Abstract
Objective:
Elevated white blood cell (WBC) count is considered to be prospectively and positively associated with cardiovascular diseases, particularly hypertension. Also, the positive role of exercise in the management of hypertension has been well and long established. However the relationship between WBC count and hypertensive management particularly in the nonpharmacological technique is ambiguous and unclear. Therefore the purpose of the present study was to determine the effect of interval training program on WBC count and cardiovascular parameters in male hypertensive patients.
Materials and Methods:
A total of 245 male patients with mild to moderate (systolic blood pressure (SBP) between 140 mmHg and 179 mmHg and diastolic blood pressure (DBP) between 90 mmHg and 109 mmHg) essential hypertension were age matched and grouped into experimental and control groups. The experimental (n=140; 58.90±7.35 years) group involved in an 8-week interval training (60-79% HR max reserve) program of between 45 minutes to 60 minutes, while the age-matched controls hypertensive (n=105; 58.27±6.24 years) group remain sedentary during this period. Cardiovascular parameters (SBP, DBP, and VO2 max) and WBC count were assessed. Student's t and Pearson correlation tests were used in data analysis.
Results:
Findings of the study revealed a significant effect of the interval training program on VO2max, SBP, and DBP and WBC count at P<0.05 and VO2max is negatively related to the WBC count (r=–0.339) at P<0.01.
Conclusions:
It was concluded that the interval training program is an effective adjunct nonpharmacological management of hypertension and the therapeutic effect of exercise programs may be mediated through suppression of inflammatory (WBC count) reaction.
Keywords: Exercise, hypertension, inflammation, white blood cell
INTRODUCTION
Several prospective studies have shown a positive and independent association between white blood cell (WBC) count and coronary heart disease, hypertension, and ischemic stroke incidence and mortality.1–6 According to Lee and Associates,3 the relationship is irrespective of sex, ethnic, and smoking status. Chronic low-grade inflammation is believed to be the mechanism behind this association whereby WBC-derived macrophages and other phagocytes contribute to vascular injury, endothelial dysfunction, and atherosclerotic disease progression.7–9 However, inflammation may also contribute to increasing microvascular capillary resistance, initiation of platelet aggregation, increased cathecolamine levels, and there is considerable evidence of a link between inflammation and hypertension.2,9–12
Another possibility for the relationship between WBC and hypertension might be the close relationship between leucocytes count and activity of corticosteroids in hypertension and other vascular diseases. This has been reported previously in both animal models and humans.13,14 The total leucocytes count and blood pressure are increased by cortisol administration in normal human subjects.15 Previous studies have shown that elevated WBC count is associated with a small, but significant increase in the risk of hypertension among white men.10,16 Nakanishi and co-workers17 also found this association to be true among Japanese men irrespective of their smoking status.
Previous reports18–21 show that moderate to high levels of cardiorespiratory fitness are protective against Cardiovascular disease (CVD) and all-cause mortality, even in individuals with CVD risk factors. Regular exercise induces anti-inflammatory actions. Geffken et al.22 found WBC to be inversely related to physical activity in a large sample (n=5201) composed of men and women from diverse racial backgrounds. Nieto et al.23 reported sport activity index to be inversely related to WBC in whites but not in blacks.
Studies that investigated the association between WBC count and hypertension management particularly the nonpharmacological are few and the exact nature of this relationship is ambiguous and unclear.24 Therefore, the purpose of the present study was to investigate the relationship between interval training program and WBC in the noninvasive management of chronic essential hypertension
MATERIALS AND METHODS
Research design
In the present study, age-matched randomized independent pretest–posttest–control group design was used to determine the influence of the interval training program on WBC count.
Subjects
The population for the study was male essential hypertensive subjects attending the hypertensive clinic of Murtala Muhammed Specialist Hospital Kano Nigeria. Subjects were fully informed about the experimental procedures, risk, and protocol, after which they gave their informed consent.
Inclusion criteria
Only those who volunteered to participate in the study were recruited. Subjects between the age range of 45-70 years with chronic mild to moderate and stable (>1 year duration) hypertension (systolic blood pressure (SBP) between 140 mmHg and 179 mmHg and diastolic blood pressure (DBP) between 90 mmHg and 109 mmHg) were selected. Only those who had stopped taking antihypertensive drugs or on a single-antihypertensive medication (monotherapy) were recruited.25 They were sedentary and have no history of psychiatry or psychological disorders or abnormalities.
Exclusion criteria
Obese or underweight (body mass index (BMI) between 20 and 30 kg/m2), smokers, alcoholic, diabetic, other cardiac, renal, respiratory disease patients were excluded. Those involved in vigorous physical activities and above averagely physically fit (VO2 max >27 and >33 ml/kg/min for over 60 and 50 years old respectively) were also excluded.
A total of 323 chronic and stable, essential mild to moderate male hypertensive patients satisfied the necessary study criteria. Subjects were aged matched and randomly grouped into experimental (162) and control (161) groups. They were fully informed about the experimental procedures, risk, and protocol, after which they gave their informed consent in accordance with the American College of Sports Medicine (ACSM) guidelines, regarding the use of human subjects26 as recommended by the human subject protocol. Ethical approval was granted by the Ethical Committee/Board of Kano State Hospitals Management Board.
