Abstract
Background:
Blood and blood products are scarce commodities. The demand often outweighs the supply. This study is directed at investigating the blood procurement sources and the risk of viral transfusion transmissible infection.
Materials and Methods:
The records of the blood transfusion unit of a tertiary health facility in south-south Nigeria were studied. The procurement and screening records from 1 January to 31 December 2009 were analyzed.
Results:
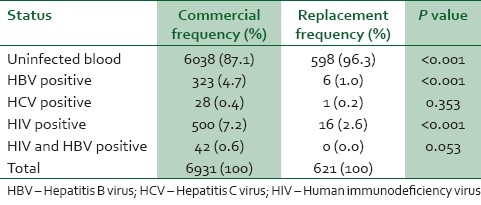
7,552 donor records were analyzed, 6,931 were commercial donor and 621 replacement donors. 891 commercial donors were infected, 500 (7.2%) were HIV positive, 323 (4.7%) HBV positive, 42 (0.6%) had HIV and HBV co-infection, while 28 (0.4%) were HCV positive. Twenty-three replacement donors were infected, 16 (2.6%) were HIV positive, 6 (1%) were HBV positive, while 1 (0.2%) were HCV positive. None of the replacement donors had co-infection. The risk of infection was significantly higher with commercial donor procurement (X2=45.07, P<0.001, OD=3.845).
Conclusion:
Commercial blood donors are still the major source of blood to the hospital and they also have the highest prevalence of transfusion transmissible viral infections in this region thus constitute a major risk transmitting infections to potential recipients.
Keywords: Blood donors, procurement, transfusion transmissible infections
INTRODUCTION
The demand for blood and blood products in Nigeria is high due to road traffic accidents, surgical and obstetrical blood loss, and anemia from other causes. Despite several researches to provide alternatives to blood transfusion, no perfect one has been found. The goal of clinical blood transfusion is to provide qualitative, safe, and adequate blood to recipient.1 The challenges in achieving these goals are enormous and serious efforts are been made globally, by government, donor agencies, and voluntary organizations towards its actualization.
The main sources of blood procurement are commercial (remunerated) blood donors, voluntary donors, and replacement donors. A commercial blood donor offers a unit of blood for a fee paid by a contracted hospital vendor. A replacement blood donor is a family member or relative of a patient, donating a unit of blood to be used for a specific patient, while a voluntary blood donor is a well-meaning member of the society who donates his or her blood without any inducement for use by a recipient not known to him or her. There is a general policy on blood screening before utilization in which a donor blood is screened for transfusion transmissible viral pathogens to ensure that only safe blood is transfused. It is a well-established fact that the safest source of blood is from a voluntary donor.2–4 In 1997, world health organization (WHO) set a goal of achieving 100% voluntary donation by 20205,6 but as at 2010 only 57 of 126 countries surveyed had established this as a standard.7
Blood transfusion is a known risk factor for transmission of infectious diseases including viral infections such as human immunodeficiency virus (HIV), hepatitis B virus (HBV), and hepatitis C virus (HCV). The risk of transmission is highest with blood procured from commercial donors.2–4,8,9 There is still a high dependence on remunerated donors especially in developing nations such as Nigeria. Several nations have made frantic efforts and are making significant improvement towards achieving 100% voluntary donor blood procurement but not so much has been achieved in Nigeria. Only 5% of voluntary donation has been achieved in some major donor centers in Nigeria.7 The objective of this investigation is to evaluate donor blood procurement and the associated risk of transfusion transmissible viral infections in a tertiary health facility in south-south Nigeria.
MATERIALS AND METHODS
Records of the blood transfusion unit of the health facility from 1 January 2009 to 31 December 2009 were reviewed in order to identify the methods of donor blood procurement and screening results for HIV, HBV and HCV. For every unit of blood, we studied the method of procurement (commercial, replacement or volunteer blood donation) and screening outcome. In this study, voluntary and replacement donors were grouped together due to the lack of proper differentiation in some of the records, the number of voluntary donors were very few and their blood were actually directed to specific recipients.
