Abstract
Objective:
The objective of this study was to delineate the prevalence and characterize the histologic pattern of benign breast diseases (BBDs) in the University of Benin Teaching Hospital, Benin City, Nigeria.
Materials and Methods:
A 25-year-old (1985-2009) retrospective study of all patients presenting with BBD.
Results:
During the 25-year-old study period, 1864 cases of BBD constituting 72.4% of all breast lesions were seen. The female to male ratio was 28.6:1. An increasing incidence of BBDs was observed. The overall mean age for BBD was 27.5 years, SD±11.3 with an age range of 9-84 years and a peak age occurrence in the third decade. The single most common lesion was fibroadenoma accounting for 43.1% of cases, followed by fibrocystic change (23.8%) with mean ages of 22.3 years and 30.2 years, respectively. Both lesions had a peak occurrence in the third decade. Other major lesions encountered were sclerosing adenosis (7.3%), atypical ductal hyperplasia (3.6%), and blunt duct adenosis (2.3%). Gynecomastia (2.1%) was the predominant lesion in males. Inflammatory lesions constituted 8.1% of cases while stromal and skin lesions accounted for 1.1% and 0.9% of cases respectively.
Conclusion:
BBDs constituted 70% of breast lumps and were mostly fibroadenoma and fibrocystic change. BBDs occurred predominantly in young females with a peak in the third decade. Though premalignant lesions of atypical hyperplasia were less common, biopsy of all BBDs should be done to exclude these lesions and routine mammographic screening of at risk individuals instituted to increase their detection.
Keywords: Benign breast disease, benign breast lesion in Africans, fibroadenoma and fibrocystic change in an African population
INTRODUCTION
The presence of a lump in the breast is a great cause of anxiety, apprehension, and uncertainty to most patients. This may be accrued to the increasing public awareness of breast cancer which is presently the most common female malignancy worldwide.1 Nevertheless the vast majority of breast lesions are benign.2–6 Benign breast diseases (BBD), however, constitute a heterogeneous group of disorders including developmental abnormalities, epithelial and stromal proliferations, inflammatory lesions, and neoplasms.7
While most reports indicate that breast lumps are predominantly benign and mostly nonproliferative epithelial lesions, there has, however, been increasing recognition of the risk implications of the various forms of premalignant lesions. Researchers widely believe that cancer risk is increased in patients with atypical ductal and atypical lobular hyperplasia.8–10 Dupont et al.8 reported a relative risk of 3.1 for subsequent breast cancer in women with atypical lobular hyperplasia. A four- to fivefold increased risk for breast cancer has been associated with atypical ductal hyperplasia mostly in the ipsilateral breast within 10-15 years of diagnosis.9
It is therefore pertinent for pathologists, oncologists, and radiologists not only to recognize and distinguish BBD from breast cancer but also to have in depth knowledge of the pattern of occurrence of these disorders in their geographical locale.
Though a few studies have been done on BBD in Nigeria, there remains a definite paucity of information on this group of disorders in this center. This 25-year study of BBD aims at defining the hospital incidence and evaluating the histopathologic pattern in the University of Benin Teaching Hospital, Benin City, Nigeria.
MATERIALS AND METHODS
All breast specimens (biopsy or mastectomy) received at the Department of Pathology, University of Benin Teaching Hospital, Benin City, Nigeria from January 1, 1984 to December 31, 2009 were reviewed. The cases of benign breast lesions formed the focus of this retrospective study.
Clinical and demographic data regarding age, sex, and clinical information were obtained from request cards and the surgical day books of the Department. Slides were retrieved from the archives of the Department of Pathology. When necessary, new slides were made from formalin-fixed, paraffin-embedded blocks and the lesions classified.7
Statistical analysis was done using the SPSS version 16 statistical package.
Ethical clearance was obtained from the Ethical Committee of the University of Benin Teaching Hospital to carry out this study.
RESULTS
Of the 2575 breast lesions histologically diagnosed in the Department during the 25-year review period, 1864 (72.4%) cases were benign. The ratio of benign to malignant breast lesions was thus 2.6:1.
Figure 1 shows the yearly distribution of BBDs as a group. Also shown is the yearly distribution of the major forms of BBDs (fibroadenoma and fibrocystic change). An increasing incidence of benign breast diseases was observed.