Pretest procedure
Wash out period
All subjects on antihypertensive drugs were asked to stop all forms of medication and in replaced, were given placebo tablets (consisted of mainly lactose and inert substance) in a single blind method.27,28 All subjects including those not on any antihypertensive medications were placed on placebo tablets for 1 week (7 days); this is known as “wash out period.” The purpose of the wash out period was to get rid of the effects of previously taken antihypertensive drugs/medications. During the wash out period all subjects were instructed to report to the hypertensive clinic for daily blood pressure monitoring and general observation. The pretest procedure was conducted at the last day of the wash out period, and in the Department of Physiotherapy of Murtala Mohammed Specialist Hospital (MMSH), Kano between 8:00 and 10:00 am.
Physiological measurement
Subjects resting heart rate (HR), SBP, and DBP were monitored from the right arm as described by Walker et al.29 and Musa et al.30 using an automated digital electronic BP monitor (Omron digital BP monitor, Medel 11 EM 403c; Tokyo, Japan). These measurements were monitored between 8:00 and 10:00 am each test day.
Anthropometric measurement
Subjects’ physical characteristics (%body fat, weight [kg] and height [m]) and body composition (body mass index [BMI] (kg/m-2)) assessment were done in accordance with the standardized anthropometric protocol.31,32
Blood sample collection (venipuncture method)
Both pre- and post-treatment venous blood samples were obtained between 8: 00 and 10:00 pm after about 12-hour overnight fast (fasting blood sample). A 5 ml syringe was used for blood sample collection, using the procedure described by Bachorik.33 One milliliter of blood sample was immediately transferred into a special container containing anticoagulant (heparin, 75 U/ml) for WBC count. All samples were stored in a refrigerator at –80°C until analysis.34
Stress test
The Young Men Christian Association (YMCA) submaximal cycle ergometry test protocol was used to assess subject's aerobic power.35,36 The YMCA protocol uses two to four 3-minute stages of continuous exercise, two HR-power output data points will be needed (steady state HR) of between 110 and 150 beat/min. The two steady state HR were plotted against the respective workload on the YMCA graph sheet. A straight line was drawn through the two points and extended to the subjects predicted maximum HR (220 age). The point at which the diagonal line intersects the horizontal-predicted HR max line represents the maximal working capacity for the subject. A perpendicular line was dropped from this point to the baseline where the maximal physical workload capacity was read in kg/m/min which was used to predict the subjects VO2 max. This procedure was done for both pre- and post-test stress test.
Test procedures
The test procedures were conducted in the Department of Physiotherapy of MMSH, Kano between 8:00 and 10:00 am.
Training program
Following the stress test and prior to the exercise training, all subjects in both control and interval groups were reassessed by the physicians and were prescribed with methyldopa (500 mg-1 g daily in divided doses of 2 to 4 times) based on the subject's responses and tolerance to therapy. Methyldopa was preferred because it does not alter normal hemodynamic responses to exercise,37 and it is a well-tolerated antihypertensive drug in Africa.38–40 In addition, it is the drug prescribed the most in Kano, where the study was conducted and had proved useful in the treatment of mild to moderately severe hypertension either as monontherapy or combination therapy.39–41 Subjects maintained these prescriptions with regular medical consultation and observation throughout the period of exercise training.
The interval group (group 1)
Subjects in the interval group exercised on a bicycle ergometer at a low intensity of between 60–79% of their HR max reserve that was estimated from 220 minus the age of a subject as recommended by ACSM.42 The starting workload was 100 kg (17 watts) which was increased at a pedal speed of 50 rpm to obtain a HR max reserve 60% was increased in the first 2 weeks to and level up at 79% HR max reserve throughout the remaining part of the training period at a work/rest ratio of 1:1of 6 minutes each. The initial of exercise session was increased from 45 minutes in the first 2 weeks of training to and leveled up at 60 minutes throughout the remaining part of the training. Exercise session of three times per week was maintained throughout the 8 weeks period of training for the interval group.
The control group (group 2)
Subjects in the control group were instructed not to undertake any vigorous physical activity during the 8 weeks period of the study.
White blood cell count
The WBC count was analyzed using the Turks method as described by Dacie and Lewis.43
Posttest procedure
Wash out period
At the end of the 8-week training period, all subjects was asked to stop methyldopa (Aldomet) and subjects were prescribed with placebo tablets in a single-blinded method for 1 week in order to get rid the effect of the methyldopa taken during the training period.
Blood sample collection
Immediately after the post-training wash out period, fasting blood samples were collected as earlier described.
Post-training SBP, DBP, WBC count assessment, and stress test were conducted as earlier described in the pretest procedures using standardized protocols, techniques, and methods.
All pre- and post-test measurements were recorded on a data sheet. A total of 257 subjects (140 from the interval, and 105 from the control groups) completed the 8 weeks training program. Seventy-eight subjects (22 from interval, and 56 from the control groups) had dropped out because of noncompliance, unfavorable responses to methyldopa, and exercise training or had incomplete data; therefore, the data of 245 subjects were used in the statistical analysis [Figure 1].
Figure 1.
