Abstract
Schistosomiasis-associated pulmonary arterial hypertension (PAH) is one of the most common causes of pulmonary hypertension worldwide. A potential contributing mechanism to the pathogenesis of this disease is a localized immune reaction to retained and persistent parasite-derived antigens. We sought to identify Schistosoma-derived egg antigens present in the lungs of individuals who died of the disease. We obtained 18 lung samples collected at autopsy from individuals who died of schistosomiasis-associated PAH in Brazil. A rabbit polyclonal antibody was created to known Schistosoma mansoni-soluble egg antigen (SEA). Histologic assessment and immunostaining of the human tissue was performed, along with immunostaining and immunoblotting of lung tissue from mice experimentally infected with S. mansoni. All 18 lung samples had evidence of pulmonary vascular remodeling with plexiform lesions and arterial medial thickening, but no visible eggs were seen. The anti-SEA antibody detected S. mansoni egg antigens in visible eggs in mouse lung and human intestine specimens, but did not identify a significant amount of egg antigen in the human lung specimens. In mouse granulomas containing degraded eggs, we observed colocalization of egg antigens and macrophage lysosomes. In conclusion, there is unlikely to be a significant amount of persistent parasite-derived antigens within the lungs of individuals who die of schistosomiasis-associated PAH. This suggests that retained and persistent parasite proteins are not contributing to a localized immune response in the pathogenesis of this disease.
Keywords: pulmonary arterial hypertension, pulmonary hypertension, schistosomiasis
INTRODUCTION
Schistosomiasis affects over 200 million people in 74 countries, where it causes more than 250,000 deaths and up to 4.5 million disability-adjusted years lost annually.[1] Approximately 10% of those chronically infected with Schistosoma mansoni develop hepatosplenic schistosomiasis, a syndrome of preportal fibrosis and portocaval shunting.[2] Approximately 10–20% of those with hepatosplenic disease, or 2–5 million people worldwide, develop pulmonary arterial hypertension (PAH), a progressive and fatal pulmonary vascular disease.[3,4] The pathology of schistosomiasis-associated PAH is similar to other forms of PAH, with smooth muscle cell hypertrophy and intimal thickening.[5]
The pathogenic mechanism by which this parasitic infection results in pulmonary vascular remodeling is unclear. Potential contributing factors include portal hypertension with resulting portopulmonary hypertension and/or egg embolism, and a host immune responses that may be systemic and/or locally directed at parasite antigens in the lung. Such a localized inflammatory response in particular could be directed at persistent antigens or, once initiated, could continue despite clearance of the inciting antigenic material.
Histologic examination of lung tissue from individuals with schistosomiasis-associated PAH reveals a dark pigment that is often located adjacent to sites of vascular remodeling, the nature of which is unclear, and which has historically been variously speculated to be derived from red blood cells,[6] “bile pigment,”[7] a component of scar tissue[7,8] or remnants of the parasite.[6,8–10] To clarify the nature of this pigment and potentially identify antigens that could be the target of a localized host inflammatory response, we sought to detect parasite egg antigens in the lung tissue from individuals who had died of schistosomiasis-associated PAH.
MATERIALS AND METHODS
Sources of human tissue
Tissue from patients who died of schistosomiasis-associated PAH was obtained from two centers in Brazil: Memorial S. Jose Hospital, Universidade de Pernambuco in Recife, Pernambuco; and Hospital Prof. Edgard Santos, Universidade Federal da Bahia, Salvador. This tissue had been previously collected at autopsy and was formalin fixed and paraffin embedded. As the material was derived from deceased persons, no Institutional Review Board approval was required.
Sources of mouse tissue
We developed an experimental mouse model of schistosomiasis-associated pulmonary hypertension.[11] Briefly, wild-type C57Bl6/J mice (Taconic) receive 5,000 S. mansoni eggs injected intraperitoneally, followed 2 weeks later by challenge with 5,000 S. mansoni eggs injected intravenously. The eggs had been purified from the homogenized livers of S. mansoni-infected mice provided by the Biomedical Research Institute (BRI, Rockville, MD, USA); the strain of S. mansoni, the NMRI strain, was originally from Puerto Rico.[12] One week after intravenous challenge, the mice are sacrificed and the lung tissue is harvested and analyzed. The blood is flushed out of the lungs, the right bronchus sutured and 2% agarose instilled into the left lung through a transtracheal catheter. The left lung is removed, formalin fixed and processed for paraffin embedding, and the right lung is removed and frozen for subsequent protein analysis. All mice were bred and housed under specific pathogen-free conditions in an American Association for the Accreditation of Laboratory Animal Care-approved facility. All experimental procedures in rodents were approved by the Animal Care and Use Committees at the University of Colorado Denver.