Figure 1.
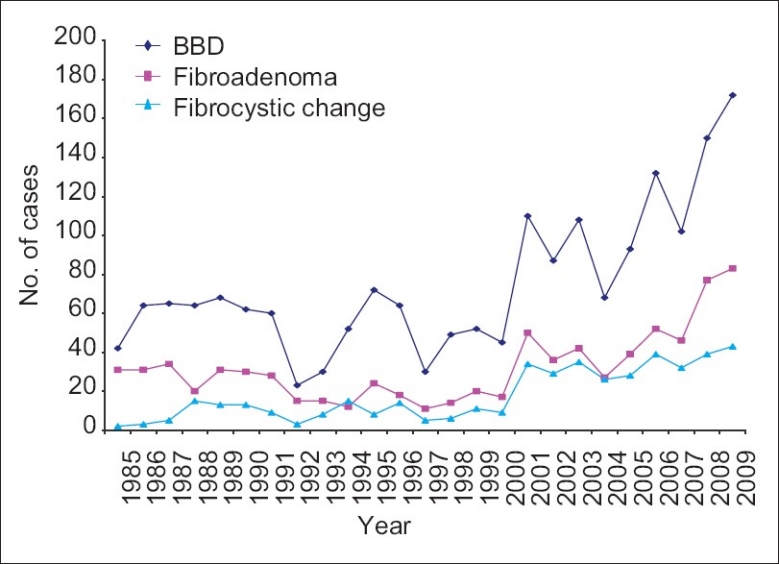
Yearly distribution of benign breast diseases
The age and sex distribution of BBDs is shown in Tables 1–4 while Figure 2 and Table 5 depict the age group distribution of the various histological types of BBDs. Benign breast diseases occurred predominantly in females (1801 cases [96.6%]) with only 63 cases (3.4%) in males giving a female to male ratio of 28.6:1.
Table 1.
Age and sex distribution of benign epithelial lesions
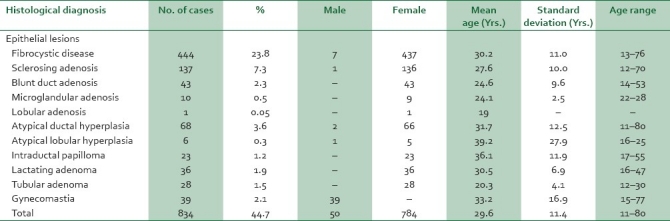
Table 4.
Age and sex distribution of inflammatory breast lesions
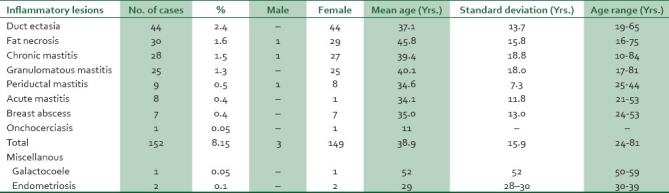
Figure 2.
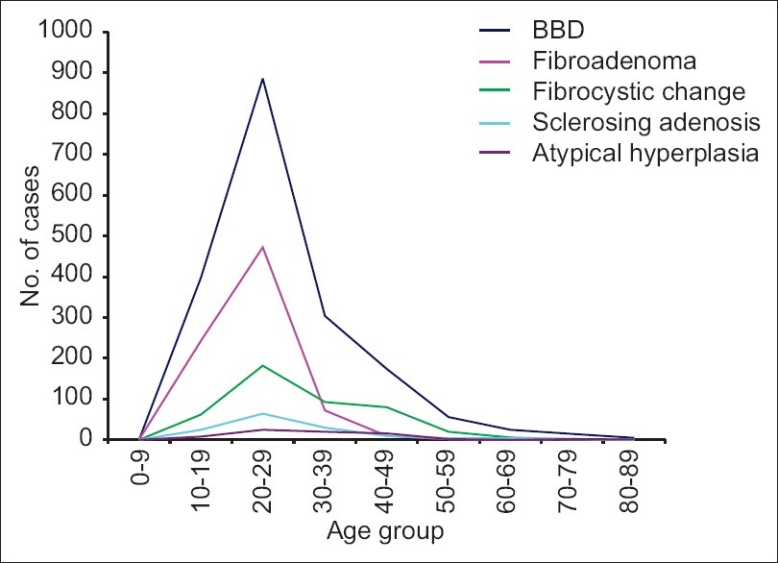
Age distribution of BBD (overall) and the major BBDs
Table 5.