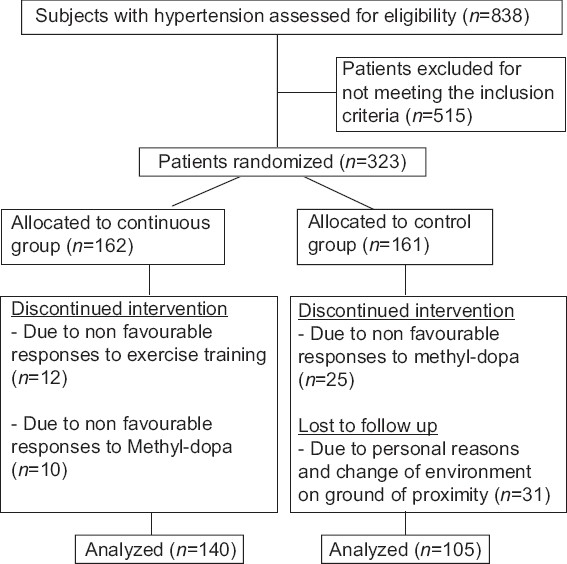
Study design flow chat
Statistical analysis
Following data collection, the measured and derived variables were statistically analyzed. The descriptive statistics (means and standard deviations) of the subject's physical characteristics, estimated VO2 max, WBC count, and cardiovascular parameters were determined. Analysis of covariance (ANCOVA) was used to assess the outcome variables; in the ANCOVA, the post-test values were the outcome variables and the co-covariates were the pretest values, age, baseline BMI, and % body fat. The Pearson product moment correlation test was also computed for the variables of interest; in the correlation test, the difference between subjects post-training and pretraining measurements (changed score) were used as dependent measures. All statistical analysis was performed on a Toshiba compatible microcomputer using the statistical package for the social science (SPSS), (Windows Version 16.0 Chicago, IL, USA). The probability level for all the above tests was set at 0.05 to indicate significance.
RESULTS
The subject's age ranged between 45 and 70 years. The mean age, height, weight, BMI, and % body fat±SD were as follows, respectively: the interval group (58.40±6.91 years, 167.78±7.81 cm, 70.18±11.37 kg, 24.96±3.88 kg/m–2, and 17.69±6.50%) and the control group (58.27±6.24 years, 167.89±5.31 cm, 68.47±17.07 kg, 24.16±4.91 kg/m–2, and 22.27±9.82%). There was no significant difference in age between the groups (t=0.156, P=0.876).
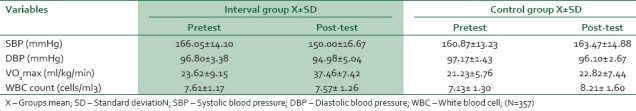
Table 1 shows pre- versus (vs.), post-treatment mean BP±SD mmHg, WBC (cells/ml3) count and VO2 max (ml/kg/min) for the exercise (SBP166.05±14.10; DBP, 96.80±3.38; WBC count 7.61±1.17, and VO2 max 23.62±9.15 vs. SBP, 150.00±16.67; DBP, 94.98±5.40; WBC count 7.57±1.26, and VO2 max 37.46±7.42) group and the control (SBP160.87±13.23; DBP, 97.17±1.43; WBC count 7.13±1.30, and VO2 max 21.23±5.76 vs. SBP, 163.47±14.88; DBP, 96.10±2.67; WBC count 8.21±1.60, and VO2 max 22.82±7.44) group.
Table 1.
Groups mean and standard deviation for pre- and post-test values
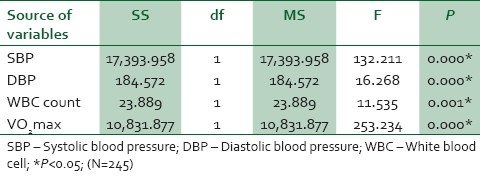
The Table 2 ANCOVA test results indicated a significant reduction in the exercise groups over control in SBP (P=0.000), DBP (P=0.000), WBC (P=0.001), and VO2 max (P=0.000) at P<0.05.
Table 2.
Analysis of the covariance test between the interval and control groups
The results also showed significant negative correlation Figures 2 and 3 between changes in VO2 max and changes in other parameters such as WBC count (r=–0.339); SBP (r=–0.304); and DBP (r=–0.289) at P<0.01. Changes in the WBC count positively and significantly correlated with both SBP (r=0.200) and DBP (r=0.263) [Figure 4].
Figure 2.
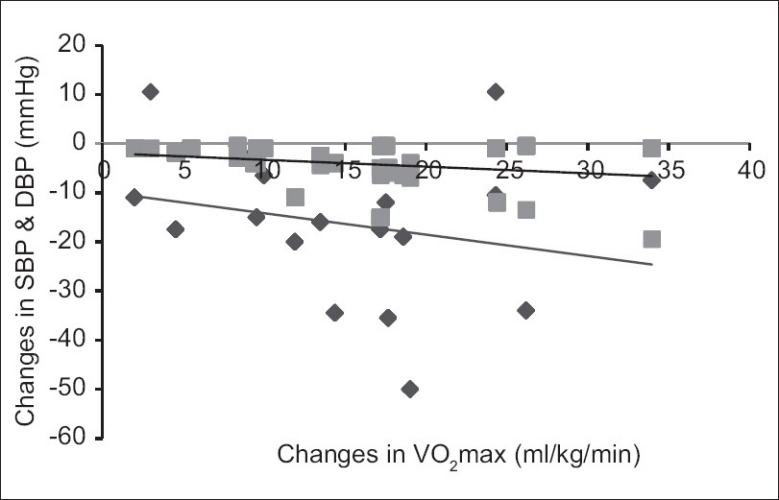
Correlation between training changes in VO2max and BP
Figure 3.