Polyclonal antibody production and immunoblotting
A rabbit polyclonal antibody was prepared to hold S. mansoni-soluble egg antigen (SEA). The SEA was prepared using a published protocol.[13] The S. mansoni eggs were of the NMRI strain provided by the BRI. The antibody was produced by GenicBio Limited, Shanghai, China. Sera from two rabbits were collected before and after immunization to SEA.
The ability of the generated antibody to detect proteins in SEA was tested by probing a Western blot of purified SEA. Pre- and post-immunization serum was applied to the Western blot membrane at a concentration of 0.1 ug/mL overnight at 4°C. The immunoblot secondary antibody was HRP-labeled goat anti-rabbit (Vector, Burlingame, CA, PI-1000), used at a concentration of 1:5,000 for 1 h at room temperature, and detected using enhanced chemiluminescence(GE Healthcare, Little Chalfont, UK, RPN2106, RPN2106). Mouse whole lung lysates prepared by macerating and sonicating samples of the frozen right lung tissue in buffer containing antiproteases were also probed using the anti-SEA antibody.
Tissue immunostaining
Sections of large intestine and lung from individuals with schistosomiasis-associated PAH and sections of lung from mice infected with S. mansoni were stained using the anti-SEA antibody. The sections were heated at 100°C in citrate buffer for 20 min (Vector H-3300), blocked with 10% horse serum in phosphate-buffered saline (PBS) for 1 h, followed by the antibody (either preimmunization serum as a negative control or the postimmunization serum containing anti-SEA antibodies) applied at a concentration of 100 μg/mL overnight at 4°C and a secondary antibody of AF594-labeled donkey anti-Rabbit (Invitrogen A21207) diluted 1:200 applied for 1 h.
Sections of infected mouse lung tissue were stained with both the rabbit anti-SEA antibody and a rat anti-Mac-3 antibody to identify macrophage lysosomes.[14] The sections were heated at 100°C in Borg buffer for 20 min (Biocare #BD1000G1); blocked with a mixture of 10% horse serum, 10% goat serum, 40% Superblock (ScyTek AAA5000) and 40% of 5% bovine serum albumin reconstituted in PBS for 1 h; the combination of anti-SEA antibody (either preimmunization serum as a negative control or the postimmunization antibody) at a concentration of 5 μg/mL and anti-Mac-3 antibody (BD Pharmingen #550292) or rat IgG (negative control) at a dilution of 1:50 applied for 1 h at room temperature; and a combination of secondary antibodies of AF594-labeled donkey anti-rabbit (Invitrogen A21207) and AF488-labeled goat anti-rat (Invitrogen A11006) each diluted 1:200 applied for 1 h.
Human and mouse immunofluorescence-stained sections were counterstained with DAPI and imaged with a Nikon Eclipse E800 microscope with either a color camera (Nikon) or a black and white CCD camera (Photometrics). The double immunofluorescence stain of anti-SEA and anti-Mac-3 was imaged using a Zeiss LSM 510 META confocal microscope system with a 100× oil-immersion objective.
