.91 | −20 | −2 | −24 | |
| Fearful > Neutral | Superior Parietal Gyrus | R | 200 | 3.60 | 18 | −80 | 50 |
| Happy > Neutral | Supramarginal Gyrus | R | 152 | 3.60 | 74 | −20 | 16 |
| Ventral Striatum | R | 240 | 2.93 | 6 | 8 | −10 | |
| Motor-Visual > Rest | |||||||
| Parahippocampal Gyrus | R | 224 | 4.28 | 16 | −18 | −28 | |
| Inferior Occipital Gyrus | R | 200 | 4.03 | 40 | −84 | −16 | |
| Angular Gyrus | R | 80 | 3.56 | 52 | −76 | 34 | |
| Postcentral Gyrus | R | 136 | 3.49 | 74 | −2 | 18 | |
Significant activations across whole-brain search are presented at p < 0.001, minimum cluster size of 10 voxels (80mm3). Activations shown in bold are within a priori ROIs, thresholded at p < 0.005.
DISCUSSION
MDMA attenuated amygdala reactivity to angry, but not fearful, faces, while enhancing ventral striatum response to happy faces. As expected, MDMA also increased subjective sociability at 1.5mg/kg. These findings suggest that MDMA alters processing of emotionally salient social information in at least two ways, by reducing responses to threat and by enhancing responses to reward.
Converging lines of evidence in humans and nonhumans suggest that MDMA increases sociability. Controlled MDMA administration to humans increases subjective ratings of sociability (Johanson et al. 2006; Tancer et al. 2007; Tancer et al. 2003; Vollenweider et al. 1999), and recreational users describe feeling sociable after ecstasy (Sumnall et al. 2006). In rodents, MDMA produces a unique pattern of adjacent lying behavior that is interpreted as sociability (Morley et al. 2005; Thompson et al. 2007). Our finding that MDMA (1.5 mg/kg) increased self-reported sociability is consistent with these reports.
Here, we investigated neural mechanisms that may mediate this increase in sociability, by studying neural responses to emotional stimuli. We found that MDMA both dampened neuronal responses to threat-related social stimuli and increased responses to positive social images, suggesting that these processes may contribute to the drug’s prosocial effects. The findings regarding threat-related stimuli are consistent with other imaging studies investigating responses to socioemotional material. Two other drugs that purportedly decrease social anxiety, THC (Phan et al. 2008) and alcohol (Gilman et al. 2008), also attenuate limbic responses to social threat (Gilman et al. 2008; Phan et al. 2008). Individuals with social anxiety, who typically avoid social interactions, have heightened amygdala response to threat signals (Phan et al. 2006); whereas those genetically predisposed towards exaggerated sociability have dampened amygdala reactivity to social threat (Meyer-Lindenberg et al. 2005). OT administration attenuates limbic threat response (Kirsch et al. 2005) and increases behavioral indicators of trust (Kosfeld et al. 2005). Together, these previous findings suggest that dampened neural response to social threat leads to greater sociability. Our findings regarding increased responses to positive social stimuli are relatively novel, because there is little existing evidence regarding effects of drugs on social reward. However, the finding is consistent with a large body of evidence that activation of dopaminergic reward circuitry is critically important in human socioaffiliative behavior (Skuse and Gallagher, 2008), supporting the hypothesis that enhanced social reward processing would also lead to heightened sociability.
The effects of MDMA were dose-dependent on some, but not all, measures. As has been reported previously (see Dumont et al. 2006), the drug increased subjective sociability only at a higher dose. Similarly, MDMA attenuated amygdala response to angry faces only at the higher dose. Of note, however, was the apparent dissociation between self-report and VS recruitment in response to happy facial expressions. At the lower dose, MDMA enhanced neural response to happy faces without changing subjective sociability ratings. It is possible that brain responses to social stimuli are more sensitive indicators of prosocial effects, and that higher doses may be needed for individuals to report feeling more sociable. Moreover, it is not known whether the subjective experience of feeling social is essential for individuals to actually behave more sociably. Further research is required to clarify relationships between behavioral, subjective and neural dimensions of MDMA’s effects, and to better characterize active doses of MDMA in terms of sociability.