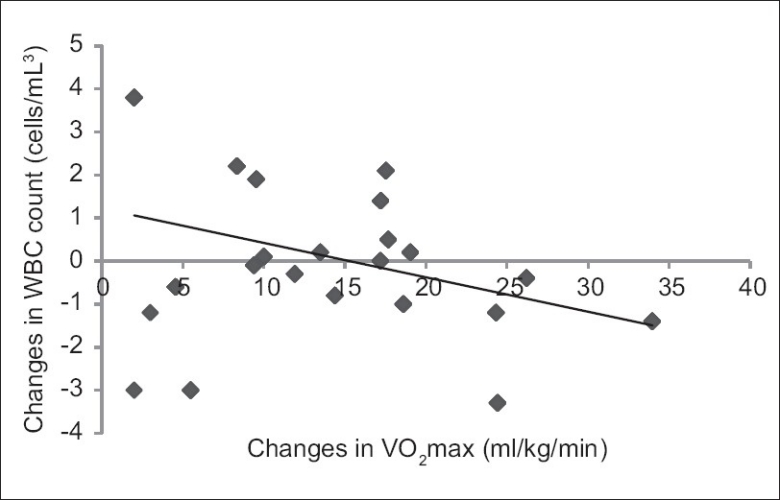
Correlation between training changes in VO2max and WBC count
Figure 4.
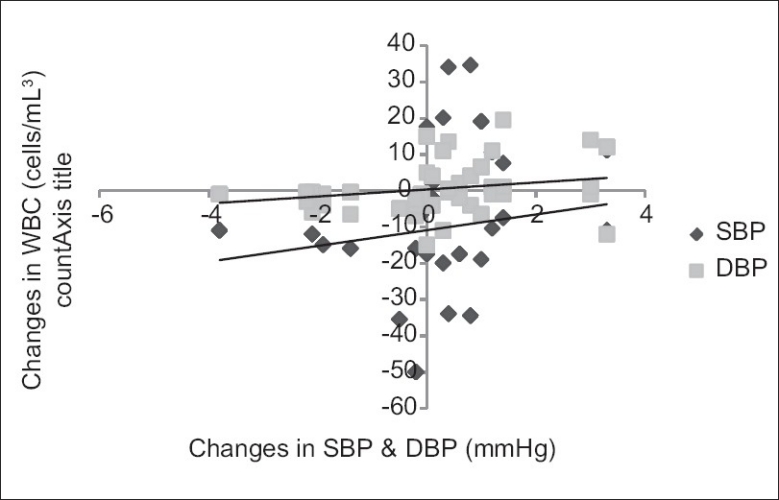
Correlation between training changes in WBC count and BP
DISCUSSION
Findings from the present study revealed a significant decrease in SBP, DBP, and increase in VO2 max in the experimental group over the control group. The favorable changes resulting from aerobic training on both SBP and DBP demonstrated in the present study is consistent with previous studies.44–51 Also, the result of the present study indicated a significant reduction in the WBC count in the experimental group over control. There was a negative significant correlation between changes in VO2 max and WBC count. This finding is in agreement with the report of Kullo et al.,52 they investigated the association between WBC count and VO2 max in men without CHD. In their study, 172 asymptomatic men (age, 51±9.3 years) engaged in a symptomless graded treadmill aerobic exercise. They reported an inverse association between WBC count and VO2 max (r=–0.22, P=0.004). Another study in support of the present study was conducted by Church et al.1 in their study, they investigated 4057 men, after age-adjusted resting levels and risk of having a clinically significant elevation of the WBC count across nine fitness body fatness combinations. They reported that fitness as measured by VO2 max was inversely related to the age-adjusted values of WBC count (P for trend P<0.0001).
A contradictory finding was reported by Shankar et al.24 they studied the relationship between WBC count and physical activity in the development of hypertension. They studied 2459 hypertension-free women and men participated after adjusting and stratifying by smoking and several other potential confounding factors. They reported a nonsignificant effect of moderate physical activity of twice per week on the WBC count (P=0.06).
Kim et al.53 investigated the relationship between WBC count and cardiorespiratory fitness (VO2 max) after adjusting for several well-known cardiovascular risk factors. Subjects who visited the health promotion center for a medical checkup and treadmill test (n=8241; age: median, 48 years; range, 16–79 years) were classified into three groups based on their WBC counts (group 1, 2200–5300 μl, n=2823; group 2, 5301–6500 μl, n=2709; group 3, 6501–10,000 μl, n=2709). After adjusting for age, body mass index, body fat percentage, smoking history, systolic blood pressure, diastolic blood pressure, serum lipid profile, and fasting plasma glucose, VO2 max still showed a significant association with WBC count (partial r=–0.11, P<0.001). They concluded that the WBC count in the normal concentration range is independently related to cardiorespiratory fitness in Korean men.
