Abstract
Introduction:
Intermittent preventive treatment of malaria with sulfadoxine-pyrimethamine is a recommendation of the World Health Organization as part of the malaria control strategy in pregnancy in areas with malaria burden.
Aim:
This study set out to appraise the effectiveness of this regimen in the prevention of placental parasitemia among parturients in Calabar, Nigeria.
Materials and Methods:
Pretested, precoded questionnaires were administered to eligible women at the antenatal clinic and later updated at the labor ward. Intermittent preventive treatment was administered under direct observation at the clinic, while packed cell volume, placental parasitemia, and other laboratory tests were measured at the labor ward.
Results:
The gross presence of placental malaria in the intermittent preventive treatment (IPT)-treated and the control groups was 10.6% and 11.3% respectively (P=0.76). Anemia occurred in 3.1% of the IPT-treated group compared to 11.7% among the control group (P=0.000). Only 7.9% of the IPT-treated women had moderate to severe placental parsitemia whereas as many as 53.2% of women in the control group had moderate to severe parasitemia (P=0.000).
Conclusion:
Intermittent preventive treatment of malaria with sulfadoxine-pyrimethamine was associated with significant reduction in the degree of placental parasitemia among women in the IPT-treated group, although it did not completely eradicate placental malaria in the treatment group.
Keywords: Intermittent preventive treatment, malaria in pregnancy, placental parasitemia
INTRODUCTION
Malaria poses a serious public health problem in most tropical and subtropical countries of the world. About 40% of the world's population in over 100 countries are at risk of malaria infection.1,2 Sub-Saharan Africa has about 90% of the world's burden of malaria.1–3 At least 30 million women living in malaria-endemic areas of Africa become pregnant yearly with attendant high maternal morbidity and mortality resulting in 200,000 neonatal deaths.3,4 The main burden of malaria in these areas results from infection with plasmodium falciparum.4–6
Malaria is highly endemic in Nigeria with a stable transmission of infection all year round.7,8 It accounts for 60% of outpatient consultations and contributes to 11% of maternal deaths in the country.7,8 Pregnant women are vulnerable to malaria infection partly due to the associated depression of cell-mediated immunity and the presence of the placenta, which serves as a seat for sequestration of parasitized erythrocytes.1,4,8 Parasitization of the placenta is a common occurrence with a higher risk in the first pregnancy than that in subsequent pregnancies.9–11 Placental parasitization has been recognized as the most important factor responsible for most complications that occur in pregnancy due to malaria.8–10 Placental parasitization is characterized by inflammatory response and pathological changes, which disrupt placental function.8,12
The World Health Organization (WHO) Expert Committee on malaria in 2000 recommended intermittent preventive treatment (IPT) of malaria in pregnancy with sulfadoxine--pyrimethamine combination.13,14 This recommendation was a sequel of the emergence of chloroquine resistant falciparum malaria and the lack of efficacy of pyrimethamine to prevent malaria infection in pregnancy. The University of Calabar Teaching Hospital adopted and commenced treatment with intermittent preventive treatment of malaria in pregnancy with sulfadoxine–pyrimethamine in the year 2004. Four years have elapsed and the effectiveness of this regimen as well as its benefits to the pregnant mothers has not been systematically evaluated in this center. This study therefore seeks to assess the effects of intermittent preventive treatment of malaria with sulfadoxine–pyrimethamine combination on placental parasitemia among parturients in the University of Calabar Teaching Hospital, Calabar, Nigeria.
MATERIALS AND METHODS
Study design and study area
This was a cross-sectional analytical study with the aim of assessing the effects of intermittent preventive treatment of malaria in pregnancy with sulfadoxine–pyrimethamine combination on placental parasitemia among parturients in the University of Calabar Teaching Hospital, Calabar. This study was conducted on pregnant women who attended antenatal clinic and delivered in the maternity annex of the University of Calabar Teaching Hospital, Calabar.