Neurobiological substrates of MDMA’s unusual effects are poorly understood (Thompson et al. 2007). However, there is some evidence that they may be mediated through effects on both oxytocin and dopaminergic (DA) neurocircuitry. OT plays a key role in modulating attachment and affiliation, and OT antagonism attenuates the prosocial effects of MDMA in rodents (Thompson et al. 2007). Ecstasy self-administration in humans is also associated with increased plasma OT levels (Wolff et al. 2006). It has been suggested that the effects of OT on social behavior may involve interactions between OT systems and DA reward circuitry (Skuse and Gallagher, 2008). This possibility has not been directly assessed in relation to MDMA. However, a recent study found that in rodents, MDMA administration in a social context produced greater neural activation than MDMA in isolation. Regions preferentially activated included some high in OT receptors and implicated in social behavior, as well as regions involved in the mesolimbic DA system, known to subserve reward (Thompson et al. 2009). Thus, it may be hypothesized that MDMA’s reinforcing effects involve enhancement of the rewarding value of social stimuli through interactions between OT and DA circuits (Thompson et al. 2009). Other studies reporting interactions between social reward and cocaine reward in rats (Thiele et al. 2008) suggest that interactions between OT and DA may be involved more broadly in addictive behaviors (McGregor et al. 2008).
It is also likely that MDMA’s well-described effects on serotonergic (5-HT) signaling play a role in alterations to social processing and behavior. MDMA’s acute pharmacodynamic actions include carrier-mediated release of 5-HT from presynaptic vesicles and monoamine oxidase inhibition, resulting in sudden increases in extracellular 5-HT levels (for review, see Green et al. 2003). A substantial body of literature implicates 5-HT neurotransmission in social processing and behavior (e.g. Del-Ben et al. 2008; Young and Leyton, 2002). Selective 5-HT manipulations alter identification (Del-Ben et al., 2008) and neural processing of emotional faces (Harmer et al. 2006), as well as affiliative behavior (Tse and Bond, 2002). It is unclear to what extent these effects would be expected to overlap with those of MDMA, given that MDMA also affects a range of other systems (see Green et al. 2003). Future research, perhaps employing pretreatment with selective 5-HT agents, is needed to clarify the role of 5-HT in social effects of MDMA in humans. Individual and interactive effects of 5-HT, OT and DA signaling will provide a rich source for future investigations of MDMA and the neural circuitry underlying social behavior and addiction.
Although these findings suggest that MDMA’s apparently specific effects relate to altered social threat and reward processing, effects of drugs of abuse in general on processing of salient material are poorly understood. It may be that other drugs also exert some of their effects by altering threat and reward processing; indeed, both cannabinoids (Phan et al., 2008) and alcohol (Gilman et al. 2008) appear to attenuate social threat responding. To our knowledge, no other drug has been found to increase subjective sociability while both attenuating responses to social threat and increasing social reward responding. However, further research is required to better assess whether other drugs also affect processing of socio-emotionally salient material.
The present findings should be regarded as preliminary; there are a number of limitations remaining to be addressed. The sample was small; these findings require replication in a larger sample. Due to the sample size, we restricted analyses to two a priori identified brain region; larger samples would facilitate detailed examination of whole brain. Further, MDMA may have actions on cerebral blood flow that affected either global or regionally-specific BOLD signal. Indeed, a previous study employing [H215O] Positron Emission Tomography (PET) to assess effects of MDMA on regional cerebral blood flow (rCBF) in humans found the drug reduced rCBF in left amygdala, independent of task (Gamma et al. 2000). Thus, although our interpretation of these findings is supported by their emotion-specific nature, potentially confounding acute effects of MDMA on cerebrovasculature cannot be ruled out. We observed decreased activation with MDMA in both primary visual and motor cortex during a simple motorvisual task, also indicating a need for caution due to the possibility of generalized activation decreases arising from MDMA. However, a global decrease in activation would not give rise to the present finding of increased VS activation to happy versus neutral faces on MDMA. Moreover, decreased visual cortex activation might be expected in the context of decreased amygdala response, given functional connections between the two in processing socially relevant material (Skuse and Gallagher, 2008).