RESULTS
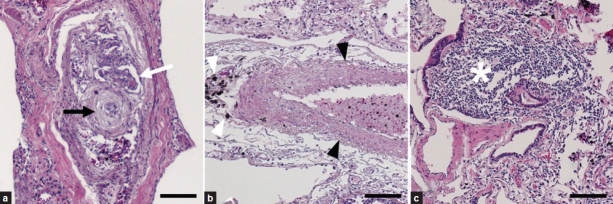
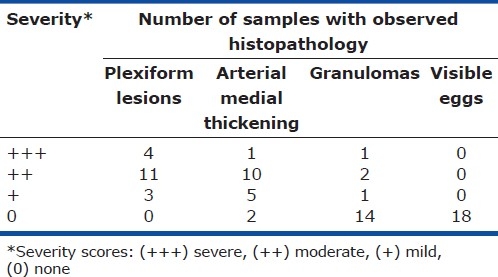
We obtained and analyzed pulmonary tissue from 18 patients who had died of schistosomiasis-associated PAH. Histologically, all lung samples had evidence of pulmonary vascular remodeling (Fig. 1). All the samples (100%) contained plexiform lesions, and 16 of 18 (89%) had evidence of arterial medial thickening. Only four of the 18 (22%) samples had granulomas. Although described by others,[15,16] we did not observe any intact S. mansoni eggs in the pulmonary samples, even within granulomas. The distribution of severity of histopathologic findings is listed in (Table 1). All specimens contained a granular pigment, which was often located adjacent to vascular lesions (Fig. 1b), but appeared indistinguishable from anthracotic pigment.
Figure 1.
Representative pulmonary pathology of schistosomiasis-associated pulmonary arterial hypertension. (a) Plexiform lesion (white arrow) in close proximity to concentric (onion-skinned) intimal thickening (black arrow). (b) Increased medial thickness (black arrowheads) adjacent to dark pigment (white arrowheads). (c) A perivascular granuloma (white star). All stains are hematoxylin and eosin. Scale bars are 100 μm.
Table 1.
Range of histopathologic findings in 18 autopsy specimens from patients who died of schistosomiasis-associated PAH
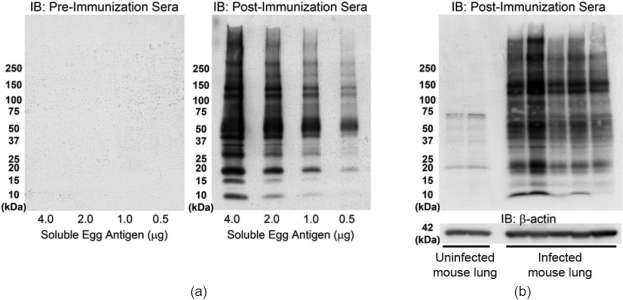
To identify antigenic material derived from S. mansoni eggs, we created a polyclonal rabbit antibody targeted to known soluble SEA. Two rabbits were inoculated and the serum samples from the two rabbits gave identical results. The ability of the antibody to detect proteins in SEA was confirmed by immunoblot and immunostain. Western blots of purified SEA were probed with the serum collected from the rabbit preimmunization (serving as a negative control) or postimmunization (Fig. 2a). Only the anti-SEA antibodies in the postimmunization serum detected proteins in the SEA, across a wide range of molecular weights.
Figure 2.
Immunoblots using anti-Schistosoma mansoni-soluble egg antigen (SEA) antibody identifies egg antigens in vitro and in vivo. (a) Serum collected from a rabbit before immunization to SEA fails to detect purified SEA proteins, while serum collected after the same rabbit has been immunized to SEA detects multiple proteins in the SEA at many molecular weights. (b) Postimmunization serum detects multiple antigenic proteins in whole-lung lysates of S. mansoni infected mice (each lane is a single animal).
We had previously developed an experimental mouse model of schistosomiasis-associated pulmonary hypertension, which utilizes intraperitoneal sensitization with S. mansoni eggs followed by an intravenous challenge with S. mansoni eggs.[11] Anti-SEA antibody in the postimmunization serum detected egg antigens present in immunoblots of whole-lung lysates of S. mansoni-infected mice (Fig. 2b). Immunostaining the infected mouse tissue with the anti-SEA antibodies in the postimmunization serum readily identified visible eggs in the mouse tissue, while the preimmunization serum failed to detect the eggs (Fig. 3a and b).
Figure 3.
