Abstract
Objectives:
The aim of this work is to determine the prevalence of intestinal parasites and bacteria among the food handlers.
Materials and Methods:
Two hundred food-handlers were subjected to a cross-sectional study working in the kitchen of a tertiary care hospital, i.e., Alnoor Specialist Hospital, Makkah, Saudi Arabia from February 2 to 27, 2009. The stool samples were examined for intestinal parasites following direct microscopic examination, formol ether concentration (Ritchie), and staining with modified acid fast staining techniques. For enteropathogenic bacteria samples were inoculated onto MacConkey's agar, deoxycholate citrate agar, xylose lysine deoxycholate agar as per the World Health Organization protocol. Fingernail materials were examined microscopically for enteropathogenic bacteria and parasites.
Results:
The majority (80%) of the food-handlers were young adults aged from 22 to 42 years. No intestinal parasites were detected from fingernail contents. Forty six (23%) stool specimens were positive for intestinal para¬sites. Giardia lamblia 18 (9%) was most frequent among the 10 different types of detected intestinal parasites followed by Entamoeba histolytica 9 (4.5%). No pathogenic bacteria were detected in all stool samples, whereas finger nails showed isolation of microorganisms as coagulase-negative staphylococci 79 (39.5%), followed by Staphylococcus aureus 35 (17.5%).
Conclusion:
The findings emphasized the importance of food handlers as potential sources of infections and suggested health institutions for appropriate hygienic and sanitary control measures.
Keywords: Entamoeba histolytica, food-handler, intestinal parasite, stool
INTRODUCTION
Intestinal parasites and protozoan infections are among the most common infections worldwide. It is estimated that some 3.5 billion people are affected, and that 450 million are ill as a result of these infections, the majority being children.1 Transmission of intestinal parasites and enteropathogenic bacteria is affected directly or indirectly through objects, i.e., food, water, nails, and fingers, indicating the importance of fecal-oral human-to-human transmission. Food handlers with poor personal hygiene could be potential sources of infections of many intestinal helminths, protozoa, and enteropathogenic bacteria.2 Compared to other parts of the hand, the area beneath fingernails harbors the most microorganisms and is most difficult to clean.3 The spread of disease via food handlers is a common and persistent problem worldwide.4,5
The centers for disease control and prevention have stated that poor personal hygiene is the third most commonly reported food preparation practice contributing to food-borne diseases.6 Parasitic infections in food handlers may pose a real threat to those who are more susceptible to infection like hospitalized patients especially those who suffered from immune deficient conditions,7 justifying the importance of a proper food handling in the hospital environment. Therefore, a proper screening procedure may be needed in order to diagnose food handlers, thus preventing possible morbidity and protecting the health of the consumers.
This study was aimed at assessing the prevalence of intestinal parasites and bacteria among food handlers working in the dietary section of a tertiary care hospital, Makkah, Saudi Arabia.
MATERIALS AND METHODS
Study design and settings
A cross-sectional study was conducted in the kitchen of a tertiary care hospital, i.e., (Alnoor Specialist Hospital, Makkah, Saudi Arabia) from February 2 to 27, 2009. Two hundred food handlers who did not take treatment for any intestinal element within 3 months prior to the study were included. A pretested structured questionnaire was used for collecting information on age, sex, educational level, and hand-washing practices of each food handler.