For ethical reasons, all participants had prior ecstasy exposure. There was substantial variability in degree and recency of exposure. This may have affected results, as it is possible that heavy ecstasy use causes long-term alterations to serotonergic neurotransmission (McCann et al. 2000; see also Bedi et al., 2008 for discussion of methodological issues). Moreover, due to involvement of serotonin in vasoconstriction (Cohen et al. 1996), it is possible that prior or recent MDMA exposure might have affected BOLD responses. To assess this possibility, we examined correlations between extracted BOLD response, lifetime ecstasy use and time since most recent use. There were no correlations between ecstasy-use variables and extracted BOLD signals, suggesting past ecstasy use did not affect results. This may have been because the majority of participants had used MDMA less than 50 times, and were not current users at the time of participation.
For safety reasons, MDMA doses were administered in ascending order, which may have influenced outcomes. In addition, we allowed 6 days between sessions; preclinical findings suggest that in non-human primates serotonin depletion may persist for up to two weeks after single doses (Ricaurte et al. 1988) or repeated oral doses (Mechan et al. 2005). Thus, it is possible that a longer period between sessions was required to ensure the absence of carry-over effects. However, although the minimum period between sessions was 6 days, sessions were an average of 10.72 (±7.15) days apart. The doses we employed were substantially less than those used in the primate studies. In addition, assessment of subjective and cardiovascular measures and extracted BOLD signal responses revealed no effect of session order, suggesting that the partial randomization protocol and length of time between sessions did not affect outcomes.
Finally, although the fMRI protocol was designed to coincide with peak drug effects, we did not obtain blood plasma measurements ensuring that imaging data collection coincided with peak plasma MDMA levels. Subjective effects support the timing of the imaging protocol. However, future studies could valuably measure plasma MDMA levels to confirm that imaging data collection coincided with peak plasma MDMA levels. Moreover, assessment of plasma MDMA levels may cast light on the dose-dependency of the sociability-altering effects of MDMA.
Such limitations notwithstanding, these data provide the first evidence that the unusual subjective profile of MDMA may be related to alterations in neural processing of social signals of threat and reward. This possibility has important implications in terms of recreational use and abuse of ecstasy, and potential use of MDMA as a psychotherapeutic agent.
Acknowledgments
Thanks to Gina Beguhn, David Hyman, Patricia Kriegal, Robert Lyons and Rosemary McCarron for technical assistance, to Royce Lee for medical support, and Emmanuel Semmes for pharmaceutical support. Thanks also to participants. This research was supported by National Institute of Drug Abuse (DA02812).
Footnotes
Disclosures/Conflict of Interest: None.
This experiment complied with the current laws of the country in which it was performed (USA).