In cross-sectional analyses, Volpatoe et al.54 found IL-6 levels to be inversely related to exercise tolerance in disabled older women, while Taaffe et al.55 reported an inverse relationship between accumulated moderate and strenuous activity with IL-6 in 880 adults aged 70-79 years. Smith et al.44 found that a 6-month-exercise program reduced Tissue necrosis factor (TNF)- (n=43, average age=49.0 years). Tsukui et al.56 reported exercise training in 29 obese women (average age=56 years) reduced TNF with only modest weight loss.
It is generally at large accepted that the physiological mediator of low-grade chronic inflammation and raised WBC count is the TNF-alpha, which has been proven to be downregulated by regular physical activities. Another mechanism is that the postexercise hypotension which is accompanied by a decrease in serum cathecolamines, norepinephrine, dopamine, cortisol, sympathetic nervous system, plasma rennin activity,57–60 thus, suppressing inflammatory reaction and finally downregulating WBC count.
Reasons for diversities in findings between the present study and several others might not be unconnected to types of exercise, plasma IL-6 during exercise increase with the intensity and duration of exercise,61 interracial differences that might exist in exercise responses to WBC count.62,63 The effect of subjects’ condition cannot be ruled out, previous studies utilized normotensive subjects compared to hypertensive patients in the present study.
CONCLUSION
Based on the results of the present study, it was concluded that interval training program is an effective adjunct nonpharmacological management of hypertension. The therapeutic effect of interval training program on blood pressure and VO2 max (aerobic fitness) may be mediated through suppression of inflammatory (WBC count) reaction.
LIMITATIONS
The present study demonstrated a rationale bases for the role of interval exercise training in the down regulation of the blood pressure and WBC count. However, there are limitations of the study, including failure to investigate the effect of severity of hypertension and other markers of inflammation on the outcome variables. It is however unlikely that a single biomarker reflects all health risk.64 These limitations warrant attention in future studies.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Church TS, Finley CE, Earnest CP, Kampert JB, Gibbons LW, Blair SN. Relative associations of fitness and fatness to fibrinogen, white blood count, uric and metabolic syndrome. Int J Obes Relat Metab Disord. 2002;26:805–13. doi: 10.1038/sj.ijo.0802001. [DOI] [PubMed] [Google Scholar]
- 2.Bautista LE. Inflammation, endothelial dysfunction, and the risk of high blood pressure: Epidemiologic and biological evidence. J Hum Hypertens. 2003;17:223–30. doi: 10.1038/sj.jhh.1001537. [DOI] [PubMed] [Google Scholar]
- 3.Lee CD, Folsom AR, Nieto FJ, Chamless LE, Shahar E, Wolfe DA. White blood cell count and incidence of coronary heart disease and ischemic stroke and mortality from cardiovascular disease in African-American and White men and women: Atherosclerosis risk in communities study. Am J Epidemiol. 2001;154:758–64. doi: 10.1093/aje/154.8.758. [DOI] [PubMed] [Google Scholar]
- 4.Fang J, Alderman MH. Serum uric acid and cardiovascular mortality the NHANES I epidemiologic follow-up study, 1971-1992. National Health and Nutrition Examination Survey. JAMA. 2000;283:2404–10. doi: 10.1001/jama.283.18.2404. [DOI] [PubMed] [Google Scholar]
- 5.Kannel WB, Anderson K, Wilson PW. White blood cell count and cardiovascular disease. Insights from the Framingham Study. JAMA. 1992;267:1253–6. [PubMed] [Google Scholar]
- 6.Zalokar JB, Richard JL, Claude JR. Leukocyte count, smoking and myocardial intarction. N Engl J Med. 1981;304:465–8. doi: 10.1056/NEJM198102193040806. [DOI] [PubMed] [Google Scholar]
- 7.Pederson BK. The anti-inflammatory effect of exercise: Its role in diabetes and cardiovascular disease control. Essays Biochem. 2006;42:105–17. doi: 10.1042/bse0420105. [DOI] [PubMed] [Google Scholar]
- 8.Sinisalo J, Paronen J, Mattila KJ, Syrjälä M, Alfthan G, Palosuo T, et al. Relation of Inflammation to vascular friction in patients with coronary heart disease. Atherosclerosis. 2000;149:403–11. doi: 10.1016/s0021-9150(99)00333-0. [DOI] [PubMed] [Google Scholar]