Blood from commercial donors is usually obtained from outside of the hospital through several vendors. Collection by the vendor is usually not supervised by hospital personnel but adheres to routine guidelines for phlebotomy. The vendor brings the units of blood to the hospital in fulfillment of a contractual agreement. Blood from replacement donor or panel donor is collected at the blood transfusion unit of the hospital. Blood (450 ml) is usually collected into a bag containing 63 ml citrate phosphate dextrose with adenine (CPDA) and stored at 2-8°C. Prior to phlebotomy, the donor's blood group is determined and the packed cell volume (PCV) ascertained.
Units of blood from commercial donors are inspected to determine good anticoagulation and exclude hemolysis. Thereafter, they were screened for HIV, HCV and HbsAg. HIV screening is conducted using the determine HIV I and II strips. The strip has two horizontal lines labeled control and patient bars. At room temperature, and using automatic micropipette, 50 μl of plasma or serum is added to the sample pad. The test strip is read within 15-60 min. A single red line at position C (control) on the strip indicates a valid control. A similar line is found in patient bar if positive for either HIV-1 or HIV-2. The sample is negative for HIV if there is no colour change in the patient bar.
Hepatitis B surface antigen screening is carried out using the Clinotech's one-step hepatitis B antigen test strip. An already anticoagulated blood sample is collected into a clean tube and centrifuged at 3000 rpm for 10 min to obtain clear plasma at room temperature. A test strip is then inserted into the test plasma to a maximum indicated by the manufacturers for 10 s and laid face up on a flat non-absorbent surface. The result is read after 15 min. A test is positive if two transverse bands (T=test and C=control) are seen and negative when only the one band at control (C) is seen. Only units of donor blood found to have good collection and storage and free from these viral infections are offered for transfusion to patients. Others that fail any of the above screening tests are discarded.
HCV antibody screening is carried out using Clinotech's diagnostic test strips. The test procedure and interpretation is similar to that of hepatitis B surface antigen. All infected commercial donor blood was discarded, while infected replacement donors are not bled.
The results are presented in cross tabulation and frequency tables. Statistical analysis was conducted using the statistical package for social sciences (SPSS) software. The unpaired Student t-test was used to test the statistical significance between commercial and replacement donor status. The test of association and risk factor for viral transfusion transmissible infection calculated using the chi-square test and odd ratios. A P<0.05 was considered significant.
RESULT
A total of 7,552 donors record were analyzed comprising 6,931(91.8%) commercial and 621(8.2%) replacement donors. 893 (11.8%) commercial donor blood and 23(0.3%) replacement donors test positive for viral transmissible infections [Table 1]. The percentage of commercial donors tested positive was 12.9% comprising 7.2% for HIV only, 4.7% for HBV, 0.6% for HIV and HBV and 0.4% for HCV. While 3.8% of replacement donors tested positive for viral infection comprising of 2.6%, 1% and 0.2% for HIV, HBV and HCV respectively [Table 2]. None of the replacement donor was positive for more than one viral infection. The chi square test was used to test the statistical significance of infection between commercial and replacement donors (χ2=45.07, P<0.001, OD=3.845).
Table 1.
Sources of blood procured
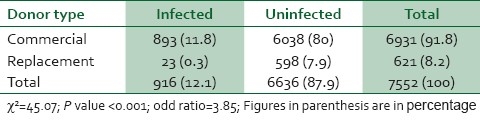
Table 2.
Viral status of donors
DISCUSSION
The hospital blood demand and utilization has been on the increase. In this 12-month transfusion record study, 7552 donors were recorded. A related work in the same hospital by Enosolease et al.8 recorded 11,021 over a 3 years period (January 2000 to December 2002). The increase is due to the increasing capacity and expansion in services rendered. The hospital is still highly dependent on commercial donors as procurement from these donors’ accounted for 91.8%. Currently the center has no policy to encourage the voluntary donor scheme. A majority of the replacement donors are medical students and doctors who donate to their unit patients; some replacement donors are actually commercial donors recruited from commercial donor centers who disguise as patient relatives.