References
- Anderson IM, Del-Ben CM, Mckie S, Richardson P, Williams SR, Elliott R, et al. Citalopram modulation of neuronal responses to aversive face emotions: A functional MRI study. NeuroReport. 2007;18:1351–1355. doi: 10.1097/WNR.0b013e3282742115. [DOI] [PubMed] [Google Scholar]
- APA. DSM-IV: Diagnostic and Statistical Manual of Mental Disorders. American Psychiatric Association; MD, US: 1994. [Google Scholar]
- Bedi G, Redman J. Ecstasy use and higher-level cognitive function: Weak effects of ecstasy after control for potential confounds. Psychol Med. 2008;38:1319–30. doi: 10.1017/S0033291708002730. [DOI] [PubMed] [Google Scholar]
- Cami J, Farre M, Mas M, Roset PN, Poudevida S, Mas A, et al. Human pharmacology of 3,4-methylenedioxymethamphetamine (“Ecstasy”): Psychomotor performance and subjective effects. J Clin Psychopharmacol. 2000;20:455–466. doi: 10.1097/00004714-200008000-00010. [DOI] [PubMed] [Google Scholar]
- Cohen Z, Bonvento G, Lacombe P, Hamel E. Serotonin in the regulation of brain microcirculation. Prog Neurobiol. 1996;50:335–62. doi: 10.1016/s0301-0082(96)00033-0. [DOI] [PubMed] [Google Scholar]
- Cole JC, Bailey M, Sumnall HR, Wagstaff GF, King LA. The content of ecstasy tablets: Implications for the study of their long-term effects. Addiction. 2002;97:1531–1536. doi: 10.1046/j.1360-0443.2002.00222.x. [DOI] [PubMed] [Google Scholar]
- Del-Ben CM, Deakin JFW, McKie S, Delvai NA, Williams SR, Elliot R, et al. The effect of citalopram pretreatment on neuronal responses to neuropsychological tasks in normal volunteers: An fMRI study. Neuropsychopharmacology. 2005;30:1724–1734. doi: 10.1038/sj.npp.1300728. [DOI] [PubMed] [Google Scholar]
- Del-Ben CM, Ferreira CAQ, Alves-Neto WC, Graeff FG. Serotonergic modulation of face-emotion recognition. Braz J Med Biol Res. 2008;41:63–9. doi: 10.1590/s0100-879x2008000400002. [DOI] [PubMed] [Google Scholar]
- Douglas LA, Varlinskaya EI, Spear LP. Rewarding properties of social interactions in adolescent and adult male and female rats: Impact of social versus isolate housing of subjects and partners. Dev Psychobiol. 2004;45:153–162. doi: 10.1002/dev.20025. [DOI] [PubMed] [Google Scholar]
- Dumont GJH, Verkes RJ. A review of acute effects of 3,4-methylenedioxymethamphetmine in healthy volunteers. J Psychopharmacol. 2006;20:176–187. doi: 10.1177/0269881106063271. [DOI] [PubMed] [Google Scholar]
- Ekman P, Friesen WV. Pictures of Facial Affect. Consulting Psychologists Press; Palo Alto, CA: 1976. [Google Scholar]
- Fitzgerald DA, Angstadt M, Jelsone LM, Nathan PJ, Phan KL. Beyond threat: Amygdala reactivity across multiple expressions of facial affect. NeuroImage. 2006;30:1441–1448. doi: 10.1016/j.neuroimage.2005.11.003. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Luria R. Reliability, validity, and clinical application of the Visual Analogue Mood Scale. Psychol Med. 1973;3:479–486. doi: 10.1017/s0033291700054283. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Holmes AP, Worsley KJ, Poline JB, Frith CD, Frackowiak RS. Statistical parametric maps in functional imaging: A general linear approach. Hum Brain Mapp. 1995;2:189–210. [Google Scholar]
- Gamma A, Buck A, Berthold T, Hell D, Vollenweider FX. 3,4-Methylenedioxymethamphetamine (MDMA) modulates cortical and limbic brain activity as measured by [H215O]-PET in healthy humans. Neuropsychopharmacology. 2000;23:388–395. doi: 10.1016/S0893-133X(00)00130-5. [DOI] [PubMed] [Google Scholar]