Sample collection, transport, and microscopic examination
Samples of fingernail contents were collected from both hands of each subject using sterile-moistened cotton-tipped swab and placed into a sterile test tube. A stool specimen was collected from each food handler in a clean stool cup by medical laboratory technicians and transported into the laboratory. The fingernail contents examined microscopically for parasites and/or bacteria following direct wet mount preparations in normal saline and iodine solution.5
Direct smear examination for stool samples
On a glass microscope slide, about 1–2 mg of stool was emulsified in a drop of normal saline (0.85% NaCl) on the left hand side of the slide, and in Lugol's iodine on the right side of the slide. A cover-slip was then placed on each side, and the slides were scanned under 10× and 40× objective lenses of a light microscope, as required. Saline direct smear is used mainly for detection of motility of intestinal protozoan trophozoites, which are seen in liquid or semi-liquid specimens. Iodine direct smear shows the characteristic features of the diagnostic stages in more details.8
Formol ether sedimentation concentration technique Ritchie
Although, this formol ether technique cannot detect trophozoites, it is considered as the best concentration technique used in diagnostic parasitology laboratories for detection of cysts, ova, and larvae.9,10 Generally, 10% formal saline is used in the Ritchie technique to kill and preserved diagnostic stages. Diethyl ether collects most of debris in a separate layer. All diagnostic stages that are applicable with the Ritchie technique will be concentrated at the bottom of the analysis centrifuge tube. However, safety precaution should be taken, as formalin is carcinogenic, and diethyl ether is flammable and explosive. Quantitatively, one slide from the Ritchie technique is a substitute of about 1000 slides or more from the direct smear technique. Thus the greater the amount of stool used, the greater the chance of recovery of diagnostic stages. The Ritchie sedimentation technique was performed by emulsifying about 2 g of stool in 10-15 ml of 10% formal saline. The suspension was allowed to stand for 30 minutes, and then strained through two layers of gauze into a 15 ml conical centrifuge tube and centrifuged at 2000 rpm for 5 minutes. When needed, the washing step was repeated until supernatant becomes clear. The sediment was resuspended with 10 ml of 10% formal saline and allowed to stand for 5-10 minutes. A total of 3 ml of diethyl ether was added, and then the tube was shaken vigorously for 30 seconds and centrifuged at 2000 rpm for 5 minutes. After centrifugation, the applicable diagnostic stages were sedimented in the bottom of the tube. The fecal debris was separated in a layer between the diethyl ether and the 10% formal-saline layers. A fecal debris layer was loosened by wooden stick and the tube rapidly inverted to discard the top three layers while the sediment remained at the bottom. One to two drops of iodine were added to the sediment and mixed well. Then, part of the sediment was transferred to a microscope slide, covered with a cover glass and scanned microscopically under low and high objective lenses.11
Modified acid fast staining techniques
Smears are prepared after concentration, air dried and then fixed in methanol, stained with Kinyoun carbol-fuchsin for 4-5 minutes, distained with 1% aqueous sulfuric acid for 2-3 minutes, rinsed with distilled water and then counterstained with Loeffler's alkaline methyline blue for 1 minute. Smears are rinsed with distilled water drained and dried.10
Culture and identification
Full microbiological examination was done to all samples including culture on Selenite-F broth and incubates for 24 hours at 37°C then xylose lysine deoxycholate agar plates and re-incubation for 2 hours at 37°C. Examination of the plates for significant colonies of Salmonila and Shigela species was done. Other plates of MacConkey's agar were inoculated to identify the Gram-negative organisms present in the samples. Identification of Gram-negative bacilli was done using the automated system (microsn) that determined the organism and minimum inhibitory concentration for antibiotics. Sorbitol-Mackonky were added to identify enteroxogenic E. coli (O157).
Statistical analysis
Data were analyzed by using Microsoft excel version 2007. Categorical data were analyzed as frequency and percentage.
Ethical consideration
The data were collected after written informed consent was obtained from all study participants. The results were kept confidential, and food handlers who were found positive for any parasitic infection were given appropriate treatment.
The Institutional Review Board of Alnoor Specialist Hospital, Makkah, granted us permission to con-duct this study. We declare that we have no financial or personal relationship(s) which may have inappropriately influenced us in writing this paper.