Cross River State where the University of Calabar Teaching Hospital is situated is in the rain forest belt of Nigeria with an annual rainfall of 1500–2500 mm. The State has a stable malaria transmission, which occurs all year round. Calabar has an estimated population of 371,022 people with women accounting for 50% of the population. (Final Report of the 2006 Nigerian Census.)
Recruitment and data collection
All eligible pregnant women who attended antenatal clinic in the University of Calabar Teaching Hospital during the period of the study who gave their informed consent were recruited into the study. They comprised pregnant women who received two therapeutic doses of sulfadoxine–pyrimethamine combination administered at the antenatal clinic 6 weeks apart under direct observation between 20 and 36 weeks of gestation. Laridox, a brand of sulfadoxine–pyrimethamine combination manufactured by Ipca Laboratories Ltd. India, was used for this study. This information was entered into the subject's questionnaire and a special code marked on the clinical folders of the women so treated for confirmation at the labor ward during delivery. The control group comprised booked mothers who did not receive any dose of sulfadoxine-pyrimethamine combination during the course of their pregnancy for various reasons. Mothers who had known allergy to the sulfonamide component of the drug, those who booked late and those who declined to take the drug were recruited into the control group. Subjects in the control group were matched for age, parity, and educational status with their counterparts in the study group. All pregnant women who receive antenatal care in our hospital receive iron and folate supplementation. A vast majority of the women had commenced this supplementation at home before booking.
The questionnaires of patients who had been recruited into both arms of the study were reviewed on admission of the parturient into the labor ward. The packed cell volume of parturients in each group was measured using the Hawksley's micro-hematocrit reader before delivery. Anemia was defined as a packed cell volume of less than 30%.15 Following delivery of the placenta, blood was aspirated from the substance of the placenta through the maternal surface and a blood smear was prepared on a clean glass slide for each subject. Microscopy for malaria parasite detection and counting was done after staining with Giemsa stain. The counting of the malaria parasites was done by counting the number of parasites in a blood film per 500 white blood cells as recommended by the World Health Organization. The number of parasites was divided by the number of white blood cells counted and multiplied by a factor of 6000. This gave the number of parasites per deciliter of blood. Mild parasitemia was defined as malaria parasite count of 1–999/dl of blood; moderate to severe parasitemia was defined by a count of 1000 or more per deciliter of blood.
Data analysis
The data obtained were analyzed with the Statistical Package for Social Sciences version 15 Inc. Chicago, Ilinois, USA. The test of statistical significance in outcome and the difference in outcomes between IPT-treated and the control groups were computed using Student's t-test and the chi-squared test (χ2 test) accordingly. Pearson coefficient was used to assess for any correlation between placental parasitemia and maternal packed cell volume as well as between placental parasitemia and cord parasitemia. A P value of <0.05 was considered statistically significant.
RESULTS
A total of 640 parturients participated in the final stage of the study, which comprised 358 women in the study group and 282 women in the control group. The overall prevalence of placental malaria in the study population was 10.9%. Plasmodium falciparum was the predominant specie detected among patients in the study population.
Table 1 shows the gross presence of placental parasitemia among women in the study and control groups. The prevalence of placental parasitemia among the IPT-treated and control groups were comparable, 10.6% and 11.3% respectively (χ2=0.09, df=1, P=0.76). The packed cell volume of women who were treated with IPT and those in the control group are shown in Table 2. The mean packed cell volume of IPT-treated parturients was 34.37%±3.12 compared to the mean packed cell volume of 33.34% ± 4.17 among the control group (t-test=12.77, df=1; P=0.000). Anemia occurred in 3.1% of the IPT-treated group compared to the prevalence of anemia of 11.7% among the control group. The difference in prevalence of anemia in both groups was highly statistically significant (χ2=25.34, df=1; P=0.000). The degrees of placental parasitemia among women in the IPT-treated and the control groups are shown in Table 3. Most (92.1%) of the IPT treated had mild placental parasitemia; only a minority (7.9%) of them had moderate to severe degree of placental parasitemia (>1000) whereas as many as 53.2% of women in the control group had moderate to severe parasitemia (t-test=22.79, df=1; P=0.000).