Age distribution of the major benign breast lesions
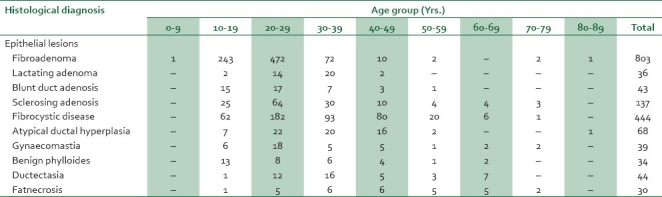
Table 2.
Age and sex distribution of fibroepithelial/stromal lesions of the breast
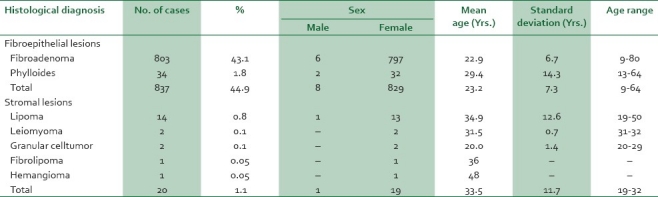
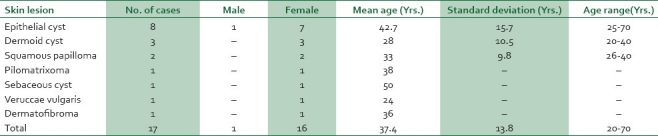
Table 3.
Age and sex distribution of skin lesions of the breast
The overall mean age for BBD was 27.5, SD±11.3 with a wide age range of 9-84 years, and a peak age occurrence in the third decade [726 (38.9%) cases].
Fibroadenoma was the most common BBD and fibroepithelial tumor accounting for 803 (43.1%) cases with ages ranging from 9 to 80 years, mean age of 22.3 years SD±6.7 years, and a peak occurrence in the third decade. Phylloides tumor, the other fibroepithelial tumor in the series comprised 34 (1.8%) cases occurring predominantly in the second decade with a mean age of 29.4 years Fibrocystic change, the second commonest lesion, accounted for 444 (23.8%) cases with a mean age of 30.2 years SD±11 years, age range of 18-76 years, and a peak incidence in the third decade.
Inflammatory lesions accounted for 152 (8.1%) cases. Of these, duct ectasia, fat necrosis, and chronic mastitis accounted for 44 (2.5%), 30 (1.6%), and 28 (1.5%) cases respectively. A case of onchocerciasis was seen in an 11-year-old female.
Stromal tumors comprised 20 (1.1%) cases. These were predominantly lipomas (14 [0.8%] cases). Granular cell tumor and leiomyoma occurred in 2 (0.1%) cases each. Fibrolipoma and hemangioma accounted for one case each.
Of the 63 cases of BBD encountered in males, gynecomastia predominated accounting for 39 cases (2.1%). The age range of patients with gynecomastia was 15-77 years, with a mean of 33.2 years±16.9 years and a peak in the third decade. Other lesions encountered in males included fibrocystic disease (7 [0.4%] cases) and fibroadenoma (6 [0.3%] cases). Only two cases each of atypical ductal hyperplasia and low-grade phylloides were seen in males.
DISCUSSION
Breast lesions pose health and cosmetic hazards predominantly in females. The anxiety and fear associated with increased awareness of breast cancer has significantly improved the health seeking behavior of patients with breast lumps. This might partially explain the increasing incidence of BBDs observed in this study. Other factors attributed to the increasing incidence of BBDs include an observed overall rise in the patient population possibly influenced by a general increase in national population, an improving economy and literacy and an expansion in the three-tier levels of healthcare facilities.
Previous reports indicate that the incidence of BBD far outnumber that of breast cancer. In Enugu, Kano, Calalar, and Ibadan, BBD accounted for 68.8, 73, 73.4, and 89.4% of all breast lumps respectively.3–6 This was also our observation. In this study, BBDs accounted for 72.5% of all breast lesions.