RESULTS
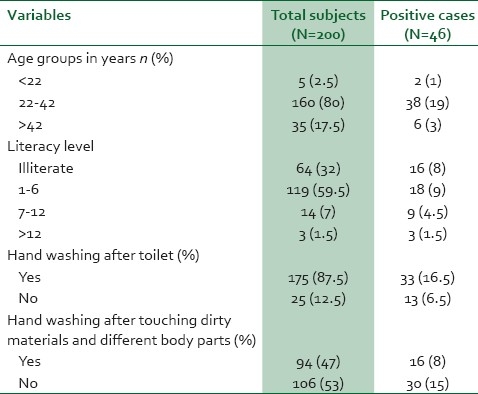
The study included all the 200 food handlers. The majority (80%) of the food handlers were young adults aged from 22 to 42 years. Only 8.5% of the food handlers had education above elementary (>6 standard) school level. In hand washing practices, 175 (87.5%) food handlers had a habit of hand washing after toilet and 5 (2.5%) participants had medical checkup including stool examination previously [Table 1].
Table 1.
Sociodemography and literacy level of food handlers versus positivity for intestinal parasites
Macroscopic examination included color, consistency of stool, and the presence of any macroscopic diagnostic stage of intestinal parasites. The color of specimens ranged from yellowish, light brown, brown to greenish or dark brown. The consistency ranged from formed, soft to loose, three samples were watery and none was bloody. Only one sample contained live adult female worm of Enterobius vermicularis.
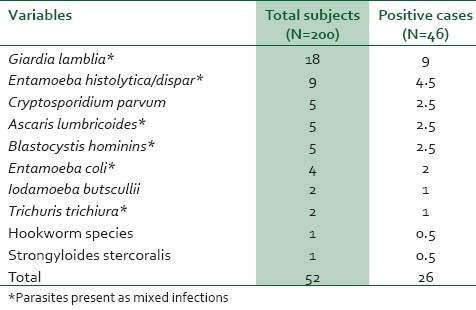
Fourty-six (23%) stool specimens were positive for intestinal para-sites. Giardia lamblia 18 (9%) was most frequent among the 10 different types of detected intestinal parasites followed by Entamoeba histolytica 9 (4.5%). Mixed intestinal parasite infections were detected in 3% of the samples, i.e., 0.5% Ascaris lumbricoides and Trichuris trichiura, 0.5% Trichuris trichuira and Giardia lamblia, 1% Entamoeba histolytica and Giardia lamblia, and 1% Entamoeba coli and Blastocystis hominis [Table 2].
Table 2.
Stool samples infected by one or more parasites
By using direct smears, 21 (10.5%) samples out of the total 200 samples were positive to different parasites that represented 45.7% out of the 46 positive samples. It has shown trophozoite stages of E. histolytica, E. coli, G.lamblia, B.hominis, and eggs of helminths clearly in some samples. Using the Ritchie technique, 45 (22.5%) samples were positive to parasitic infection that represented 97.8% out of the total 46 positive samples, as this technique was able to detect all the parasites, except the trophozoite stages of the intestinal protozoan parasites. Modified acid fast stain was performed for all liquid or semiliquid stool samples to detect the Cryptosporidium oocysts.
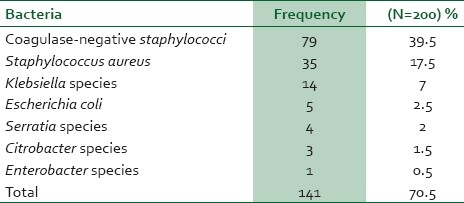
Bacterial species isolated from cultures of fingernail contents of 200 samples were found to be coagulase-negative staphylococci 79 (39.5%) followed by Staphylococcus aureus 35 (17.5%). However, no intestinal parasites were detected from the samples of fingernail contents [Table 3].
Table 3.
Frequency of the bacteria isolated from fingernail contents of food handlers
DISCUSSION
Several authors from all over the world have stressed out the importance of food handlers as threats in the transmissions of parasitic and bacterial diseases.12 In this study, the overall prevalence (23%) of intestinal parasites was high and other species of bacteria were also isolated from their fingernail contents. These indicated the health status and very poor hygiene practices of the food handlers working in our kitchen. The high (23%) prevalence of intestinal parasites in our study was in agreement with the findings of other stud-ies, i.e., 29.1% in the study of Andargie et al., and 41.1% in the study of Aberal.5,13 Such a high prevalence of intestinal parasites is largely due to poor personal hygiene practices and environmen-tal sanitation, lack of supply of safe water, and ignorance of health-promotion practices.