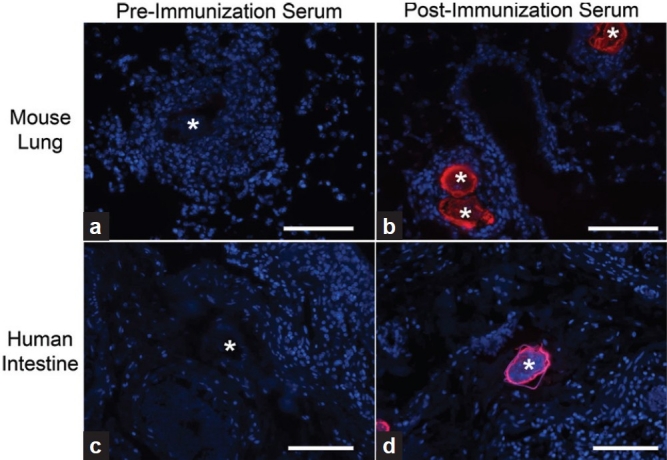
Anti-Schistosoma mansoni-soluble egg antigen (SEA) antibody detects S. mansoni eggs in infected mouse lung and human intestine. (a and b) Mice with S. mansoni eggs administered intravenously develop peri-egg granulomas. The eggs can be visualized with the polyclonal anti-SEA antibody in postimmunization serum, but no significant signal is seen when antibodies in preimmunization rabbit serum are used. (c and d) Similarly, postimmunization serum but not preimmunization serum detects S. mansoni eggs in infected human intestines. Eggs are marked with a “*”; blue is DAPI; all scale bars are 100 μm.
We performed anti-SEA immunostaining of human tissue to detect S. mansoni antigens present in situ. One of the tissue specimens from a patient who had died of schistosomiasis-associated PAH had visible S. mansoni eggs in the colonic submucosa, and the anti-SEA antibody in the postimmunization serum readily detected SEA present in these eggs, while the preimmunization serum did not identify the eggs (Fig. 3c and d). We then probed human lung specimens with the anti-SEA antibody. No significant staining was observed throughout the lung tissue using either the post- or preimmunization serum (Fig. 4). We did identify rare fragments of S. mansoni eggs in human lung specimens using the anti-SEA antibody, which were only present within granulomas, and were not apparent by conventional staining. However, there was no significant staining adjacent to the pulmonary vascular lesions.
Figure 4.
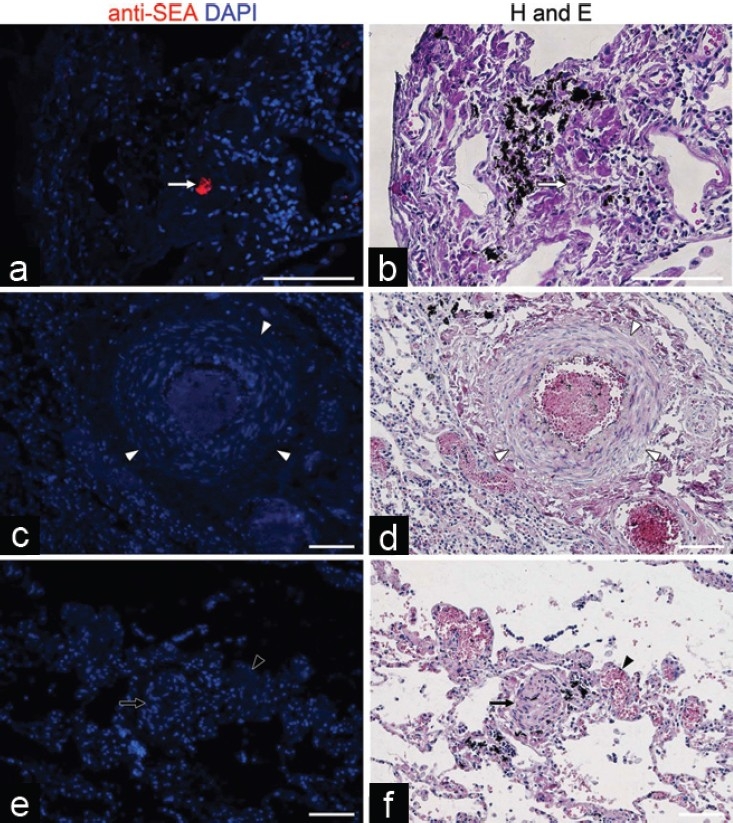
Anti-Schistosoma mansoni-soluble egg antigen (SEA) antibody identifies only a small amount of egg antigen within human lung granulomas, and not adjacent to vascular lesions. (a and b) Although there is no visible egg by hematoxylin and eosin stain, the anti-SEA antibodies in the postimmunization serum identify a small fragment of egg antigen (white arrow) present in a granuloma. (c-f) No Schistosoma egg antigens are found adjacent to pulmonary vascular lesions, including increased medial thickness (white arrowheads in c and d), a plexiform lesion (black arrow in e and f) and a dilated or angiomatoid lesion (black arrowhead in e and f). All scale bars are 100 μm.