Table 1.
Placental parasitemia among women in the study and control groups
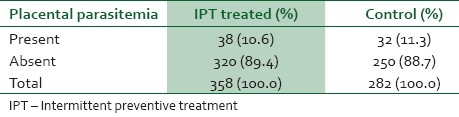
Table 2.
Packed cell volume of women in the study and control groups
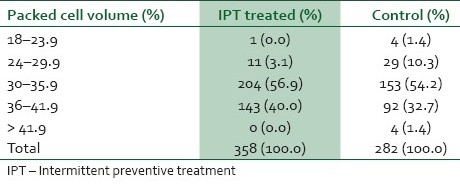
Table 3.
Degree of placental parasitemia among women in the study and control groups
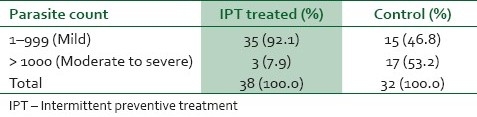
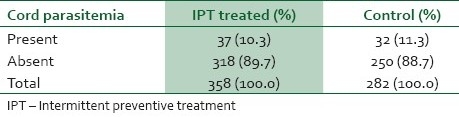
Table 4 shows the association between placental parasitemia and packed cell volume as well as placental parasitemia and cord parasitemia. There was a weak negative correlation between placental parasitemia and the packed cell volume. There was also a weak-positive correlation between placental parasitemia and cord parasitemia.
Table 4.
Umbilical cord parasitemia among women in the study and control groups
DISCUSSION
The occurrence of placental malaria in malaria-endemic areas is a recognized phenomenon in pregnancy. This study revealed an overall prevalence of placental malaria among patients in the study population of 10.9%. Comparable prevalence of 10.3% and 10.5% were obtained by Bassey et al.16 and Falade et al.17 in Calabar and Ibadan respectively. This finding has confirmed that placental malaria is a constant feature in pregnancy in areas where malaria is endemic. The prevalence of placental malaria among IPT-treated patients was 10.6% while that among the control was 11.3% (P=0.76). There was no statistically significant difference in prevalence between the two groups. This was an interesting finding which has revealed that although IPT reduces the degree of placental parasitemia, it does not completely eradicate placental malaria.18,19 It is also reminiscent of the fact that current malaria control strategies are targeted at disease control rather than disease eradication.19 The mean packed cell volume among IPT-treated parturients was 34.37%±3.12 compared to the mean packed cell volume of 33.34%±4.17 among the control group (P=0). The prevalence of anemia among the control group was 11.7% compared to 3.1% among the IPT-treated group. The difference was highly statistically significant (P=0). These results have confirmed the effectiveness of IPT in the prevention of maternal anemia in pregnancy.16,17 Anemia from placental malaria results largely from sequestration of parasitized red blood cells at the placental bed.11,12 Falade et al.17 in their study observed an equally significant difference in prevalence of anemia among the group that received IPT and other groups that did not receive IPT in their randomized controlled trial. The degree of placental parasitemia among the IPT-treated group was significantly lower than in the control group. The mean parasitemia in the control group was 1951.25±1969.26 compared to 297.05±764.04 among the IPT-treated group (P=0). This result has once again confirmed the role of IPT in the reduction of placental parasitemia. This result is in agreement with results obtained by Falade et al.17 and Parise et al.20 in their studies. There was virtually no correlation between placental parasitemia and packed cell volume (r=–0.09). This finding was revealing as it tended to suggest that the resulting anemia had no significant correlation with the degree of placental parasitemia among parturients in the study group. This rather surprising result may not be unrelated to the fact that the pathophysiology of placental malaria and its effects are not completely understood at the moment. Studies done from different centers have never produced consistent results; while Bergstrom et al.21 and McGregor22 also found weak negative correlations between placental parasitemia and packed cell volume in their studies, Falade et al.17 obtained a strong negative correlation between placental parasitemia and packed cell volume in their study. Other workers have opined that the relationship between placental malaria and packed cell volume was unclear.23,24 There was a weak positive correlation between placental parasitemia and cord parasitemia. The ratio of cord parasitemia in the IPT treated and control was 1:1. This finding was also revealing as it demonstrated that IPT had no influence on the transplacental transmission of malaria in the study population. This result suggests that IPT may not be effective in the prevention of congenital malaria. This finding however contrasted with results from Lagos and Maputo-Mozambique where the ratio of cord parasitemia between the IPT treated and control were 1:3 and 1:12 respectively.21,25 The reason for this disparity is not clear. It should also be noted that the mechanism of action by which transplacental transmission of malaria occurs is not very clear at the moment.