It was found in Kenya that the most common intestinal parasites affecting the food handlers were A. lumbricoides (13.1%) and E. histolytica (11.9%).14 One study in Makkah during Hajj season reported low prevalence of intestinal parasites and the most common detected pathogenic protozoa were E. histolytica (2.78%) and G. lamblia (1.98%).11 Alkalani et al. in Libya reported (2.5%) of G. lamblia.15 On the other hand Koshak and Zakai reported that the most common parasite were Trichuiris trichuira (39.1%) followed by hookworm (34.2%) and E. histolytica (16.1%).16
Also the E. histolytica and G. lamblia were the most frequent parasites in Yemen.17 Another study indicated clearly the relatively high frequency of occurrence of pathogenic organisms (30.1%) among food handlers in the city of Omdurman and its suburbs.18 Stages of intestinal parasites were not detected in the fingernails of food handlers in our study while earlier reports showed the presence of ova, larvae, and cysts of intestinal parasites under fingernails of study participants.19
In this study, most food handlers working in the kitchens were inexperienced with low educational levels that agreed with previous studies.13,20 Assessment of hand washing practices revealed varied results. Food handlers’ hand washing practices after toilet (87.5%) was in parallel with the previous reports.5,13 However, fewer numbers had practices of hand washing after touching dirty materials and different body parts between handling of food items. These reflected that food handlers lack awareness about food contamination with poor hygienic practices.
In our study, majority of cultures of fingernail contents were found to be positive for coagulase-negative staphylococci (39.5%) followed by S. aureus (17.5%). This high prevalence of isolation of coagulase-negative staphylococci was because it is the normal commensal of the skin. Few cases showed isolation of different species of enterobacteria from finger nails that reflected low personal hand-wash of food handlers. E. coli and Serratia species were isolated from fingernail contents in one study supporting the concept of contamination by fecal bacteria due to inadequate hand-washing of the food handlers.5 This study suggested that the poor hygiene practice might have been confounded by the fact that most food handlers were individuals from the lower socioeconomic class with low level of education. In addition, none of the food handlers at the facilities had been appropriately trained in safe food-handling practices. In fact, a prerequisite for the control and prevention of intestinal parasitosis require clear understanding of their epidemiology.10
CONCLUSION
The prevalence of intestinal para-sites of the food handlers was high in this study. An effective means of preventing the transmission of pathogens from food-handling personnel via food to consumers is strict adherence to good personal hygiene and to hygienic food-handling practices. Health education, raising awareness, and strengthening the existing screening methods especially for food handlers are among the ways to control the problem of intestinal parasitic infections. So we are in need for constant epidemiological surveillance through periodical surveys parallel with development of healthcare toward the problem of parasitic infections.