We sought to localize the degraded egg fragments within granuloma cells. Costaining the mouse lung tissue for Mac-3/CD107b (a marker of macrophage plasma membranes and lysosomes[14] ) and anti-SEA revealed that in granulomas encompassing degraded eggs, there were macrophage lysosomes containing intracellular egg antigens (Fig. 5).
Figure 5.
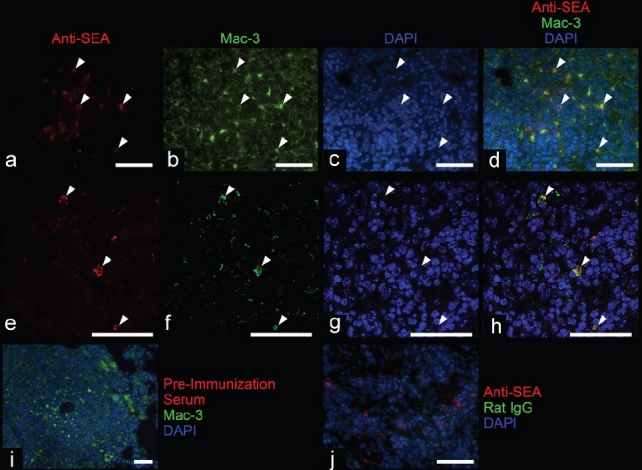
Immunofluorescence staining demonstrates colocalization of an anti-Schistosoma mansoni-soluble egg antigen (SEA) antibody and anti-Mac-3 antibody in infected mouse lung. (a– h) The anti-SEA antibody colocalizes with Mac-3 within many macrophages (white arrowheads) within granulomas surrounding largely degraded S. mansonieggs; (e– h) imaged using a confocal microscope with a 100x oil-immersion objective. (i and j) Costaining with isotype controls does not demonstrate significant colocalization. All scale bars are 50 μm.
DISCUSSION
We sought to identify parasite proteins within the lungs of individuals who had died of schistosomiasis-associated PAH using a polyclonal antibody generated to known soluble SEA. The antibody was validated by identifying egg antigens in vitro and in mouse lung tissue and human intestinal tissue. We did not find evidence of egg antigens in the human lung tissue except for a very small amount within visible granulomas, which are presumably eggs being degraded by the host immune system. In particular, the commonly seen black granular pigment did not contain apparent schistosomal antigen, and given its identical appearance to anthracotic pigment, may represent carbon fragments from environmental smoke inhalation.
Although unlikely, we cannot entirely exclude the possibility that there may be a more extensive distribution of parasite-derived antigens, such as entirely insoluble proteins (not included in the soluble egg antigen inoculation of the rabbits) or nonegg parasite proteins left behind during the initial passage of the schistosomula through the lungs in acute schistosomiasis infection (Katayama syndrome).[17] Similarly, the dark pigment frequently observed adjacent to pulmonary vascular lesions in schistosomiasis-associated PAH, and which has been speculated to be derived from the parasite,[6,8–10] is unlikely to be parasite derived.
Numerous investigators have postulated that an underlying cause of pulmonary vascular remodeling is inflammation.[18,19] Historically, Schistosoma ova were frequently seen in the lungs of individuals who died of schistosomiasis-associated PAH. For example, in 1954, de Faria reported histologically visible eggs present in 18 of 18 (100%) autopsy cases, with the number of eggs seen in a single 2.5 cm2 section ranging from four to approximately 250.[16] The report by Crosby et al. that mice chronically infected with schistosomiasis develop PH only when eggs are present in the lung, and that treatment with the anti-helmenthic praziquantel prevents or reverses this PH,[20] suggests that the localized parasite antigens trigger inflammation-mediated vascular remodeling, which stops once the antigenic material is eliminated.