The risk of viral transfusion transmissible infection is highest with blood procured from commercial donors. The prevalence of HIV infection was highest followed by HBV infection and HCV. HBV and HIV co-infection was reported in commercial donors. The findings are different from reports of earlier studies8 in the same centre but the prevalence of HIV in this study is comparable to that of the national HIV survey for 2010 which showed a rate of 7.5% in Benin.10 This shows that the prevalence of HIV infection has increased over the years. Ejele et al.6 in a survey in Niger Delta Nigeria reported lower prevalence rates, but the survey showed that commercial donors have the highest prevalence rates for viral transfusion transmissible infections. The unacceptably high rate of infections among commercial donors may be attributed to the fact that most of the donors may belong to the high risked group. It brings to fore the need to adhere to the strict practice of screening potential donors prior to donation. Furthermore, hospitals and donation centers should embrace and implement policies that will encourage voluntary blood donor scheme.
Study limitation
The donor blood samples that tested positive were not subjected to further testing to validate the results.
CONCLUSION
Commercial blood donors are still the major source of blood to the hospital and they also have the highest prevalence of transfusion transmissible viral infections in this region thus constitute a major risk transmitting infections to potential recipients.
Recommendation
Commercial donors should be screened for transfusion transmissible infections prior to blood donation. Alternative safer donor procurement method should be explored. Concerted efforts should be made towards encouraging voluntary blood donation, retention of donors through campaign and media enlightenment programmes so that reasonable progress can be made to achieve the WHO vision 2020 goal.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Nigerian National Blood Policy. Revised Ed 2005. Nov, National Blood Transfusion Service; Federal Ministry of Health Abuja. [Google Scholar]
- 2.Eastland T. Monetary blood donation incentives and the risk of transfusion transmitted infections. Transfusion. 1998;38:874–82. doi: 10.1046/j.1537-2995.1998.38998409009.x. [DOI] [PubMed] [Google Scholar]
- 3.Van der Poel CL, Seifried E, Schaasberg WP. Paying for blood donations: Still a risk? Vox Sang. 2002;83:285–93. doi: 10.1046/j.1423-0410.2002.00239.x. [DOI] [PubMed] [Google Scholar]
- 4.Glynn SA, Smith JW, Schreiber GB, Kleinman SH, Nass CC, Bethel J, et al. Repeat whole-blood and plateletpheresis donors: Unreported deferrable risks, reactive screening tests, and response to incentive programs. Transfusion. 2001;41:736–43. doi: 10.1046/j.1537-2995.2001.41060736.x. [DOI] [PubMed] [Google Scholar]
- 5.Blood safety and donation: fact sheet No. 279. 2008. Jun, Available from: http://www.who.int/mediacentre/factsheets/fs279/en/
- 6.World Health Organization: The Melbourne declaration on 100% voluntary non-remunerated donation of blood and blood components. 2009. [Accessed on 27 January 2009]. Available at http://www.who.int/entity/worldblood donorday/MelbourneDeclaration2009.doc .
- 7.Ajayi A. Nigeria: Nigerian Vanguard Newspaper; 2010. Jun 15, Voluntary donation is main source of safe blood. Available on All Africa.com . [Google Scholar]
- 8.Enosolease ME, Imarengiaye CO, Awodu OA. Donor blood procurement and utilization at the University of Benin Teaching Hospital, Benin City. Afr J Reprod Health. 2004;8:59–63. [PubMed] [Google Scholar]
- 9.Ejele OA, Erhabor O, Nwauche CA. The risk of transfusion-transmissible viral infections in the Niger-Delta area of Nigeria. Sahel Med J. 2005;8:16–19. [Google Scholar]
- 10.Department of Public Health, National AIDS/STI Control Programme. National HIV Sero-prevalence Sentinel Survey 2010 (Technical Report) 2010 [Google Scholar]