Fibroadenoma was the most common BBD in this study accounting for 43% of cases. This was also the finding in most previous studies done in Nigeria. In Ilesha, Enugu, Port Harcourt, and Ife, fibroadenoma accounted for 46.2, 44, 51, and 59.1% of cases of BBD respectively.3,6,11–13 Fibroadenoma was also the commonest BBD in Ghana and USA accounting for 70 and 48% of BBDs respectively.14,15 However, in Kano, fibroadenoma was documented as the second commonest BBD comprising 28.8% of cases, a finding consistent with reports from Pakistan and Jamaica where fibroadenoma accounted for 29.4, 33% respectively.4,16,17 A racial predilection of Negroes to fibroadenoma has however, been previously documented.15 This may account for the higher frequency of fibroadenoma in this and other African studies. This increased frequency may be modulated by genetic, environmental and socio-cultural factors.
Fibrocystic change, the second most common BBD in this study, accounted for 23.8% of cases, a figure consistent with the 22.9 and 27.7% documented in Enugu and Port Harcourt respectively.3,12 A much higher figure of 42.2% was however reported in Ilesha and of note, fibrocystic change was reported as the commonest BBD in Kano comprising 34.3% of cases.4,11 In Italy and in another study in the USA, fibrocystic change was also the commonest BBD accounting for 43.2 and 47% of cases respectively.18,19 Fibrocystic change seems relatively more common in Pakistanis as Memmon16 reported a high frequency of 66.3% and observed a changing trend of benign tumors from fibroadenoma to fibrocystic change. This trend was however not observed in this study as fibroadenoma remained the commonest BBD throughout the 25-year study period.
Fibrocystic change consists of a spectrum of morphological changes comprising cysts, adenosis epithelial hyperplasia, and fibrosis and occurs predominantly between the ages of 30 and 50 years.18,19 The age range of patients with fibrocystic change in this study was 13-76 years, with a mean age of 30 years which is in consonance with the 32, 33, and 37 years documented in Ibadan, Kano, and Ife, respectively.4,6,13 Though both fibroadenoma and fibrocystic disease had a peak occurrence in the third decade, in contrast to fibrocystic disease which showed a relatively high prevalence up to the fifth decade, a sharp decline in the occurrence of fibroadenoma was observed after the third decade.
Previous studies indicate that atypical ductal hyperplasia and atypical lobular hyperplasia are proliferative lesions with atypia and therefore have premalignant potential.8,9 Dupont et al.8 reported a fourfold increased risk than that of the general population for subsequent invasive carcinoma. This risk increasing to 10-fold if the patient has a first-degree relative with breast cancer. More so, studies have shown that atypical hyperplasia confers a bilateral risk for subsequent invasive carcinoma. In this study, atypical ductal hyperplasia and atypical lobular hyperplasia comprised 47 (2.4%) and 6 (0.3%) cases respectively. These figures are relatively low considering the fact that breast cancer is the commonest malignancy in Nigeria. However, most breast cancer patients in Nigeria present late with advanced stage of disease. The observed low prevalence of BBDs having significantly increased risk for carcinoma may be attributed to the fact that most patients were referrals for clinically detected lesions. Institution of routine mammographic screening of high risk groups aimed at detecting microscopic lesions may increase the detection of atypical hyperplasia. Early diagnosis with appropriate management of premalignant lesions should significantly decrease the prevalence and mortality associated with invasive cancer.
Sclerosing adenosis has been classified as a proliferative lesion without atypia, having a relative risk of 1.3-1.9 for invasive carcinoma.10 This lesion accounted for 137 (7.3%) of the cases in this study. Sclerosing adenosis, a lobulocentric lesion of disordered acini, myoepithelial, and connective tissues, may be difficult to distinguish grossly from infiltrating carcinoma and may occur in association with other epithelial hyperplasia including epithelial hyperplasia and intraductal papilloma. It may also coexist with invasive and in situ carcinoma.10,20 A biopsy with histological diagnosis is therefore indicated in such cases particularly in low resource settings as ours where mammographic services may not be available.