In comparison with the historical reports, it is striking that we saw no intact intrapulmonary ova in similarly sized sections from any of our 18 samples. A potential explanation for this difference is the introduction of antihelmenthic therapies such as praziquantel, which was developed in the late 1970s.[21] However, although praziquantel is effective at killing the parasite (and thereby preventing further eggs from being laid), clinically, praziquantel is largely ineffective at treating the vascular remodeling in those who have developed PAH after chronic and repeated infection with S. mansoni.[22] Similarly, our finding of vascular remodeling in patients who died of this disease, despite the absence of immunohistochemically identified antigenic material, suggests that after an initial acute inflammatory response, vascular lesions are established and follow a course of persistence or even progression. Beyond this “point of no return,” processes such as altered cellular bioenergetics[23] or clonal proliferation[24] may drive the lesions to continue.
Although alternatively activated (M2) macrophages have been described to be present in the inflammatory granulomas surrounding Schistosoma eggs,[25] to our knowledge, it has not been previously directly demonstrated that some of the macrophages contain degraded S. mansoni egg antigens. The specific colocalization of egg antigens to macrophage lysosomes suggests that macrophages phagocytose and digest the fragments of parasite after degradation by extracellular proteins secreted by the host immune response.
Overall, there is unlikely to be a significant amount of persistent parasite-derived antigens within the lungs of individuals who die of schistosomiasis-associated PAH. This suggests that retained and persistent parasite proteins are not driving a localized immune response contributory to the pathogenesis of pulmonary vascular remodeling.
Footnotes
Source of Support: This study was funded by a research grant from the Pulmonary Vascular Research Institute (PVRI; BBG), NIH/NHLBI 1K08HL105536-01A1 (BBG), a Parker B Francis Career Development Grant (BBG), and a grant from the Cardiovascular Medical Research Fund (CMREF; RMT)
Conflict of Interest: None declared.
REFERENCES
- 1.Chitsulo L, Loverde P, Engels D. Schistosomiasis. Nat Rev Microbiol. 2004;2(1):12–3. doi: 10.1038/nrmicro801. [DOI] [PubMed] [Google Scholar]
- 2.Warren KS. Hepatosplenic schistosomiasis: A great neglected disease of the liver. Gut. 1978;19:572–7. doi: 10.1136/gut.19.6.572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de Cleva R, Herman P, Pugliese V, Zilberstein B, Saad WA, Rodrigues JJ, et al. Prevalence of pulmonary hypertension in patients with hepatosplenic Mansonic schistosomiasis-prospective study. Hepatogastroenterology. 2003;50:2028–30. [PubMed] [Google Scholar]
- 4.Lapa M, Dias B, Jardim C, Fernandes CJ, Dourado PM, Figueiredo M, et al. Cardiopulmonary manifestations of hepatosplenic schistosomiasis. Circulation. 2009;119:1518–23. doi: 10.1161/CIRCULATIONAHA.108.803221. [DOI] [PubMed] [Google Scholar]
- 5.Tuder RM. Pathology of pulmonary arterial hypertension. Semin Respir Crit Care Med. 2009;30:376–85. doi: 10.1055/s-0029-1233307. [DOI] [PubMed] [Google Scholar]
- 6.Sadigursky M, Andrade ZA. Pulmonary changes in schistosomal cor pulmonale. Am J Trop Med Hyg. 1982;31:779–84. doi: 10.4269/ajtmh.1982.31.779. [DOI] [PubMed] [Google Scholar]
- 7.Macieira-Coelho E, Duarte CS. The syndrome of portopulmonary schistosomiasis. Am J Med. 1967;43:944–50. doi: 10.1016/0002-9343(67)90253-7. [DOI] [PubMed] [Google Scholar]
- 8.Girgis B, Guirguis S, Mowfaty R, El-Katib H. Bilharzial cor pulmonale: A clinicopathological report of two cases. Am Heart J. 1953;45:190–200. [Google Scholar]