- Gilman JM, Ramchandani VA, Davis MB, Bjjork JM, Hommer DW. Why we like to drink: A functional Magnetic Resonance Imaging study of the rewarding and anxiolytic effects of alcohol. J Neurosci. 2008;28:4583–4591. doi: 10.1523/JNEUROSCI.0086-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green AR, Mechan AO, Elliott JM, O’Shea E, Colado MI. The pharmacology and clinical pharmacology of 3,4-methylenedioxymethamphetamine (MDMA, “Ecstasy”) Pharmacol Rev. 2003;55:463–508. doi: 10.1124/pr.55.3.3. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Mattay VS, Tessitore A, Fera F, Smith WG, Weinberger DR. Dextroamphetamine modulates the response of the human amygdala. Neuropsychopharmacology. 2002;27:1036–1040. doi: 10.1016/S0893-133X(02)00373-1. [DOI] [PubMed] [Google Scholar]
- Harmer CJ, Makcay CE, Reid CB, Cowen PJ, Goodwin GM. Antidepressant drug treatment modifies the neural processing of nonconscious threat cues. Biol Psychiatry. 2006;59:816–20. doi: 10.1016/j.biopsych.2005.10.015. [DOI] [PubMed] [Google Scholar]
- Harris DS, Baggott M, Mendelson JH, Mendelson JE, Jones RT. Subjective and hormonal effects of 3,4-methylenedioxymethamphetamine (MDMA) in humans. Psychopharmacology. 2002;162(4):396–405. doi: 10.1007/s00213-002-1131-1. [DOI] [PubMed] [Google Scholar]
- Holmes AP, Friston KJ. Generalisability, random effects and population inference. NeuroImage. 1998;7:S754. [Google Scholar]
- Johanson CE, Kilbey M, Gatchalian K, Tancer M. Discriminative stimulus effects of 3,4-methylenedioxymethamphetamine (MDMA) in humans trained to discriminate among d-amphetamine, meta-chlorophenylpiperazine and placebo. Drug Alcohol Depend. 2006;81:27–36. doi: 10.1016/j.drugalcdep.2005.05.003. [DOI] [PubMed] [Google Scholar]
- Johanson CE, Uhlenhuth EH. Drug preference and mood in humans: Diazepam. Psychopharmacology. 1980;71:269–273. doi: 10.1007/BF00433061. [DOI] [PubMed] [Google Scholar]
- Kirsch P, Esslinger C, Chen Q, Mier D, Lis S, Siddhanti S, et al. Oxytocin modulates neural circuitry for social cognition and fear in humans. J Neurosci. 2005;25:11489–11493. doi: 10.1523/JNEUROSCI.3984-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson B, Cooper JC. Functional magnetic resonance imaging of reward prediction. Curr Opin Neurol. 2005;18:411–417. doi: 10.1097/01.wco.0000173463.24758.f6. [DOI] [PubMed] [Google Scholar]
- Kolbrich EA, Goodwin RS, Gorelick DA, Hayes RJ, Stein EA, Huestis MA. Physiological and subjective responses to controlled oral 3,4-methylenedioxymethamphetamine administration. J Clin Psychopharmacol. 2008;28:432–440. doi: 10.1097/JCP.0b013e31817ef470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosfeld M, Heinrichs M, Zak PJ, Fischbacher U, Fehr E. Oxytocin increases trust in humans. Nature. 2005;435:673–676. doi: 10.1038/nature03701. [DOI] [PubMed] [Google Scholar]
- Liechti ME, Saur MR, Gamma A, Hell D, Vollenweider FX. Psychological and physiological effects of MDMA (“Ecstasy”) after pretreatment with the 5-HT-sub-2 antagonist ketanserin in healthy humans. Neuropsychopharmacology. 2000a;23(4):396–404. doi: 10.1016/S0893-133X(00)00126-3. [DOI] [PubMed] [Google Scholar]
- Liechti ME, Vollenweider FX. Acute psychological and physiological effects of MDMA (“Ecstasy”) after haloperidol pretreatment in healthy humans. Eur Neuropsychopharmacol. 2000b;10(4):289–295. doi: 10.1016/s0924-977x(00)00086-9. [DOI] [PubMed] [Google Scholar]