- 9.Mugge A, Lopex JA. Do leucocytes have a role in hypertension? Hypertension. 1991;17:331–3. doi: 10.1161/01.hyp.17.3.331. [DOI] [PubMed] [Google Scholar]
- 10.Friedman GD, Selby JV, Qveseroberry CP., Jr The leukocyte count: A predictor of hypertension. J Clin Epidemiol. 1990;43:907–11. doi: 10.1016/0895-4356(90)90074-y. [DOI] [PubMed] [Google Scholar]
- 11.Panza JA, Quyyum , AA , Brush JE, Epstein SE. Abnormal endothelium-dependent vascular relaxation in patients with essential hypertwension. N Engl J Med. 1990;323:22–7. doi: 10.1056/NEJM199007053230105. [DOI] [PubMed] [Google Scholar]
- 12.Lipowski HH, Usami S, Chien S. In vivo measurements of “apparent viscosity” and microvessel hematocrit in the mesentery of the cat. Microvasc Res. 1980;19:297–319. doi: 10.1016/0026-2862(80)90050-3. [DOI] [PubMed] [Google Scholar]
- 13.Slowik A, Turaj W, Pankiewicz J, Dziedzic T, Szermer P, Szczudlik A. Hypercortisolemia in acute stroke is related to the inflammatory response. J Neurol Sci. 2002;196:27–32. doi: 10.1016/s0022-510x(02)00018-7. [DOI] [PubMed] [Google Scholar]
- 14.Suzuki H, Zweifach BW, Forrest MJ, Schmid-Schönbein GW. Modification of leukocyte adhesion in sportaneously hypertensive rats by ardent corticostriids. J Leukoc Biol. 1995;57:20–6. [PubMed] [Google Scholar]
- 15.Whiteworth JA, Saines D, Scoggins BA. Blood pressure and metabolic effects of cortisol and deoxycorticossride in man. Cli Exp Hypertens A. 1984;6:795–809. doi: 10.3109/10641968409044039. [DOI] [PubMed] [Google Scholar]
- 16.Gillum RF, Mussolino ME. White blood cell count and hypertension incidence.The NHANES 1 Epidemiologic follow-up study. J Clin Epidemiol. 1994;47:911–9. doi: 10.1016/0895-4356(94)90195-3. [DOI] [PubMed] [Google Scholar]
- 17.Nakanishi N, Sato M, Shirai K, Suzuki K, Tatara K. White blood cell count as a risk factor for hypertension: a study of Japanese male office workers. J Hypertens. 2002;20:851–7. doi: 10.1097/00004872-200205000-00018. [DOI] [PubMed] [Google Scholar]
- 18.Blair SN, Kohl HW, 3rd, Paffenbarger RS, Jr, Clark DG, Cooper KH, Gibbons LW. Physical fitness and all-cause mortality: A prospective study of healthy men and women. JAMA. 1989;262:2395–401. doi: 10.1001/jama.262.17.2395. [DOI] [PubMed] [Google Scholar]
- 19.Ekelund LG, Haskell WL, Johnson JL, Whaley FS, Criqui MH, Sheps DS. Physical fitness as a predictor of cardiovascular mortality in asymptomatic North American men: The Lipid Research Clinics Mortality Follow-up Study. N Engl J Med. 1988;319:1379–84. doi: 10.1056/NEJM198811243192104. [DOI] [PubMed] [Google Scholar]
- 20.Blair SN, Kampert JB, Kohl HW, 3rd, Barlow CE, Macera CA, Paffenbarger RS, Jr, et al. Influences of cardiorespiratory fitness and other precursors on cardiovascular disease and all-cause mortality in men and women. JAMA. 1996;276:205–10. [PubMed] [Google Scholar]
- 21.Blair SN, Kohl HW, 3rd, Barlow CE, Paffenbarger RS, Jr, Gibbons LW, Macera CA. Changes in physical fitness and all-cause mortality: A prospective study of healthy and unhealthy men. JAMA. 1995;273:1093–8. [PubMed] [Google Scholar]
- 22.Geffken DF, Cushman M, Burke GL, Polak JF, Sakkinen PA, Tracy RP. Association between physical activity and markers of inflammation in a healthy elderly population. Am J Epidemiol. 2001;153:242–50. doi: 10.1093/aje/153.3.242. [DOI] [PubMed] [Google Scholar]
- 23.Nieto FJ, Szklo M, Folsom AR, Rock R, Mercuri M. Leukocyte count correlates in middle-aged adults: The Atherosclerosis Risk in Communities (ARIC) Study. Am J Epidemiol. 1992;136:525–37. doi: 10.1093/oxfordjournals.aje.a116530. [DOI] [PubMed] [Google Scholar]
- 24.Shankar A, Klein BE, Klien R. Relationship between white blood cell count and incident hypertension. Am J Hypertens. 2001;17:233–9. doi: 10.1016/j.amjhyper.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 25.Stewart KJ, Bacher AC, Turner KL, Fleg JL, Hees PS, Shapero EP, et al. Effects of exercise on blood pressure in older person. Arch Intern Med. 2005;165:756–62. doi: 10.1001/archinte.165.7.756. [DOI] [PubMed] [Google Scholar]
- 26.Guide lines for exercise testing and Prescription. 4th ed. Philadelphia: Lea and Febiger; 1991. American College of Sport Medicine; pp. 120–5. [Google Scholar]
- 27.Townsend RR, Mcfadden TC, Ford V, Cadee JA. A randomized double blind, placebo-controlled trial of casein protein hydrolysnte (C12 peptide) in human essential hypertension. Am J Hypertension. 2004;17:1056–8. doi: 10.1016/j.amjhyper.2004.06.018. [DOI] [PubMed] [Google Scholar]