- 9.Andrade ZA, Andrade SG. Pathogenesis of schistosomal pulmonary arteritis. Am J Trop Med Hyg. 1970;19:305–10. doi: 10.4269/ajtmh.1970.19.305. [DOI] [PubMed] [Google Scholar]
- 10.Gelfand M. Cor-pulmonale and cardiopulmonary schistosomiasis. Trans R Soc Trop Med Hyg. 1957;51:533–40. doi: 10.1016/0035-9203(57)90043-3. [DOI] [PubMed] [Google Scholar]
- 11.Graham BB, Mentink-Kane MM, El-Haddad H, Purnell S, Zhang L, Zaiman A, et al. Schistosomiasis-induced experimental pulmonary hypertension: Role of interleukin-13 signaling. Am J Pathol. 2010;177:1549–61. doi: 10.2353/ajpath.2010.100063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lewis FA, Stirewalt MA, Souza CP, Gazzinelli G. Large-scale laboratory maintenance of Schistosoma mansoni, with observations on three schistosome/snail host combinations. J Parasitol. 1986;72:813–29. [PubMed] [Google Scholar]
- 13.Boros DL, Warren KS. Delayed hypersensitivity-type granuloma formation and dermal reaction induced and elicited by a soluble factor isolated from Schistosoma mansoni eggs. J Exp Med. 1970;132:488–507. doi: 10.1084/jem.132.3.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen JW, Murphy TL, Willingham MC, Pastan I, August JT. Identification of two lysosomal membrane glycoproteins. J Cell Biol. 1985;101:85–95. doi: 10.1083/jcb.101.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Andrade ZA. Pathology of human schistosomiasis. Mem Inst Oswaldo Cruz. 1987;82:17–23. doi: 10.1590/s0074-02761987000800005. [DOI] [PubMed] [Google Scholar]
- 16.de Faria JL. Cor Pulmonale in Manson's Schistosomiasis I.Frequency in Necropsy Material; Pulmonary Vascular Changes Caused by Schistosome Ova. Am J Pathol. 1954;30:167–93. [PMC free article] [PubMed] [Google Scholar]
- 17.Ross AG, Vickers D, Olds GR, Shah SM, McManus DP. Katayama syndrome. Lancet Infect Dis. 2007;7:218–24. doi: 10.1016/S1473-3099(07)70053-1. [DOI] [PubMed] [Google Scholar]
- 18.Dorfmuller P, Perros F, Balabanian K, Humbert M. Inflammation in pulmonary arterial hypertension. Eur Respir J. 2003;22:358–63. doi: 10.1183/09031936.03.00038903. [DOI] [PubMed] [Google Scholar]
- 19.Pullamsetti SS, Savai R, Janssen W, Dahal BK, Seeger W, Grimminger F, et al. Inflammation, immunological reaction and role of infection in pulmonary hypertension. Clin Microbiol Infect. 2011;17:7–14. doi: 10.1111/j.1469-0691.2010.03285.x. [DOI] [PubMed] [Google Scholar]
- 20.Crosby A, Jones FM, Southwood M, Dunne DW, Morrell NW. Praziquantel prevents progression of right ventricular hypertrophy in a mouse model of Schistosomiasis. Am J Respir Crit Care Med. 2010;181:A4895. [Google Scholar]
- 21.Gonnert R, Andrews P. Praziquantel, a new board-spectrum antischistosomal agent. Z Parasitenkd. 1977;52:129–50. doi: 10.1007/BF00389899. [DOI] [PubMed] [Google Scholar]
- 22.Richter J. Evolution of schistosomiasis-induced pathology after therapy and interruption of exposure to schistosomes: A review of ultrasonographic studies. Acta Trop. 2000;77:111–31. doi: 10.1016/s0001-706x(00)00125-x. [DOI] [PubMed] [Google Scholar]
- 23.Dromparis P, Sutendra G, Michelakis ED. The role of mitochondria in pulmonary vascular remodeling. J Mol Med (Berl) 2010;88:1003–10. doi: 10.1007/s00109-010-0670-x. [DOI] [PubMed] [Google Scholar]
- 24.Tuder RM, Radisavljevic Z, Shroyer KR, Polak JM, Voelkel NF. Monoclonal endothelial cells in appetite suppressant-associated pulmonary hypertension. Am J Respir Crit Care Med. 1998;158:1999–2001. doi: 10.1164/ajrccm.158.6.9805002. [DOI] [PubMed] [Google Scholar]
- 25.Wilson MS, Mentink-Kane MM, Pesce JT, Ramalingam TR, Thompson R, Wynn TA. Immunopathology of schistosomiasis. Immunol Cell Biol. 2007;85:148–54. doi: 10.1038/sj.icb.7100014. [DOI] [PMC free article] [PubMed] [Google Scholar]