- McBride D, Barrett SP, Kelly JT, Aw A, Dagher A. Effects of expectancy and abstinence on the neural response to smoking cues in cigarette smokers: An fMRI study. Neuropsychopharmacology. 2006;31:2728–2738. doi: 10.1038/sj.npp.1301075. [DOI] [PubMed] [Google Scholar]
- McCann UD, Eligulashvili V, Ricaurte GA. ±3,4-Methylenedioxymethamphetamine (‘Ecstasy’)-induced serotonin neurotoxicity: Clinical studies. Neuropsychobiology. 2000;42:11–6. doi: 10.1159/000026665. [DOI] [PubMed] [Google Scholar]
- McGregor IS, Callaghan PD, Hunt GE. From ultrasocial to antisocial: A role for oxytocin in the acute reinforcing effects and long-term adverse consequences of drug use? Br J Pharmacol. 2008;154:358–68. doi: 10.1038/bjp.2008.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNair DLM, Droppleman L. Profile of Mood States. Educational and Industrial Testing Service; San Diego: 1971. [Google Scholar]
- Mechan A, Yuan J, Hatzidimitriou G, Irvine RJ, McCann UD, Ricaurte GA. Pharmacokinetic profile of single and repeated oral doses of MDMA in squirrel monkeys: Relationship to lasting effects on brain serotonin neurons. Neuropsychopharmacology. 2005;31:339–50. doi: 10.1038/sj.npp.1300808. [DOI] [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Hariri AR, Munoz KE, Mervis CB, Mattay VS, Morris CA, et al. Neural correlates of genetically abnormal social cognition in William’s syndrome. Nat Neurosci. 2005;8:991–993. doi: 10.1038/nn1494. [DOI] [PubMed] [Google Scholar]
- Morley KC, Arnold JC, McGregor IS. Serotonin (1A) receptor involvement in acute 3,4-methylenedioxymethamphetamine (MDMA) facilitation of social interaction in the rat. Prog Neuro-Psychopharmacol Biol Psychiatry. 2005;29:648–657. doi: 10.1016/j.pnpbp.2005.04.009. [DOI] [PubMed] [Google Scholar]
- Parrott A. The psychotherapeutic potential of MDMA (3,4-methylenedioxymethamphetamine): An evidence-based review. Psychopharmacology. 2007;191:181–193. doi: 10.1007/s00213-007-0703-5. [DOI] [PubMed] [Google Scholar]
- Phan KL, Angstadt M, Golden J, Onyewuenyi I, Popovska A, de Wit H. Cannabinoid modulation of amygdala reactivity to social signals of threat in humans. J Neurosci. 2008;28:2313–2319. doi: 10.1523/JNEUROSCI.5603-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan KL, Fitzgerald DA, Nathan PJ, Tancer ME. Association between amygdala hyperactivity to harsh faces and severity of social anxiety in generalized social phobia. Biol Psychiatry. 2006;59:424–429. doi: 10.1016/j.biopsych.2005.08.012. [DOI] [PubMed] [Google Scholar]
- Ricaurte GA, DeLanney LE, Irwin I, Langston JW. Toxic effects of MDMA on central serotonergic neurons in the primate: Importance of route and frequency of administration. Brain Res. 1988;446:165–8. doi: 10.1016/0006-8993(88)91309-1. [DOI] [PubMed] [Google Scholar]
- Schott BH, Minuzzi L, Krebs RM, Elmenhorst D, Lang M, Winz OH, et al. Mesolimbic functional Magnetic Resonance Imaging activations during reward anticipation correlate with reward-related ventral striatum dopamine release. J Neurosci. 2008;28:14311–14319. doi: 10.1523/JNEUROSCI.2058-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skuse DH, Gallagher L. Dopaminergic-neuropeptide interactions in the social brain. Trends Cogn Sci. 2008;13:27–35. doi: 10.1016/j.tics.2008.09.007. [DOI] [PubMed] [Google Scholar]