Similar to the observation in all other previous studies, gynecomastia was the most commonly encountered breast disease in males constituting 2.1% of all cases. This is consistent with the 1% documented in Caucasians and comparable to the 3.9, and 4% recorded in Ife and Kano.4,13 A much higher figure of 14% reported from Port Harcourt is however similar to the 12% observed in Uganda.12,20 Gynecomastia in this study showed a mean age of 33.2 years, with a peak incidence in the third decade and ages ranging from 15 to 77 years. Though a bimodal peak age distribution at adolescence and elderly has been described in the literature, this was not observed in this study. In Kano, no case of gynecomastia was documented in the elderly patients. However, it was the only breast disease encountered in males.4 The converse is true of this study as many of the breast diseases encountered in females also occurred in males. Fibrocystic disease, fibroadenoma, atypical ductal hyperplasia, and benign phylloides in males together constituted 0.8% of cases. Anecdotal cases of chronic mastitis, periductal mastitis, fat necrosis, sclerosing adenosis, atypical lobular hyperplasia, lipoma, and epithelial cyst were also encountered.
Low-grade (benign) phylloides, a fibroepithelial tumor, accounted for 34 (1.8%) cases. In Enugu, benign phylloides accounted for 3.9% of BBDS.3 It is documented in the literature that benign phylloides occurs predominantly in middle-aged women between 40 and 50 years with definite rarity in childhood and adolescence in the Western countries.21,22 In this study, the peak age occurrence of benign phylloides was 10-19 years with a mean age of 29.4 years; 11 of these 34 cases were in adolescence. In Ibadan, the mean age of benign phylloides was even lower (18.8 years).6 It would appear that benign phylloides occurs in a relatively younger population in this environment. The predominant occurrence of phylloides tumor in young Nigerian females needs further research. It is noteworthy that in a similar study in Pakistan, benign phylloides also occurred mostly in adolescents.16
Consistent with previous reports from Nigeria and in the World literature,2–6,11–13 stromal tumors were rarely encountered, accounting for only 20 (1.1%) cases and were predominantly lipoma (14 [0.8%] cases). In Pakistan, stromal tumors comprised 0.68% of BBDs.16
Inflammatory lesions accounted for 8.1% of cases of BBD in this study. Though slightly higher than the 4.6% and 6% recorded in Ife and Kano, it is much lower than the 14.8% documented in Port Harcourt.4,12,13 An observation in this study was the markedly low-incidence of breast abscess in this study which is not reflective of the clinical incidence of the disease. This may be attributed to the fact that most breast abscesses are incised and drained without histopathologic diagnosis. Moreover, most patients with breast abscesses are treated in the secondary centers and are not often referred to tertiary hospitals such as ours.
Lesions of the skin overlying the breast may also present as lumps clinically indistinguishable from primary breast lesions. In this study, there were 17 (0.9%) lumps arising from the skin and these were predominantly epithelial cysts (8 [0.4%] cases) and dermoid cysts (3 [0.2%] cases). In Ibadan, skin lesions accounted for 0.3% of cases.6 The excision biopsy is often required to determine the benignity of these lesions.