- 28.Waeber B, Nussberger J, Brunner HR. The rennin angiotension system: Role in experimental and human hypertension. In: Zanchetti A, Tarazi RC, editors. Pathophysiology of hypertension: regulatory mechanisms. Amsterdam: Elsevier; 1986. pp. 489–519. [Google Scholar]
- 29.Walker AJ, Bassett DR, Jr, Duey WJ, Howley ET, Bond V, Torok DJ, et al. Cardiovascular and plasma cathecolamae responses to exercise in blacks and whites. Hypertension. 1992;20:542–8. doi: 10.1161/01.hyp.20.4.542. [DOI] [PubMed] [Google Scholar]
- 30.Musa DI, Ibrahim DM, Toriola AL. Cardiorespiratory fitness and risk factors of CHD in pre-adolescent Nigerian girls. J Hum Mov Stud. 2002;42:455–5. [Google Scholar]
- 31.International standards for anthropometric assessment. Patche Fstroom, South Africa: ISAK; 2001. International Society for the Advancement of Kinanthropometry (ISAK) [Google Scholar]
- 32.Ross WD, Marfell-Jones MJ. Physiological testing of the high performance athletes. In: MacDugall JD, Wenger A, Green HJ, editors. Kinanthropometry Champaign IL: Human Kinetics Books; 1991. pp. 223–308. [Google Scholar]
- 33.Bachorik PS. Collection of blood sample for lipoprotein analysis. Clin Chem. 1982;28:1375–8. [PubMed] [Google Scholar]
- 34.Barbieri M, Ferrucci L, Corsi AM, Macchi C, Lauretani F, Bonafè M, et al. Is chronic inflammation a determinant of blood pressure in the elderly? Am J Hypertens. 2003;16:537–43. doi: 10.1016/s0895-7061(03)00861-6. [DOI] [PubMed] [Google Scholar]
- 35.ASCM's guidelines for exercise testing and prescription. 5th ed. Baltimore: Williams and Wilkins; 1995. American College of Sports Medicine; pp. 140–5. [Google Scholar]
- 36.Golding LA, Meyers CR, Sinniny WE. The complete carnote to fitness testing and instruction. 3rd Ed. Champaign IL: Human Kinetics Publishers; 1995. Way to physical fitness. [Google Scholar]
- 37.Katzung BG. 7th ed. New York: Lange Medical Books/Craw Hill; 1998. Basic and clinical pharmacology. [Google Scholar]
- 38.Mancia G, Ferari L, Gregorini L, Leonett L, Terzoli L, Biachini C, Zanchetti A. Effects of treatment with methyldopia on basal haemodynamic and on rural control. In: Robertson JS, Pickering GW, Goldwell ADS, editors. The therapeutics of hypertension. London: Royal Society of Medicine and Academic Press Inc. Ltd; 1980. pp. 70–8. [Google Scholar]
- 39.Adigun AQ, Ishola DA, Akintomide AO, Ajayi AA. Shifting trends in the pharmacologic treatment of hypertension in a Nigerian tertiary hospital: a real-world evaluation of the efficacy, safety, rationality and pharmaco-economics of old and newer antihypertensive drugs. J Hum Hypertens. 2003;17:277–85. doi: 10.1038/sj.jhh.1001538. [DOI] [PubMed] [Google Scholar]
- 40.Oyewo EA, Ajayi AA, Ladipo GO. A therapeutic audit in the management of hypertension in Nigerians. East Afr Med J. 1989;66:458–67. [PubMed] [Google Scholar]
- 41.Salako LA. Ibadan: Ciba Geigy Ltd; 1976. Treatment of hypertension: cardiovascular disease in Africa. [Google Scholar]
- 42.American College of Sport Medicine. Physical activity, physical fitness and hypertension. Medicine and Science in Sports and Exercise. 1993;25:1–10. [PubMed] [Google Scholar]
- 43.Dacie JV, Lewis SM. 5th Ed. London: Churchill Livingstone; 1975. Practical hematology. [Google Scholar]
- 44.Smith PJ, Blumenthal JA, Babyak MA, Georgiades A, Hinderlister A, Sherwood A. Effects of exercise and weight loss on depressive symptoms among men and women with hypertensive. J Psychosome Res. 2007;63:463–9. doi: 10.1016/j.jpsychores.2007.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Westhoff TH, Franke N, Schmidt S, Vallbracht-Israng K, Meissner R, Yildirim H, et al. Too old benefit from sports? The cardiovascular effects of exercise training in elding subjects treated for isolated systolic hypertension. Kidney Blood Press Res. 2007;30:240–7. doi: 10.1159/000104093. [DOI] [PubMed] [Google Scholar]
- 46.Laterza MC, de Matos LD, Trombetta IC, Braga AM, Roveda F, Alves MJ, et al. Exercise training restores baroreflex sensitivity in never trained hypertensive patients. Hypertension. 2007;49:1298–306. doi: 10.1161/HYPERTENSIONAHA.106.085548. [DOI] [PubMed] [Google Scholar]
- 47.Sohn AJ, Hasnain M, Sinacore JM. Impact of exercise (walking) on blood pressure levels in African American adults with newly diagonised hypertension. Ethn Dis. 2007;17:503–7. [PubMed] [Google Scholar]