CONCLUSION
Intermittent preventive treatment of malaria in pregnancy with sulfadoxine–pyrimethamine combination was associated with significant reduction in placental parasitemia among women in the IPT-treated group, although it did not completely eradicate placental malaria in the treatment group. There was no significant correlation between the degree of placental parasitemia and packed cell volume. Similarly, there was no significant correlation between the degree of placental parasitemia and cord parasitemia. The need for a study that would assess the effect of placental parasitemia on perinatal outcome has become imperative. The use of suphadoxine–pyrimethamine combination in intermittent preventive treatment of malaria in pregnancy should however be encouraged till a better substitute is found.
ACKNOWLEDGMENT
We acknowledge with thanks the effort of Dr. (Mrs.) Angela Oyoita for her patience and painstaking analysis of the data of this research work.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Harrison KA. Malaria in pregnancy. In: Lawson JB, Harrison KA, Bergstrom S., editors. Maternity care in developing countries. London: RCOG Press; 2001. pp. 99–11. [Google Scholar]
- 2.Guyatt HL, Snow RW. The epidemiology and burden of Plasmodium falciparum related anaemia among pregnant women in Sub-saharan Africa. Am J Trop Med Hyg. 2001;55:100–6. doi: 10.4269/ajtmh.2001.64.36. [DOI] [PubMed] [Google Scholar]
- 3.WHO technical report. Geneva: WHO; 1999. World Health Organisation: Rolling back malaria; pp. 49–63. [Google Scholar]
- 4.Gravett MG, Sampson JE. Malaria in high-risk pregnancy. In: James DK, Steer PJ, Weiner CP, editors. High-risk pregnancies. Vol. 1. Mosby: Churchill Livingstone, Saunder's Company Ltd; 1994. pp. 541–50. [Google Scholar]
- 5.WHO Africa malaria report. Vol. 1. Geneva: WHO; 2003. World Health Organisation. Malaria during pregnancy; pp. 38–44. [Google Scholar]
- 6.Al-Hajjaj D. Malaria in pregnancy. PostGrad Doct Africa. 2005;3:50–60. [Google Scholar]
- 7.Edugie A, Mosanya ME, Amajoh C. Essential actions to support the attainment of the Abuja targets. Nigeria RBM country consultative mission, 2003;1:1–30. [Google Scholar]
- 8.Opare-Addo HS, Odoi AT. Malaria in pregnancy. In: Kwawukume KE, Emuveyan EE, editors. Comprehensive Obstetrics for the tropics. Dansoman: Asante and Hittscher Printing Press Ltd; 2002. pp. 250–6. [Google Scholar]
- 9.Matteelli A, Caligaris S, Castelli F, Carosi GS. The placenta and malaria. Ann Trop Med Parasitol. 1997;91:803–10. doi: 10.1080/00034989760563. [DOI] [PubMed] [Google Scholar]
- 10.Ibhanesbhor SE, Okolo AA. Placental malaria and pregnancy outcome. Int J Gynaecol Obstet. 1992;37:247–53. doi: 10.1016/0020-7292(92)90324-c. [DOI] [PubMed] [Google Scholar]
- 11.Allen SJ, Raiko A, O’Donnell A, Alexander ND, Clegg JB. Causes of preterm delivery and intrauterine growth retardation in a malaria endemic region of Papua, New Guinea. Arch Dis Child Fetal Neonatal Ed. 1998;79:F135–40. doi: 10.1136/fn.79.2.f135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sowunmi A. Malaria during pregnancy. In: Okonofua F, Odunsi K, editors. Contemporary Obstetric and Gynaecology for Developing countries. Ibadan-Nigeria: Intec Printers Ltd; 2003. pp. 498–51. [Google Scholar]