CONCLUSION
The prevalence of benign breast diseases in this study was on the increase and diseases were mostly fibroadenoma and fibrocystic change occurring in young females with a peak in the third decade. A low prevalence of premalignant lesions, not reflective of the high incidence of breast cancer in this environment, was observed. Routine mammographic screening of high risk groups aimed at early detection of these premalignant lesions is therefore highly indicated. A biopsy with histological diagnosis of all breast lumps is also recommended as this will aid in the detection of premalignant lesions particularly in low resource settings.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 2.Yusufu LM, Odigie VI, Mohammed A. Breast masses in Zaria, Nigeria. Ann Afr Med. 2003;2:13–6. [Google Scholar]
- 3.Anyikam A, Nzegwu MA, Ozumba BC, Okoye I, Olusina DB. Benign breast lesions in Eastern Nigeria. Saudi Med J. 2008;29:241–4. [PubMed] [Google Scholar]
- 4.Ochicha O, Edino ST, Mohammed AZ, Amin SN. Benign breast lesions in Kano. Nig J Surg Res. 2002;4:1–5. [Google Scholar]
- 5.Otu AA. Benign breast diseases in an African population. J R Col Surg Edin. 1990;35:373–5. [PubMed] [Google Scholar]
- 6.Irabor DO, Okolo CA. An audit of 149 consecutive breast biopsies in Ibadan, Nigeria. Pak J Med Sci. 2008;24:257–62. [Google Scholar]
- 7.Tavassoli FA, Devilee P. France: IARC; 2003. World Health Organization histological classification of tumours of the breast in pathology and genetics of tumours of the breast and female genital organs. [Google Scholar]
- 8.Dupont WD, Parl FF, Hartmann WH, Brinton LA, Winfield AC, Worrell JA, et al. Breast cancer risk associated with proliferative breast disease and atypical hyperplasia. Cancer. 2006;71:1258–65. doi: 10.1002/1097-0142(19930215)71:4<1258::aid-cncr2820710415>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 9.Hartmann LC, Sellers TA, Frost MH, Lingle WL, Degnim AC, Ghosh K, et al. Benign breast disease and the risk of breast cancer. N Eng J Med. 2005;353:229–37. doi: 10.1056/NEJMoa044383. [DOI] [PubMed] [Google Scholar]
- 10.Jensen RA, Page DL, Dupont WD, Rogers LW. Invasive breast cancer risk in women with sclerosing adenosis. Cancer. 1989;64:1977–83. doi: 10.1002/1097-0142(19891115)64:10<1977::aid-cncr2820641002>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 11.Adesunkanmi AR, Agbakwuru EA. Benign breast lesions in Wesley Guild Hospital, Ilesha, Nigeria. West Afr J Med. 2001;20:146–51. [PubMed] [Google Scholar]
- 12.Kathcy KC, Datubo-Brown DD, Gogo-Abite M, Iweha UU. Benign breast lesions in Nigerian women in Rivers State. East Afr Med J. 1990;67:201–4. [PubMed] [Google Scholar]
- 13.Adeniji KA, Adelusola KA, Odesanmi WO. Benign disease of the breast in Ile-Ife: A 10 year experience and literature review. Cent Afr J Med. 1997;43:140–3. [PubMed] [Google Scholar]
- 14.Bewtra C. Fibroadenoma in women in Ghana. Pan Afri Med J. 2009;2:11. doi: 10.4314/pamj.v2i1.51710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oluwole SF, Freeman HP. Analysis of benign breast lesions in blacks. Am J Surg. 1979;137:786–9. doi: 10.1016/0002-9610(79)90094-1. [DOI] [PubMed] [Google Scholar]
- 16.Memon A, Parveen S, Sangrarasi AK, Malik AM, Laghari A. Changing pattern of benign breast lumps in young females. World J Med Sci. 2007;2:21–4. [Google Scholar]
- 17.Shirley SE, Mitchell DI, Soares DP, James M, Escoffery CT, Rhoden AM, et al. Clinicopathologic features of breast disease in Jamaica: Findings of the Jamaican Breast Disease Study, 2000-2002. West Indian Med J. 2008;57:90–4. [PubMed] [Google Scholar]
- 18.Ciatto S, Bonardi R, Ravaioli A, Canuti D, Foglietta F, Modena S, et al. Benign breast disease surgical biopsies, are they always justified? Tumori. 1998;84:521–4. doi: 10.1177/030089169808400502. [DOI] [PubMed] [Google Scholar]
- 19.Donegan WL, Spratt JS. Cancer of the breast. 4th ed. Vol. 9. Philadelphia: Saunders WB; 1995. Cancer Prev; pp. 1–15. [Google Scholar]
- 20.Cheng J, Qiu S, Raju U, Wolman SR, Worsham MJ. Benign breast disease heterogeneity: Association with histopathology, age and ethnicithy. Breast Cancer Res Treat. 2008;111:289–96. doi: 10.1007/s10549-007-9775-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Niewoehner CB, Nuttal FQ. Gynaecomastia in a hospitalized male population. Am J Med. 1984;77:633–8. doi: 10.1016/0002-9343(84)90353-x. [DOI] [PubMed] [Google Scholar]
- 22.Juan Rosai. Rosai and Ackerman's surgical pathology. 9th ed. USA: Elsevier; 2004. The breast; p. 1829. [Google Scholar]