- 48.Jones JM, Dowling TC, Park JJ, Phares DA, Park JY, Obisesan TO, et al. Differential aerobic exercise-induced changes in plasma aldosterol between Africa Americans and Caucasians. Exp Physiol. 2007;92:871–9. doi: 10.1113/expphysiol.2007.037408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dengel DR, Brown MD, Reynolds TH, Kuskowski MA, Supiano MA. Effect of aerobic training on blood pressure sensitivity to dietary sodium in older hypertensives. J Hum Hypertens. 2006;20:372–8. doi: 10.1038/sj.jhh.1001989. [DOI] [PubMed] [Google Scholar]
- 50.Ketelhut RG, Franz IW, Scholze J. Regular exercise as an effective approach in antihypertensive therapy. Med Sci Sports Exerc. 2004;36:4–8. doi: 10.1249/01.MSS.0000106173.81966.90. [DOI] [PubMed] [Google Scholar]
- 51.Ferrier KE, Waddell TK, Gatzka CD, Cameron JD, Dart AM, Kingwell BA. Aerobic exercise treining does not modify large artery compliance in isolated systolic hypertension. Hypertension. 2001;38:222–6. doi: 10.1161/01.hyp.38.2.222. [DOI] [PubMed] [Google Scholar]
- 52.Kullo IJ, Khaleghi M, Hensrud DD. Markers of inflammation are inversely associated with VO2 max in asymptomatic men. J Appl Physiol. 2007;102:1374–9. doi: 10.1152/japplphysiol.01028.2006. [DOI] [PubMed] [Google Scholar]
- 53.Kim DJ, Noh JH, Lee BW, Choi YH, Jung JH, Min YK, et al. A white blood cell count in the normal concentration range is independently related to cardiorespiratory fitness in apparently healthy Korean men. Metabolism. 2003;54:1448–52. doi: 10.1016/j.metabol.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 54.Volpato S, Guralnik JM, Ferrucci L, Balfour J, Chaves P, Fried LP, et al. Cardiovascular disease, interleukin-6, and risk of mortality in older women: the women's health and aging study. Circulation. 2001;103:947–53. doi: 10.1161/01.cir.103.7.947. [DOI] [PubMed] [Google Scholar]
- 55.Taaffe DR, Harris TB, Ferrucci L, Rowe J, Seeman TE. Cross-sectional and prospective relationships of interleukin-6 and C-reactive protein with physical performance in elderly persons: MacArthur studies of successful aging. J Gerontol A Biol Sci Med Sci. 2000;55:M709–15. doi: 10.1093/gerona/55.12.m709. [DOI] [PubMed] [Google Scholar]
- 56.Tsukui S, Kanda T, Nara M, Nishino M, Kondo T, Kobayashi I. Moderate-intensity regular exercise decreases serum tumor necrosis factor-alpha and HbA1c levels in healthy women. Int J Obes Relat Metab Disord. 2000;24:1207–11. doi: 10.1038/sj.ijo.0801373. [DOI] [PubMed] [Google Scholar]
- 57.Brooks GA, Fahey TD, White TP. 2nd ed. Mountain View: May Field Publishing Company; 1996. Exercise physiology, human bioenergetics and its application. [Google Scholar]
- 58.Hagberg JM. Physical activity, fitness, health and aging. In: Bouchard C, Shepard R, Stephens T, editors. Physical activity, fitness and health international proceedings and consensus statement. Champaign IL: Human Kinetics Publishers; 1994. [Google Scholar]
- 59.Duncan JJ, Farr JE, Upton SJ, Hagan RD, Oglesby ME, Blair SN. The effects of aerobic exercise on plasma cathecplamines and blood pressure in patients with essential hypertension. JAMA. 1985;254:2609–13. [PubMed] [Google Scholar]
- 60.Nelson L, Jannings GL, Esler MD, Korner PI. Effect of changing levels of physical exercise on blood pressure and haemodynamics in essential hypertension. Lancet. 1986;2:437. doi: 10.1016/s0140-6736(86)90354-5. [DOI] [PubMed] [Google Scholar]
- 61.Ostrowski K, Schjerling P, Pedersen BK. Physical activity and plasma interleukin-6 in human-effect of intensity of exercise. Eur J Appl Physiol. 2000;83:512–5. doi: 10.1007/s004210000312. [DOI] [PubMed] [Google Scholar]
- 62.Geffken DF, Cushman M, Burke GL, Polak JF, Sakkinen PA, Tracy RP. Association between physical activity and markers of inflammation in a healthy elderly population. Am J Epidemiol. 2001;153:242–50. doi: 10.1093/aje/153.3.242. [DOI] [PubMed] [Google Scholar]
- 63.Nieto FJ, Szklo M, Folsom AR, Rock R, Mercuri M. Leukocyte count correlates in middle-aged adults: The Atherosclerosis Risk in Communities (ARIC) Study. Am J Epidemiol. 1992;136:525–37. doi: 10.1093/oxfordjournals.aje.a116530. [DOI] [PubMed] [Google Scholar]
- 64.Beavers KM, Hsu FC, Isom S, Kritchevsky SB, Church T, Goodpaster B, et al. Long-term Physical Activity and Inflammatory Biomarkers in Older Adults. Med Sci Sports Exerc. 2010;42:2189–96. doi: 10.1249/MSS.0b013e3181e3ac80. [DOI] [PMC free article] [PubMed] [Google Scholar]