Recommendations
It is recommended that local health authorities should implement interventions such as food handler's training on food safety, institute periodic focused medical checkup for food handlers, and improve human waste disposal. It is recommended that education and training about good-hygiene practices should be provided to all food handlers.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Control of tropical diseases. Geneva: WHO; 1998. World health organization. [Google Scholar]
- 2.Kaferstein F, Abdussalam M. Food safety in the 21st century. Bull World Health Organ. 1999;77:347–51. [PMC free article] [PubMed] [Google Scholar]
- 3.Lin CM, Wu FM, Kim HK, Doyle MP, Michael BS, Williams LK. A comparison of hand washing techniques to remove Escherichia coli and caliciviruses under natural or artificial fingernails. J Food Prot. 2003;66:2296–301. doi: 10.4315/0362-028x-66.12.2296. [DOI] [PubMed] [Google Scholar]
- 4.Zain MM, Naing NN. Sociodemographic characteristics of food handlers and their knowledge, attitude and practice towards food sanitation: A preliminary report. Southeast Asian J Trop Med Public Health. 2002;33:410–7. [PubMed] [Google Scholar]
- 5.Andargie G, Kassu A, Moges F, Tiruneh M, Huruy K. Prevalence of bacteria and intestinal parasites among food-handlers in Gondar town, northwest Ethiopia. J Health Popul Nutr. 2008;26:451–5. doi: 10.3329/jhpn.v26i4.1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lillquist DR, McCabe ML, Church KH. A comparison of traditional hand washing training with active hand washing training in the food handler industry. (28).J Environ Health. 2005;67:13–6. [PubMed] [Google Scholar]
- 7.Robinson RD, Murphy EL, Wilks RJ, Neva FA, Terry SI, Hanchard B, et al. Gastrointestinal parasitic infection in healthy jamaican carriers of HTLV-I. J Trop Med Hyg. 1991;94:411–5. [PubMed] [Google Scholar]
- 8.Kuo HY, Chiang DH, Wang CC, Chen TL, Fung CP, Lin CP, et al. Clinical significance of Blastocystis hominis: Experience from a medical center in northern Taiwan. J Microbiol Immunol Infect. 2008;41:222–6. [PubMed] [Google Scholar]
- 9.Wakid MH. Distribution of intestinal parasites among food handlers in Jeddah, Saudi Arabia. J Parasit Dis. 2006;30:146–52. [Google Scholar]
- 10.Garcia LS. Washington: ASM Press; 2007. Diagnostic Medical Parasitology. [Google Scholar]
- 11.Wakid MH, Azhar EI, Zafar TA. Intestinal parasitic infection among food handlers in the Holy City of Makkah during Hajj Season 1428 Hegira. JKAU Med Sci. 2009;16:39–52. [Google Scholar]
- 12.Feglo PK, Frimpong EH, Essel-Ahun M. Salmonellae carrier status of food vendors in Kumasi, Ghana. East Afr Med J. 2004;81:358–61. doi: 10.4314/eamj.v81i7.9191. [DOI] [PubMed] [Google Scholar]
- 13.Abera B, Biadegelgen F, Bezabih B. Prevalence of Salmonella typhi and intestinal parasites among food handlers in Bahir Dar Town, Northwest Ethiopia. Ethiop J Health Dev. 2010;24:46–50. [Google Scholar]
- 14.Nyarango RM, Aloo PA, Kabiru EW, Nyanchongi BO. The risk of pathogenic intestinal parasite infections in Kisii Municipality, Kenya. BMC Public Health. 2008;14(8):237. doi: 10.1186/1471-2458-8-237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Al Kilani MK, Dahesh SM, El Taweel HA. Intestinal parasitosis in Nalout popularity, western Libya. J Egypt Soc Parasitol. 2008;38:255–64. [PubMed] [Google Scholar]
- 16.Koshak E, Zakai H. A spectrum of pathogenic and non-pathogenic intestinal parasites in pre-employment medical check-up for workers and their families. J Family Community Med. 2003;10:47–53. [PMC free article] [PubMed] [Google Scholar]
- 17.Baswaid SH, AL-Haddad AM. Parasitic infections among restaurant workers in Mukalla (Hadhramout/Yemen) Iran J Parasitol. 2008;3:37–41. [Google Scholar]
- 18.Saeed HA, Hamid HH. Bacteriological and parasitological assessment of food handlers in the Odurman area of Sudan. J Microbiol Immunol Infect. 2010;43:70–3. doi: 10.1016/S1684-1182(10)60010-2. [DOI] [PubMed] [Google Scholar]
- 19.Sahlemariam Z, Mekete G. Examination of finger nail contents and stool for ova, cyst and larva of intestinal parasites from food handlers working in student cafe-terias in three higher institutions in Jimma. Ethiop J Health Sci. 2001;11:131–8. [Google Scholar]
- 20.Zeru K, Kumie A. Sanitary conditions of food establishments in Mekelle town, Tigray, north Ethiopia. Ethiop J Health Dev. 2007;21:3–11. [Google Scholar]