- Sumnall HR, Cole JC, Jerome L. The varieties of ecstasy experience: An exploration of the subjective experiences of ecstasy. J Psychopharmacol. 2006;20:670–682. doi: 10.1177/0269881106060764. [DOI] [PubMed] [Google Scholar]
- Tancer M, Johanson CE. The effects of fluoxetine on the subjective and physiological effects of 3,4-methylenedioxymethamphetamine (MDMA) in humans. Psychopharmacology. 2007;189:565–573. doi: 10.1007/s00213-006-0576-z. [DOI] [PubMed] [Google Scholar]
- Tancer ME, Johanson CE. Reinforcing, subjective, and physiological effects of MDMA in humans: A comparison with d-amphetamine and mCPP. Drug Alcohol Depend. 2003;72(1):33–44. doi: 10.1016/s0376-8716(03)00172-8. [DOI] [PubMed] [Google Scholar]
- Thiele KJ, Okun AC, Neisewander JL. Social reward-conditioned place preference: A model revealing an interaction between cocaine and social context rewards in rats. Drug Alcohol Depend. 2008;96:202–212. doi: 10.1016/j.drugalcdep.2008.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson MR, Callaghan PD, Hunt GE, Cornish JL, McGregor IS. A role for oxytocin and 5-HT(1A) receptors in the prosocial effects of 3,4 methylenedioxymethamphetamine (“ecstasy”) Neuroscience. 2007;146:509–514. doi: 10.1016/j.neuroscience.2007.02.032. [DOI] [PubMed] [Google Scholar]
- Thompson MR, Hunt GE, McGregor IS. Neural correlates of MDMA (“Ecstasy”)-induced social interaction in rats. Soc Neurosci. 2009;4:60–72. doi: 10.1080/17470910802045042. [DOI] [PubMed] [Google Scholar]
- Tse WS, Bond AJ. Serotonergic interventions affects both social dominance and affiliative behavior. Psychopharmacology. 2002;161:324–30. doi: 10.1007/s00213-002-1049-7. [DOI] [PubMed] [Google Scholar]
- Tsukiura T, Cabeza R. Orbitofrontal and hippocampal contributions to memory for face-name associations: The rewarding power of a smile. Neuropsychologia. 2008;46:2310–2319. doi: 10.1016/j.neuropsychologia.2008.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vollenweider FX, Remensberger S, Hell D, Geyer MA. Opposite effects of 3,4-methylenedioxymethamphetamine (MDMA) on sensorimotor gating in rats versus healthy humans. Psychopharmacology. 1999;143(4):365–372. doi: 10.1007/s002130050960. [DOI] [PubMed] [Google Scholar]
- Walter B, Blecker C, Kirsch P, Sammer G, Schlienle A, Stark R, et al. MARINA: An easy to use tool for the creation of MAsks for Region of INterest Analyses. Paper presented at Ninth International Conference on Functional Mapping of the Human Brain; New York, NY. June.2003. [Google Scholar]
- Whalen PJ, Rauch SL, Etcoff NL, McInerney SC, Lee MB, Jenike MA. Masked presentations of emotional facial expressions modulate amygdala activity without explicit knowledge. J Neurosci. 1998;18:411–418. doi: 10.1523/JNEUROSCI.18-01-00411.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff K, Tsapakis EM, Winstock AR, Hartley D, Holt D, Forsling ML, et al. Vasopressin and oxytocin secretion in response to the consumption of ecstasy in a clubbing population. J Psychopharmacol. 2006;20:400–410. doi: 10.1177/0269881106061514. [DOI] [PubMed] [Google Scholar]
- Young SN, Leyton M. The role of serotonin in human mood and social interaction: Insight from altered tryptophan levels. Pharmacol Biochem Behav. 2002;71:857–65. doi: 10.1016/s0091-3057(01)00670-0. [DOI] [PubMed] [Google Scholar]
- Zald DH. The human amygdala and the emotional evaluation of sensory stimuli. Brain Res Rev. 2003;41:88–123. doi: 10.1016/s0165-0173(02)00248-5. [DOI] [PubMed] [Google Scholar]