- 13.WHO technical report. Geneva: WHO; 2001. World Health Organisation. Antimalarial drug combination therapy; pp. 1–35. [Google Scholar]
- 14.Federal Ministry of Health, Nigeria. Use of IPT in pregnancy. National guidelines and strategies for malaria prevention and control during pregnancy. 2005:1–50. [Google Scholar]
- 15.Harrison KA. Anaemia in pregnancy. In: Harrison KA, Bergstrom S, editors. Maternity care in developing countries. London: RCOG Press; 2001. pp. 112–28. [Google Scholar]
- 16.Bassey AE, Ejezie GC, Alaribe AA, Useh MF, Udoh JJ, Ekanem AD. Congenital malaria in Calabar, Nigeria. Mary Slessor J Med. 2005;5:37–40. [Google Scholar]
- 17.Falade CO, Yusuf BO, Fadero FF, Mokuolu OA, Hamer DH, Salako LA. Intermittent preventive treatment with sulphadoxine-pyrimethamine is effective in preventing maternal and placental malaria in Ibadan, south-western Nigeria. Malar J. 2007;6:88. doi: 10.1186/1475-2875-6-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roberts DR. DDT and Malaria Control. Past, present and future. Century Sci Technol J. 2002;7:8–11. [Google Scholar]
- 19.Shulman CE, Dorman EK. Importance and prevention of malaria in pregnancy. Trans R Soc Trop Med Hyg. 2003;97:30–5. doi: 10.1016/s0035-9203(03)90012-5. [DOI] [PubMed] [Google Scholar]
- 20.Parise ME, Ayisi JG, Nahlen BL, Schultz LJ, Roberts JM, Misore A, et al. Efficacy of sulphadoxine-pyrimethamine for prevention of placental malaria in an area of Kenya with a high prevalence of malaria and human immunodeficiency virus infection. Am J Trop Med Hyg. 1998;59:813–22. doi: 10.4269/ajtmh.1998.59.813. [DOI] [PubMed] [Google Scholar]
- 21.Lamikanra OT. A study of malaria parasitaemia in pregnant women, placenta, cord blood and newborn in Lagos, Nigeria. West Afr J Med. 1993;12:213–7. [PubMed] [Google Scholar]
- 22.McGregor IA, Wilson ME, Billewics WZ. Malaria infection of the placenta in the Gambia, West Africa; its incidence and relationship to stillbirth, birthweight and placental weight. Trans R Soc Trop Med Hyg. 1993;77:232–44. doi: 10.1016/0035-9203(83)90081-0. [DOI] [PubMed] [Google Scholar]
- 23.Menendez C, Ordi J, Ismail MR, Ventura PJ, Aponte JJ, Kahigwa E, et al. The impact of placental malaria on gestational age and birth weight. J Infect Dis. 2000;181:1740–5. doi: 10.1086/315449. [DOI] [PubMed] [Google Scholar]
- 24.Reinhardt MC, Ambroise-Thomas P, Cavallo-Serra R, Meylan C, Gautier R. Malaria at delivery at Abidjan. Helv Paediatr Acta Suppl. 1998;41:65–84. [PubMed] [Google Scholar]
- 25.Bergstrom S, Fernandes A, Schwalbach J, Perez O, Miyar R. Materno-fetal transmission of pregnancy malaria: an immunoparasitological study on 202 parturients in Maputo. Gynaecol Obstet Invest. 1993;35:103–7. doi: 10.1159/000292675. [DOI] [PubMed] [Google Scholar]


